Abstract
Stable cytoplasmic bridges (or ring canals) connecting the clone of spermatids are assumed to facilitate the sharing of haploid gene products and synchronous development of the cells. We have visualized these cytoplasmic bridges under phase-contrast optics and recorded the sharing of cytoplasmic material between the spermatids by a digital time-lapse imaging system ex vivo. A multitude of small (ca. 0.5 μm) granules were seen to move continuously over the bridges, but only 28% of those entering the bridge were actually transported into other cell. The average speed of the granules decreased significantly during the passage. Immunocytochemistry revealed that some of the shared granules contained haploid cell-specific gene product TRA54. We also demonstrate the novel function for the Golgi complex in acrosome system formation by showing that TRA54 is processed in Golgi complex and is transported into acrosome system of neighboring spermatid. In addition, we propose an intercellular transport function for the male germ cell-specific organelle chromatoid body. This mRNA containing organelle, ca. 1.8 μm in diameter, was demonstrated to go over the cytoplasmic bridge from one spermatid to another. Microtubule inhibitors prevented all organelle movements through the bridges and caused a disintegration of the chromatoid body. This is the first direct demonstration of an organelle traffic through cytoplasmic bridges in mammalian spermatogenesis. Golgi-derived haploid gene products are shared between spermatids, and an active involvement of the chromatoid body in intercellular material transport between round spermatids is proposed.
INTRODUCTION
A characteristic feature of spermatogenesis is that the dividing germ cells fail to complete cell division resulting in formation of stable cytoplasmic bridges that interconnect a large number of cells (Burgos and Fawcett, 1955; Fawcett et al., 1959). Obviously the function of cytoplasmic bridges is to facilitate the sharing of cytoplasmic constituents and to allow germ cell differentiation to be directed by the products of both parental chromosomes (Erickson, 1973). Despite all the spermatids (step 1–19 in rats) contain only half of the genome; each spermatid will finally develop into fully maturing spermatozoa. It is obvious that the spermatids need an efficient intercellular trafficking system where the gene products of haploid cells are shared between the neighbor cells. Braun et al. (1989) showed with a transgenic mouse strain that chimeric gene products expressed only by postmeiotic cells are evenly distributed between genotypically haploid spermatids. However, it has not been previously possible to study either the mechanisms of this material sharing or the functions of the cytoplasmic bridges during spermiogenesis. Which gene products are shared between neighbor male germ cells is not known. Recent findings that there exist many genes that are expressed only in haploid cells, such as TRA54 (Pereira et al., 1998), make the role of cytoplasmic bridges in sharing of gene products even more intriguing.
Another evolutionally conserved feature at haploid phase of germ cell differentiation is a large perinuclear cytoplasmic organelle, the cheromatoid body. In Drosophila oocytes, an analogous organelle is called sponge body (Wilsch-Bräuninger et al., 1997) or yolk nucleus in human fetal oocytes (Hertig and Adams, 1967). Recent investigations have suggested similar functions for these organelles in both sexes. Antibodies against conserved germline-specific, RNA-binding VASA proteins demonstrated immunostaining in both yolk nucleus (Castrillon et al., 2000) and the chromatoid body (Toyooka et al., 2000). However, the functions of these organelles are not clearly known.
Chromatoid body has been suggested to have a role in transport and storage of RNA in haploid cells. It has been demonstrated to contain radioactivity derived from tritiated uridine (Söderström and Parvinen, 1976a), actin (Walt and Armbruster, 1984), and RNA (Figueroa and Burzio, 1998). It moves rapidly in the cytoplasm of living early spermatids (Parvinen and Jokelainen, 1974) in both parallel and perpendicular manner related to the nuclear envelope, suggesting for a transport function of haploid gene products (Parvinen and Parvinen, 1979). Obviously these perinuclear organelles of both sexes have an important role during gametogenesis, because targeted disruption of VASA or its homologue genes results in sterility and serious defects in germ cell development in both sexes (Styhler et al., 1998; Tanaka et al., 2000).
Here we demonstrate a novel approach to study the transport of small granules between early round spermatids through cytoplasmic bridges ex vivo. We also describe the location of a haploid specific gene product (TRA54) inside the Golgi complex of early spermatids and its transportation in small granules through cytoplasmic bridge into neighbor spermatid. With an inhibition study we demonstrate that the expression of TRA54 is Golgi complex dependent. The subcellular localization of TRA54 in late spermatids was also studied. First we demonstrate a movement of the chromatoid body through the cytoplasmic bridge and propose an intercellular transport function for this organelle. Also new data about the close contacts between the chromatoid body and the nuclear envelope were obtained. Finally, we show that both the movement of cytoplasmic organelles and the integrity of chromatoid body are microtubule dependent.
MATERIALS AND METHODS
Animals and Tissue Preparation
Adult Sprague Dawley rats were used as donors of the testes. After decapsulation, the seminiferous tubules were dissected free from the interstitial tissue in a Petri dish containing phosphate-buffered saline solution, pH 7.4. The transillumination pattern (Parvinen and Vanha-Perttula, 1972) was identified under stereomicroscope, and tubules at stages I–IV of the cycle (Leblond and Clermont, 1952) were selected for further analysis. All animal experiments were approved by the Turku University Committee of Ethics and Animal Experimentation (permission no. 848/98).
Light Microscopic Evaluation
Studying the Living Male Germ Cells. After identification by transillumination technique, tubule segments of 0.5–1 mm in length from stages I-IV were cut under a stereomicroscope and transferred with a pipette on microscope slides in 15 μl of medium. A coverslip (20 × 20 mm) was lowered carefully onto the tubule segment, avoiding the formation of air bubbles. The excess fluid was removed by blotting, which allowed the cells of the seminiferous epithelium to float out from the tubule. This was monitored under 40× phase-contrast optics to adjust an optimal slightly flattened monolayer. The edges of the coverslip were then sealed with paraffin oil to immobilize the cells. The cells and the cytoplasmic bridges were examined under a 100× oil immersion phase-contrast optics. The accurate stages of the cycle of the seminiferous epithelium were identified as described earlier (Toppari et al., 1985, Kangasniemi et al., 1990). Altogether, 16 cytoplasmic bridges were analyzed for cytoplasmic material exchange.
Image Acquisition and Quantitative Image Analysis. A Kappa CF 8/1 FMC CCD black/white video camera (Kappa, Gleichen, Germany) was attached to a Leica DMRB phase-contrast microscope (Wetzlar, Germany) with a 15-cm extraadapter tube to allow a maximal geometric enlargement. Image sequences were directly digitized and stored into a hard disk for 300 s at a rate of 4–6 pictures per second using a FAST image grabbing system (FAST Multimedia AG, Munich, Germany). The frames from original AVI-files were first converted to bitmap (bmp) format. A custom-made image analysis program developed for Windows95 platform was used in granule and organelle movement analyses by recording the coordinates of the organelles in consecutive frames. The distances were determined in pixels and converted to metric scale (328 pixels = 10 μm). The distances, movement paths, and velocities of the organelles were plotted using Microsoft Excel spreadsheet program (version 97, Microsoft Corporation, Redmond, WA).
Immunofluorescence. After observation of living squash preparation, it was snap-frozen in liquid nitrogen and fixed with +4°C ethanol (97%). Then the coverslip was removed and the slides were stored in cold PBS. Fixed cells were permeabilized with 0.5% Triton X for 10 min followed by two washes with PBS and PBS/gelatin for 5 min. The cells were then incubated either with mAb TRA54 1:50 (Pereira et al. 1998), polyclonal anti-Mvh antibody (mouse VASA-homologue, Toyooka et al. 2000) 1:1000, or anti–heat schock factor (HSF)-2 antibody 1:150 (Alastalo et al. 1998) for 1–10 h. Control slides were incubated with normal nonimmunized appropriate animal serum. After two washes with PBS and PBS/gelatin, the slides were incubated for 1–10 h with fluorescein-conjugated anti-mouse, -rat, or -rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). From three to six parallel experiments were performed for each group.
Confocal laser scanning microscope Selected stage I-IV seminiferous tubule segments (ca. 2 mm in length) were fixed in + 4°C 4% paraformaldehyde for 4 h. After fixation, the seminiferous tubule segments were stored in PBS at + 4°C. Fixed seminiferous tubules were permeabilized with 0.5% Triton X for 10 min followed by two washes with PBS and PBS/gelatin for 5 min. The seminiferous tubules were then double-labeled with mAb TRA54 (1:50) and HSF-2 (1:150) as described above. Control slides were incubated with normal nonimmunized rat (TRA54) or rabbit (HSF-2) serum. Then fixed and labeled seminiferous tubules were whole-mounted on the microscope slide and observed under a Leica TCS-SP confocal laser scanning microscope equipped with argon-krypton laser (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany).
Electron Microscopy
Tubule segments (ca. 2 mm in length) from stages I–IV of the cycle were isolated by transillumination (Parvinen and Vanha-Perttula, 1972) and fixed in 5% glutaraldehyde in s-collidine-HCl buffer (0.16 M, pH 7.4) at 20°C, postfixed with 1% osmium tetroxide in 1.5% potassium ferrocyanide, and embedded in Epoxy resin (Glycidether 100, Merck, Darmstadt, Germany). They were sectioned at 70 nm with a Reichert E ultramicrotome (Reichert Jung, Vienna, Austria). Uranyl acetate and lead citrate were used for staining of the sections, before examination with a Jeol 100 SX (Jeol, Tokyo, Japan) electron microscope.
Immunoelectron Microscopy. Seminiferous tubules of stage I–IV were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.08 M sodium cacodylate buffer containing 0.05% calcium chloride, pH 7.3, for 4 h. After dehydration in ascending series of ethanol, samples were embedded in LR-White at 50°C for 72 h. Sections of ∼100 nm were cut with Reichert Ultracut E microtome, placed on nickel grids, and incubated in the presence of monoclonal rat anti-TRA54 antibodies (Pereira et al., 1998) or nonimmunized normal rat serum (control slides) in 0.01 M phosphate buffer, pH 7.4, 0.1% BSA (Sigma), for 60 min at 1:10. This was followed by goat anti-rat IgG-conjugated 15-nm colloidal gold (Electron Microscopy Sciences, Fort Washington, PA) labeling (1:30) and counterstaining with uranyl acetate and lead citrate. Finally, samples were examined with a Jeol 100 SX (Jeol) electron microscope at 60 kV.
Snap-frozen Method. After observation in living condition the squash preparations were frozen in liquid nitrogen and processed for electron microscopy as described earlier (Parvinen et al., 1997). Briefly, after dipping in liquid nitrogen, the coverslip was removed in frozen condition and immediately dipped in 5% glutaraldehyde in s-collidine-HCl buffer (0.16 M, pH 7.4) at 20°C. The slides were washed with collidine buffer, dehydrated in graded ethanol series, and treated with propylene oxide for 2× 5 min and finally infiltrated with propylene oxide and resin (1:1). The squash preparation was covered with an 8-mm Beem capsule containing epoxy resin (Glycidether 100; Merck) and polymerized at 60°C for 36 h. The slides with resin blocks were then heated on 70°C plate for 5 min. The capsule was removed from the slide by dipping into liquid nitrogen. The preparation was localized to make pyramids including appropriate areas. Sections were cut close to parallel to the surface, and the subsequent staining process was continued as described above.
Inhibition Studies with Nocodazole, Vincristine, Cytochalasin D, and Brefeldin A
The drugs were dissolved in ethanol. Staged tubule segments (I–IV) were transferred to Petri dishes containing either Dulbecco's MEM alone supplemented with 25 μl/ml ethanol and 1.0, 10, or 100 μg/ml concentrations of nocodazole, vincristine, cytochalasin D to disrupt microtubules and microfilaments or 1.0 and 5.0 μg/ml brefeldin A to destroy the Golgi complex. The tubules were incubated for 48 h at 34°C in an atmosphere containing 5% CO2 in air.
Statistical Methods
The velocities inside the cytoplasm and cytoplasmic bridge were compared by the paired Student's t test. Mann-Whitney U-test was adopted to compare the distances of the movement path of the analyzed granules and organelle. p < 0.05 was considered significant.
RESULTS
Round Spermatids Share Intracellular Material via Cytoplasmic Bridges
The cytoplasm of living early spermatids contained a multitude of granules with diameters of ∼0.5 μm. They moved along defined paths in a nonrandom manner in varying speeds and had frequent contacts with each other and with larger organelles, such as Golgi complex and the chromatoid body. We focused our interest in granules that were close to the intercellular bridges.
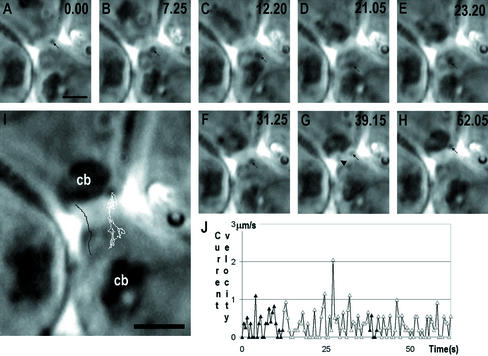
Altogether 20 cytoplasmic bridges were analyzed, 16 of which had exchange of cytoplasmic material during the observation time. The diameter of the bridges was 1.9–3.0 μm. Figure 1 shows a characteristic step 2 spermatid, where a small granule migrates through the bridge and moves back and forth through the cytoplasmic bridge during 62.05-s observation time. Figure 1I shows another granule, which moved through the cytoplasmic bridge and migrated from the vicinity of chromatoid body of upper spermatid. The average velocity of the granules transported through the cytoplasmic bridge was 0.44 μm/s inside the cytoplasm, and the average transit velocity inside the cytoplasmic bridge was 0.23 μm/s, which is ∼47.7% lower than that in the cytoplasm (p < 0.001).
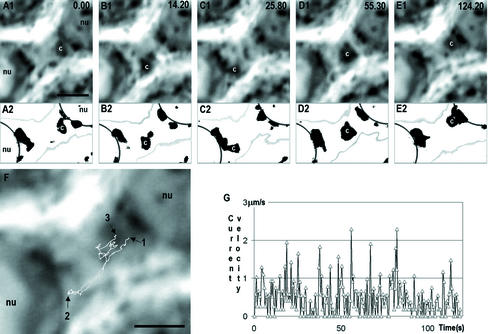
Figure 1.
A successive series of time-scaled (in seconds, upper right corner) of images (A–H) of step 2 spermatid ex vivo, showing a two-directional movement of granules through the cytoplasmic bridge. Representative positions of the granule are shown by arrows. The path of movement is shown by white line at I. Another granule (arrowhead in G), traced by black line in I shows how it dissociate from the vicinity of chromatoid body and moves uni-directionally over the bridge. The changing velocity of granule at various time points is summarized in J; the movement inside the bridge is indicated with solid triangles. cb, chromatoid body, Bars, 2 μm.
Spermatids Share a Haploid Cell-specific Gene Product TRA54
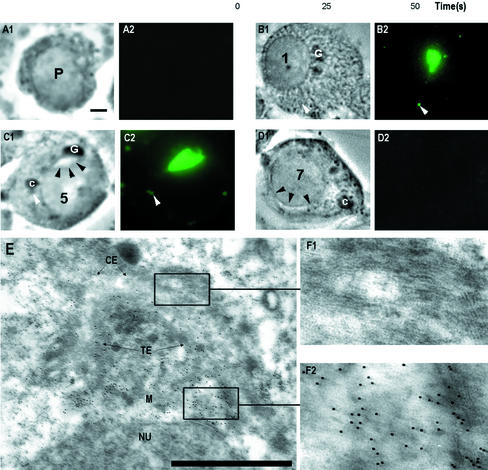
To study the content of transported granules, germ cell-specific markers and antibodies against cytoskeleton and haploid cell-specific gene products were used. Immunostaining of an antigen recognized by a haploid cell-specific mAb TRA54 was localized in the granules. Translation or addition of a sugar moiety of the antigenic epitope of TRA54 starts at early spermiogenesis (Figure 2). Golgi complexes of step 1–3 spermatids were highly TRA54 positive. Also small granules inside the cytoplasm of early spermatids were TRA54 positive. Accumulation of TRA54 into acrosome system is clearly seen in step 3–5 spermatids. Medulla and trans-element of the Golgi complex of early round spermatids were TRA54 positive in immunoelectron microscopic analysis (Figure 2E).
Figure 2.
TRA54 localization in early spermatids. Squash preparation from different stages were fixed and immunostained with TRA54 (A–D). Phase contrast (1), immunofluorescence of TRA54 (2). Pachytene spermatocytes (P) were negative (A). Translation of TRA54 started in the early spermatids (B). Numbers indicate the differentiation steps of spermatids. Golgi complex (G) was highly TRA54 positive. Small granules inside the cytoplasm were TRA54 positive (white arrowhead). At step 5 spermatids acorosome system (black arrowheads, C) is TRA54 positive. Also small granules in the vicinity of chromatoid body (c) were positive (white arrowhead). Golgi complex was TRA54 negative at step 5 spermatids. (D) A control slide from step 7 spermatid incubated with rat nonimmunized serum. Acrosome system is marked with black arrowheads. Bar, 2 μm. Immunoelectron microscopy (E) of early spermatids revealed strongest TRA54 labeling in trans-element (TE) of the Golgi complex. cis-element (CE) contains no TRA54 labeling (enlargement, F1). The strongest labeling is seen at the trans-saccular element (TE) and in the medulla (M), where proacrosomic granules are formed (enlargement, F2). Bar, 1 μm.
To study whether the transported granules seen ex vivo contain TRA54, the same cell was studied after fixation and double immunostaining with TRA54 and Mvh (Mouse VASA-homologue; Toyooka et al., 2000). The same granule that was seen to move through the cytoplasmic bridge between living cells was TRA54 positive after immunostaining (Figure 3).
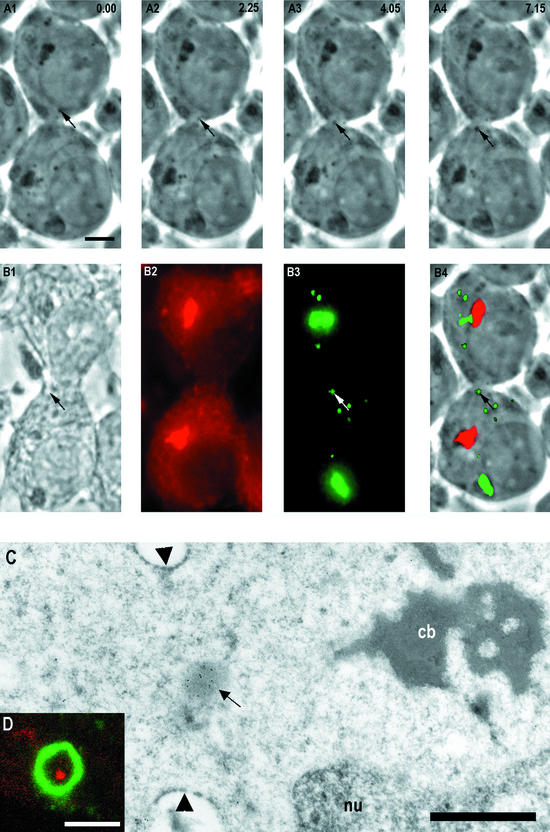
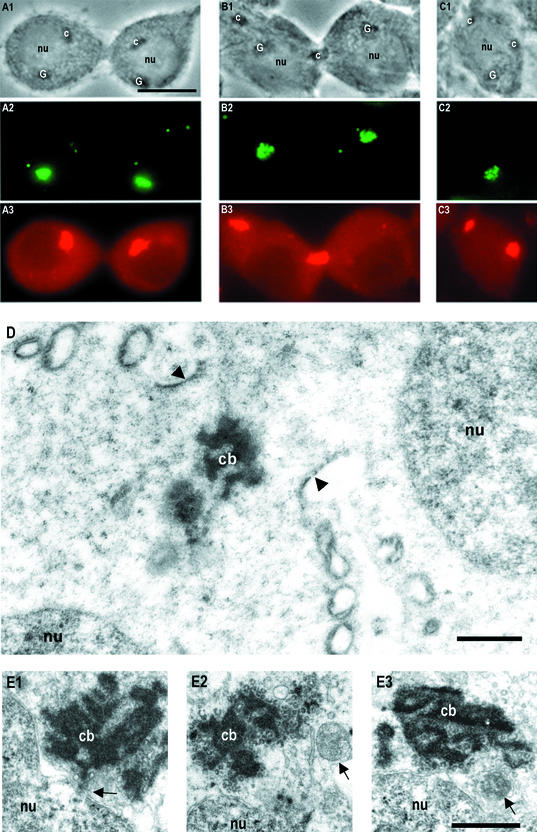
Figure 3.
A successive time-scaled series of phasecontrast images ex vivo showing granule (arrows) transport between neighbor spermatids (A1–4). The time points in seconds are indicated at top right corner. (B1) Phase-contrast image from the same cells as in A after quick fixation, immunoreaction of anti-Mvh (mouse VASA-homologue; B2), TRA54 (B3), and superimposed image (B4) where granules and Golgi complex were TRA54 positive (green) and chromatoid body anti-Mvh positive (red). The same granule passing through the cytoplasmic bridge ex vivo (A) was TRA54 positive (arrows in B3 and B4). Bar, 5 μm. (C) Immunoelectron microscopy revealed a TRA54-positive granule (arrow) inside the cytoplasmic bridge (arrowheads). The chromatoid body (cb) was seen close to the cytoplasmic bridge. nu, nucleus. Bar, 1 μm. (D) Close-up view of a cytoplasmic bridge from a whole-mounted step 1–4 spermatid under confocal microscope double-immunostained with heat shock factor 2 (HSF-2) and TRA54 antibodies. HSF-2 (green) labeled the cytoplasmic bridge. TRA54-positive granule (red) was seen inside the cytoplasmic bridge. Bar, 2 μm.
The exact localization of the TRA54-positive granule inside the cytoplasmic bridge was confirmed by immunoelectron microscopy (Figure 3C). TRA54-positive granules were also demonstrated inside the cytoplasmic bridge by confocal laser scanning microscopy of whole mounted seminiferous tubule segments after double-immunostaining with TRA54 and HSF-2 antibodies (Figure 3D). HSF-2 was previously shown to be present in the walls of the cytoplasmic bridges between spermatids (Alastalo et al., 1998).
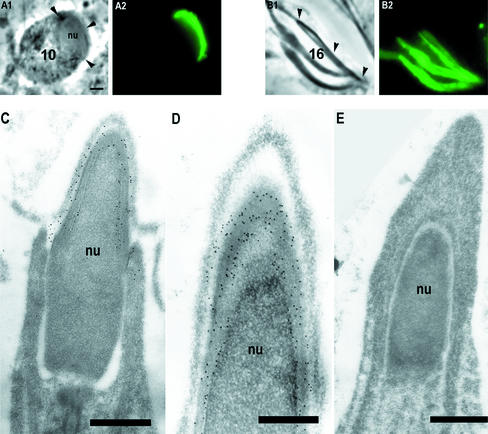
Localizations of TRA54 in late spermatids were studied after immunostaining (Figure 4). The number of TRA54-positive granules inside the cytoplasm decreased after step 5, and at step 10 no TRA54-positive cytoplasmic granules were found. In the elongated spermatids TRA54 was localized at the edge of the acrosome system but the Golgi complexes remained unstained. Immunoelectron microscopy showed the TRA54-positive structure in the middle part of the membranes surrounding the acrosomic system (Figure 4, C and D).
Figure 4.
TRA54 location during later spermatid development. In step 10 spermatids only acrosome system (arrowheads in A1) was TRA54 positive (A2). In step 16 spermatids acrosome systems (arrowheads in B1) were highly TRA54 positive (B2). Bar, 2 μm. Electron microscopy of step 15–17 spermatids (C–E) revealed TRA54 labeling in acrosome system (C) specifically in the middle part of the acrosomal membranes (D). Controls incubated with nonimmune normal rat serum were negative (E). Bar, 1 μm.
Chromatoid Body Moves through the Cytoplasmic Bridge between Neighbor Spermatids
The chromatoid body was demonstrated to move back and forth through the cytoplasmic bridge between neighbor spermatids with transient contacts with nuclei of both spermatids (Figure 5). The thresholded image series (Figure 5, A2–E2) shows the changing lobulated morphology of the chromatoid body and the close transient interactions between the chromatoid body and both nuclei. The speed of the movement of chromatoid body inside the bridge was rather constant, with a 0.20 μm/s average. Location of chromatoid body inside the cytoplasm and inside the cytoplasmic bridge was also studied after double-immunostaining with Mvh (Mouse VASA-homologue) and TRA54 antibodies (Figure 6). The locations of the chromatoid body next to the nucleus (Figure 6A), inside the cytoplasmic bridge (Figure 6B), and next to the nucleus of neighbor spermatid (Figure 6C) were demonstrated. The transient position of the chromatoid body inside the cytoplasmic bridge was confirmed by electron microscopy (Figure 6D).
Figure 5.
Time-scaled (top right corner in seconds) series of phase-contrast images showing two-directional movement of the chromatoid body (c) between two step 1 spermatids (A1–E1) through the cytoplasmic bridge. The same series is thresholded in A2–E2 to show in detail the chromatoid body and associated granules at the nuclear (nu) envelope. Moreover, in threshold image series the edges of the cells (gray) and nucleus (black) are presented. The movement path of the centroid of the chromatoid body is shown in F. Arrow 1 shows the starting point of the movement and arrow 2 the point where chromatoid body was close to the nucleus of the lower cell. Arrow 3 indicates the end point of chromatoid body movement during the 124.2-s recording period. The velocity of chromatoid body at various time points is shown in G. Bars, 2 μm.
Figure 6.
Different localizations of the chromatoid body in early spermatids in fixed squash preparations (A–C) seen under phase-contrast optics (1) and after double-immunostaining with TRA54 (2) and Mvh (3) antibodies. Chromatoid body (c) located next to the nucleus (nu; A1–A3), inside the cytoplasmic bridge (B1–B3) and C1-C3 shows two chromatoid bodies inside the same spermatid. Bar, 10 μm. Electronmicrograph of the chromatoid body (cb) in the cytoplasmic bridge (arrowheads) of step 1–4 spermatids (D). nu, nucleus. (E1–E3) Electron micrographs showing the interrelationships between chromatoid body and the nuclear envelope in snap-frozen preparations (see MATERIALS AND METHODS). A large contact area was seen between nucleus and chromatoid body, and a nuclear pore complex with material continuities to chromatoid body (arrow in E1). (E2) A membranous structure (arrow) closely associated with the chromatoid body. (E3) A chromatoid body close to the nucleus and a multivesicular body (arrow). Bars, 1 μm.
The snap-frozen electron microscopy shows a very close relationship between nuclear pore complex with a large contact area between chromatoid body and the nuclear envelope (Figure 6E1). This was observed several (>20) times. Tubule-like structures were found in the space between chromatoid body and nucleus, and they are apparently associated with the outer membrane of the nuclear envelope (Figure 6E2). Figure 6E3 shows how the chromatoid body is connected to nuclear envelope at its sharp outpocketing. A membrane-bound 0.5-μm structure that resembles multive-sicular body is seen in the space between chromatoid body and the nucleus.
Expression of Antigenic Epitope Recognized by TRA54 is Golgi Complex Dependent In Vitro
To study the role of the Golgi complex in TRA54 expression, a 48-h in vitro incubation study was performed. Tubule segments (0.5–1 mm) from stage I of the cycle were selected for incubation. In step 1 spermatids the acrosome system are not yet developed (Figure 7A), but TRA54 and Mvh immunoreactions showed well-developed Golgi complex and chromatoid body. In control culture conditions, step 1 spermatids differentiated to step 3 during 48 h (Figure 7B); acrosome system and TRA54-positive granules inside the cytoplasm were seen. However, if stage I seminiferous tubules were incubated for 48 h in the presence of 1.0 μg/ml brefeldin A, the Golgi complex was disrupted, and the development of the acrosome system was prevented (Figure 7C). The spermatids showed no labeling with TRA54, but the chromatoid body remained intact and showed normal Mvh immunoreaction.
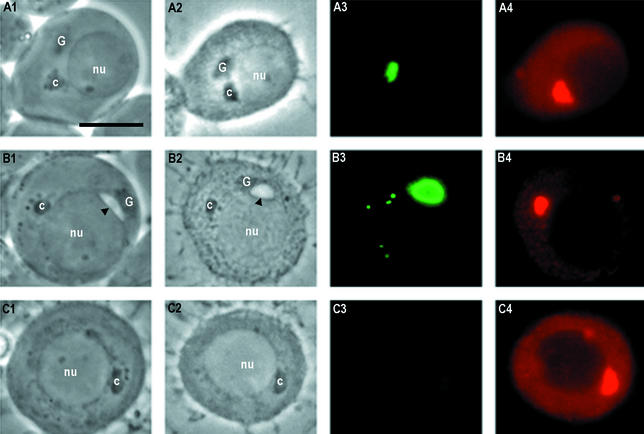
Figure 7.
Phase contrast (1), fixed (2), TRA54 (3), and VASA (4) immunostained images showing an early step 1 spermatids at a starting point of incubation (A) and after a 48-h incubation in control conditions (B) and with 1.0 μg/ml Golgi complex-disturbing agent brefeldin A (C). In early step 1 spermatids (A) only faint labeling of TRA54 was seen in the Golgi complex (A3). The chromatoid body was stained with Mvh antibody (A4). After 48 h of incubation in control conditions (B) acrosome system is developed (arrowheads in B1 and B2). After TRA54 immunostaining the acrosome system and some cytoplasmic granules were positive (B3). When step 1 spermatids were incubated for 48 h with brefeldin A, no Golgi complex was seen (C1 and C2). Neither the acrosome system nor TRA54-positive granules were seen in spermatids (C3). The chromatoid body was intact after treatment with brefeldin A (C4). Bar, 10 μm.
Microtubule Inhibitors Prevented TRA54 Transportation
To study the roles of microfilaments and microtubules in cytoplasmic organelle movements, seminiferous tubule segments of stage I of the cycle were incubated for 48 h with microfilament inhibitor, cytochalasin D, and the microtubule inhibitors, nocodazole and vincristine. Nocodazole and vincristine at concentrations of 10 μg/ml turned the nonrandom cytoplasmic granules movement into random Brownian motion, and the chromatoid body disintegrated to form several small spheres (Figure 8A1). Electron microscopic analysis revealed that these spheres contained ribosome-like granules covered by a thin layer of chromatoid material (Figure 8A2). Although concentration of 100 μg/ml cytochalasin D was toxic to the cells, the chromatoid body remained intact (unpublished data). The distance between the initial and the most distant point during the granule movement was measured to distinguish the Brownian motion from nonrandom granule movement. This value was significantly smaller (p < 0.01) after incubation of 10 μg/ml nocodazole when compared with controls. Cytochalasin D at concentrations of 10 μg/ml had also a significant inhibitory effect (Figure 8A3). After incubation of stage I tubule segments for 48 h with nocodazole (10 μg/ml), two types of spermatids developed (Figure 8B): one showed TRA54 immunostaining in Golgi complex, whereas the other lacked TRA54 labeling. This suggests that microtubule inhibitors block the transportation of TRA54 between the spermatids. Ca. 90% of the spermatids were TRA54 positive and 10% negative. The Mvh immunoreaction after nocodazole or vincristine incubation confirmed the disintegration of the chromatoid body (Figures 8, B4 and C4), respectively.
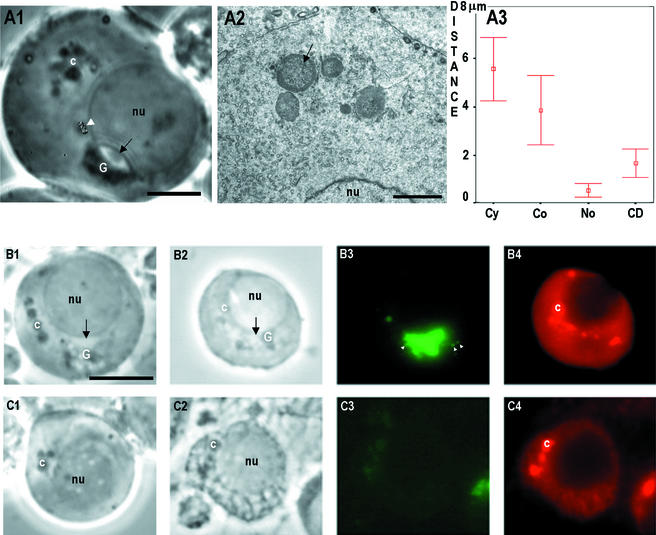
Figure 8.
Effect of microtubule inhibitors. Stage I seminiferous tubules segments were incubated for 48 h in the presence of 10 μg/ml nocodazole or vincristine. (A1) Step 3 spermatid after a 48-h incubation in 10 μg/ml vincristine. The chromatoid body (c) was disintegrated into several separate granules. Normal cytoplasmic granule movement was changed into random Brownian motion. Movement path of one granule is indicated at the white arrowhead. The morphology of the Golgi complex (G) remained intact under phase-contrast optics. Th acrosome system is indicated with black arrow. Bar, 5 μm. Electron microscopic appearance of the chromatoid body after 48-h incubation with 10 μg/ml vincristine. Several spheres of chromatoid material containing ribosome-like granules were seen (arrow). Bar, 2 μm. Phase contrast ex vivo (B1 and C1), phase contrast after fixation (B2 and C2), TRA54 immunofluorescence (B3 and C3), and Mvh immunostaining (B4 and C4) after incubation step 1 spermatids for 48 h in the presence of 10 μg/ml nocodazole. The chromatoid body was converted into several spheres (B1). Also the morphology of the Golgi complex (G) was altered. After TRA54 immunostaining two types of spermatids were found: spermatids that contained the TRA54-positive acrosome system (B3) and small TRA54-positive granules in the vicinity of the Golgi complex (white arrowheads) and spermatids where the acrosome system was absent (C3). Disintegrated morphology of the chromatoid body was seen after immunostaining with Mvh (B4 and C4). Bar, 10 μm.
DISCUSSION
The observation of Braun et al. (1989) about sharing of gene products between spermatids has been recently recognized to be of central importance. Very likely the products of recently found haploid cell-specific genes (Penttilä et al., 1995) are shared between haploid cells. Moreover, certain Y chromosomal genes are highly transcribed in spermatids (Hendriksen et al., 1995) and are demonstrated to have a role in controlling spermiogenesis (for review, see Delbridge and Graves, 1999). These data support the hypothesis that also the products of sex chromosomes are effectively transported between spermatids ensuring the synchronous development of the germ cells (Fawcett et al., 1959). Therefore it is important to understand how this gene product sharing is regulated and what is the role of certain macromolecules, such as Golgi complex and chromatoid body, in this process. This study is the first showing visually in mammals the sharing of cytoplasmic material between developing spermatids. We report here the sharing of haploid cell-specific gene product (TRA54; Pereira et al., 1998) and the transport of the mRNA-rich organelle, chromatoid body (Figueroa and Burzio, 1998), between the neighbor spermatids. Also the regulation of the transport and the evaluation on electron microscopic level is demonstrated in the present study.
Active Role of Cytoplasmic Bridges in Gene Product Sharing
Transient intercellular bridges are seen among a wide variety of cells before the completion of cytokinesis (Sanders and Field, 1994). However, these are distinct from stable intercellular bridges that remain persistent after incomplete cytokinesis (for review see Robinson and Cooley, 1996). Stable intercellular bridges exist both in female and male gametogenesis, in which the incomplete cytokinesis gives a possibility for sharing of relatively large cytoplasmic granules. The functions and components of the cytoplasmic bridges between mammalian gametes are not well understood. Cytoplasmic bridges show morphological differences in various phases during spermatogenesis (Weber and Russell, 1987). Diameter of the bridge increases during spermatogenesis, as spermatogonial bridges between spermatogonia are 1.0–1.3 μm and 3.0 μm between step 18 spermatids in rat. At step 1 spermatids the diameter of the bridge is ∼1.8 μm, which is efficient to allow the passage of the chromatoid body as demonstrated in the present study. According to passage of cytoplasmic material and morphological alterations during spermatogenesis in cytoplasmic bridges, it seems that these channels are active organelles between the cells. Similarly as in cytoplasmic bridge connecting the ovary and nurse cells in Drosophila (so-called ring canals-Bohrmann and Biber, 1994), only few (ca. 30%) of the granules circulating in the immediate vicinity of the bridge actually were seen to pass through. This suggests that selection and regulation of material takes place in the cytoplasmic bridges. This may be due the proteins found inside the cytoplasmic bridge. In mammals only few proteins have been demonstrated in the walls of the cytoplasmic bridges: cytoskeletal proteins actin (Russell et al., 1987), sak 57 (Tres et al., 1996), and HSF-2 (Alastalo et al., 1998). However, molecular structure and the development of intercellular bridges in Drosophila germ cells are better understood (Robinson and Cooley, 1996). Interestingly, mutations in the specific gene, cheerio (Robinson et al., 1997), prevent the expansion of cytoplasmic bridges during oogenesis, resulting obvious malfunction of cytoplasmic material sharing, and decrease of the size of oocyte and female sterility. On the basis of these observations and according to the function of the found proteins, it can be suggested that regulatory mechanisms of the transport and possibly packaging (chaperone) functions for transported materials occur in the cytoplasmic bridges. This might explain the decrease of the velocity of the granules, which we demonstrated to occur inside the cytoplasmic bridges.
An essential question is what gene products are packed in organelles passing through the cytoplasmic bridges. It is obvious that Golgi complex plays a main role in vesicle and granule trafficking (for review, see Lippincott-Schwartz et al., 2000; Moreno et al., 2000a). In round spermatids the role of the Golgi complex is crucial, because of its role in formation of acrosome system, which contains hydrolytic enzymes involved in fertilization and penetration through zona pellucida (Hermo et al. 1980; Moreno et al., 2000b). When the Golgi complex was disturbed by brefeldin A, the formation of the acrosome system was inhibited (Ventelä et al., 2000). This was confirmed here by inhibition of TRA54 immuno-staining. Also no TRA54-positive granules were visible inside the cytoplasm after disrupting the Golgi complex. This finding emphasizes the role of the Golgi complex in male germ cell differentiation and in the sharing of gene products between the spermatids. The lack of TRA54 immunostaining inside the cytoplasm after addition of brefeldin A supports the idea that the antigen recognized by the TRA54 antibody is a haploid cell-specific sugar moiety added in the Golgi complex. Under the immunoelectron microscope the TRA54 labeling is present only at the trans face of the Golgi complex. It is known that cisternae of the Golgi complex are highly organized as a series of processing compartments: the phosphorylation of oligosaccharides exist in cis-element and completion of glycosylation in trans-element. Also the sorting of proteins according to their final destination is performed at the trans-element of the Golgi complex. On the basis of these data it seems that TRA54 is formed in trans face of the Golgi complex of early spermatids either after glycosylation or alteration of configuration of recognized glycoprotein. These findings demonstrate that the Golgi complex has a novel role during spermiogenesis by sorting the granules needed not only for formation of one acrosome system but possibly in several spermatids. The average velocity of the granules moving in a nonrandom salutatory manner was 0.4 μm/s inside the cytoplasm and 0.2 μm/s inside the cytoplasmic bridge. Similar movement has been demonstrated in peroxisomes in vivo (Rapp et al., 1996). As in the present study and also in peroxisomes, the movements were changed into Brownian movement after microtubule-depolymerizing agent nocodazole. The effects of nocodazole and vincristine also demonstrated the importance of the microtubules during spermatogenesis, probably in part due to the fact that spermatids become insufficient in their capacity to share cytoplasmic material.
The Function of the Chromatoid Body
It has been proposed that male germ cell-specific organelle chromatoid body might have a role in storage and transport of haploid cell-specific gene products (Söderström and Parvinen, 1976a). However, the ultimate function of the chromatoid body is still obscure. The concept about storage of haploid gene products in chromatoid body, originally based on observation about radioactivity incorporation after incubation with tritiated uridine in this organelle (Söderström and Parvinen, 1976b) has obtained support from several studies. Figueroa and Burzio (1998) have showed that isolated chromatoid bodies contained a complex population of RNAs; mRNA, 5.8 and 5 S rRNA, but no tRNA. Previously it has also been demonstrated that chromosomal protein histone H4 (Werner and Werner, 1995), germ cell-specific DNA and RNA binding protein p48/52 (Oko et al., 1996), hnRNPs and ribosomal proteins (Biggiogera et al., 1990), mouse VASA-homologue (Tanaka et al., 2000; Toyooka et al., 2000), and actin (Walt and Armbruster, 1984) are localized in the chromatoid body.
Previous time-lapse cinemicrographic observations have shown two main components of the rapid movement of the chromatoid body in early spermatids, directed either parallel or perpendicular in relation to the nuclear envelope (Parvinen and Parvinen, 1979; Parvinen et al., 1997). The significance of these movements is not clear but it is possible that the parallel movements over the haploid nucleus are needed for collection of gene products from various parts of the haploid nucleus from different chromosome territories (Cremer et al. 1993). The significance of the perpendicular component of the movement of chromatoid body has been more difficult to understand. However, the demonstration that chromatoid body moves through the intercellular bridge suggests for the significance of this type of movement. Finally, our electron microscopic study demonstrated for the first time the location of chromatoid body inside the cytoplasmic bridge between two spermatids. Previously, a special RNA-rich structure called sponge body has been found from Drosophila oocytes (Wilsch-Bräuninger et al., 1997). It located inside the nurse cells, inside the oocytes and inside the cytoplasmic bridge between nurse cells and oocytes, suggesting that this subcellular structure is transported between these cells. Therefore the authors propose that sponge body is needed in assembly and transport of mRNA during Drosophila oogenesis. These observations suggest for a number of similarities between chromatoid body and sponge body.
The snap-freeze-fixed preparations revealed a multitude of details in the interrelationship between chromatoid body and early haploid nucleus that have not observed earlier. Parvinen et al. (1997) showed that in living condition, chromatoid body has pinocytosis-like transient engulfments toward nuclear pale chromatin areas. Snap-frozen preparations revealed a large contact area between the chromatoid body and nuclear envelope, and several intermediate organelles that obviously are sensitive for conventional fixation processes. In the present study we found that the chromatoid body has close contacts with several multivesicular body- or lysosome-like organelles that may be mediators of both nucleus-chromatoid body and spermatid-spermatid material transport. All these phenomena were observed in multiple (>20) specimens in several different cells. This study further demonstrated that the space between the chromatoid body and nuclear envelope is rich of vesicles and granules. The existence of these structures was anticipated by observations on early spermatids ex vivo.
It has been reported previously that the movement of chromatoid body is inhibited with vincristine in a dose-dependent manner (Mali et al., 1990). In both light and electron microscopic studies we were able to demonstrate that both microtubule inhibitors vincristine and nocodazole disintegrates the chromatoid body and induces a clear separation of chromatoid material and ribosome-like granules. Surprisingly, after α-tubulin immunocytochemistry, no microtubules were found inside the chromatoid body (unpublished data). However, after FITC-phalloidin staining chromatoid bodies were positive (unpublished data), supporting the previous data that actin is a component of chromatoid body (Walt and Armbruster, 1984). It might be that actin inside the chromatoid body is involved in mRNA binding to the cytoskeletal framework, which has been demonstrated to exist in many type of cells (for review, see Jansen, 1999; Ornelles et al., 1986). Actin is not involved in the normal movement of chromatoid body, because in our experiments cytochalasin D was ineffective. These results suggest that microtubules are involved in the normal integrity of the chromatoid body, in its movements, and in the normal Golgi complex–derived granule traffic in the cytoplasm of spermatids and between neighbor cells. Recently Morales et al. (1998) presented immunocytochemical evidence that testis-brain RNA-binding protein (TB-RBP) moves from the nucleus to the cytoplasm and through intercellular bridges in rat spermatids, supporting the hypothesis that small granules may be involved in transport of gene products at mRNA level. TB-RBP has also been demonstrated to suppress in vitro the translation of mRNA and to bind specific mRNAs to microtubules (Han et al., 1995), which further supports the importance of microtubules in inter- and intracellular material transport.
The present study demonstrates that novel digital techniques for quantification of organelle movements in living spermatogenic cells are useful for clarification of the possible function of certain organelles, such as the chromatoid body and Golgi complex in living cells. Particularly the demonstration that whole chromatoid body is able to pass the cytoplasmic bridge opens new insights about the function of this organelle.
Supplementary Material
Acknowledgments
We thank Urpo Reunanen at the laboratory of Electron Microscopy, University of Turku for generous help, Dr. Mika Mulari for advice in immunoelectron microscopy. Marko Räsänen, M.Sc., and Olli Nevalainen at the Department of Computer Science, University of Turku gave valuable advices and help in computer work. We are indebted to Drs. Yoshitake Nishimune and Hiromitsu Tanaka for TRA54, Dr. Toshiaki Noce for VASA, and Drs. Marko Kallio and Lea Sistonen for HSF-2 antibodies. Financial support was obtained from Turku University Foundation, The Sigrid Juselius Foundation, The Finnish Medical Society Duodecim, and Scandinavian Sasakawa Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–10–0647. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0647.
Online version of this article contains video material. Online version is available at www.molbiolcell.org.
References
- Alastalo, T.P., Lönnström, M., Leppä, S., Kaarniranta, K., Pelto-Huikko, M., Sistonen, L., and Parvinen, M. (1998). Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell Res. 240, 16–27. [DOI] [PubMed] [Google Scholar]
- Biggiogera, M., Fakan, S., Leser, G., Martin, T.E., and Gordon, J. (1990). Immunoelectron microscopical visualization of ribonucleoproteins in the chromatoid body of mouse spermatids. Mol. Reprod. Dev. 26, 150–158. [DOI] [PubMed] [Google Scholar]
- Bohrmann, J., and Biber, K. (1994). Cytoskeleton-dependent transport of cytoplasmic particles in pre-vitellogenic to mid-vitellogenic ovarian follicles of Drosophila: time-lapse analysis using video-enhanced contrast microscopy. J. Cell Sci. 107, 849–858. [DOI] [PubMed] [Google Scholar]
- Braun, R.E., Behringer, R.R., Peschon, J.J., Brinster, R.L., and Palmiter, R.D. (1989). Genetically haploid spermatids are phenotypically diploid. Nature 337, 373–376. [DOI] [PubMed] [Google Scholar]
- Burgos, M.H., and Fawcett, D.W. (1955). Studies on the fine structure of the mammalian testis. J. Biophys. Biochem. Cytol. 1, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon, D.H., Quade, B.J., Wang, T.Y., Quigley, C., and Crum, C.P. (2000). The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 97, 9585–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer, T. et al. (1993). Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold. Spring Harb. Symp. Quant. Biol. 58, 777–792. [DOI] [PubMed] [Google Scholar]
- Delbridge, M.L., and Graves, L.A. (1999). Mammalian Y chromosome evolution and the male-specific functions of Y chromosome-borne genes. Rev. Reprod. 4, 101–109. [DOI] [PubMed] [Google Scholar]
- Fawcett, D.W., Ito, S., and Slautterback, D.L. (1959). The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J. Biophys. Biochem. Cytol. 5, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, J., and Burzio, L.O. (1998). Polysome-like structures in the chromatoid body of rat spermatids. Cell Tissue Res. 291, 575–579. [DOI] [PubMed] [Google Scholar]
- Erickson, R.P. (1973). Haploid gene expression versus meiotic drive: the relevance of intercellular bridges during spermatogenesis. Nat. New Biol. 243, 210–212. [DOI] [PubMed] [Google Scholar]
- Han, J.R., Yiu, G.K., and Hecht, N.B. (1995). Testis/brain RNA-binding protein attaches translationally repressed and transported mRNAs to microtubules. Proc. Natl. Acad. Sci. USA 92, 9550–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen, P.J., Hoogerbrugge, J.W., Themmen, A.P., Koken, M.H., Hoeijmakers, J.H., Oostra, B.A., van der Lende, T., and Grootegoed, J.A. (1995). Postmeiotic transcription of X and Y chromosomal genes during spermatogenesis in the mouse. Dev. Biol. 170, 730–733. [DOI] [PubMed] [Google Scholar]
- Hermo, L., Rambourg, A., and Clermont, Y. (1980). Three-dimensional architecture of the cortical region of the Golgi apparatus in rat spermatids. Am. J. Anat. 157, 357–373. [DOI] [PubMed] [Google Scholar]
- Hertig, A.T., and Adams, E.C. (1967). Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage. J. Cell Biol. 34, 647–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.P. (1999). RNA-cytoskeletal associations. FASEB J. 13, 455–466. [PubMed] [Google Scholar]
- Kangasniemi, M., Kaipia, A., Mali, P., Toppari, J., Huhtaniemi, I., and Parvinen, M. (1990). Modulation of basal and FSH-stimulated cyclic AMP production in rat seminiferous tubules staged by an improved transillumination technique. Anat. Rec. 227, 62–76. [DOI] [PubMed] [Google Scholar]
- Leblond, C.P., and Clermont, Y. (1952). Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. NY Acad. Sci. 55, 548–573. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Roberts, T.H., and Hirschberg, K. (2000). Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 16, 557–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, P., Toppari, J., Parvinen, L.M., and Parvinen, M. (1990). The chromatoid body in spermatogenesis: nucleo-cytoplasmic transport of haploid gene products and its cytoskeletal regulation. In: Nuclear Structure and Function. J.R. Harris and IB. Zbarsky, editors. New York: Plenum Press, 473–476.
- Morales, C., Wu, X., and Hecht, N. (1998). The DNA/RNA-binding protein, TB-RBP, moves from the nucleus to the cytoplasm and through intercellular bridges in male germ cells. Dev. Biol. 201, 113–123. [DOI] [PubMed] [Google Scholar]
- Moreno, R.D., Ramalho-Santos, J., Sutovsky, P., Chan, E.K., and Schatten, G. (2000a). Vesicular traffic and golgi apparatus dynamics during mammalian spermatogenesis: implications for acrosome architecture. Biol. Reprod. 63, 89–98. [DOI] [PubMed] [Google Scholar]
- Moreno, R.D., Ramalho-Santos, J., Chan, E.K., Wessel, G.M., and Schatten, G. (2000b). The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during rhesus spermiogenesis: structural alterations. Dev. Biol. Mar. 219, 334–349. [DOI] [PubMed] [Google Scholar]
- Oko, R., Korley, R., Murray, M.T., Hecht, N.B., and Hermo, L. (1996). Germ cell-specific DNA and RNA binding proteins p48/52 are expressed at specific stages of male germ cell development and are present in the chromatoid body. Mol. Reprod. Dev. 44, 1–13. [DOI] [PubMed] [Google Scholar]
- Ornelles, D.A., Fey, E.G., and Penman, S. (1986). Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol. Cell. Biol. 6, 1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen, M., and Jokelainen, P.T. (1974). Rapid movements of the chromatoid body in living early spermatids of the rat. Biol. Reprod. 11, 85–92. [DOI] [PubMed] [Google Scholar]
- Parvinen, M., and Parvinen, L.-M. (1979). Active movements of the chromatoid body: a possible transport mechanism for haploid gene products. J. Cell Biol. 80, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen, M., Salo, J., Toivonen, M., Nevalainen, O., Soini, E., and Pelliniemi, L.J. (1997). Computer analysis of living cells: movements of the chromatoid body in early spermatids compared with ultra-structure in snap-frozen preparations. Histochem. Cell Biol. 108, 77–81. [DOI] [PubMed] [Google Scholar]
- Parvinen, M., and Vanha-Perttula, T. (1972). Identification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat. Rec. 174, 435–450. [DOI] [PubMed] [Google Scholar]
- Pereira, L.A., Tanaka, H., Nagata, Y., Sawada, K., Mori, H., Chimelli, L.M., and Nishimune, Y. (1998). Characterization and expression of a stage specific antigen by monoclonal antibody TRA 54 in testicular germ cells. Int. J. Androl. 21, 34–40. [DOI] [PubMed] [Google Scholar]
- Penttilä, T.L., Yuan, L., Mali, P., Hoog, C., and Parvinen, M. (1995). Haploid gene expression: temporal onset and storage patterns of 13 novel transcripts during rat and mouse spermiogenesis. Biol. Reprod. 53, 499–510. [DOI] [PubMed] [Google Scholar]
- Rapp, S., Saffrich, R., Anton, M., Jakle, U., Ansorge, W., Gorgas, K., and Just, W.W. (1996). Microtubule-based peroxisome movement. J. Cell Sci. 109, 837–849. [DOI] [PubMed] [Google Scholar]
- Robinson, D.N., and Cooley, L. (1996). Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 6, 474–479. [DOI] [PubMed] [Google Scholar]
- Robinson, D.N., Smith-Leiker, T.A., Sokol, N.S., Hudson, A.M., and Cooley, L. (1997). Formation of the Drosophila ovarian ring canal inner rim depends on cheerio. Genetics 145, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, L.D., Vogl, A.W., and Weber, J.E. (1987). Actin localization in male germ cell intercellular bridges in the rat and ground squirrel and disruption of bridges by cytochalasin D. Am. J. Anat. 180, 25–40. [DOI] [PubMed] [Google Scholar]
- Sanders, S.L., and Field, C.M. (1994). Cell division. Septins in common? (1994). Curr. Biol. 4, 907–910. [DOI] [PubMed] [Google Scholar]
- Styhler, S., Nakamura, A., Swan, A., Suter, B., and Lasko, P. (1998). Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125, 1569–1578. [DOI] [PubMed] [Google Scholar]
- Söderström, K.-O., and Parvinen, M. (1976a). Incorporation of (3H)uridine by the chromatoid body during rat spermatogenesis. J. Cell Biol. 70, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström, K.-O., and Parvinen, M. (1976b). Transport of material between the nucleus, the chromatoid body and the Golgi complex in the early spermatids of the rat. Cell Tissue Res. 168, 335–342. [DOI] [PubMed] [Google Scholar]
- Tanaka, S.S., Toyooka, Y., Akasu, R., Katoh-Fukui, Y., Nakahara, Y., Suzuki, R., Yokoyama, M., and Noce, T. (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841–853. [PMC free article] [PubMed] [Google Scholar]
- Toyooka, Y., Tsunekawa, N., Takahashi, Y., Matsui, Y., Satoh, M., and Noce, T. (2000). Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149. [DOI] [PubMed] [Google Scholar]
- Toppari, J., Eerola, E., and Parvinen, M. (1985). Flow cytometric DNA analysis of defined stages of rat seminiferous epithelial cycle during in vitro differentiation. J. Androl. 6, 325–333. [DOI] [PubMed] [Google Scholar]
- Tres, L.L., Rivkin, E., and Kierszenbaum, A.L. (1996). Sak 57, an intermediate filament keratin present in intercellular bridges of rat primary spermatocytes. Reprod. Dev. 45, 93–105. [DOI] [PubMed] [Google Scholar]
- Ventelä, S., Mulari, M., Okabe, M., Tanaka, H., Nishimune, Y., Toppari, J., and Parvinen, M. (2000). Regulation of acrosome formation in mice expressing green fluorescent protein as a marker. Tissue Cell. 32, 501–507. [DOI] [PubMed] [Google Scholar]
- Walt, H., and Armbruster, B.L. (1984). Actin and RNA are components of the chromatoid bodies in spermatids of the rat. Cell Tissue Res. 236, 487–490. [DOI] [PubMed] [Google Scholar]
- Weber, J., and Russell, L. (1987). A study of intercellular bridges during spermatogenesis in the rat. Am. J. Anat. 180, 1–24. [DOI] [PubMed] [Google Scholar]
- Werner, G., and Werner, K. (1995). Immunocytochemical localization of histone H4 in the chromatoid body of rat spermatids. J. Submicrosc. Cytol. Pathol. 27, 325–330. [PubMed] [Google Scholar]
- Wilsch-Bräuninger, M., Schwarz, H., and Nusslein-Volhard, C. (1997). A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis. J. Cell Biol. 139, 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.