Abstract
In order to create an extended map of chromatin features within a mammalian multigene locus, we have determined the extent of nuclease sensitivity and the pattern of histone modifications associated with the mouse β-globin genes in adult erythroid tissue. We show that the nuclease-sensitive domain encompasses the β-globin genes along with several flanking olfactory receptor genes that are inactive in erythroid cells. We describe enhancer-blocking or boundary elements on either side of the locus that are bound in vivo by the transcription factor CTCF, but we found that they do not coincide with transitions in nuclease sensitivity flanking the locus or with patterns of histone modifications within it. In addition, histone hyperacetylation and dimethylation of histone H3 K4 are not uniform features of the nuclease-sensitive mouse β-globin domain but rather define distinct subdomains within it. Our results reveal a complex chromatin landscape for the active β-globin locus and illustrate the complexity of broad structural changes that accompany gene activation.
In eukaryotic nuclei, regions of the genome containing active genes are more sensitive to digestion with DNase I than other regions (59). For a number of gene loci that have been examined, this relative sensitivity extends far outside transcribed sequences (1, 29, 31, 32, 52) and can still be observed in the absence of active transcription (46, 51). Insofar as sensitivity to nuclease digestion implies increased accessibility of the DNA to DNase I, it has long been assumed that nuclease-sensitive gene loci are packaged in a less condensed and more open chromatin structure that represents a necessary preliminary step in the process of active transcription. Thus, it has been postulated that active or potentially active genes reside within broad domains that are more open than other regions harboring only inactive genes.
Core histone amino-terminal tails are known to be required for higher-order chromatin folding (10, 24, 28) and are also subject to a wide array of covalent modifications that have the potential to affect chromatin condensation. In particular, it has been shown that high levels of acetylation of histones H3 and H4 can interfere with salt-induced compaction of nucleosomal arrays (55). Recently, the term domain has been extended to refer to large regions encompassing at least some active genes that exhibit high levels of histone modification (9, 18). These considerations have led to the hypothesis that covalent modifications of core histones might be the cause of nuclease sensitivity. There is reason to doubt this, however, since it has been shown that not all actively transcribed genes exhibit domains of histone hyperacetylation (41). Thus, it remains to be determined whether nuclease sensitivity is a result of covalent modifications of histone tails or of other alterations to chromatin structure.
A related question regarding domains concerns how they are organized. Every domain, whether defined by sensitivity to DNase I digestion or by core histone modification, must have a boundary with or transition to insensitive or unmodified or differentially modified chromatin. At least some gene loci appear to contain specific regulatory sequences that are involved in this organization and function to define the transition points (11, 43, 45, 53).
The mammalian β-globin loci have been intensely investigated for several decades as a model system of gene activation. In both mouse and human, the β-globin genes occur in a single cluster, and both naturally occurring deletions (17) and studies with transgenic animals (23) have implicated a large (20- to 30-kb) region 5′ of the genes, termed the locus control region (LCR), as a crucial component of their activation. Consisting structurally of several nuclease hypersensitive sites (HSs), the LCR was thought not only to be responsible for stimulating high-level transcription of the β-globin genes but also to be required for the formation of the active β-globin gene domain as defined by sensitivity to DNase I digestion.
It was therefore surprising that deletion of the LCR from the endogenous mouse β-globin locus, while significantly reducing β-globin expression levels, did not affect the establishment of nuclease sensitivity (5). The results of the LCR deletion led immediately to the question of what other elements at the β-globin locus might represent candidates for sequences required to form the nuclease-sensitive domain in the absence of the LCR. Given the reasonable assumption that elements involved in domain formation will be within the domain or at its boundaries, this question then demands that we know the extent of the active domain and that we ascertain, as a corollary, whether boundary elements are involved in its organization.
We have determined the extent of the nuclease-sensitive β-globin gene domain in adult mouse erythroid tissue, and we have identified several candidate regulatory elements, marked by DNase I HSs, within this domain. We found that although sequences with canonical boundary activity can be identified within the β-globin locus, they do not coincide with the limits of the nuclease-sensitive domain. Similarly, we have mapped histone modifications—specifically, hyperacetylation of histones H3 and H4 and dimethylation of lysine 4 of histone H3—within the β-globin locus and have found that rather than corresponding to the nuclease-sensitive domain, these modifications define distinct subdomains within it. These results indicate that domain organization is more complex than previously thought and that phenomena associated with domain formation and organization are not necessarily related.
MATERIALS AND METHODS
Nuclease digestion of mouse spleen cell nuclei.
DNase I HS mapping and generalized sensitivity assays were performed using the same nuclease digestion series. Cells were isolated from mouse spleens 6 days after the onset of a phenylhydrazine-induced hemolytic anemia, when ∼80% of cells are erythroid (47). Brains, thymuses, and livers were dissected from the same mice. Nuclei were isolated as previously described (50). Treatment of nuclei with DNase I, restriction enzyme digestion of genomic DNA, and Southern blotting were performed as previously described (17). Evaluation of relative sensitivities of different fragments was carried out both by visual inspection of films and by quantitation of phosphorimager scans.
Restriction enzymes and probes for Southern blot analysis.
All coordinates given below for restriction fragments and probes within the β-globin locus reflect relative positions, with the cap site of the Ey-globin gene (the first A in the sequence 5′-ACGTACTTGCTTCTGACACT…) assigned the coordinate bp 1. Sequences within and flanking the mouse β-globin locus used to design the nuclease sensitivity and PCR assays have been published previously (7, 8) or were taken from the mouse genome project database (http://www.ncbi.nlm.nih.gov/genome/guide/mouse/). Coordinates given below for probes within the mouse T-cell receptor β (TCRβ)-trypsinogen locus are from GenBank entry AE000663.
For analysis of DNase I HSs within or flanking the β-globin locus, enzyme-probe combinations were as follows: PstI with an SpeI/PstI fragment from bp −81335 to −80723 for an HS at kb −85.5; EcoRI with an HindIII/EcoRI fragment from bp −60553 to −59598 for HSs at kb −62.5 and −60.6; BamHI with an NheI/HpaI fragment from bp −3469 to −2867 for an HS at kb −1.8; EcoRI with an HpaI/EcoRI fragment from bp 34166 to 34902 for a region 3′ of the βmaj gene; EcoRV with an EcoRV/EcoRI fragment from bp 39496 to 39984 for a region 5′ of the βmin gene; BamHI with an SspI/MspI fragment from bp 50568 to 51294 for a region 3′ of the βmin gene; and EcoRV with an AccI/NsiI fragment from bp 69906 to 70470 (AN) for an HS located 3′ of the β-globin locus (3′HS1).
For analysis of generalized nuclease sensitivity within and flanking the β-globin locus, enzyme-probe combinations were as follows: AccI with a PCR fragment from bp −90473 to −89939 (M5B6), a BglII/XhoI fragment from bp −74603 to −73908 (BX), a BamHI/NsiI fragment from bp −63922 to −63235, a PstI/EcoRI fragment from bp −45946 to −45427, an EcoRV/EcoRI fragment from bp 39496 to 39984, and AN; BamHI with a SacI/ApaI fragment from bp 13755 to 14440 (3′βh1) and an XbaI/SacI fragment from bp 82712 to 83249; BglII with BX, an EcoRI/BglII fragment from bp −66300 to −65706, and an AccI fragment from bp 2411 to 2898 (3′Ey); EcoRI with 3′Ey, AN, a SacI/EcoRV fragment from bp 74086 to 74811, a PCR fragment from bp 77487 to 78058, a PCR fragment from bp 81610 to 82323, and a PstI/HincII fragment from bp 118250 to 118930; EcoRV with BX, an HpaI/EcoRI fragment from bp 34166 to 34902, a PstI/Sau3AI fragment from bp 48175 to 49029, and an XbaI/SacI fragment from bp 82712 to 83249; HincII with an HindIII fragment from bp −79780 to −79064, a PstI/EcoRI fragment from bp −45946 to −45427, a PCR fragment from bp −29652 to −28998 (P1), and AN; PstI with a PCR fragment from bp −71792 to −71267, P1, 3′βh1, and a PstI/HincII fragment from bp 118250 to 118930; SacI with an XbaI fragment from bp −59343 to −58293, a SacI/EcoRV fragment from bp 74086 to 74811, and an HpaI/ScaI fragment from bp 106093 to 106752; and MscI with M5B6. Within the mouse TCRβ-trypsinogen locus, enzyme-probe combinations were as follows: EcoRI with a PCR fragment from bp 62090 to 62762, BglII with a PCR fragment from bp 170004 to 170526, PstI with a PCR fragment from bp 92589 to 93297 (mTCR2; this fragment occurs within a large region that is duplicated and so also corresponds to an identical PstI fragment with a probe from bp 115432 to 116140), and AccI and MscI digests with mTCR2 (which again hybridizes to two identical restriction fragments within this locus).
K562 colony assay.
Constructs for the colony assay consisted of several distinct sequence elements in various combinations. Common to all constructs were the pBSKSII(+) vector backbone and the γβgeo reporter gene. The latter consists of the βgeo marker, a fusion of the lacZ and neo marker genes (20), driven by the γ-globin promoter (sequences from bp −262 to 47 relative to the transcription start site). The human 5′HS2 fragment consisted of an SmaI/BglII restriction fragment corresponding to bp −11261 to −10264 relative to the ɛ-globin transcription start site. The mouse 3′HS1 fragment used was an AseI/MfeI restriction fragment spanning bp 66589 to 67524 relative to the Ey gene transcription start site, while the mouse HS-62.5 fragment consisted of an XbaI/HincII restriction fragment spanning bp −63420 to −61822.
Mutation of the CTCF binding site of mouse 3′HS1 was accomplished using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's directions. The template for mutagenesis was a plasmid consisting of the AseI/MfeI fragment in the pBSKSII(+) vector, and primers were 5′-TCATTTCTCTAATGATCCTGTTGCATAAAAGTCTGTTGCAGAAGTGTTCCACTGATTTCCGCCCTCCTCTCC-3′ and 5′-GGAGAGGAGGGCGGAAATCAGTGGAACACTTCTGCAACAGACTTTTATGCAACAGGATCATTAGAGAAATGA-3′.
K562 cells for the colony assay were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM glutamine. Cells were grown to a density of 0.5 × 106 to 1 × 106/ml, spun down, washed in ice-cold phosphate-buffered saline, and spun down again. Final resuspension was in phosphate- buffered saline at 2 × 107 cells/ml. Cells (0.5 ml) were added to 0.4-cm-gap electrocuvettes containing 5 μg of γβgeo or equivalent molar amounts of the other plasmids, along with 0.1 μg of supercoiled pCMV-HGH, encoding human growth hormone (HGH), for later assay (see below). Electroporations were performed at 200 V and 960 μF with no resistor, and cells were plated in 20 ml of medium. After 24 h at 37°C, 5 ml of cells was diluted to 20 ml and G418 was added to a concentration of 0.9 μg/ml (active). Cells were plated in 100-μl aliquots into 96-well plates, and all samples were incubated at 37°C. Remaining cells were maintained at 37°C for an additional 48 h, after which the supernatants were assayed for HGH levels by using the HGH-TGES radioisotopic assay kit (Nichols Institute, San Juan Capistrano, Calif.), according to the manufacturer's directions, to normalize colony counts for transfection efficiency. Colonies were counted 12 to 14 days after plating. Each construct was transfected in duplicate for each experiment; the data in Fig. 3 represent the average of results from three to seven experiments for each construct.
FIG. 3.
Enhancer-blocking by 3′HS1 but not HS-62.5 shown by colony assays. (A) 3′HS1 colony assay. Test constructs are illustrated to the left. The bar graph shows relative colony numbers obtained for each construct, normalized to the number of colonies obtained with the γβgeo reporter alone (construct 1). HS2 is 5′HS2 from the human β-globin LCR; HS1 is 3′HS1 from the mouse locus; HS1x is the 3′HS1 fragment containing the mutation of the CTCF binding site. (B) HS-62.5 colony assay.
ChIP.
Preparation of single-cell suspensions derived from phenylhydrazine-treated mouse spleens, formaldehyde cross-linking, chromatin purification, and immunoprecipitation were performed as previously described (49). In addition to the mouse pancreatic amylase gene, the mouse necdin gene was used as a control sequence in the quantitative duplex PCRs. Duplex PCRs using the necdin and amylase genes revealed no difference in histone modifications between the two loci. For CTCF chromatin immunoprecipitation (ChIP), values given for fold enrichments reflect the average of results from three separate immunoprecipitations. Values shown in Fig. 5 for fold enrichments determined by ChIPs using antibodies against histone modifications are from results of a representative set of quantitative duplex PCRs from a single ChIP, while values shown in Fig. 6 represent averages of results from three to five separate ChIPs. Rabbit polyclonal antibodies against panacetylated histone H4, histone H3 acetylated at lysines 9 and 14, and histone H3 dimethylated at lysine 4 were obtained from Upstate Biotechnology (Lake Placid, N.Y.); rabbit polyclonal antibodies against CTCF were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). PCR primer sequences and locations are available as supplemental information and can be downloaded at http://www.fhcrc.org/labs/groudine/publications/MCB2003.pdf.
FIG. 5.
Histone modifications across the β-globin and neighboring ORG clusters. Shown in bar graph format are enrichments, relative to control sequences from the amylase and/or necdin genes, of cross-linked chromatin samples immunoprecipitated using antibodies against the indicated products of histone modifications of specific sequences, as determined by quantitative duplex PCR. Values are normalized to test sequence/control sequence ratios for input chromatin samples and for control immunoprecipitations using nonspecific antibody (see Materials and Methods). The data shown are results of a single representative ChIP. The β-globin locus is represented to scale at the top of the figure as in Fig. 1, with the region of nuclease sensitivity indicated by the bracket underneath. The locations of test sequences are shown by the short bars immediately beneath the representation of the β-globin locus and by the bar graphs. In the bar graphs, y-axis values measure relative enrichments (e.g., fivefold and 20-fold). The border between shaded and unshaded regions in each panel represents an enrichment of 1.0-fold, i.e., no measurable enrichment.
FIG. 6.
Histone acetylation at the transitions in nuclease sensitivity flanking the β-globin locus. Relative enrichments with acetylated histones H3 (H3Ac) and H4 (H4Ac) are shown in bar graph format as in Fig. 5. The data shown for each probe are averages of results from three to five immunoprecipitations; data for probes assayed in less than three ChIPs are not shown. The values for the two probes for the kb −60 HSs are beyond the limits depicted in the figure. Shaded vertical bars mark the approximate regions of transition in nuclease sensitivity as determined by the data in Fig. 4.
RESULTS
Nuclease HS mapping across the β-globin locus.
In erythroid cells, the active β-globin locus contains several DNase I HSs, which have been shown to map to sequences with regulatory function (Fig. 1A). These include the β-globin gene promoters and the elements that make up the β-globin LCR (HS1 to HS6; in mouse, HS5 is a minor site, so our representations of the mouse β-globin locus include only HS1 to HS4, HS6, and two weaker sites located between HS4 and HS6). In a previous study, we found that 3′HS1 was conserved between mouse and human, as was HS6 of the LCR (7). Conservation was defined both by the presence of an HS and by sequence similarity.
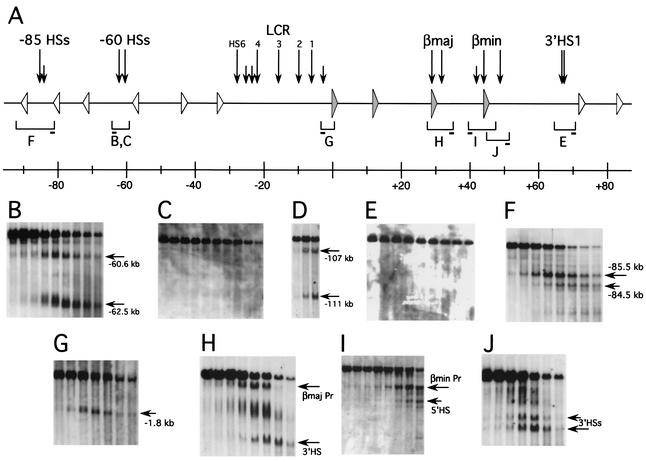
FIG. 1.
DNase I HSs within and flanking the β-globin locus. (A) Summary of DNase I HSs over the region. The locus is represented to scale, with closed triangles indicating the positions of the β-globin genes and open triangles indicating the positions of the open reading frames of ORGs. HSs are indicated by vertical arrows; shorter arrows are used to mark minor or less intense sites. Brackets beneath the locus correspond to the restriction fragments analyzed in panels B to J, as labeled, with the short bars indicating the positions of the probes used. Numbers on the scale beneath indicate coordinates in kilobases, with bp 1 marking the transcription start site of the Ey-globin gene. (B) Region of kb −60 in SacI digest of nuclei from mouse spleen. Major HSs are marked by horizontal arrows, and their approximate positions on the map in panel A are indicated. (C) Region of kb −60 in SacI digest of nuclei from mouse thymus. (D) Region of kb −111 upstream of human β-globin locus in EcoRI digest of nuclei from MEL cells harboring human chromosome 11. Positions indicated are relative to the human ɛ-globin gene promoter. (E) 3′HS1 region in EcoRV digest of nuclei from mouse thymus. (F) Region of kb −85 in PstI digest of nuclei from mouse spleen. (G) Region upstream of Ey-globin gene promoter in BamHI digest of nuclei from mouse spleen. (H) βmaj-globin gene and downstream region in EcoRI digest of nuclei from mouse spleen. (I) βmin-globin gene and upstream region in EcoRV digest of nuclei from mouse spleen. βmin Pr, βmin gene promoter. (J) βmin-globin gene and downstream region in BamHI digest of nuclei from mouse spleen.
Much of the conservation of mouse-human sequences flanking the β-globin locus is associated with conserved olfactory receptor genes (ORGs) within these regions, but a few blocks of sequence similarity are outside ORG transcription units (8). These are candidate β-globin regulatory sequences. We have now extended HS mapping to include the regions flanking the β-globin locus in both mouse (Fig. 1) and human (Fig. 1D).
Downstream of the β-globin locus, we have found no HSs other than 3′HS1, with our mapping extending to kb 134 relative to the Ey-globin transcription start site. Upstream, HSs have been reported that map to kb −62.5 and −60.6 (14). We also detected these HSs in DNase I digestions of nuclei from mouse spleen (Fig. 1B) and murine erythroleukemia (MEL) cells (data not shown). Of these sites, the HS at kb −62.5 falls within a 1.6-kb block of sequence homology (50 to 85% identity throughout) with a corresponding block in the human locus. The corresponding location in human is located ∼111 kb upstream of the human ɛ-globin gene promoter, and we found an HS there in the human erythroid cell line K562 (data not shown) as well as in MEL cells containing human chromosome 11 (Fig. 1D) (46). An additional HS at kb −107 in human maps within a large retroviral insertion.
Notably, we do not detect 3′HS1 or either of the kb −60 HSs in mouse thymus (Fig. 1C and E), brain, or adult liver (data not shown), suggesting that these structures are erythroid-specific. As a control for the quality of the DNase I digestions, we have hybridized the brain, thymus, and liver blots with a probe from the mouse TCRα-Dad1 locus, which reveals the constitutive HS present at this region (data not shown) (42).
We have also found DNase I HSs at kb −85.5 (HS-85.5) and −84.5 in mouse (Fig. 1F), but there is no corresponding sequence or structure in human for these HSs. These sites are not present in brain or thymus (data not shown). Another HS is located at kb −1.8 in mouse, between 5′HS1 of the LCR and the inactive Ey-globin gene, both in spleen cells (Fig. 1G) and in MEL cells (data not shown).
Finally, we have mapped HSs near the βmaj- and βmin-globin genes, which are the active genes in erythroid cells of adult mice. The most prominent of these sites are associated with the gene promoters, but we also found strong HSs 3′ of the genes, ∼1.8 kb downstream of the βmaj gene coding region and ∼3.5 kb downstream of the βmin gene (Fig. 1H and J). These downstream HSs map to sequences that are similar to each other (62% identity over 170 bp), while sequence similarity between the regions containing the βmaj and βmin genes is otherwise restricted to the genes themselves and their promoters. These sequences are not conserved in human, however. We also found a weaker HS 1.4 kb 5′ of the βmin gene promoter (Fig. 1I) but no corresponding HS 5′ of the βmaj gene promoter.
The mammalian β-globin locus is flanked by putative boundary elements.
Evidence from several studies suggests that HS5 from the human β-globin LCR behaves as an enhancer-blocking element (13, 35). The term enhancer-blocking activity refers to the ability of some DNA sequences to prevent enhancer-mediated promoter activation but only when placed between the enhancer and promoter. It has been demonstrated that HS5 and 3′HS1 from mouse and human can be bound in vitro by the transcription factor CTCF, which is required for the enhancer-blocking activity of some boundary elements (3, 4). The CTCF binding sites from HS5 and 3′HS1 mediate enhancer-blocking activity in colony assays (13).
The conserved sequences corresponding to 3′HS1, 5′HS5 of the LCR, the HS located at kb −62.5 in mice and kb −111 in humans, and the HS at kb −85.5 in mice resemble the CTCF binding sites from previously characterized enhancer-blocking elements (Fig. 2A). To determine whether CTCF binds to these sequences in vivo, we performed ChIP analysis using antibodies to CTCF. Upon immunoprecipitation with CTCF antibodies, we found that in erythroid tissue HS-62.5, 5′HS5, and 3′HS1 are significantly enriched (Fig. 2B). By comparison, neither the active βmaj-globin gene promoter nor a region located ∼1 kb from HS5 shows significant enrichment. HS-85.5 exhibits a modest (twofold) enrichment (data not shown).
FIG. 2.
CTCF binding at sites within and near the β-globin locus. (A) Alignment of selected sequences of known or putative CTCF binding sites. Igf2-H19 m1 and Igf2-H19 h1 are selected sites within the imprinting control regions of the Igf2-H19 loci from the mouse and human loci, respectively (3). DM1 site 1 is a site from the human DM1 locus (17). The remaining sites are derived from β-globin loci in the indicated organisms and are described in the text. Point mutations introduced into the CTCF binding site of mouse 3′HS1 are in boldface and underlined. (B) Results of ChIP assay using antibodies to CTCF. The panel shows products of a representative quantitative duplex PCR with formaldehyde-cross-linked chromatin derived from mouse spleen. I, input DNA; B, antibody-bound fraction; −, control immunoprecipitation with no antibody. Fold enrichments for the test sequence (lower band in each lane) in bound versus input samples are shown at the bottom; this figure combines the results from three separate immunoprecipitations. The control sequence (upper band in each lane) is derived from the mouse pancreatic amylase gene.
We have assayed a 1-kb fragment containing mouse 3′HS1 in colony assays with the human erythroid cell line K562 (Fig. 3A). In our assays the presence of an erythroid enhancer (5′HS2 from the human β-globin LCR) results in a 5.5-fold increase in the number of G418-resistant colonies (compare constructs 1 and 2). Flanking the reporter gene with 3′HS1, with or without the enhancer, results in a mild (twofold) reduction in colony number (compare construct 1 with 3 and 2 with 4), which could represent silencing activity and/or protection from positive position effects. Interposition of 3′HS1 between the enhancer and the reporter gene, however, produces an additional threefold reduction in the number of colonies (compare constructs 4 and 5), which is consistent with enhancer-blocking activity.
We have also tested the same 1-kb 3′HS1 fragment after introducing six point mutations within the putative CTCF binding site (Fig. 2A and 3A). The mutation does not appear to have any effect on silencing activity (compare constructs 1, 3, and 6 as well as 2, 4, and 7) but diminishes enhancer-blocking activity (compare constructs 4, 5, 7, and 8). The data suggest that CTCF binding to 3′HS1 mediates most of its enhancer-blocking properties and also demonstrate that the enhancer-blocking effect does not result from increased distance between HS2 and the reporter gene.
We have also tested HS-62.5 in colony assays with K562 cells (Fig. 3B). Despite the presence of bound CTCF at this site in vivo, HS-62.5 exhibits no significant enhancer-blocking activity in the colony assay (compare constructs 4 and 5) and appears to act as a mild enhancer when two copies flank the reporter gene construct (compare constructs 1 and 3 or 2 and 4). This activity is reproduced by a single copy of HS-62.5 upstream of the promoter (data not shown) and so is probably a result of placing the element close to the promoter. HS-62.5 had no activity in transient transfection assays, and no function could be assigned in either assay to the HS at kb −60.6, whether alone or in combination with HS-62.5 (data not shown). The activity of HS-62.5 demonstrates that CTCF binding alone is not sufficient to confer enhancer-blocking activity and thus that elements such as 3′HS1 must have other sequence determinants to produce such an effect.
Generalized nuclease sensitivity within the mouse β-globin locus.
We have determined the extent of generalized nuclease sensitivity at the β-globin locus in mouse (Fig. 4A). The assay involves the isolation of erythroid nuclei, followed by treatment with various concentrations of DNase I. Genomic DNA is then purified, cut with restriction enzymes, and probed by Southern blotting. We compare only the digestion profiles of restriction fragments that are of similar sizes and that do not contain HSs or transcribed gene sequences, which could complicate the digestion of fragments that contain them. As controls, we used regions between the β-globin genes as examples of nuclease-sensitive sequences and regions within the mouse TCRβ-trypsinogen locus, an extensive region that contains no genes expressed in erythroid cells, as examples of nuclease-insensitive sequences.
FIG. 4.
Generalized nuclease sensitivity at the β-globin locus in erythroid cells. (A) The β-globin locus is represented to scale as in Fig. 1. Underneath this, restriction fragments determined to be relatively sensitive to DNase I are shown as thin lines, and those determined to be relatively insensitive are shown as thick lines. Specific fragments are identified and correspond to those shown in panels B to D. (B) Nuclease digestion profiles for BglII restriction fragments. mTCR is a fragment from the mouse TCRβ-trypsinogen locus. Numbers in parentheses indicate sizes in base pairs of each fragment. The graph at the bottom shows the results of phosphorimager analysis of the Southern blots, with the intensity of the sample band in each lane normalized to that of the first lane. (C) Nuclease digestion profiles and phosphorimager analysis of PstI restriction fragments. (D) Nuclease digestion profiles and phosphorimager analysis of EcoRI restriction fragments.
We found that the transition in nuclease sensitivity 5′ of the β-globin genes occurs far 5′ of the LCR and ∼10 kb further upstream from HS-62.5 (Fig. 4A to C). Since the region in which the transition occurs does not contain any nuclease HS to demarcate it, we can only roughly assign it to a position at or near the open reading frame of the ORG MOR5′β4, which has also been termed MOR3-1. We have been able to define two partially overlapping fragments in this region, one of which is nuclease sensitive and one of which is nuclease insensitive (B1 and P1 in Fig. 4A to C). Given the limited resolution of the assay, however, we cannot rule out an area of gradual transition of up to 3 kb within this region. Notably, the region encompassing the erythroid-specific HSs at kb −85.5 and −84.5 is nuclease insensitive, demonstrating that HS formation by itself is not a reliable indicator of open chromatin.
We found a transition in sensitivity 3′ of the β-globin locus, ∼5 kb further downstream from 3′HS1. As with the 5′ transition, no nuclease HS maps to this region but the transition maps roughly to a position at or near the open reading frame of an ORG, in this case MOR3′β1, which has also been termed MOR31-1 or Olfr67 (Fig. 4A and D).
Between these transitions, we have not detected any fragment that is nuclease insensitive. The difference in degrees of digestion at any given time point, as quantified by phosphorimager analysis, is only two- to threefold (Fig. 4B to D), but as shown in Fig. 4A, the behaviors of multiple restriction fragments at or near the transition regions are consistent. Still, it is possible that our assay would not reveal either differences in nuclease sensitivity within the β-globin locus that are more subtle than the difference between this region and the control insensitive loci or a gradual change in sensitivity at the transition areas spanning several kilobases of DNA.
The domain therefore encompasses roughly 145 kb of DNA, including the β-globin genes and their associated regulatory elements (the LCR and 3′HS1). Five of the neighboring ORGs fall within the domain as well. Although the open reading frames of two ORGs roughly coincide with the transitions, their promoters and the majority of their transcribed regions lie within the sensitive domain. Notably, the novel HSs located at kb −62.5 and −60.6 are also located within this domain. The HSs at kb −85.5 and −84.5 are not and appear to be embedded within an insensitive region.
β-globin-proximal ORGs are not expressed in erythroid cells.
The presence of ORGs within the active β-globin domain raises the question of whether they are expressed in erythroid cells. Although such expression was reported in an earlier study (15), we have been unable to detect expression of the β-globin-proximal mouse ORGs by reverse transcription-PCR in a variety of erythroid tissues and cell lines (data not shown). This was the case whether we used intron-spanning primers or primers within the intronless open reading frames of these genes, although all of the primer sets were capable of amplifying products from cDNA from olfactory epithelium. Reverse transcription employed random hexamers as primers, so our PCR analysis would also have revealed any nongenic transcription across these regions.
The lack of ORG expression in erythroid tissue could result from the enhancer-blocking activity of 5′HS5 of the LCR and 3′HS1, which could insulate the ORGs from the β-globin LCR. Mouse lines containing a deletion of HS5 and HS6 of the mouse LCR have previously been shown to have no effect on β-globin gene expression (6; see also reference 14). By reverse transcription-PCR, we have now determined that this deletion does not result in the activation of any of the ORGs located 5′ of the LCR (MOR5′β1 to MOR5′β4) (data not shown). The results show that 5′HS5 of the mouse β-globin LCR is not required to insulate the 5′ ORGs from erythroid regulatory elements of the β-globin locus.
Histone modifications within the β-globin locus.
One hallmark of active domains is enrichment with modified histones, usually hyperacetylated histones H3 and H4 but also including histone H3 dimethylated at K4 (9, 18). ChIP assays have previously been used to evaluate enrichments from specific histone modifications within the mouse β-globin locus (19, 49). The data from these studies, while differing in some details of interpretation, agree in general: in adult mouse erythroid tissue, three broad regions of histone hyperacetylation are evident, encompassing (respectively) the LCR, the βmaj-globin gene, and the βmin-globin gene. Sequences between these three regions are either lacking in hyperacetylation or are only modestly hyperacetylated (two- to fourfold increase in hyperacetylation levels) compared to control inactive loci.
We have extended these studies to encompass histone acetylation across the regions flanking the mouse β-globin locus and dimethylation of lysine 4 of histone H3 throughout (Fig. 5). We have also used a more comprehensive set of probes within the β-globin locus itself. Regarding histone acetylation, our results agree with those of earlier studies of the mouse β-globin locus. Outside the previously characterized regions, the only new region exhibiting significant enrichment with acetylated histones is that containing HSs at kb −62.5 and −60.6. No trend in histone acetylation levels is detectable in comparisons of regions on either side of these HSs, those on either side of 3′HS1, or the regions of transition in nuclease sensitivity on either side of the locus (Fig. 6). Notably, histone acetylation levels at DNA sequences located between HSs at kb −62.5 and −60.6 and the LCR, or between the βmaj and βmin genes, do not differ significantly from those at the inactive controls (the mouse amylase or necdin gene). Between the LCR and the βmaj gene, where the inactive Ey- and βh1-globin genes reside, all sequences tested exhibit modest (two- to fourfold) enrichments with acetylated histones compared to control inactive loci.
In general, the pattern of dimethylation of histone H3 K4 follows that of histone H3 and H4 acetylation. In the case of ChIP with this antibody, however, no enrichment is detectable between the LCR and the active βmaj-globin gene. The sensitivity of the anti-dimethyl H3 K4 antibody in our assays was lower than that of the antibodies to acetylated H3 and H4, however, as shown by the lower fold enrichments within the LCR and active globin genes.
A single exception to the pattern of histone modification is worth noting. At HS-85.5, we observe a modest enrichment (∼threefold) with dimethylated histone H3 K4, compared to an apparent depletion in acetylated histones H3 and H4.
DISCUSSION
In this study, we have described the patterns of nuclease sensitivity and histone modifications across the β-globin locus and the regions that flank it. We have also identified binding sites for the protein factor CTCF within the locus, two of which (HS5 and 3′HS1) are also associated with enhancer-blocking activity. We found, however, that the enhancer-blocking elements do not coincide with transitions in generalized nuclease sensitivity or histone modification patterns, which in turn do not correspond with each other. The data do not appear to conform to existing models of domain formation and demonstrate an unexpected complexity associated with the active β-globin domain.
Nuclease-sensitive mouse β-globin domain.
The observation that regions of the genome harboring active or potentially active genes are more sensitive to digestion by DNase I than other regions provided one of the first indications that higher-order structural modifications, presumably in chromatin, were involved in eukaryotic gene regulation. Some, though not all, nuclease-sensitive regions appear to be bounded by abrupt transitions, spanning 1 kb or less, to nuclease-insensitive regions, suggesting the existence of discrete boundary elements that might organize and define chromatin domains (26, 43).
The chicken β-globin locus in particular appears to fulfill such expectations, since it exhibits a strict concordance between transitions in nuclease sensitivity and histone modification patterns on the 5′ side (26, 36, 37) and also contains a sequence element, 5′HS4, that behaves in functional assays as an enhancer-blocking element and as a barrier against transgene position effects (11, 45). Another element located on the 3′ side of the locus also has enhancer-blocking, but not barrier, activity, and the concordance between its location and the transitions in nuclease sensitivity and histone modification patterns is not as obviously strict (48). Nevertheless, the chicken β-globin domain has been the locus characterized in the most detail and as such represents the major empirical model for domain organization.
The mouse β-globin domain differs from that of chicken in several key respects. First, the nuclease-sensitive region includes a number of ORGs that are not expressed in erythroid cells, and erythroid-specific DNase I HSs (those at kb −85.5, −84.5, −62.5, and −60.6) are located among them. Although we have been unable to assign a function to the novel HSs located at kb −62.5 and −60.6, they nevertheless represent excellent candidates for sequences that act in β-globin gene regulation in addition to the LCR. The evidence for this assertion includes the conservation of the sequences and structures of the HS at kb −62.5 and an HS at kb −111 in the human β-globin locus; the formation of both sites in erythroid tissue but not in brain, thymus, or liver; and the presence of these sites within the nuclease-sensitive β-globin gene domain. In addition, a recent study has shown that these sites colocalize in the nucleus with the β-globin LCR and the active β-globin genes (54). The fact that the β-globin gene complex is not organized into a domain separate from that of the neighboring ORG cluster has ample precedent. Examples of this sort of genomic mixing are well known, including the presence of major regulatory elements of the α-globin locus within introns of a neighboring, ubiquitously expressed gene (2, 56).
Second, the transitions in nuclease sensitivity do not map to DNase I HSs; conversely, elements that possess enhancer-blocking activity (5′HS5 of the LCR and 3′HS1) are not located at these transitions. Recently, it has been shown that enhancer-blocking and barrier activities are separable functions of chicken 5′HS4 (45). In principle, either of these activities might be related to the transition in nuclease sensitivity at 5′HS4, but our results show that enhancer blocking does not coincide with transitions to the nuclease-sensitive structure at the mouse locus. The 5′HS5 of the human β-globin LCR has also been associated with barrier activity in assays with transgenic animals (34, 59); although we have not defined the nuclease-sensitive domain at the human β-globin locus, this association implies that barrier activity also does not correlate with patterns of DNase I sensitivity within this region. Therefore, the mouse β-globin locus might represent a weak domain, one defined not by sequences that function as boundaries but rather by the distribution of other regulatory elements (12). Analysis of the deletion of HS5 and HS6 from the mouse β-globin LCR also brings into question the relevance of enhancer-blocking activity or barrier activity to the function of HS5 at the endogenous locus. HS5 is clearly not required for the normal pattern of expression of either the β-globin genes (6) or the neighboring ORGs. Mouse HS5, however, exhibits very weak enhancer-blocking activity relative to other sequences, including human HS5 (13), and so it is possible that HS5 plays a distinct role at β-globin loci in other mammals.
Third, patterns of histone acetylation, or of dimethylation of lysine 4 of histone H3, do not correspond either to the pattern of nuclease sensitivity or to the location of enhancer-blocking elements. While all regions enriched by histone acetylation and H3 K4 dimethylation are within the nuclease-sensitive domain, it does not follow that all nuclease-sensitive sequences are enriched by these histone modifications. It has previously been shown that the coding regions of some actively transcribed genes are not hyperacetylated (41). Similarly, the α-globin locus, which occurs within a region containing a number of constitutively expressed genes, is hyperacetylated only in erythroid cells (2). Our study is the first, however, to demonstrate the lack of concordance between histone modification and nuclease sensitivity directly.
Basis of nuclease sensitivity and domain organization.
The question then remains, what is the molecular basis of nuclease sensitivity? A long-standing assumption has been that nuclease sensitivity is the result of a more open or decondensed chromatin structure, but to date no direct molecular evidence supports this. Studies using limited trypsin digestion (38) or extraction under high salt conditions (21) suggested that chromatin higher-order structure, or at least higher-order structure dependent upon linker histones or high-mobility-group proteins, was not related to nuclease sensitivity. While our results show that histone acetylation and H3 K4 dimethylation may also be ruled out, other histone modifications that we have not tested might better correlate with the nuclease-sensitive structure; the list of known covalent histone modifications is already very long and continues to grow. It is also conceivable that the antibodies against acetylated histones H3 and H4 that we have used might not be sensitive enough to discern changes in the distribution of an acetylation event occurring at only one histone tail lysine.
Alternatively, nuclease sensitivity may reflect a feature of chromatin structure other than histone modification, such as the binding of nonhistone chromosomal proteins. Some studies have suggested that there may be more than one mode of chromatin condensation, facilitated by different chromatin-binding proteins (24).
Subdomains enriched by histone modifications within the nuclease-sensitive domain.
Our results, combined with those of previous studies (19, 49), define four subdomains within the nuclease-sensitive β-globin domain that exhibit hyperacetylation of histones H3 and H4 and dimethylation of lysine 4 of histone H3. One of these is relatively small, encompassing only ∼2 kb at and near HSs at kb −62.5 and −60.6. A larger subdomain is located over the LCR and extends for more than 26 kb. The other two subdomains encompass the active βmaj- and βmin-globin genes, respectively, and extend for ∼10 kb in each case. These subdomains do not exhibit uniform levels of histone modification, but sequences outside them are clearly deficient in these modifications and so are qualitatively distinct.
The subdomains of histone hyperacetylation that we have defined within the mouse β-globin locus may be related to subdomains defined by intergenic transcription within the human β-globin locus (22). While we have not investigated intergenic transcription in mouse, it is possible that tracking of associated histone acetyltransferases by elongating RNA polymerase complexes could account for the histone modification patterns we observe. An alternative model would be the nucleation and spread of acetyltransferase-containing chromatin-binding complexes, analogous to deacetylase-containing complexes involved in the formation of silenced chromatin in Saccharomyces cerevisiae and Drosophila (40). In this case, we would predict a role for either the gene promoters or other elements, such as the HSs 3′ of each of the genes, in the formation of the subdomains encompassing the βmaj and βmin genes. Recent studies have suggested that the erythroid transcription factors GATA-1 and NF-E2 are required for histone modification at some but not all of these regions, and so the mechanism by which subdomains are formed could be complex (30, 33).
The function of domains of histone hyperacetylation is unknown. A study of gene expression in chicken erythrocytes noted that the β-globin genes are transcribed at extremely high levels compared to other genes and that this might correlate with domain formation (41). With mouse alleles in which the β-globin LCR is deleted, however, β-globin gene expression is only 1 to 4% of normal—yet histone acetylation patterns are unaffected (49). We had previously suggested that domains of histone hyperacetylation correlate with regulation by distal enhancers (9), and this remains a viable hypothesis if such enhancers reside within the β-globin gene subdomains. At HGH gene loci in transgenic mice, the formation of a 32-kb domain defined by histone hyperacetylation is dependent upon binding sites for the transcription factor Pit-1 located within a single HS and disruption of the domain correlates with loss of gene expression (27). In addition, activation of c-myc expression by the immunoglobulin H gene LCR is also associated with generalized increases in histone acetylation levels (39). Thus, at the β-globin locus, subdomains defined by histone modifications may represent a significant clue to an important component of gene activation.
β-globin loci in chicken and mammals.
The differences between the organizations of the active β-globin domains in chicken and mouse provide additional evidence that they are not orthologous (25). Sequencing of β-globin cDNA from a marsupial revealed two separate sets of β-globin genes, one more closely related to β-globin genes in other mammals, the other more closely related to those in chicken (58). This finding suggests that chicken and most mammals have inherited different products of a duplication of an ancestral β-globin locus. In chicken, the neighboring locus on the 5′ side of the β-globin genes contains a folate receptor gene that is expressed in erythroid cells but has a different profile during erythroid differentiation (44), and the two loci are separated by a 16-kb region that exhibits DNA hypermethylation, histone hypoacetylation, and methylation of lysine 9 of histone H3. Thus, the chicken β-globin domain has evolved within a different genomic context than the extant mammalian loci, which are embedded within a larger cluster of ORGs. This could explain the distinct manners in which the active mouse and chicken β-globin gene domains are organized.
Acknowledgments
We thank A. West, G. Felsenfeld, and members of the Groudine lab for comments and for critical reading of the manuscript.
M.B. was supported by a postdoctoral fellowship from the Helen Hay Whitney Foundation and is currently supported by a career award from the Burroughs Wellcome Fund. D.S. is supported by a fellowship from the Rett Syndrome Research Foundation. M.A.B. is supported by a grant from the Cooley's Anemia Foundation and by an American Society of Hematology Scholar Award. R.C.H. is supported by NIH grants DK27635 and HG02238. M.G. is supported by NIH grants DK44746 and HL57620.
REFERENCES
- 1.Alevy, M. C., M. J. Tsai, and B. W. O'Malley. 1984. DNase I sensitive domain of the gene coding for the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 23:2309-2314. [DOI] [PubMed] [Google Scholar]
- 2.Anguita, E., C. A. Johnson, W. G. Wood, B. M. Turner, and D. R. Higgs. 2001. Identification of a conserved erythroid specific domain of histone acetylation across the alpha-globin gene cluster. Proc. Natl. Acad. Sci. USA 98:12114-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, A. C., and G. Felsenfeld. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405:482-485. [DOI] [PubMed] [Google Scholar]
- 4.Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387-396. [DOI] [PubMed] [Google Scholar]
- 5.Bender, M. A., M. Bulger, J. Close, and M. Groudine. 2000. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol. Cell 5:387-393. [DOI] [PubMed] [Google Scholar]
- 6.Bender, M. A., A. Reik, J. Close, A. Telling, E. Epner, S. Fiering, R. Hardison, and M. Groudine. 1998. Description and targeted deletion of 5′ hypersensitive site 5 and 6 of the mouse beta-globin locus control region. Blood 92:4394-4403. [PubMed] [Google Scholar]
- 7.Bulger, M., J. H. van Doorninck, N. Saitoh, A. Telling, C. Farrell, M. A. Bender, G. Felsenfeld, R. Axel, and M. Groudine. 1999. Conservation of sequence and structure flanking the mouse and human beta-globin loci: the beta-globin genes are embedded within an array of odorant receptor genes. Proc. Natl. Acad. Sci. USA 96:5129-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulger, M., M. A. Bender, J. H. van Doorninck, B. Wertman, C. M. Farrell, G. Felsenfeld, M. Groudine, and R. Hardison. 2000. Comparative structural and functional analysis of the olfactory receptor genes flanking the human and mouse beta-globin gene clusters. Proc. Natl. Acad. Sci. USA 97:14560-14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulger, M., T. Sawado, D. Schübeler, and M. Groudine. 2002. ChIPs of the beta-globin locus: unraveling gene regulation within an active domain. Curr. Opin. Genet. Dev. 12:170-177. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers, L. M., and J. C. Hansen. 2000. The core histone N termini function independently of linker histones during chromatin condensation. J. Biol. Chem. 275:37285-37290. [DOI] [PubMed] [Google Scholar]
- 11.Chung, J. H., M. Whiteley, and G. Felsenfeld. 1993. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74:505-514. [DOI] [PubMed] [Google Scholar]
- 12.Dillon, N., and P. Sabbattini. 2000. Functional gene expression domains: defining the functional unit of eukaryotic gene regulation. Bioessays 22:657-665. [DOI] [PubMed] [Google Scholar]
- 13.Farrell, C. M., A. G. West, and G. Felsenfeld. 2002. Conserved CTCF insulator elements flank the mouse and human beta-globin loci. Mol. Cell. Biol. 22:3820-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell, C. M., A. Grinberg, S. P. Huang, D. Chen, J. G. Pichel, H. Westphal, and G. Felsenfeld. 2000. A large upstream region is not necessary for gene expression or hypersensitive site formation at the mouse beta-globin locus. Proc. Natl. Acad. Sci. USA 97:14554-14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feingold, E. A., L. A. Penny, A. W. Nienhuis, and B. G. Forget. 1999. An olfactory receptor gene is located in the extended human beta-globin gene cluster and is expressed in erythroid cells. Genomics 61:15-23. [DOI] [PubMed] [Google Scholar]
- 16.Filippova, G. N., C. P. Thienes, B. H. Penn, D. H. Cho, Y. J. Hu, J. M. Moore, T. R. Klesert, V. V. Lobanenkov, and S. J. Tapscott. 2001. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat. Genet. 28:335-343. [DOI] [PubMed] [Google Scholar]
- 17.Forrester, W. C., E. Epner, M. C. Driscoll, T. Enver, M. Brice, T. Papayannopoulou, and M. Groudine. 1990. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 4:1637-1649. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg, E. C., and E. H. Bresnick. 2001. Histone acetylation beyond promoters: long-range acetylation patterns in the chromatin world. Bioessays 23:820-830. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg, E. C., K. M. Downs, H. M. Christensen, H. Im, P. A. Nuzzi, and E. H. Bresnick. 2000. Developmentally dynamic histone acetylation pattern of a tissue-specific chromatin domain. Proc. Natl. Acad. Sci. USA 97:14494-14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513-1523. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin, G. H., R. H. Nicolas, P. N. Cockerill, S. Zavou, and C. A. Wright. 1985. The effect of salt extraction on the structure of transcriptionally active genes; evidence for a DNAseI-sensitive structure which could be dependent on chromatin structure at levels higher than the 30 nm fibre. Nucleic Acids Res. 13:3561-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gribnau, J., K. Diderich, S. Pruzina, R. Calzolari, and P. Fraser. 2000. Intergenic transcription and developmental remodeling of chromatin subdomains in the human beta-globin locus. Mol. Cell 5:377-386. [DOI] [PubMed] [Google Scholar]
- 23.Grosveld, F., G. B. van Assendelft, D. R. Greaves, and G. Kollias. 1987. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 51:975-985. [DOI] [PubMed] [Google Scholar]
- 24.Hansen, J. 2002. Conformational dynamics of the chromatin fiber in solution: determinants, mechanisms and functions. Annu. Rev. Biophys. Biomol. Struct. 31:361-392. [DOI] [PubMed] [Google Scholar]
- 25.Hardison, R. C. 2001. New views of evolution and regulation of vertebrate beta-like globin gene clusters from an orphaned gene in marsupials. Proc. Natl. Acad. Sci. USA 98:1327-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebbes, T. R., A. L. Clayton, A. W. Thorne, and C. Crane-Robinson. 1994. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 13:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho, Y., F. Elefant, N. Cooke, and S. Liebhaber. 2002. A defined locus control region determinant links chromatin domain acetylation with long-range gene activation. Mol. Cell 9:291-302. [DOI] [PubMed] [Google Scholar]
- 28.Horn, P. J., and C. L. Peterson. 2002. Chromatin higher order folding—wrapping up transcription. Science 297:1824-1827. [DOI] [PubMed] [Google Scholar]
- 29.Jantzen, K., H. P. Fritton, and T. Igo-Kemenes. 1986. The DNase I sensitive domain of the chicken lysozyme gene spans 24 kb. Nucleic Acids Res. 14:6085-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiekhaefer, C. M., J. A. Grass, K. D. Johnson, M. E. Boyer, and E. H. Bresnick. 2002. Hematopoietic-specific activators establish an overlapping pattern of histone acetylation and methylation within a mammalian chromatin domain. Proc. Natl. Acad. Sci. USA 99:14309-14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson, G. M., M. J. Tsai, and B. W. O'Malley. 1980. Deoxyribonuclease I sensitivity of the nontranscribed sequences flanking the 5′ and 3′ ends of the ovomucoid gene and the ovalbumin and its related X and Y genes in hen oviduct nuclei. Biochemistry 19:4403-4441. [DOI] [PubMed] [Google Scholar]
- 32.Lawson, G. M., B. J. Knoll, C. J. March, S. L. Woo, M. J. Tsai, and B. W. O'Malley. 1982. Definition of 5′ and 3′ structural boundaries of the chromatin domain containing the ovalbumin multigene family. J. Biol. Chem. 257:1501-1507. [PubMed] [Google Scholar]
- 33.Letting, D. L., C. Rakowski, M. J. Weiss, and G. A. Blobel. 2003. Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1. Mol. Cell. Biol. 23:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Q., M. Zhang, H. Han, A. Rohde, and G. Stamatoyannopoulos. 2002. Evidence that DNase I hypersensitive site 5 of the human beta-globin locus control region functions as a chromosomal insulator in transgenic mice. Nucleic Acids Res. 30:2484-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Q., and G. Stamatoyannopoulos. 1994. Hypersensitive site 5 of the human beta locus control region functions as a chromatin insulator. Blood 84:1399-1401. [PubMed] [Google Scholar]
- 36.Litt, M. D., M. Simpson, M. Gaszner, C. D. Allis, and G. Felsenfeld. 2001. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science 293:2453-2455. [DOI] [PubMed] [Google Scholar]
- 37.Litt, M. D., M. Simpson, F. Recillas-Targa, M. N. Prioleau, and G. Felsenfeld. 2001. Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J. 20:2224-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundell, M., and H. G. Martinson. 1989. The DNase I sensitive state of “active” globin gene chromatin resists trypsin treatments which disrupt chromatin higher order structure. Biochemistry 28:9757-9765. [DOI] [PubMed] [Google Scholar]
- 39.Madisen, L., A. Krumm, T. R. Hebbes, and M. Groudine. 1998. The immunoglobulin heavy chain locus control region increases histone acetylation along linked c-myc genes. Mol. Cell. Biol. 18:6281-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moazed, D. 2001. Common themes in mechanisms of gene silencing. Mol. Cell 8:489-498. [DOI] [PubMed] [Google Scholar]
- 41.Myers, F. A., D. R. Evans, A. L. Clayton, A. W. Thorne, and C. Crane-Robinson. 2001. Targeted and extended acetylation of histones H4 and H3 at active and inactive genes in chicken embryo erythrocytes. J. Biol. Chem. 276:20197-20205. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz, B. D., F. Harrow, D. Cado, B. Santoso, and A. Winoto. 2001. Function and factor interactions of a locus control region element in the mouse T cell receptor-alpha/Dad1 gene locus. J. Immunol. 167:3836-3845. [DOI] [PubMed] [Google Scholar]
- 43.Phi-van, L., and W. H. Strätling. 1988. The matrix attachment regions of the chicken lysozyme gene co-map with the boundaries of the chromatin domain. EMBO J. 7:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prioleau, M. N., P. Nony, M. Simpson, and G. Felsenfeld. 1999. An insulator element and condensed chromatin region separate the chicken beta-globin locus from an independently regulated erythroid-specific folate receptor gene. EMBO J. 18:4035-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Recillas-Targa, F., M. J. Pikaart, B. Burgess-Beusse, A. C. Bell, M. D. Litt, A. G. West, M. Gaszner, and G. Felsenfeld. 2002. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc. Natl. Acad. Sci. USA 99:6883-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reik, A., A. Telling, G. Zitnik, D. Cimbora, E. Epner, and M. Groudine. 1998. The locus control region is necessary for gene expression in the human beta-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol. Cell. Biol. 18:5992-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reitman, M., and G. Felsenfeld. 1990. Developmental regulation of topoisomerase II sites and DNase I-hypersensitive sites in the chicken beta-globin locus. Mol. Cell. Biol. 10:2774-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitoh, N., A. C. Bell, F. Recillas-Targa, A. G. West, M. Simpson, M. Pikaart, and G. Felsenfeld. 2000. Structural and functional conservation at the boundaries of the chicken beta-globin domain. EMBO J. 19:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schübeler, D., M. Groudine, and M. A. Bender. 2001. The murine beta-globin locus control region regulates the rate of transcription but not the hyperacetylation of histones at the active genes. Proc. Natl. Acad. Sci. USA 98:11432-11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sierra, F. 1990. Biomethods, vol. 2. A laboratory guide to in vitro transcription. Birkhauser Verlag, Basel, Switzerland.
- 51.Stalder, J., M. Groudine, J. B. Dodgson, J. D. Engel, and H. Weintraub. 1980. Hb switching in chickens. Cell 19:973-980. [DOI] [PubMed] [Google Scholar]
- 52.Stalder, J., A. Larsen, J. D. Engel, M. Dolan, M. Groudine, and H. Weintraub. 1980. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNase I. Cell 20:451-460. [DOI] [PubMed] [Google Scholar]
- 53.Stief, A., D. M. Winter, W. H. Strätling, and A. E. Sippel. 1989. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature 341:343-345. [DOI] [PubMed] [Google Scholar]
- 54.Tolhuis, B., R.-J. Palstra, E. Splinter, F. Grosveld, and W. de Laat. 2002. Looping and interaction between hypersensitive sites in the active β-globin locus. Mol. Cell 10:1453-1465. [DOI] [PubMed] [Google Scholar]
- 55.Tse, C., T. Sera, A. P. Wolffe, and J. C. Hansen. 1998. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol. Cell. Biol. 18:4629-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyas, P., M. A. Vickers, D. J. Picketts, and D. R. Higgs. 1995. Conservation of position and sequence of a novel, widely expressed gene containing the major human alpha-globin regulatory element. Genomics 29:679-689. [DOI] [PubMed] [Google Scholar]
- 57.Weintraub, H., and M. Groudine. 1976. Chromosomal subunits in active genes have an altered conformation. Science 193:848-856. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler, D., R. Hope, S. B. Cooper, G. Dolman, G. C. Webb, C. D. Bottema, A. A. Gooley, M. Goodman, and R. A. Holland. 2001. An orphaned mammalian beta-globin gene of ancient evolutionary origin. Proc. Natl. Acad. Sci. USA 98:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu, J., J. H. Bock, J. L. Slightom, and B. Villeponteau. 1994. A 5′ beta-globin matrix-attachment region and the polyoma enhancer together confer position-independent transcription. Gene 139:139-145. [DOI] [PubMed] [Google Scholar]