Abstract
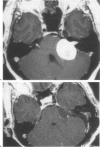
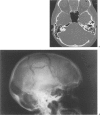
Surgical reconstruction of the skull base and cranium adjacent to open paranasal sinuses with alloplastic materials is problematic secondary to an increased risk of implant infection in these locations. The authors report their initial experience with the use of a porous polyethylene implant for closure of defects in these locations in 20 patients, in 14 of these with the implant placed in direct contact with the mastoid or paranasal simuses. The implant is flexible, which facilitates surgical reconstruction of the cranial base, and porous in nature, which enhances soft tissue and bone ingrowth in decrease the risk of infection. The implant is radiolucent on plain roentgenograms and CT, and produces no imaging artifact on MRI. The implant was utilized for a variety of skull base of cranium adjacent to sinus reconstructive applications with no infectious complications, with a follow-up period ranging from 8 to 50 months. This preliminary experience suggests that the alloplast may be a useful adjunct in skull base reconstruction, and further evaluation of its use in this application is warranted.
Full text
PDF
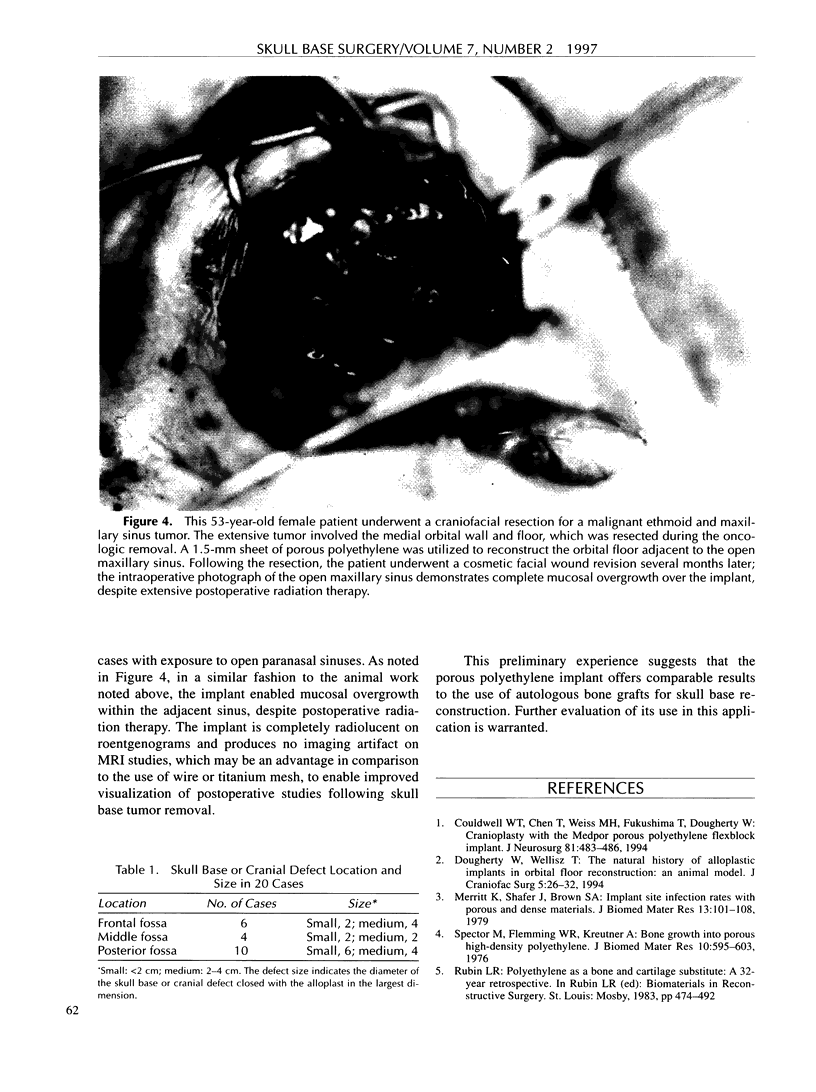

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couldwell W. T., Chen T. C., Weiss M. H., Fukushima T., Dougherty W. Cranioplasty with the Medpor porous polyethylene flexblock implant. Technical note. J Neurosurg. 1994 Sep;81(3):483–486. doi: 10.3171/jns.1994.81.3.0483. [DOI] [PubMed] [Google Scholar]
- Dougherty W. R., Wellisz T. The natural history of alloplastic implants in orbital floor reconstruction: an animal model. J Craniofac Surg. 1994 Feb;5(1):26–33. doi: 10.1097/00001665-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Homsy C. A. Bio-compatibility in selection of materials for implantation. J Biomed Mater Res. 1970 Sep;4(3):341–356. doi: 10.1002/jbm.820040306. [DOI] [PubMed] [Google Scholar]
- Merritt K., Shafer J. W., Brown S. A. Implant site infection rates with porous and dense materials. J Biomed Mater Res. 1979 Jan;13(1):101–108. doi: 10.1002/jbm.820130111. [DOI] [PubMed] [Google Scholar]
- Romano J. J., Iliff N. T., Manson P. N. Use of Medpor porous polyethylene implants in 140 patients with facial fractures. J Craniofac Surg. 1993 Jul;4(3):142–147. doi: 10.1097/00001665-199307000-00007. [DOI] [PubMed] [Google Scholar]
- Rubin P. A., Bilyk J. R., Shore J. W. Orbital reconstruction using porous polyethylene sheets. Ophthalmology. 1994 Oct;101(10):1697–1708. doi: 10.1016/s0161-6420(94)31113-4. [DOI] [PubMed] [Google Scholar]
- Spector M., Flemming W. R., Kreutner A. Bone growth into porous high-density polyethylene. J Biomed Mater Res. 1976 Jul;10(4):595–603. doi: 10.1002/jbm.820100416. [DOI] [PubMed] [Google Scholar]
- Wellisz T., Dougherty W., Gross J. Craniofacial applications for the Medpor porous polyethylene flexblock implant. J Craniofac Surg. 1992 Sep;3(2):101–107. doi: 10.1097/00001665-199209000-00009. [DOI] [PubMed] [Google Scholar]