Abstract
The epidermal growth factor receptor (EGFR) is often amplified and rearranged structurally in tumors of the brain, breast, lung, and ovary. The most common mutation, EGFRvIII, is characterized by an in-frame deletion of 801 base pairs, resulting in the generation of a novel tumor-specific epitope at the fusion junction. A murine homologue of the human EGFRvIII mutation was created, and an IgG2a murine mAb, Y10, was generated that recognizes the human and murine equivalents of this tumor-specific antigen. In vitro, Y10 was found to inhibit DNA synthesis and cellular proliferation and to induce autonomous, complement-mediated, and antibodydependent cell-mediated cytotoxicity. Systemic treatment with i.p. Y10 of s.c. B16 melanomas transfected to express stably the murine EGFRvIII led to long-term survival in all mice treated (n = 20; P < 0.001). Similar therapy with i.p. Y10 failed to increase median survival of mice with EGFRvIII-expressing B16 melanomas in the brain; however, treatment with a single intratumoral injection of Y10 increased median survival by an average 286%, with 26% long-term survivors (n = 117; P < 0.001). The mechanism of action of Y10 in vivo was shown to be independent of complement, granulocytes, natural killer cells, and T lymphocytes through in vivo complement and cell subset depletions. Treatment with Y10 in Fc receptor knockout mice demonstrated the mechanism of Y10 to be Fc receptor-dependent. These data indicate that an unarmed, tumor-specific mAb may be an effective immunotherapy against human tumors and potentially other pathologic processes in the “immunologically privileged” central nervous system.
Keywords: central nervous system neoplasms, epidermal growth factor receptor, immunotherapy
The epidermal growth factor receptor (EGFR) gene is often amplified and mutated in human neoplasms (1–4). The most frequently observed mutation, EGFRvIII, enhances tumorigenicity (5, 6) and is found on a high percentage of malignant gliomas (2, 7), breast carcinomas (7, 8), nonsmall cell lung carcinomas (9), and ovarian tumors (7). This mutation is characterized by a consistent in-frame deletion of 801 base pairs from the extracellular domain that splits a codon and produces a novel glycine at the fusion junction (1, 10). This fusion junction encodes a tumor-specific protein sequence expressed on the surface of tumor cells that is not present in normal tissues (1, 7–9), making it an ideal target for antitumor immunotherapy.
Previously, we described the production of murine mAbs that recognize the tumor-specific antigen EGFRvIII (8). In this report, we demonstrate that one of these antibodies, Y10, specifically recognizes a murine homologue of EGFRvIII; inhibits DNA synthesis and cellular proliferation; induces autonomous, complement-mediated, and antibody-dependent cell-mediated cytotoxicity in vitro; and mediates potent Fc receptor (FcR)-dependent antitumor effects in vivo (11).
i.p. injections of unarmed Y10 consistently caused rejection of s.c. B16 melanomas expressing the murine EGFRvIII and induced long-lasting antitumor immunity. Similarly, direct injection of Y10 into established B16 melanomas in the “immunologically privileged” central nervous system (CNS) prolonged survival and led to complete tumor rejection in almost one-third of tumor-bearing mice. These data demonstrate that unarmed mAbs can serve as specific and potent therapeutic agents within the CNS.
Materials and Methods
Animal Models and Tumor Cell Lines.
All experiments used 6- to 12-week-old female mice that were maintained in a virus-free environment in accordance with the Laboratory Animal Resource Commission standards. The B16F10 murine melanoma cell line (12) was provided by I. Fidler (M. D. Anderson Cancer Center, Houston). The U87MGΔEGFR malignant glioma cell line, derived from transfection of the U87 malignant glioma cell line with human EGFRvIII (5), was provided by W. Cavanee (Ludwig Institute, San Diego). All cell lines were grown in antibiotic-free zinc option medium (Life Technologies, Grand Island, NY) containing 10% (vol/vol) heat-inactivated FCS and were shown to be free of Mycoplasma contamination as described (13).
Murine EGFRvIII.
A murine homologue of the human EGFRvIII mutation was created by using cDNA sequences spanning the murine EGFR (EMBL X78987) kindly provided by A. Dunn (Ludwig Institute for Cancer Research, Royal Melbourne Hospital, Victoria, Australia). The precise cDNA sequences cloned into pMFG as described (14) to construct the murine EGFRvIII (msEGFRvIII) were as follows: base pairs 60–147, GT, 951-3737. This cloning procedure creates a junctional peptide LEEKKGNYVVTDH identical to that found in human EGFRvIII (1). CRIP packaging cell lines (15) producing replication-defective retroviral vectors containing the murine EGFRvIII were then generated and used to transfect B16 cells (16). Transfected cells were single-cell cloned twice, and positive clones were selected by flow cytometry. EGFRvIII expression was quantitated by Quantum Simply Cellular Microbeads and software (Flow Cytometry Standards, San Juan, PR; ref. 17). Expression of msEGFRvIII in the B16-msEGFRvIII clone was stable through 20 passages in vitro and 20 days of intracerebral (i.c.) and s.c. growth in vivo and was shown to be expressed on the cell surface as well as in the cytoplasm.
mAbs.
The production and characterization of Y10, a murine IgG2a mAb specific for EGFRvIII, has been described (8). Y10 specifically recognizes both the human and murine equivalents of EGFRvIII. For this study, Y10 ascites was induced in athymic mice, purified on a protein A column, concentrated on an ABX ion-exchange column, and checked for quality by size-exclusion chromatography and ELISA (1, 18). M22.1 is a murine IgG2a mAb specific for methylguanine methyltransferase that is used as an isotype-matched control. Binding affinity was confirmed by BiaCore (Piscataway, NJ) analysis.
In Vitro Cytotoxicity Assays.
Murine effector cells were harvested from the peritoneal cavity of mice primed 3 days earlier with Brewer's thioglycolate. Human effector cells were isolated from peripheral blood by using density gradient centrifugation with Cappel lymphocyte separation medium (ICN). Effector cells were washed, resuspended at 2.5 × 106 cells per ml in RPMI medium 1640 containing 10% (vol/vol) FCS, plated into 48-well plates (5 × 105 cells per well), and incubated at 37°C and 5% CO2. After 2 h, each well was rinsed twice with 500 μl of Hanks' balanced salt solution to remove nonadherent cells. Target cells were incubated for 24 h with methyl-[3H]thymidine (New England Nuclear), washed twice, and resuspended at 2 × 105 cells per ml in Dulbecco's PBS (DPBS) containing 100 μg/ml of mAb. After 1 h, target cells were plated into 48-well plates at indicated dilutions and incubated at 37°C and 5% CO2. Plates were centrifuged, and 150 μl of supernatant was harvested from triplicate wells at indicated time points, mixed with 10 ml of Safety-Solve (Research Products International) in scintillation vials, and counted. Maximal lysis was induced by 1 M NaOH.
Complement-mediated lysis was evaluated against target cells incubated with 51Cr (Amersham Pharmacia) for 1 h, washed in DPBS, resuspended in DPBS at 2 × 105 cells per ml, and plated into 96-well plates (1 × 104 cells per well). Serial dilutions of mAb or polyclonal antiserum were then added to each well in 50-μl volumes. Rabbit complement (Cedarlane Laboratories), titrated by hemolysis of sensitized sheep erythrocytes (Sigma), was then added to each well in a volume of 50 μl at a final dilution of 1/15. Incubation was performed at 37°C for 2 h, after which plates were centrifuged, and supernatants were counted.
In Vitro Proliferation Assays.
B16-msEGFRvIII cells were incubated with 10 μg/ml of mAb as described above. Cells were then harvested with 0.125% trypsin, counted or washed twice in DPBS with 0.1% glucose, and fixed overnight with 70% (vol/vol) ethanol. Cells were then centrifuged at 1,333 × g for 10 min, resuspended at a concentration of 1 × 106 cells per ml in DPBS with 0.1% glucose, 50 μg/ml propidium iodide (Sigma), and 100 units/ml of RNase A (Sigma), and analyzed by flow cytometry.
Tumor Therapy.
B16-msEGFRvIII cells were harvested with 0.125% trypsin/0.02% EDTA and washed once in serum-containing medium and twice in DPBS. Trypan blue-resistant cells were counted. For s.c. tumors, 1.5 × 104 cells were injected in the right flank in a volume of 500 μl. For i.c. tumors, resuspended cells were mixed with an equal volume of 10% (vol/vol) methylcellulose in zinc option medium and loaded into a 250-μl syringe (Hamilton) with an attached 25-gauge needle. The needle was positioned at the bregma, 2 mm to the right of the midline suture and 4 mm below the surface of the skull by using a Kopf stereotactic frame (Kopf Instruments, Tujunga, CA). In a volume of 5 μl, 500 cells were implanted into the right caudate nucleus of the brain. Systemic therapy with Y10 commenced with an i.p. injection of 500 μg of mAb in a volume of 250 μl of DPBS on the day of tumor implantation. Subsequent i.p. injections of 200 μg of mAb in a volume of 100 μl were delivered every other day for 20 days. For direct therapy of i.c. tumors, a single dose of 10 μg of mAb in a volume of 5 μl of DPBS was injected at the previous tumor-injection site 1 day after tumor inoculation. Mice were rechallenged in the contralateral caudate nucleus.
In Vivo Complement Depletion.
Cobra Venom Factor (Sigma) was injected i.p. in 250 μl of DPBS 12 h before tumor challenge at a dose predetermined to deplete serum C3, an essential component of the complement cascade. Whole blood was collected from the retro-orbital plexus to confirm depletion of C3 with a sandwich ELISA.
Leukocyte Subset Depletions.
CD4+, CD8+, granulocyte, and natural killer (NK) cell subsets were depleted in vivo as described (16). Anti-CD4 (GK1.5; ref. 19) and anti-CD8 (2.43; ref. 20) mAbs were produced as described (16). RB6, an antibody used for granulocyte depletion, was provided by R. Coffman (DNAX; ref. 21). Polyclonal rabbit anti-asialo GM1 antibody against murine NK cells was obtained commercially (Wako Chemicals, Richmond, VA). All antibodies were injected once i.v. 3 days before tumor challenge and i.p. every 5 days thereafter with pretitrated amounts of antibody as described (16). Flow cytometric analysis of splenocytes with fluorescein isothiocyanate-labeled anti-CD3 (145–2C11), anti-CD4 (GK1.5), and anti-CD8 (53–6.72) antibodies (BD PharMingen, San Diego) confirmed a >97% depletion of the targeted subset and a normal level of the other subsets. NK cell depletion was confirmed by immunohistochemical staining of the spleen, and granulocyte depletion was confirmed by analysis of peripheral blood smears.
Immunohistochemistry.
Brains from tumor-bearing animals were snap frozen in Tissue-Tek O.C.T. (Miles), sectioned at 4 μm onto gelatin-coated slides, and fixed in cold acetone. Immunoperoxidase staining was performed by using the avidin-biotin-peroxidase Vectastain Elite ABC kit (Vector Laboratories). Primary antibodies were GK 1.5 (anti-CD4; ref. 19), 2.43 (anti-CD8; ref. 20), and F4/80 (anti-macrophage; ref. 22), and affinity-purified and absorbed rabbit polyclonal antibody sera raised against EGFRvIII (1). Secondary antibodies were biotinylated goat α-rat Ig and goat α-rabbit Ig (Vector Laboratories). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride, counterstained with 1% hematoxylin, and permanently mounted. Results were analyzed by an observer blinded to the therapy.
Statistical Analysis.
Survival curves were estimated for each treatment group by using the product-limit estimator of Kaplan and Meier (23). Survival data were compared by using the proportional hazards regression model described by Cox (24). Student's t test was used to assess statistical significance of other data. Statistical significance was determined at the 0.05 level.
Results
Murine mAb Y10 has been shown to be specific for the EGFRvIII (8). Reactivity as examined by flow cytometry, ELISA, RIA, immunoprecipitation, and immunoblotting against whole cells, membrane preparations, and cell lysates demonstrated that Y10 specifically recognized EGFRvIII while completely failing to recognize the wild-type EGFR.
Y10 Mediates Autonomous, Complement-Mediated, and Cell-Mediated Cytolysis with Murine and Human Effector Cells in Vitro.
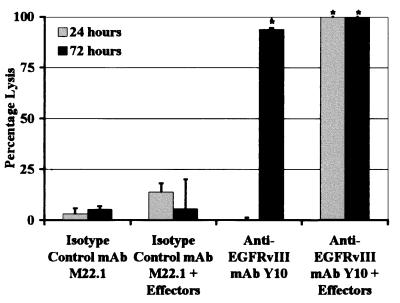
To determine whether unarmed Y10 could mediate autonomous or cell-mediated cytolysis of tumor cells expressing EGFRvIII, in vitro cytotoxicity assays were performed in the absence and presence of effector cells. Both Y10 and isotype-matched control mAb M22.1 failed to induce significant cytolysis in a 24-h assay in the absence of effector cells (Fig. 1). At this time point, the presence of murine effector cells did not significantly enhance lysis produced by M22.1 (P = 0.67) but significantly enhanced lysis mediated by Y10 from undetectable levels to a level of lysis equal to that produced by 1 M NaOH (maximal lysis; P < 0.0018). After 72 h of incubation, M22.1 still failed to mediate significant lysis in the absence or presence of effector cells, whereas Y10 in the absence of effector cells produced lysis equal to 93.9% of maximal lysis (P = 0.0014). In the presence of effector cells, Y10 again mediated target cell lysis to a degree similar to that produced by 1 M NaOH. In similar assays with human effector cells and the EGFRvIII-expressing human-malignant glioma cell line U87MGΔEGFR as a target, M22.1 again did not significantly lyse target cells under any conditions. In this system, unarmed Y10 also did not significantly lyse target cells in the absence of effector cells but, in the presence of effector cells, produced lysis equal to 73% of that induced by 1 M NaOH within 72 h (data not shown). Both assays were performed at least two times and produced similar results in each experiment.
Figure 1.
Lysis of B16-msEGFRvIII cells in vitro by anti-EGFRvIII mAb Y10 with and without murine peritoneal macrophages. B16-msEGFRvIII cells are B16 murine melanoma cells stably transfected to express a murine homologue of the human tumor-specific EGFR mutation EGFRvIII. Y10 is a murine IgG2a mAb that specifically recognizes EGFRvIII. M22.1 is an isotype-matched control murine mAb. B16-msEGFRvIII cells are labeled with 3H, incubated on ice in a solution of DPBS containing 0.1 mg/ml of mAb, and then added to wells containing peritoneal macrophages harvested from C57BL6/J mice and purified by plastic adherence. Degree of lysis is expressed as a percentage of maximal possible lysis as induced by 1 M NaOH. Effector-to-target ratio is 10:1. Gray bars indicate lysis after 24 h, and black bars indicate lysis after 72 h. In the absence of effector cells, unarmed Y10 mediates no lysis within 24 h but nearly complete lysis of all target cells within 72 h. In the presence of effector cells, complete lysis is achieved within 24 h. Asterisks indicate conditions where Y10 produced significantly greater lysis than M22.1 (*, P < 0.05). Error bars show one standard deviation from mean values.
To determine whether Y10 could produce complement-mediated lysis in vitro, lysis of B16-msEGFRvIII cells was examined in the presence of EGFRvIII-specific antibodies and rabbit complement. In this assay, titrations of affinity-purified and absorbed EGFRvIII-specific polyclonal rabbit antisera (1) produced lysis equivalent to 86% and 48% of maximal lysis at dilutions of 1/50 and 1/250, respectively. Similarly, titrations of Y10 produced 63% and 14% of maximal lysis at concentrations of 5 and 0.02 μg/ml, respectively. All other isotype-matched control antibody combinations on EGFRvIII-expressing targets and all negative cell targets exhibited lysis less than 5.6% of maximal lysis.
Y10 Inhibits DNA Synthesis and Tumor Cell Proliferation in Vitro.
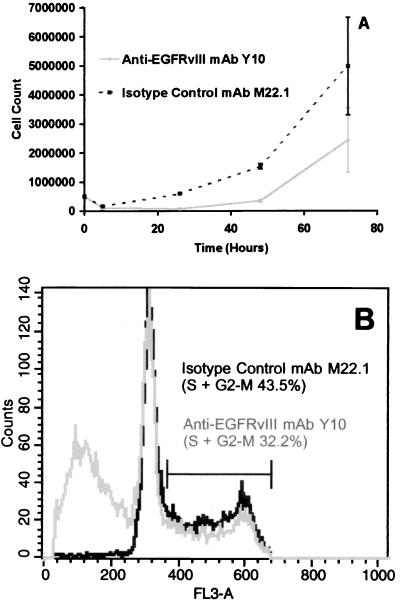
To confirm whether autonomous cytolysis was induced by Y10 and to determine whether Y10 had antitumor effects aside from cytolysis, B16-msEGFRvIII cells incubated with mAbs as described above, in the absence of effector cells, were counted and stained with propidium iodide and studied by flow cytometry. As expected, incubation with isotype-matched control mAb M22.1 failed to induce significant cell death, whereas incubation with Y10 produced a decrease in cell number (Fig. 2A) and increased the mean percentage of subcellular events indicating cell death from 0.52% with M22.1 to 45.99% (P < 0.001; Fig. 2B). Similarly, Y10 reduced the mean percentage of cells in the S, M, and G2 phases of the cell cycle from 42.71% to 32.86% (P < 0.001) indicating a decrease in DNA synthesis and cellular proliferation. These findings were confirmed by population growth curve analysis as well. Based on the results of these initial studies, we chose to evaluate Y10 for antitumor effects in vivo.
Figure 2.
(A) Cell growth curve after exposure to anti-EGFRvIII mAb Y10. (B) Flow cytometry plot of B16-msEGFRvIII cell-cycle analysis after exposure to anti-EGFRvIII mAb Y10. B16-msEGFRvIII cells are B16 murine melanoma cells stably transfected to express a murine homologue of the human tumor-specific EGFR mutation EGFRvIII. Y10 is a murine IgG2a mAb that specifically recognizes EGFRvIII. M22.1 is an isotype-matched control murine mAb. B16-msEGFRvIII cells incubated with 10 μg/ml mAb. Triplicate 10-mm tissue culture plates were harvested for counting and staining with propidium iodide for flow cytometric analysis at each time point. Error bars show one standard deviation from the mean for each count.
Systemic Treatment with Y10 Protects Mice Against s.c. Tumor Challenge and Induces Long-Lasting Antitumor Immunity.
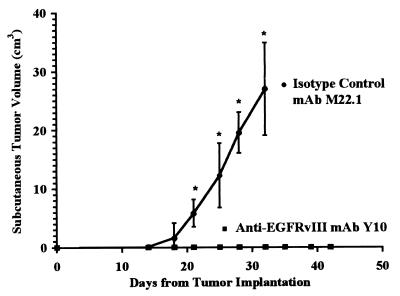
To determine whether Y10 could mediate effective antitumor responses in vivo, we challenged C57BL6/J mice with a lethal dose of B16-msEGFRvIII cells s.c. These mice were then treated with i.p. injections of Y10 every other day as described. In two separate experiments, all mice treated with DPBS (n = 20) or isotype-matched control mAb M22.1 (n = 20) uniformly developed lethal tumors (Fig. 3). In these same experiments, mice treated with Y10 (n = 20) never developed palpable tumor, and all mice lived >45 days without evidence of toxicity (P < 0.001). All mice initially treated with Y10 were then rechallenged with s.c. tumor. These mice survived an additional 55 days without evidence of tumor or toxicity. Thus, we chose to evaluate the therapeutic potential of Y10 further by determining its ability to eradicate tumors from the immunologically privileged CNS.
Figure 3.
Volume of s.c. B16-msEGFRvIII tumors in C57BL6/J mice treated with Y10. B16-msEGFRvIII cells are B16 murine melanoma cells stably transfected to express a murine homologue of the human tumor-specific EGFR mutation EGFRvIII. Y10 is a murine IgG2a mAb that specifically recognizes EGFRvIII. M22.1 is an isotype-matched control murine mAb. Mice were challenged with 15,000 B16-msEGFRvIII cells s.c. and treated i.p. with 500 μg of Y10 or M22.1 mAb on the day of tumor challenge and with 200 μg of mAb every other day thereafter for 20 days. Treatment with M22.1 failed to protect against tumor growth. Treatment with Y10, however, protected 100% of the mice from tumor growth, and all mice treated with Y10 survived for 45 days without evidence of tumor. All mice were rechallenged with tumor at this point but again failed to develop tumor. Asterisks indicate where mean tumor growth in mice treated with M22.1 exceeded that in mice treated with Y10 (*, P < 0.05). Error bars show one standard deviation from mean values.
Intratumoral Treatment with Y10 Prolongs Survival in Mice with i.c. Tumors.
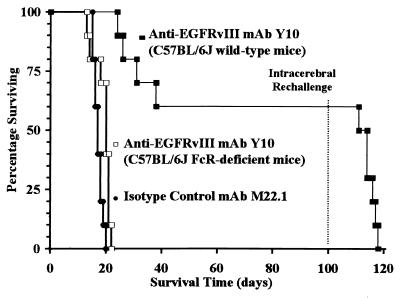
In experiments similar to those performed in mice with s.c. tumors, systemic treatment with Y10 failed to induce significant increases in median survival in mice with i.c. tumors. Therefore, we investigated the efficacy of direct intratumoral injections of Y10 for the treatment of i.c. tumors. Mice were challenged with a lethal dose of B16-msEGFRvIII cells i.c. and then treated with a single intratumoral injection of Y10 1 day after tumor implantation as described. In one experiment, mice treated with isotype-matched control mAb M22.1 (n = 10) had a median survival of 17 days, whereas mice treated with Y10 (n = 10) had a >488% increase in median survival to >100 days (P < 0.001; Fig. 4). In addition, in this experiment, 6/10 mice treated with Y10 survived without evidence of tumor. Under the same conditions, treatment with DPBS (n = 10) produced negative results similar to those obtained with M22.1. Overall, in a total of nine experiments, Y10 (n = 177) increased median survival by 286% relative to treatment with M22.1 (n = 115; P < 0.001). In addition, all mice in all experiments treated with DPBS or M22.1 succumbed to tumor, whereas across all experiments an average of 26% of mice treated with a single intratumoral injection of Y10 lived >100 days without evidence of tumor growth or toxicity. Treatment of mice 3 days after i.c. tumor challenge, when tumors were histologically evident as established tumors, produced similar results. In mice treated with Y10 that died from tumor, immunohistochemical analysis of the tumors demonstrated uniform positive staining for msEGFRvIII similar to that seen in tumors from mice treated with M22.1 or DPBS, indicating that progressive tumors in mice treated with Y10 were not antigen-loss variants. Finally, unlike mice that rejected s.c. tumors, when mice surviving i.c. tumor challenge were rechallenged with tumor in the contralateral cerebral hemisphere, all mice succumbed to tumor, with a median survival not different from that observed in control mice, indicating a failure to develop a sustained immune response sufficiently potent to affect tumors growing within the brain.
Figure 4.
Survival of C57BL6/J mice with i.c. B16-msEGFRvIII tumors treated with anti-EGFRvIII mAb Y10. B16-msEGFRvIII cells are B16 murine melanoma cells stably transfected to express a murine homologue of the human tumor-specific EGFR mutation EGFRvIII. Y10 is a murine IgG2a mAb that specifically recognizes EGFRvIII. M22.1 is an isotype-matched control murine mAb. Mice were challenged with 500 B16-msEGFRvIII cells i.c. and treated 24 h later with a single bolus of 10 μg of mAb injected directly into the tumor. In wild-type mice, intratumoral injection of Y10 significantly increased median survival by >488% relative to treatment with M22.1, and 60% of mice treated with Y10 survived for >90 days without evidence of tumor (P < 0.001). Mice that were rechallenged with i.c. tumor at this point all succumbed to tumor, however. In mice with deletions of both FcRγ and FcγRII genes, treatment with Y10 failed to increase median survival (P > 0.4).
Rejection of i.c. Tumors by Intratumoral Y10 Requires the FcR and Is Independent of Complement.
To examine the therapeutic role of the Fc portion of the mAb in this model, we used mice with deletions of both FcRγ and FcγRII genes (no. 000585-MM, Taconic Farms) that were devoid of all receptors for IgG. Treatment of i.c. tumors in these double-knockout mice with Y10 as described above produced median survival times of only 20 days (P > 0.4), indicating that the FcRs are also an absolute requirement for the therapeutic mechanism of Y10 in this model (Fig. 4).
Although these data implied that the cytolysis induced by Y10 was not mediated by complement, to confirm this implication further, wild-type C57BL6/J mice with i.c. tumors were depleted of complement factor C3 before therapy with Y10 (n = 24) or M22.1 (n = 20) as described in Materials and Methods. In these experiments, complement depletion did not significantly impair the observed efficacy of intratumoral Y10 against i.c. tumors (P = 0.4; data not shown). These data also provide additional evidence that the complement-mediated lysis induced by Y10 in vitro plays a minor role, if any, in the efficacy of Y10 against i.c. tumor in vivo.
Rejection of i.c. Tumors by Intratumoral Y10 Is Independent of Granulocytes, NK Cells, and T Lymphocytes.
To define the effector cells responsible for the FcR-dependent cytolysis mediated by Y10 in this model, mice with i.c. tumors were depleted of leukocyte subsets throughout mAb therapy (n = 10 in each group in each experiment). Two separate experiments demonstrated that the efficacy of Y10 in the i.c. tumor model was not altered significantly by the depletion of granulocytes (n = 20; P = 0.4), NK cells (n = 20; P = 0.47), CD4+ cells (n = 20; P = 0.5), or CD8+ cells (n = 20; P = 0.3) compared with Y10-treated mice (n = 23) that were not depleted (data not shown). In addition, histological and immunohistochemical analysis of tumors in mice that were not depleted of any cell subtype demonstrated a paucity of NK cells and CD4+ and CD8+ lymphocytes in and around the tumor in mice treated with either Y10 or M22.1. However, macrophages were found throughout the tumor matrix and around the tumor periphery in both Y10-treated and M22.1-treated mice (Fig. 5).
Figure 5.
Peritumoral infiltrates of F480-positive cells in mice with i.c. B16-msEGFRvIII tumors treated with anti-EGFRvIII mAb Y10. B16-msEGFRvIII cells are B16 murine melanoma cells stably transfected to express a murine homologue of the human tumor-specific EGFR mutation EGFRvIII. Y10 is a murine IgG2a mAb that specifically recognizes EGFRvIII. M22.1 is an isotype-matched control murine mAb. Brains were analyzed immunohistochemically 15 days after tumor challenge. B, brain; T, tumor; C, cavity (original magnification: ×66).
Discussion
Specific targeting of therapeutic agents to tumors has been a goal of cancer therapy since it was first suggested by Ehrlich almost a century ago (25). Although mAbs have been useful for targeting radiotherapy (26–28) and recombinant bacterial toxins (29, 30) to tumors, such therapy is still limited by the toxicity to normal tissues resulting from antibodies that are not tumor-specific and the inherently nonspecific toxicity of the therapeutic conjugate. In an attempt to reduce toxicity, unarmed antibodies that target normal cellular proteins that are overexpressed on tumor cells have been used with some success against human lymphomas (31) and breast (32) and colon (33, 34) carcinomas located outside the CNS. The most significant finding reported herein is that an unarmed mAb that targets an antigen that is tumor-specific can mediate potent therapeutic antitumor responses in the CNS. These data also illuminate the possibility that this immunotherapeutic approach may be useful in the treatment of other pathologic processes affecting the CNS, including viral encephalitides or AIDS.
We focused our attention on the utility of Y10 against brain tumors, because in many ways, unarmed anti-EGFRvIII mAbs may be an ideal adjunct for the therapy of these tumors. High-level clonal expression of EGFRvIII is evident on the most common and most malignant primary brain tumors and seems to be part of the fundamental pathway toward malignant progression of these tumors (35). EGFRvIII is also expressed on many tumors that metastasize to the brain from carcinomas of the lung and breast (7–9). In addition, unlike other organs subject to neoplastic transformation, such as the bone marrow, breast, and prostate, where crossreactivity with normal antigens can be expected to do limited harm, crossreactivity with normal CNS antigens could be devastating (36–39). Thus, a tumor-specific approach is even more important in the CNS. Furthermore, macrophages (40, 41), microglia (42–44), and astroglial cells (45), all of which contain FcR, are abundant within brain tumors (46) and throughout the substance of the brain. Passive immunotherapy is also an attractive approach to immunotherapy in patients with brain tumors, because most of these patients suffer from a profound and intrinsic immunosuppression that predominantly affects the T cell arm of the immune system (47–49). This immunosuppression combined with the intrinsic low-level expression of major histocompatibility complex antigen presentation molecules in the CNS (50) may severely limit other cell-mediated immunotherapy approaches. Furthermore, although systemic delivery of unarmed mAbs into brain tumors (51) and other solid tumors is limited by a number of physiologic barriers (52), recent advances in the direct delivery of various substrates throughout the brain by direct convection-enhanced continuous microinfusion into the brain substance bypass these obstacles and allow the delivery of high concentrations of even very large therapeutic constructs, such as mAbs, throughout the brain (29, 53).
Another attractive feature of i.c. therapy in this model is that in contrast to systemic delivery of Y10 for the treatment of s.c. tumors, our failure to induce a long-acting memory immune response after treatment in brain with Y10 indicates that immunotherapy with this approach may be self-limiting. This failure may be related to the small dose of mAb delivered into the brain or an intrinsic difference between the mechanism of the response induced in the brain, possibly related to the antiproliferative action of the mAb, and that induced systemically. For example, the possibility remains that, in the brain, FcR crosslinking acts to perturb signal transduction through the EGFRvIII that has been targeted by the mAb. In either event, although failure to induce immunologic memory is generally considered an undesirable event in immunotherapeutic approaches, for therapy of brain tumors, this approach may provide a comfort zone with less risk of inducing a self-perpetuating immune response that may produce untoward inflammation in an especially sensitive organ.
Although the results reported in this article are promising, a number of obstacles will need to be overcome before the ultimate potential of this unarmed tumor-specific mAb and other similar agents will be realized. Although tumor specificity has obvious advantages, one significant potential problem with an agent that selectively targets a single tumor-specific mutation will be the intrinsic antigenic heterogeneity of the cells that comprise malignant tumors. Although EGFRvIII seems to represent a nearly terminal branch of malignant progression for malignant brain tumors and is expressed clonally within this lineage, neoplastic cells not expressing this epitope may have a selective growth advantage under the conditions of therapy with such an agent. In the experiments reported herein, antigen loss variants did not seem to play a role in treatment failure, however. Tumors derived from mice succumbing to tumor remained uniformly antigen-positive. This finding suggests that therapy had been ineffective, perhaps because of subtle antigen expression down-regulation or because of technical issues that limit accurate targeting of tumors in the mouse brain. Still, antigenic heterogeneity will likely remain an issue in the therapy of human tumors. This traditional limitation of narrowly specific immunotherapeutic agents such as mAbs may be overcome by directing the broader repertoire of host immune cells toward the tumor through use of cytokine-based immunoconjugates. For example, Becker et al. (54) demonstrated that large tumor masses with heterogeneous expression of a targeted antigen could be eradicated by host immune cells activated by tumor-specific fusion proteins containing IL-2.
In summary, we have created a murine homologue of an important human mutation in the EGFR. Within the context of this model, we have demonstrated that systemic delivery of unarmed tumor-specific mAbs that recognize this mutation has potent antitumor activity against s.c. tumors and generates long-lasting antitumor immunity. Although systemic delivery of tumor-specific mAb fails to alter the growth of i.c. tumors, we demonstrate that direct intratumoral delivery restores the potency of the mAb against i.c. tumors and mediates potent antitumor effect that is FcR-dependent and self-limited. These data also establish passive, unconjugated antibody-based immunotherapy as a viable mechanism of defense against pathologic processes in the CNS.
Acknowledgments
This work was supported by the Pediatric Brain Tumor Foundation of the U.S., the Cancer Research Institute/Partridge Foundation, and National Institutes of Health Grants CA 74886, CA 11898, and NS 20023.
Abbreviations
- CNS
central nervous system
- DPBS
Dulbecco's PBS
- EGFR
epidermal growth factor receptor
- FcR
Fc receptor
- i.c.
intracerebral
- NK
natural killer
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130166597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130166597
References
- 1.Humphrey P A, Wong A J, Vogelstein B, Zalutsky M R, Fuller G N, Archer G E, Friedman H S, Kwatra M M, Bigner S H, Bigner D D. Proc Natl Acad Sci USA. 1990;87:4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekstrand A J, Sugawa N, James C D, Collins V P. Proc Natl Acad Sci USA. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong A J, Ruppert J M, Bigner S H, Grzeschik C H, Humphrey P A, Bigner D D, Vogelstein B. Proc Natl Acad Sci USA. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libermann T A, Nusbaum H R, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield M D, Ullrich A, Schlessinger J. Nature (London) 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa R, Ji X D, Harmon R C, Lazar C S, Gill G N, Cavenee W K, Huang H J. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra S K, Castelino-Prabhu S, Wikstrand C J, Zhu X, Humphrey P A, Friedman H S, Bigner D D. Cell Growth Differ. 1995;6:1251–1259. [PubMed] [Google Scholar]
- 7.Moscatello D K, Holgado-Madruga M, Godwin A K, Ramirez G, Gunn G, Zoltick P W, Biegel J A, Hayes R L, Wong A J. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 8.Wikstrand C J, Hale L P, Batra S K, Hill M L, Humphrey P A, Kurpad S N, McLendon R E, Moscatello D, Pegram C N, Reist C J, et al. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 9.Garcia de Palazzo I E, Adams G P, Sundareshan P, Wong A J, Testa J R, Bigner D D, Weiner L M. Cancer Res. 1993;53:3217–3220. [PubMed] [Google Scholar]
- 10.Sugawa N, Ekstrand A J, James C D, Collins V P. Proc Natl Acad Sci USA. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steplewski Z, Lubeck M D, Koprowski H. Science. 1983;221:865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- 12.Fidler I J. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 13.Kurtzberg J, Hershfield M S. Cancer Res. 1985;45:1579–1586. [PubMed] [Google Scholar]
- 14.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danos O, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson J H, Archer G E, Ashley D M, Fuchs H E, Hale L P, Dranoff G, Bigner D D. Proc Natl Acad Sci USA. 1996;93:10399–10404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wikstrand C J, McLendon R E, Friedman A H, Bigner D D. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 18.Wikstrand C J, Stanley S D, Humphrey P A, Pegram C N, Archer G E, Kurpad S, Shibuya M, Bigner D D. J Neuroimmunol. 1993;46:165–173. doi: 10.1016/0165-5728(93)90246-u. [DOI] [PubMed] [Google Scholar]
- 19.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 20.Sarmiento M, Glasebrook A L, Fitch F W. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 21.Tepper R I, Coffman R L, Leder P. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 22.Walker W S. Cell Immunol. 1987;107:417–432. doi: 10.1016/0008-8749(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E L, Meier P. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Cox D R. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 25.Ehrlich P. Collected Studies on Immunity. New York: Wiley; 1906. [Google Scholar]
- 26.Bigner D, Brown M, Friedman A H, Coleman R E, Akabani G, Friedman H S, Thorstad W L, McLendon R E, Bigner S H, Zhao X-G, et al. J Clin Oncol. 1998;16:2202–2212. doi: 10.1200/JCO.1998.16.6.2202. [DOI] [PubMed] [Google Scholar]
- 27.Riva P, Franceschi G, Arista A, Frattarelli M, Riva N, Cremonini A M, Giuliani G, Casi M. Cancer. 1997;80:2733–2742. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2733::aid-cncr53>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Larson S M, Sgouros G, Cheung N K. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 534–552. [Google Scholar]
- 29.Laske D W, Youle R J, Oldfield E H. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 30.Pai L H, Pastan I. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 521–533. [Google Scholar]
- 31.Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A, Epenetos A, Gisselbrecht C, Huhn D, Jaeger U, et al. J Clin Oncol. 1998;16:3257–3263. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 32.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz C C, Dantis L, Sklarin N T, Seidman A D, Hudis C A, Moore J, et al. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 33.Riethmuller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirche H, Pichlmayr R. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 34.Herlyn D, Herlyn M, Steplewski Z, Koprowski H. Eur J Immunol. 1979;9:657–659. doi: 10.1002/eji.1830090817. [DOI] [PubMed] [Google Scholar]
- 35.Furnari F B, Huang H J, Cavenee W K. Pediatr Neurosurg. 1996;24:41–49. doi: 10.1159/000121013. [DOI] [PubMed] [Google Scholar]
- 36.Pasteur L. C R Acad Sci. 1885;101:765–774. [Google Scholar]
- 37.Stuart G, Krikorian K. Lancet. 1930;1:1123–1125. [Google Scholar]
- 38.Bloom H J, Peckham M J, Richardson A E, Alexander P A, Payne P M. Br J Cancer. 1973;27:253–267. doi: 10.1038/bjc.1973.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trouillas P. Rev Neurol (Paris) 1973;128:23–38. [PubMed] [Google Scholar]
- 40.Herlyn D, Koprowski H. Proc Natl Acad Sci USA. 1982;79:4761–4765. doi: 10.1073/pnas.79.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams D O, Hall T, Steplewski Z, Koprowski H. Proc Natl Acad Sci USA. 1984;81:3506–3510. doi: 10.1073/pnas.81.11.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulvestad E, Williams K, Matre R, Nyland H, Olivier A, Antel J. J Neuropathol Exp Neurol. 1994;53:27–36. doi: 10.1097/00005072-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Bender H, Takahashi H, Adachi K, Belser P, Liang S H, Prewett M, Schrappe M, Sutter A, Rodeck U, Herlyn D. Cancer Res. 1992;52:121–126. [PubMed] [Google Scholar]
- 44.Sutter A, Hekmat A, Luckenbach G A. Pathobiology. 1991;59:254–258. doi: 10.1159/000163657. [DOI] [PubMed] [Google Scholar]
- 45.Nitta T, Yagita H, Sato K, Okumura K. Neurosurgery. 1992;31:83–87. doi: 10.1227/00006123-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Wood G W, Morantz R A, Tilzer S A, Gollahon K A. J Natl Cancer Inst. 1980;64:411–418. [PubMed] [Google Scholar]
- 47.Brooks W H, Netsky M G, Normansell D E, Horwitz D A. J Exp Med. 1972;136:1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott L H, Brooks W H, Roszman T L. J Clin Invest. 1990;86:80–86. doi: 10.1172/JCI114719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morford L A, Elliott L H, Carlson S L, Brooks W H, Roszman T L. J Immunol. 1997;159:4415–4425. [PubMed] [Google Scholar]
- 50.Lampson L A, Hickey W F. J Immunol. 1986;136:4054–4062. [PubMed] [Google Scholar]
- 51.Faillot T, Magdelenat H, Mady E, Stasiecki P, Fohanno D, Gropp P, Poisson M, Delattre J Y. Neurosurgery. 1996;39:478–483. doi: 10.1097/00006123-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Jain R K. Cancer Res. 1990;50:814s–819s. [PubMed] [Google Scholar]
- 53.Bobo R H, Laske D W, Akbasak A, Morrison P F, Dedrick R L, Oldfield E H. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker J C, Varki N, Gillies S D, Furukawa K, Reisfeld R A. Proc Natl Acad Sci USA. 1996;93:7826–7831. doi: 10.1073/pnas.93.15.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]