Abstract
The Lyme disease spirochete, Borrelia burgdorferi, causes a persistent infection in the vertebrate host even though infected animals mount an active immune response against the spirochete. One strategy used by the spirochete to evade vertebrate host immunity is to vary the structure and expression of outer membrane antigens. The vlsE locus represents the best-studied example of antigenic variation in B. burgdorferi. During vertebrate host infection, recombination between the active vlsE locus and silent, partial vlsE copies leads to gene conversion events and the generation of novel alleles at the expression site. In the present study, we followed a population of B. burgdorferi organisms moving through vertebrate host and tick stages to complete one transmission cycle. The major goal of the study was to determine if the vlsE locus was subject to different selective pressure and/or recombination frequency at different stages of the spirochete's life cycle. We report here that the vlsE genetic diversity generated within the rodent host was maintained through the larval and nymphal tick stages. Therefore, naturally infected ticks are likely to transmit spirochete populations with multiple vlsE alleles into naive vertebrate hosts. Although vlsE genetic diversity in mice was maintained through tick stages, the dominant vlsE alleles were different between tick stages as well as between individual ticks. We propose that population-level bottlenecks experienced by spirochetes, especially during the larval-to-nymphal molt, are responsible for individual infected ticks harboring different dominant vlsE alleles. Although vlsE genetic diversity is maintained through tick stages, the VlsE protein is unlikely to be of functional importance in the vector, because the protein was expressed by very few (<1%) bacteria in the vector.
Pathogens alter the structure of surface antigens to adapt to different niches within the vertebrate host and to evade destruction by the host's immune system (3, 38). The Lyme disease spirochete, Borrelia burgdorferi, causes a persistent infection in the vertebrate host, even in the face of a robust immune response (4). The B. burgdorferi genome codes for a large number of membrane proteins, many of which are surface exposed (12). The vlsE locus codes for a surface lipoprotein and represents the best-studied system of antigenic variation in B. burgdorferi (40-42).
B. burgdorferi vlsE is homologous to the variable membrane protein (vmp) genes of Borrelia hermsii and other relapsing fever spirochetes (40). The vls and vmp systems are similar in that they are both gene families present on linear plasmids (27, 40). However, the B. burgdorferi system in strain B31 consists of a functional expression locus flanked by 15 silent partial gene copies, whereas B. hermsii has a functional expression site and at least 40 silent copies scattered in different parts of the genome (3, 27). DNA recombination events between the silent genes and expression site lead to partial or complete gene conversion events and the production of novel alleles at the expression site (17, 27, 40-42). In the B. burgdorferi B31 strain, the vlsE locus is present near the telomere of plasmid lp28-1 (40). The expression site consists of a promoter and an open reading frame that has conserved 5′ and 3′ domains and a central variable cassette that is flanked by 17-bp direct repeat sequences (40). Directly upstream of the expression site are 15 promoterless vlsE cassettes, most of which also have the 17-bp direct repeats at the 5′ and 3′ ends (40). Recombination between the silent gene and expressed site leads to partial gene conversion events and the generation of alleles at the expression site that are a mosaic of the silent copies (41, 42). In theory, this mechanism could lead to the generation of over 1030 different alleles at the expression site, although in practice, this diversity appears to be constrained for reasons that are not understood (36).
The primary role of recombination at the vlsE locus is, most likely, immune evasion, because the variable regions in the central domain are antigenic and form epitopes on the exposed surface of the protein (11, 19, 21, 22). The vlsE locus undergoes extensive recombination over the course of an infection in the vertebrate host, which results in the generation of a bacterial population with multiple, novel vlsE alleles (40-42). In contrast, novel recombinants have not been observed among spirochetes grown in culture, indicating that the recombination mechanism may be inactive or that the rate of recombination may be too low in culture to be detected.
A few studies have focused on vlsE alleles within infected nymphal ticks (15, 24). Individual wild ticks carry spirochete populations with multiple vlsE alleles, which may be indicative of recombination within the tick or the tick acquiring a heterogeneous population from the vertebrate host (16). In studies with naturally infected nymphs, we observed a few vlsE variants in unfed nymphs and many variants in partially fed nymphs (24). This result suggested that vlsE recombination may be stimulated during nymphal feeding or that the vlsE locus may be subjected to different selective forces within unfed and partially fed nymphs. In a related study, Indest et al. (15) used capillary feeding to introduce a clonal population of spirochetes with a single vlsE allele into ticks (15). When capillary-infected nymphs were tested, only the original allele was detected, indicating that vlsE recombination did not occur within capillary-infected nymphs (15). In the present study, we have introduced a clonal population with a single vlsE allele into a mouse and followed changes in the expression and genetic structure of alleles at a population level as the spirochetes (i) moved from the rodent host into larval ticks, (ii) were transstadially maintained through the molt from larvae to nymphs, and (iii) moved from nymphal ticks back into a naive mouse to complete a single transmission cycle. The major goal of the study was to determine if the vlsE locus was subject to different selective pressures and/or recombination frequencies at different stages of the spirochete's life cycle.
MATERIALS AND METHODS
B. burgdorferi.
A low-passage B31 strain of B. burgdorferi (Centers for Disease Control and Prevention, Fort Collins, Colo.) was grown on solid Barbour-Stoenner-Kelly II (BSK II) medium (2, 18), and a single clone designated B31-C1 was isolated and used in the present study. B. burgdorferi B31-C1 was inoculated into liquid BSK II medium and grown to midexponential phase (1.45 × 107 cells per ml). This culture was frozen and used as a stock for subsequent studies.
Ixodes scapularis ticks.
The ticks used in this study originated from females collected in Bridgeport, Conn. The larvae used were the F1 generation of the wild ticks.
Study design.
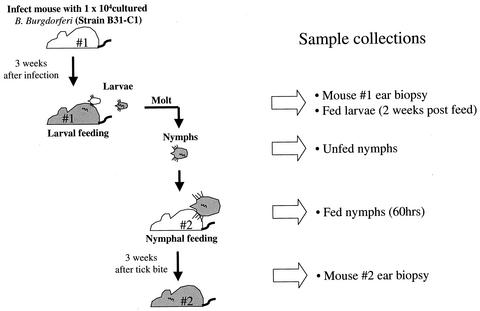
A single transmission cycle was completed in the laboratory, and samples were collected at different stages as outlined in Fig. 1. A clonal population of strain B31 spirochetes (B31-C1) was used to initiate the cycle. A C3H/HENj mouse was infected by subcutaneous injection of 104 spirochetes. Three weeks after infection, 100 larval ticks derived from a single female tick were allowed to feed on the mouse. The larvae acquired spirochetes from the mouse during the blood meal. Engorged larvae were collected and kept in a humid chamber at 21°C until they molted to the nymphal stage. Two weeks after emergence, 20 infected nymphs were placed on a naive mouse and allowed to feed to repletion. During the blood meal, the nymphs transmitted the infection to the naive mouse. Ear biopsies were collected from the first mouse 2 days before larval placement and from the second mouse 3 weeks after the nymphal blood meal. Infected ticks were collected for analysis 2 weeks after the larval blood meal (larvae), prior to the nymphal blood meal (unfed nymphs), and 60 h into the nymphal blood meal (partially fed nymphs) (Fig. 1). Three ticks were collected from each stage. To culture spirochetes, the biopsies and individual ticks were inoculated into BSK II medium containing antibiotics (fosfomycin and rifampin) and antifungal (amphotericin B) agents to reduce contamination (Sigma Chemical Co., St. Louis, Mo.). Positive cultures were plated on solid BSK II medium to obtain single colonies of spirochetes. B31-C1 used to start the cycle was also plated on BSK II plates to obtain single colonies.
FIG. 1.
Experimental design. Three weeks after infection with B. burgdorferi strain B31-C1, 100 larval ticks were placed on mouse 1. Most of the larvae fed to repletion and successfully molted to the nymphal stage. Mouse 2 was challenged with 20 infected nymphal ticks.
RFLP analysis of Borrelia clones.
Each BSK II plate with Borrelia colonies was derived from a single mouse or tick. The vlsE locus was amplified from individual colonies that were randomly picked from each plate. The numbers of colonies picked from each plate were as follows: mouse 1—24 colonies; larvae 1, 2, and 3—9, 9, and 10 colonies, respectively; unfed nymphs 1, 2, and 3—10, 14, and 20 colonies, respectively; partially fed nymphs 1, 2, and 3—19, 20, and 29 colonies, respectively; and mouse 2—19 colonies. Furthermore, 52 colonies were picked from the plate with cultured B31-C1 colonies. The colonies were picked by touching isolated colonies with a sterile toothpick. The tip of the toothpick was dipped into a PCR mixture to amplify the vlsE gene with primers vlsE-F and vlsE-R (24), which annealed to conserved regions just outside the variable central cassette domain. The amplified PCR fragments were digested with restriction enzymes AluI and MboI. The clones with identical restriction fragment length polymorphism (RFLP) patterns were grouped together, and each group was given a numerical identity.
Calculation of Borrelia vlsE allele diversity in mouse and tick stages.
The RFLP data were used to calculate the vlsE allele heterogeneity within individual mice and ticks as well as at particular life stages (mouse, larvae, unfed nymph, and fed nymph). The vlsE allele diversity was calculated by using Simpson's index of diversity (SID) (34). The index is calculated from the following equation: 1 − D = 1 − Σni (ni − 1)/N(N − 1), where D is the diversity index, ni is the number of the ith type, and N is the total number of individuals in the population. SID measures the probability of drawing a pair of individuals of different species from a sample. The statistical significance of the differences in the SID between different animals or life stages was compared by using SAS software and the Bootstrap method (JMP, version 4.0, SAS Institute, Cary, N.C.).
DNA sequencing of selected vlsE clones.
We sequenced the B31-C1 parent clone as well as 11 other clones with known RFLP patterns from unfed and partially fed nymphs to verify that the RFLP assay distinguished between clones with similar and different sequences. The vlsE variable central cassette was PCR amplified from each clone, and the PCR products were cloned into a TOPO cloning vector, pCR2.1. (Qiagen, Valencia, Calif.) for sequencing. Cloned fragments were sequenced with the vlsE-F and vlsE-R primers described above at the University of North Carolina Automated DNA Sequencing Facility. The sequences of the selected clones were aligned by using the multiple-alignment software ClustalW, version 1.4 (37). The parental B31-C1 clone used in our study had a sequence that was identical to a B31 vlsE sequence retrieved from GenBank (U76405) (40).
Artificial capillary feeding of nymphal ticks.
Borrelia clone B31-C1 was introduced into Borrelia-free nymphs by a previously described artificial capillary feeding method (6). Briefly, a suspension of spirochetes at a density of 5.0 × 107 bacteria per ml was introduced into a glass capillary tube. The tube was placed over the hypostome of nymphal ticks for 2 h at 37°C. Some of the artificially infected ticks were placed on a naive mouse and allowed to feed to repletion. Artificially infected nymphs were collected before and immediately after the blood meal for RFLP analysis. One group of infected nymphs was starved for 7 days before analysis. The ticks were cultured for Borrelia, and the vlsE alleles were analyzed as described above.
Detection of VlsE protein on spirochetes by IFA.
Cultured B. burgdorferi organisms as well as gut and salivary glands from unfed and partially fed infected nymphs were prepared for indirect immunofluorescence assay (IFA) as previously described (24). In brief, the slides were blocked with 5% fetal calf serum-phosphate-buffered saline (PBS) prior to incubation with a rabbit serum raised against the IR6 domain of the VlsE protein (20) (kindly provided by Mario Phillip, Primate Center, Tulane University). After four washes with PBS, the slides were incubated with goat anti-rabbit immunoglobulin G conjugated with Alexa 594 (MolecularProbes, Eugene, Oreg.) and fluorescein isothiocyanate (FITC)-conjugated anti-Borrelia antibody (KPL, Gaithersburg, Md.) before being viewed with an epifluorescence microscope (ECLIPSE 600, Nikon, Tokyo, Japan).
Nucleotide sequence accession number.
The vlsE nucleotide sequences of the 11 B. burgdorferi clones derived from unfed and partially fed nymphs have been deposited in the GenBank database under accession no. AY179870 (U2001), AY179871 (U2002), AY179872 (U2003), AY179873 (U2004), AY179874 (U2005), AY179875 (U2010), AY179876 (F2002), AY179877 (F2006), AY179878 (F2008), AY179879 (F3001), and AY179880 (F3007).
RESULTS
RFLP assay for genotyping vlsE alleles.
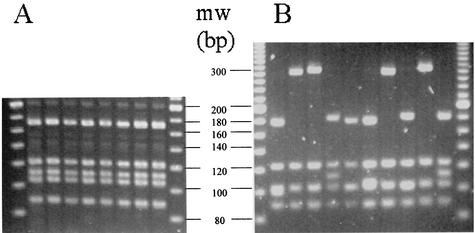
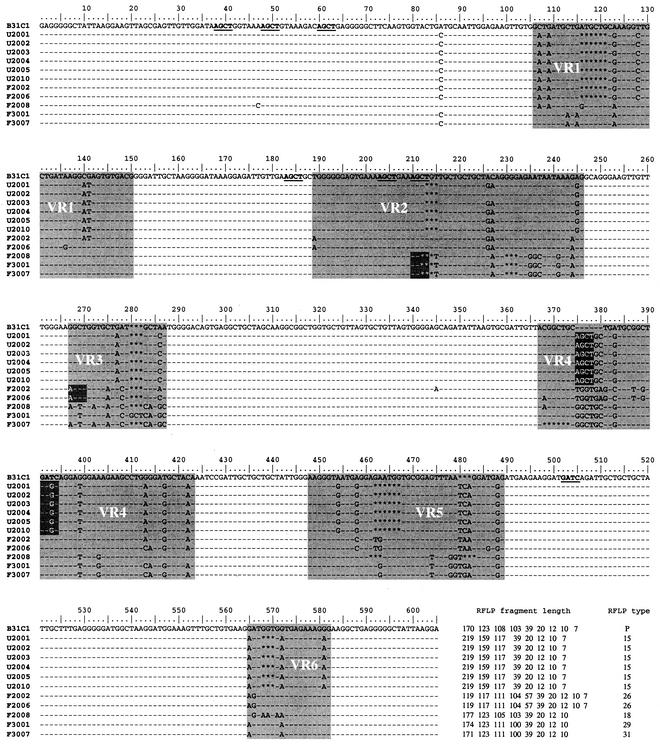
To date, investigators studying antigenic variation at the vlsE locus have used DNA sequencing to characterize different alleles. Given the large number of samples we had to analyze for the present study, we decided to use an RFLP assay to characterize vlsE alleles. The vlsE RFLP type was determined by PCR amplification of the variable central cassette region and then digesting the PCR product with the restriction enzymes AluI and MboI. All colonies (52 individual clones) selected from culture-grown B31-C1 had the same RFLP pattern, whereas the colonies derived from the infected mouse had highly variable RFLP patterns, indicating that the RFLP assay was sensitive enough to detect recombination occurring in the vertebrate host (Fig. 2). We also sequenced a subset of clones from unfed and partially fed nymphs to further validate the RFLP assay. The vlsE locus from 11 clones that had been grouped into RFLP types was sequenced. Clones with identical RFLP patterns shared >97% identity (Fig. 3). For example, six clones belonging to RFLP type 15 were identical, except for one clone (clone U2002), which differed by a two-nucleotide nonsynonymous change from the other RFLP type 15 clones at positions 226 to 227 (Fig. 3). Clones with different RFLP types were less closely related. For example clones belonging to RFLP types 26 (clone F2002) and 18 (clone F2008) shared only 91.67% nucleotide identity (Fig. 3) and 83% amino acid identity, indicating that most changes between the clones were nonsynonymous (data not shown). These results confirm that the RFLP assay is a rapid and inexpensive method for separating vlsE alleles that are closely related if not identical from those that are more distantly related.
FIG. 2.
RFLP typing of representative vlsE alleles. Borrelia colonies were individually analyzed by PCR-RFLP typing with AluI and MboI restriction enzymes. The original parent clonal B31-C1 culture was plated, and eight individual colonies were analyzed (A). Spirochetes were also cultured from a mouse 3 weeks after infection with B31-C1, and 10 individual colonies were analyzed (B). Note that all original parent B31-C1 colonies shared the same RFLP pattern, whereas the clones from the mouse have different RFLP patterns, indicating recombination at the vlsE locus. The extreme left and right lanes in each panel display a 20-bp molecular size ladder (MW).
FIG. 3.
Validation of vlsE RFLP assay by DNA sequencing. Twelve clones with known RFLP patterns were sequenced. The clones were derived from cultured B31-C1 (1 clone), unfed nymphs (6 clones: U2001, U2002, U2003, U2004, U2005, and U2010), and partially fed nymphs (5 clones: F2002, F2006, F2008, F3001, and F3007). The sequences were aligned by using a multiple-alignment software, ClustalW, version 1.4. Nucleotides that are identical to the parental B31C1 strain are marked as dashes, and changes are indicated with the letter of the new nucleotide. Gaps are indicated by asterisks. AluI (AGCT) and MboI (GATC) sites on the B31C1 sequence are underlined. Nucleotide changes in the tick clones that lead to the creation or destruction of an AluI or MboI site are indicated in white text. The variable domain consists of six hypervariable regions, which are shaded and labeled VR1 to VR6. The expected fragment sizes for each clone following AluI and MboI digestion and the designated RFLP type are indicated at the end of the sequence.
Changes in the type and diversity of vlsE alleles at different stages of the B. burgdorferi transmission cycle.
To detect changes in vlsE alleles generated over the course of the transmission cycle, spirochetes were cultured from the original donor mouse, larvae, and unfed and partially fed nymphs and from the second mouse, which acquired the infection from the nymphs (Fig. 1). Thus, all of the spirochetes analyzed were derived from a clonal population initially injected into the single donor mouse. Furthermore, all the ticks used were derived from an egg mass from one tick. A total of 183 Borrelia clones isolated from individual ticks and mice were examined by PCR-RFLP typing, and 37 (36 new types plus the parental B31-C1 type) different RFLP patterns were identified (Table 1). Twenty-four independent clones from the first, donor, mouse belonged to nine RFLP types, with the majority belonging to type 1. The larvae that fed on the mouse had vlsE RFLP types that were mostly distinct from the types in the mouse. Of the 28 clones analyzed from three larval ticks, only one clone (L1, type 6) had an RFLP type that was observed in the donor mouse (Table 1). RFLP types were shared between individual larval ticks (Table 1). Of the seven different RFLP types observed in the larvae, six were present in more than one tick.
TABLE 1.
vlsE RFLP types observed in individual ticks and mice over the course of a single transmission cyclea
| RFLP type | No. of clones with same RFLP type
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Mouse | Larvae
|
Nymphs
|
2nd Mouse | ||||||||
| Unfed
|
Fed
|
||||||||||
| L1 | L2 | L3 | U1 | U2 | U3 | F1 | F2 | F3 | |||
| P | 1 | ||||||||||
| 1 | 11 | ||||||||||
| 2 | 3 | ||||||||||
| 3 | 2 | ||||||||||
| 4 | 1 | 7 | |||||||||
| 5 | 1 | ||||||||||
| 6 | 2 | 1 | 1 | ||||||||
| 7 | 2 | ||||||||||
| 8 | 1 | ||||||||||
| 9 | 3 | 2 | 3 | 1 | 9 | 11 | 3 | 8 | |||
| 10 | 1 | 2 | |||||||||
| 11 | 3 | 4 | 2 | ||||||||
| 12 | 1 | 1 | 1 | ||||||||
| 13 | 1 | 1 | |||||||||
| 14 | 1 | 1 | 1 | ||||||||
| 15 | 10 | ||||||||||
| 16 | 12 | ||||||||||
| 17 | 6 | ||||||||||
| 18 | 2 | 4 | |||||||||
| 19 | 2 | ||||||||||
| 20 | 1 | ||||||||||
| 21 | 1 | 1 | |||||||||
| 22 | 1 | ||||||||||
| 23 | 5 | ||||||||||
| 24 | 4 | ||||||||||
| 25 | 1 | ||||||||||
| 26 | 2 | ||||||||||
| 27 | 1 | ||||||||||
| 28 | 1 | ||||||||||
| 29 | 7 | ||||||||||
| 30 | 7 | ||||||||||
| 31 | 8 | 5 | |||||||||
| 32 | 2 | ||||||||||
| 33 | 2 | ||||||||||
| 34 | 3 | ||||||||||
| 35 | 2 | ||||||||||
| 36 | 1 | ||||||||||
| Total | 24 | 9 | 9 | 10 | 10 | 14 | 20 | 19 | 20 | 29 | 19 |
A total of 183 clones derived from mouse and tick stages were analyzed, and 37 distinct RFLP patterns, including the parental type (P type), were identified.
When the larvae molted to the nymphal stage, only two of the eight RFLP types observed in the nymphs overlapped with RFLP types observed in preceding stages (donor mouse and larvae) (Table 1). Unlike the larvae, little overlap was observed in the RFLP types between individual nymphal ticks. When the nymphs fed, 16 RFLP types were observed, and only 3 of these types overlapped with preceding stages. As in the case of the unfed nymphs, little overlap was observed between individual feeding nymphs. At the completion of the cycle with the infection of the second mouse, five RFLP types were observed among 19 clones tested, and three of these types were not observed in the preceding stage. Of the 37 RFLP types, only 5 types (types 4, 6, 9, 18, and 31) were observed in more than one life stage.
We used SID (34) to calculate the genetic diversity at the vlsE locus in spirochete populations isolated from different stages (Table 2). All of the clones (52 clones) from the cultured B31-C1 parent had the same RFLP type and therefore an SID of 0 (Table 2). When B31-C-1 was injected into a mouse and spirochetes were cultured 3 weeks after infection, nine different RFLP types were detected among 24 clones tested (SID = 0.78). Thus, as expected, cultured spirochetes became genetically more diverse at the vlsE locus in the rodent host. The diversity generated in the mouse was maintained in larval ticks, since individual larvae had SIDs that ranged from 0.81 to 0.89. Following the larval molt to the nymphal stage, the SID was variable in individual nymphs. The three nymphs tested had SIDs of 0.00, 0.27, and 0.72, respectively. Following the blood meal, the diversity among the partially fed nymphs was high and ranged from 0.67 to 0.82. When the nymphs transmitted the spirochetes to the second mouse, the SID was 0.75, which was similar to the SID of the first, donor mouse. Previously we reported an increase in the number of vlsE alleles within ticks following the nymphal blood meal (24). In the present study, we observed average SIDs of 0.41 in unfed ticks and 0.76 in partially fed nymphs, indicating a possible increase in diversity following the blood meal. However, this difference was not statistically significant, possibly because of the small sample size (three ticks per group) as well as the large tick-to-tick variation among unfed nymphs. In fact, statistical analysis of the indices of diversity demonstrated no significant differences between any of the stages in ticks and mice (analysis of variance [ANOVA] followed by Tukey-Kramer highly significant differences [HSD]test; P < 0.05) (JMP, version 4.0, SAS Institute). Because the SID of the unfed nymphs was highly variable, we tested another four unfed nymphs. These four unfed nymphs had SIDs of 0.56, 0.65, 0.61, and 0.37, respectively, confirming that the SID was, indeed, highly variable between individual unfed nymphs.
TABLE 2.
Genetic diversity at the vlsE locus of spirochetes isolated from culture, tick stages, and micea
| Stage(s) | No. of clones tested | No. of RFLP typesb | Diversity indexc | Mean (SD) |
|---|---|---|---|---|
| B31C-1 | 52 | 1 | 0 | |
| 1st Mouse | 24 | 9 | 0.78 | |
| Larvae | ||||
| 1 | 9 | 6 | 0.83 | 0.84 (0.04) |
| 2 | 9 | 5 | 0.81 | |
| 3 | 10 | 6 | 0.89 | |
| Unfed | ||||
| 1 | 10 | 1 | 0 | 0.41 (0.36) |
| 2 | 14 | 3 | 0.27 | |
| 3 | 20 | 5 | 0.72 | |
| Fed | ||||
| 1 | 19 | 6 | 0.78 | 0.76 (0.07) |
| 2 | 20 | 6 | 0.67 | |
| 3 | 29 | 6 | 0.82 | |
| 2nd Mouse | 19 | 5 | 0.76 |
There is no significant difference in diversity between tick stages and between mouse and tick stages (ANOVA followed by Tukey-Kramer HSD; P < 0.05).
Number of distinct RFLP types observed in the clones tested.
SID was calculated as described in the text.
Overlapping vlsE alleles at different stages of the transmission cycle.
Several vlsE RFLP types (types 4, 6, 9, 18, and 31) were observed in more than one stage of the transmission cycle (Table 1). The most striking example of this was type 9, which was present in the larval, unfed and fed nymphal, and 2nd mouse stages (Table 1). However, when representative clones of RFLP type 9 were sequenced from different stages, the sequences were not closely related, indicating that type 9 represents a rare case in which clones with different sequences generate restriction fragments of similar sizes (data not shown). Similarly, RFLP types 4, 6, and 18 also represent cases in which restriction fragments of similar sizes were generated by divergent sequences. In contrast, RFLP type 31 represents a case in which a vlsE allele detected in a feeding tick was subsequently detected in the mouse 3 weeks after transmission. When clones of RFLP type 31 from fed nymphs (F2) and mouse 2 were sequenced, the variable domain was >99% identical at the nucleotide level (data not shown).
vlsE alleles in artificially infected nymphs.
To directly test if nymphal feeding stimulated vlsE recombination and an increase in vlsE allele diversity, we used a capillary feeding method to introduce clonal Borrelia with a single vlsE allele into unfed nymphs. Some of the artificially infected nymphs were allowed to feed on a naive mouse. All Borrelia clones from these artificially infected ticks that had not fed (44 clones from two ticks) or had partially fed (53 clones from three ticks) had only the single parental B31-C1 RFLP type, indicating that recombination or selection did not occur in capillary-fed nymphal ticks or occurred at a frequency that was too low to be detected with the sample sizes used here.
vlsE expression in the tick vector.
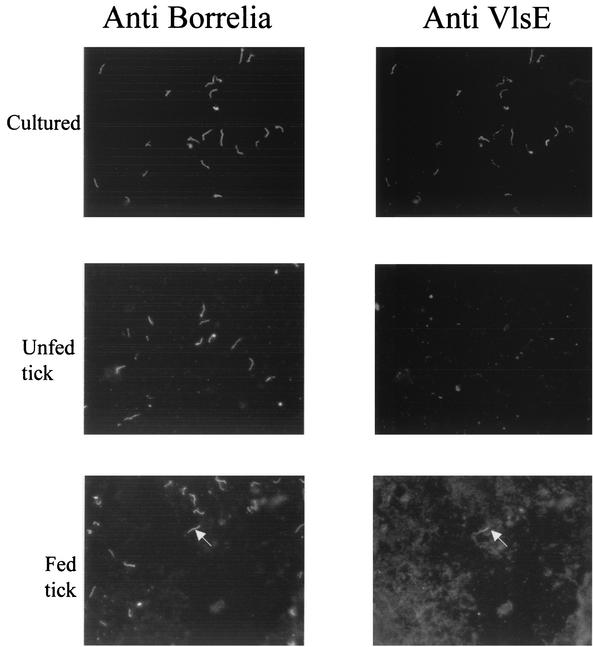
The VlsE protein is expressed and under immune selection in the vertebrate host and, therefore, is likely to be of functional importance in the vertebrate. Experiments were done to determine if the protein was produced by spirochetes within ticks too. Spirochete homogenates were prepared from unfed and partially fed nymphal ticks as well as from culture-grown bacteria. The samples were fixed and stained by double-labeling IFA using an antibody directed against a conserved region of VlsE and as well as a polyclonal antiserum raised against whole spirochetes. The majority of cultured spirochetes (>90%) produced VlsE (Fig. 4). In contrast, very few bacteria (< 1%) in the unfed or partially fed nymphs produced the protein (Fig. 4). The analysis was extended in partially fed nymphs by staining guts and salivary glands separately, and <1% of the spirochetes in each organ produced the protein. Even the few positive spirochetes had very faint fluorescent signals indicating low levels of protein compared to those in cultured bacteria.
FIG. 4.
Detection of VlsE protein on spirochetes within ticks and spirochetes grown in culture. Spirochetes in culture or within unfed or partially fed (48 h) nymphs were stained by double-labeling IFA with antibodies directed against a conserved region on the VlsE proteins and a polyclonal serum against whole Borrelia. Most spirochetes grown in culture stained with the VlsE antibody, whereas very few bacteria (an arrowhead points to a VlsE-positive bacterium) within ticks stained with the antibody.
DISCUSSION
The goal of the present study was to characterize population-level changes in the genetic structure and expression of the B. burgdorferi vlsE locus during a single transmission cycle involving the vertebrate host and invertebrate vector. A complete cycle consists of the spirochetes moving from an infected mouse into larvae, the infection being maintained transstadially through the larval-to-nymphal molt, and the spirochetes being transmitted to a new mouse during the nymphal blood meal. To minimize variation due to the genetic background of the pathogen and vector, we used a cloned population of spirochetes to initiate the cycle and used a tick population derived from a single egg mass for the experiment.
vlsE alleles in mice and larval ticks.
Fifty-two clones derived from cultured B31-C1 had the same RFLP type, indicating that recombination did not occur in vitro or occurred at a frequency that was too low to be detected in a sample of 52 clones. A similar observation has been made by others who have examined the vlsE locus of spirochetes grown in culture (15, 40-42). When these spirochetes were injected into a mouse, multiple vlsE alleles were observed, indicating that recombination and selection in the rodent host lead to the emergence of a genetically heterogeneous population of spirochetes with multiple vlsE alleles (24, 40-42). We determined if the major vlsE alleles present within a mouse were also acquired by feeding larvae. Very little overlap was observed between vlsE alleles from the mouse and larvae that fed on the mouse. Among the seven RFLP types observed within larvae, only one type overlapped with the RFLP types present in the donor, mouse 1. After 3 weeks of infection, the mouse has a population of spirochetes with a large repertoire of vlsE alleles. It is unclear how much spatial heterogeneity exists in the animal with respect to the distribution of spirochetes with different vlsE genotypes. The mouse may be infected with distinct genotypes of Borrelia at different sites in the body, and extensive spatial heterogeneity may explain the lack of overlap in vlsE alleles in spirochetes cultured from larvae and mouse ear biopsies. It is also plausible that novel vlsE alleles that appear in the mouse may rapidly distribute throughout the animal so that the same dominant alleles are present in different regions of the animal. Under this scenario, a selection event occurring within the mouse or larval tick may be responsible for the differences in the vlsE genotype of spirochetes in the donor mouse and the larvae.
We also compared the vlsE alleles dominant in each larval tick. When larvae were tested 2 weeks after infection, we observed extensive overlap of RFLP types between larvae (Table 1). Of the seven RFLP types observed, six were present in more than one larval tick. The extensive overlap in vlsE alleles between individual larvae points to cofeeding larvae sampling the same spirochete populations from a mouse or a selection event in the mouse or larvae favoring the survival of spirochetes expressing a specific subset of vlsE alleles.
vlsE alleles in nymphal ticks.
In the laboratory, larvae molt to the nymphal stage 3 to 4 weeks after the larval blood meal. We observed little overlap between RFLP types in larvae and nymphs as well as between individual nymphs. Currently, we do not understand why RFLP types were observed in common between individual larvae but not between individual nymphs (Table 1). The larval molt subjects spirochetes to unknown selective forces, because the bacterial population size drops by approximately 10-fold (from >2,500 spirochetes to <300 spirochetes) during the molt (26), and individual nymphs end up with low numbers of bacteria that widely vary from tick to tick. As the spirochete numbers drop, many vlsE alleles may become extinct, and such a population-level bottleneck may explain why the vlsE diversity index was highly variable, with some unfed nymphs having very low diversity indices and others having high diversity indices.
Previously, we reported that unfed nymphs had few vlsE alleles in comparison to partially fed nymphs that had many alleles (24). In the present study, too, we observed an increase in diversity (average SID of 0.41 in unfed ticks and 0.76 in partially fed nymphs) during nymphal feeding. However, this difference was not statistically significant. Thus, we conclude that the vlsE allele diversity does not decrease in individual unfed nymphs, but rather it becomes more variable, with some nymphs having populations with a low SID and others continuing to maintain a high SID. When the diversity indices for the vlsE locus were compared between all stages (mice, larvae, and unfed and fed nymphs), none of the stages was significantly different from any other stage. The most conservative interpretation of these results is that, at a population level, the high-level vlsE variability generated within an infected mouse is maintained through all tick stages and the tick transmits a population of spirochetes into the second mouse that is as variable as the population in the first, donor, mouse.
A vlsE allele present in fed ticks (RFLP type 31) was observed in mouse 2 3 weeks after transmission (Table 1). RFLP type 31 clones from the feeding nymph and mouse were >99% identical at the nucleotide level (data not shown). These results indicate that certain vlsE alleles can persist in the vertebrate host for as long as 3 weeks and not be subject to immune clearance. In studies with syringe-infected mice, the alleles in the inoculum have rarely been observed several weeks after infection. Further studies are needed to understand why certain vlsE alleles may persist following tick but not syringe infection.
Does the vlsE locus recombine in the tick vector?
Artificial capillary feeding experiments support the conclusion that novel vlsE alleles are not generated de novo within the tick vector. Indest and colleagues recently used an artificial capillary feeding method to infect nymphal ticks with a clonal population of spirochetes with a single vlsE allele (15). When these capillary-infected ticks were allowed to engorge to repletion and tested 10 days later, only the parental vlsE allele was recovered, indicating that recombination did not occur in the tick or occurred at a frequency that was too low to be detected by these assays (15). We also used the capillary method to infect nymphs and to test for vlsE recombination. Unlike Indest and colleagues, who tested the partially fed nymphs 10 days after the blood meal, we tested the nymphs in the process of active feeding because the recombinants may be selectively transmitted to the vertebrate host and may not be retained in the tick after the blood meal. However, we still failed to detect any evidence for vlsE recombination, indicating that novel alleles are not likely to be generated within capillary-infected nymphs. However, it is premature to conclude that recombination does not occur in the tick during natural infection, because spirochetes introduced by natural infection and capillary feeding are not the same. Naturally infected ticks acquire spirochetes as larvae, and the bacteria represent a population that has infected the vector for several months as well as a population that has survived through a tick molt. Capillary-delivered spirochetes enter the nymphal stage and have infected the vector for a relatively short time before the nymphal blood meal. We recently found that spirochetes introduced by the capillary method are less efficiently transmitted than naturally infected nymphs (6). Additional studies are needed to determine if all the vlsE variants observed in the vector were generated in the vertebrate host or if vlsE recombination occurs in naturally infected nymphs.
The VlsE protein expression data support the conclusion that the locus is unlikely to be of functional importance or directly under selective pressure in ticks. Indest and colleagues reported that the VlsE protein is produced by spirochetes within nymphs, but at low levels (15). Using double-labeled IFA microscopy, we report here that only a small fraction (<1%) of spirochetes produce VlsE, and even the few bacteria that were positive produced a faint signal indicating low-level expression. We propose that the vlsE locus falls into the growing category of Borrelia genes that are preferentially expressed in the host and not the vector (10, 13). Several studies have identified temperature and pH as signals that regulate the expression of Borrelia genes (7, 8, 28, 35, 39). These signals are likely to play a role during natural transmission, because spirochetes experience an increase in temperature and decrease in pH as they move from the vector to the vertebrate. To identify the entire set of B. burgdorferi genes differentially regulated by temperature and pH, two groups have recently probed Borrelia microarrays with cDNA prepared from spirochetes grown in culture (25, 29). These studies have led to the identification of a large number of differentially expressed genes, including those such as ospC, known to be differentially produced during tick feeding. However, the vlsE locus did not stand out as a locus that was differentially produced in response to temperature or pH (25, 29). When cultured spirochetes were compared to spirochetes harvested from chambers implanted in a mouse, the expression of the vlsE locus was increased approximately threefold in the mammal, indicating that the vlsE locus may be activated after the bacteria leave the vector and enter the rodent host (29). The only signal that has increased the expression of vlsE in vitro is derived from endothelial cell membranes, further lending support to the idea that transcription is activated in the vertebrate host and not the tick vector (14).
Antigenic variation systems similar to the vlsE system are found in other vector-borne bacteria (3). The msp2 system in Anaplasma marginale is based on partial gene conversion events leading to the generation of a large number of msp2 variants in the vertebrate host (5, 23). Although some studies have reported evidence for tick-specific msp2 variants (9, 30), other studies indicate that the dominant msp2 alleles in the vertebrate host are passively maintained in the tick (1, 31). It would not be surprising to discover that tick-specific msp2 alleles are the norm rather than the exception, because, unlike the vlsE locus, the msp2 locus is expressed by A. marginale colonizing the vector (31). The variable membrane protein (vmp) antigenic variation system of the relapsing fever spirochete, B. hermsii, consists of a tick-specific vmp33 expression site that is genetically stable and not subject to high-frequency recombination and a separate vertebrate vmp expression locus that recombines with over 40 different silent genes to generate novel alleles (3, 33). The major vmp alleles expressed in the vertebrate host at the time of tick feeding are acquired by the tick, but within the tick, transcription switches from the mammalian expression locus to the tick-specific vmp33 locus (32, 33). The mammalian vmp alleles are passively maintained through the tick, and transcription is activated again after transmission to the next vertebrate host (32, 33). Thus, the vlsE and vmp antigenic variation systems of Borrelia are active and under selection in the vertebrate host but only passively maintained through vector stages of the life cycle.
In summary, we have demonstrated here that the VlsE protein is unlikely to be of functional importance in the vector because the protein was expressed by very few bacteria in ticks. Furthermore, Borrelia strains missing the linear plasmid (lp28-1) coding for vlsE show a decreased infectivity to mice but not ticks, indicating a role for this plasmid in the vertebrate host and not the vector (15). DNA recombination and selection lead to the generation of a large number of vlsE alleles within the rodent host. We also demonstrate here that vlsE genetic diversity generated within the vertebrate host was maintained through larval and nymphal stages so that feeding nymphs are likely to inject a population of spirochetes with many different alleles into the vertebrate. Although vlsE genetic diversity in mice was maintained through tick stages, the dominant vlsE alleles were different between tick stages and individual ticks. We propose that large decreases in the spirochete population size, especially during the larva-to-nymph molt, lead to the random survival of some vlsE genotypes and not others and the observed differences in the dominant vlsE alleles in individual ticks.
Acknowledgments
This work was supported by Public Health Service grants KO1 AR02061 and RO1 AR47948 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases and an Arthritis Investigator Award from the Arthritis Foundation.
We thank Mario Philipp for generously providing the anti-VlsE polyclonal rabbit serum and members of the de Silva laboratory for advice and help with these studies. We also thank two anonymous reviewers for many constructive comments for improving the manuscript.
REFERENCES
- 1.Barbet, A. F., J. Yi, A. Lundgren, B. R. McEwen, E. F. Blouin, and K. M. Kocan. 2001. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect. Immun. 69:3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W. 2000. Lyme borreliosis, p. 281-304. In J. P. Nataro, M. J. Blaser, and S. Cunningham-Rundles (ed.), Persistent bacterial infections. ASM Press, Washington, D.C.
- 5.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 6.Broadwater, A. H., D. E. Sonenshine, W. L. Hynes, S. Ceraul, and A. M. de Silva. 2002. Glass capillary tube feeding: a method for infecting nymphal Ixodes scapularis (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi. J. Med. Entomol. 39:285-292. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Fuente, J., and K. M. Kocan. 2001. Expression of Anaplasma marginale major surface protein 2 variants in persistently infected ticks. Infect. Immun. 69:5151-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eicken, C., V. Sharma, T. Klabunde, M. B. Lawrenz, J. M. Hardham, S. J. Norris, and J. C. Sacchettini. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J. Biol. Chem. 277:21691-21696. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 14.Hudson, C. R., J. G. Frye, F. D. Quinn, and F. C. Gherardini. 2001. Increased expression of Borrelia burgdorferi vlsE in response to human endothelial cell membranes. Mol. Microbiol. 41:229-239. [DOI] [PubMed] [Google Scholar]
- 15.Indest, K. J., J. K. Howell, M. B. Jacobs, D. Scholl-Meeker, S. J. Norris, and M. T. Philipp. 2001. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect. Immun. 69:7083-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer, R., J. M. Hardham, G. P. Wormser, I. Schwartz, and S. J. Norris. 2000. Conservation and heterogeneity of vlsE among human and tick isolates of Borrelia burgdorferi. Infect. Immun. 68:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitten, T., A. V. Barrera, and A. G. Barbour. 1993. Intragenic recombination and a chimeric outer membrane protein in the relapsing fever agent Borrelia hermsii. J. Bacteriol. 175:2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtti, T. J., U. G. Munderloh, R. C. Johnson, and G. G. Ahlstrand. 1987. Colony formation and morphology in Borrelia burgdorferi. J. Clin. Microbiol. 25:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, F. T., E. Aberer, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, Jr., V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 20.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 21.Liang, F. T., J. M. Nowling, and M. T. Philipp. 2000. Cryptic and exposed invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi s1. J. Bacteriol. 182:3597-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDowell, J. V., S.-Y. Sung, L. T. Hu, and R. T. Marconi. 2002. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect. Immun. 70:4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeus, P. F., K. A. Brayton, G. H. Palmer, and A. F. Barbet. 2003. Conservation of a gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol. Microbiol. 47:633-643. [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piesman, J., J. Oliver, and R. Sinsky. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42:352-357. [DOI] [PubMed] [Google Scholar]
- 27.Plasterk, R. H., M. I. Simon, and A. G. Barbour. 1985. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature 318:257-263. [DOI] [PubMed] [Google Scholar]
- 28.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rurangirwa, F. R., D. Stiller, D. M. French, and G. H. Palmer. 1999. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc. Natl. Acad. Sci. USA 96:3171-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rurangirwa, F. R., D. Stiller, and G. H. Palmer. 2000. Strain diversity in major surface protein 2 expression during tick transmission of Anaplasma marginale. Infect. Immun. 68:3023-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280:1938-1940. [DOI] [PubMed] [Google Scholar]
- 33.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson, E. H. 1949. Measurement of diversity. Nature 163:688. [Google Scholar]
- 35.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung, S.-Y., J. V. McDowell, and R. T. Marconi. 2001. Evidence for the contribution of point mutations to vlsE variation and for apparent constraints on the net accumulation of sequence changes in vlsE during infection with Lyme disease spirochetes. J. Bacteriol. 183:5855-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wylie, J. L., and R. C. Brunham. 2000. Antigenic variation and the persistence of extracellular bacteria in vertebrate hosts, p. 13-29. In J. P. Nataro, M. J. Blaser, and S. Cunningham-Rundles (ed.), Persistent bacterial infections. ASM Press, Washington, D.C.
- 39.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, J.-R., and S. J. Norris. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 66:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 66:3689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]