Abstract
The transcriptomes of vancomycin intermediate-resistance Staphylococcus aureus (VISA) clinical isolates HIP5827 and Mu50 (MIC = 8 μg/ml) were compared to those of highly vancomycin-resistant S. aureus (VRSA; MIC = 32 μg/ml) passage derivatives by microarray. There were 35 genes with increased transcription and 16 genes with decreased transcription in common between the two VRSAs compared to those of their VISA parents. Of the 35 genes with increased transcription, 15 involved purine biosynthesis or transport, and the regulator (purR) of the major purine biosynthetic operon (purE-purD) was mutant. We hypothesize that increased energy (ATP) is required to generate the thicker cell walls that characterize resistant mutants.
Vancomycin is the treatment of choice for serious infections caused by oxacillin-resistant Staphylococcus aureus, and some isolates are reported that are susceptible only to this antibiotic (8, 12). Oxacillin-resistant S. aureus isolates with reduced susceptibility to vancomycin (vancomycin intermediate-resistance S. aureus [VISA] isolates; the vancomycin MIC increases from 1 to 8 μg/ml) have been recovered from patients receiving prolonged courses of vancomycin (1-3). A number of abnormalities have been noted among VISA isolates, including reduced rate of growth, decreased cell wall cross-linking (6, 16), increased cell wall thickness (4, 10), decreased autolysis (17), changes in penicillin binding proteins (6, 9, 15), and alterations in glutamate amidation of the peptidoglycan stem peptide (5). No unifying molecular hypothesis has been proposed that explains the VISA phenotype, but the current hypothesis is that the thickened, poorly cross-linked cell wall provides more peripheral targets for vancomycin, trapping the antibiotic before it can reach its site of lethal action at the cell membrane (17). It is also assumed that because of the long clinical vancomycin exposure times required to generate resistance, multiple genes and, probably, multiple metabolic pathways have been altered.
We sought to assess genomic changes in gene transcription (the transcriptome) by DNA microarray and took a number of approaches different from those taken by others in trying to understand genomic changes associated with the VISA phenotype. First, we used clinical VISA isolates as parents rather than vancomycin-susceptible laboratory strains. There is evidence that there is something unique about VISA isolates that allows them to become resistant to vancomycin more easily than other S. aureus isolates (13). Second, we sought to amplify the phenotype by continued exposure of VISA isolates to vancomycin in vitro, further increasing the vancomycin MIC. We hypothesized that in this way, we would create isogenic strain sets that exaggerate the changes present in clinical VISA isolates. Third, we compared stable mutants of VISA strains for which vancomycin MICs were increased and we did not grow strains in the presence of the antibiotic, removing any direct effect of the antibiotic on transcription.
Generation of mutants.
Mutants were generated from the clinical VISA isolates HIP5827 (vancomycin MIC = 8 μg/ml) and MU50 (8 μg/ml) by streaking 10 μl of an overnight culture onto vancomycin-containing brain heart infusion gradient plates (0 to 8 μg of vancomycin/ml) and incubating the plates overnight at 37°C. Colonies that grew at the highest concentration of vancomycin were grown in subcultures in broth containing a similar concentration of vancomycin. This procedure was repeated with increasing gradient concentrations of vancomycin until mutants for which the stable vancomycin MIC was 32 μg/ml were obtained for HIP5827 (mutant VP-32) and MU50 (mutant MU50-32), which usually required no more than three gradients (three vancomycin passages). In addition, a more susceptible derivative of HIP5827 (P-100; MIC = 2 μg/ml) was obtained by 100 serial passages in the absence of vancomycin. The MICs for all VISA derivatives and parents were determined by E-test strips and confirmed each time the strains underwent further analysis.
The growth characteristics of P-100, VP-32, Mu50-32, and both parents were examined in brain heart infusion broth without antibiotics. VP-32 and Mu50-32 grew more slowly in the absence of the antibiotic than did P-100, HIP5827, or Mu50. VP-32 and Mu50-32 required 9 h to reach an optical density at 600 nm (OD600) of 0.75, while HIP5827, P-100, and Mu50 reached this OD in 4 h. Growth curves as measured by OD were confirmed by determining viable cell counts at intervals during growth in the absence and presence of vancomycin for all strains.
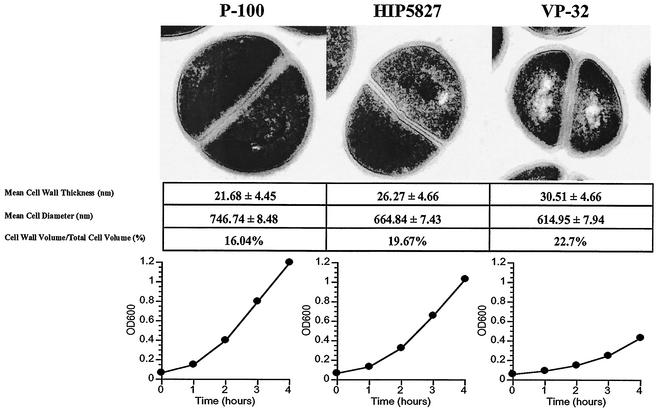
Transmission electron microscopy (EM) was performed on VP-32, P-100, and HIP5827 to assess cell wall thickness. Parent strains and their resistant derivatives were prepared for EM using standard procedures, as described by Cui et al. (4). Examples of a single cell for each isolate are shown in Fig. 1. The cell wall diameters of 30 to 40 individual cells for each isolate were measured with ImagePro (MediCybernetics, Silver Spring, Md.) software. Only cells that could be measured at three different points were selected. The mean values (in nanometers ± standard deviations) for cell wall diameters were 21.68 ± 4.45 (P-100), 26.27 ± 4.66 (HIP5827), and 30.51 ± 4.66 (VP-32). The cell wall diameter measurements were significantly different among all three strains by analysis of variance (P < 0.001). The total cellular diameters and cytoplasmic diameters were measured from EMs and converted to values for the total cellular and cytoplasmic volumes. Subtraction of these values yielded the cell wall volumes. The ratios of the cell wall volumes to the total cellular volumes were 16.04% for P-100, 19.67% for HIP5827, and 22.70% for VP-32.
FIG. 1.
Physical characteristics of P-100, HIP5827, and VP-32. The upper panel shows the three isolates as examined by transmission EM, demonstrating the progressive increase in cell wall thickness of the three strains as they become more resistant to vancomycin. The measurements of cell wall thickness, total cellular volume (nanometers ± standard deviations), and the cell wall volume-to-total cellular volume ratio are shown below the EMs. The cell wall thickness and total cellular thickness were measured directly on EMs and converted to volumes using the formula for the volume of a sphere (4/3 ○r3). The differences were all statistically significant (P < 0.001). The lower panel illustrates the progressively slower growth rate of each strain in the absence of antibiotics as the vancomycin MIC increased. Growth rates were determined by the changes in OD600 (vertical axis) with time (horizontal axis).
Cell wall composition was determined as described previously (6, 18). Separation of muropeptides was achieved by reversed-phase high-performance liquid chromatography using a Hewlett Packard 1100 series system. The degree of cross-linking of muropeptides was calculated as described by Strandén et al. (18) by the formula 0.5 × muropeptide dimers (percent) + 0.67 × muropeptide trimers (percent) + 0.9 × muropeptide oligomers (percent). Increasing resistance to vancomycin was associated with decreased cross-linking. The degree of cross-linking was 81.97% for P-100, 79.49% for HIP5827, and 78.32% for VP-32.
Microarray transcriptional profiling of mutants.
The entire microarray procedure is described in detail elsewhere (http://www.tigr.org/microarray/Vanco_Paper/). In brief, 2,688 unique PCR products (from 50 to 1,200 bp) representing 98.7% of the 2,723 open reading frames (ORFs) composing the COL genome being sequenced at The Institute for Genomic Research were printed onto UltraGAPS slides (Corning Life Sciences, Acton, Mass.) by means of a Molecular Dynamics Generation III array spotter (Amersham Biosciences, Piscataway, N.J.). Total RNA was extracted from mechanically disrupted S. aureus cells grown to mid-log phase without antibiotics. RNA (2 μg) was used for indirect labeling with either Cy3 or Cy5 dyes, leading to production of 3 μg of cDNA with 170 pmol of dye molecule incorporated per microgram of cDNA produced. TIFF images of the hybridized arrays were analyzed using TIGR-Spotfinder (http://www.tigr.org/software/) software, the data set was normalized by applying the LOWESS algorithm (block mode; smooth parameter: 0.33) and using TIGR-MIDAS (http://www.tigr.org/software/) software, and significant changes were identified with SAM (significance analysis of microarrays; http://www-stat.stanford.edu/∼tibs/SAM/index.html) software (19). Several controls were employed to ensure that the data obtained were of good quality. First, each ORF was present in duplicate on the array. Second, three independent RNA batches per strain were used. Third, the quality of the RNA samples was checked in self-hybridization experiments. Finally, each RNA preparation was used to make probes for at least two separate arrays for which the incorporated dye was reversed (dye flip). To compare the results obtained for P-100/VP-32 to those obtained for Mu50/Mu50-32, the same parameters were used for both pairs during the SAM analysis: number of permutations, 1,000; median number of falsely called significant genes, 4.6; median false discovery rate, 3%.
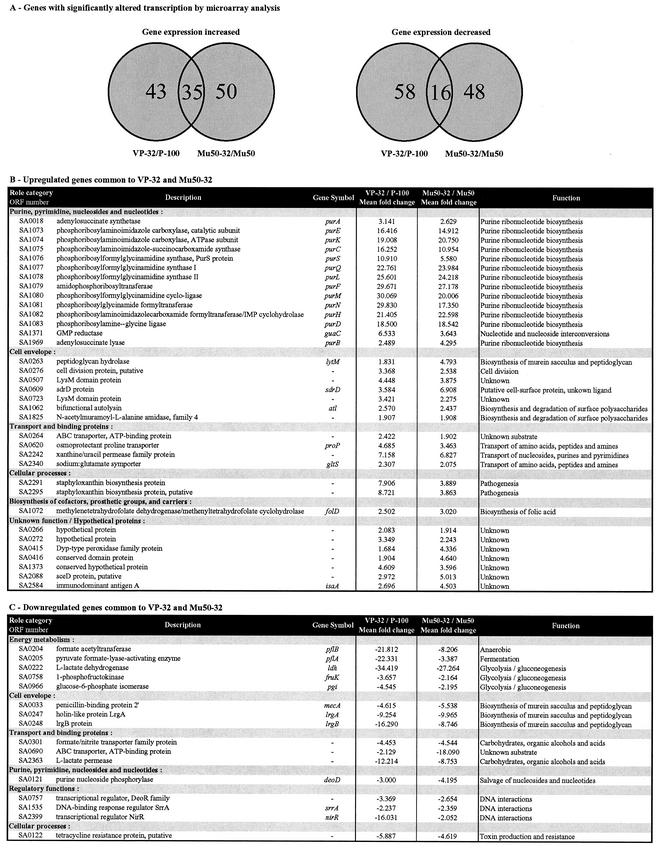
There were no differences in transcription between P-100 and HIP5827, and comparisons of each with VP-32 yielded similar data. The data in Fig. 2 show the P-100/VP-32 comparisons. Although there were approximately 150 genes with altered expression in each strain pair, only 51 genes were common to both P-100/VP-32 and Mu50/Mu50-32 (Fig. 2A): 35 genes with increased expression (Fig. 2B) and 16 with decreased expression (Fig. 2C). Those genes significantly altered in either VP-32 or Mu50-32 but not altered in the other strain are presented as supplemental data at http://www.tigr.org/microarray/Vanco_Paper/. The largest increases in transcription (from 16- to 30-fold for VP-32 and from 11- to 24-fold for Mu50-32) were seen in the 11-gene de novo purine biosynthesis operon purED (Fig. 2 and 3). The transcription of 13 out of 16 genes involved in purine biosynthesis in S. aureus (purE-purD, purA [SA0018], and purB [SA1969]) was increased in the vancomycin-resistant S. aureus strains compared to that of the genes in their isogenic parents as well as that of a xanthine/uracil permease family gene (SA2242). In addition, seven cell wall-associated genes were found to have their expression increased in VP-32 as well as in Mu50-32: three encoding peptidoglycan hydrolases (lytM, atl, and one for an N-acetylmuramoyl-l-alanine amidase family protein [SA0276]); two LysM domain protein genes (LysM domains are found in enzymes involved in bacterial cell wall degradation); one encoding a putative cell division protein (SA1062); and sdrD, encoding a protein tethered to the cell wall (7). No significant changes were seen in transcription of genes in P-100, HIP5827, and VP-32 when each strain was grown in the absence and presence of vancomycin.
FIG.2.
Significant alterations in transcription by microarray analysis. The pairs of isolates compared included P-100 versus VP-32 and Mu50 versus Mu50-32. (A) Venn diagram of genes with significantly altered transcription as determined by microarray analysis. The outside numbers are the numbers of genes with altered transcription that are unique to each comparison pair. The numbers in the overlap regions are the numbers of genes with altered transcription that are common to both comparisons. (B) Genes with increased transcription in common between VP-32 and Mu50-32. (C) Genes with decreased transcription in common between VP-32 and Mu50-32. The ORF numbers given correspond to the TIGR locus designation as detailed for the S. aureus COL genome (available at http://www.tigr.org).
FIG. 3.
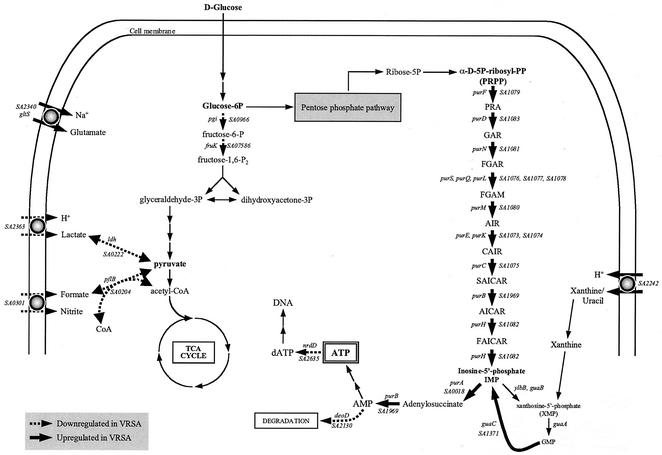
Proposed metabolic pathways with altered transcription for the highly vancomycin-resistant isolates VP-32 and Mu50-32 as determined by microarray analysis. Genes with increased transcription are indicated by solid arrows. Genes with decreased transcription are indicated by dashed arrows. VRSA, vancomycin-resistant S. aureus; TCA, tricarboxylic acid.
The transcription of purM and the xanthine/uracil permease gene was quantified by real-time reverse transcription-PCR with both VP-32 and Mu50-32 and compared to that seen with their parents. gyrA and 16S rRNA were amplified as control and standard, respectively. Amplification and detection of specific products was performed with an ABI Prism 7700 sequence detection system (PE Applied Biosystems) as previously described (11). The primer sequences used are available at http://www.tigr.org/microarray/Vanco_Paper/. Transcription of purM increased 127-fold in VP-32 and 477-fold in Mu50-32 in comparison to that of the gene in their parents. Transcription of the xanthine/uracil permease family protein gene increased 7.0- and 6.4-fold in VP-32 and Mu50-32, respectively. This compares to the 31- and 20-fold (purM) and 7.2- and 6.8-fold (xanthine/uracil permease gene) increases found by microarray for VP-32 and Mu50-32, respectively. In similarity to the microarray result, there was no difference in gyrA transcription in either strain.
Transcription of the purED operon of Bacillus subtilis is under the partial control of PurR, a repressor that binds to two specific pur boxes within the promoter-operator region of purE (14, 20). Under the assumption that increased transcription of purED in VP-32 and Mu50-32 is related to changes in purR, we sequenced purR and the upstream region of purE. The set of primers used for the PCR amplification of purR and purE is available at http://www.tigr.org/microarray/Vanco_Paper/. The upstream region of purE showed no evidence of mutations in comparison to that of its isogenic parent isolate for either VP-32 or Mu50-32. A single base pair substitution was identified in both VP-32 and Mu50-32 at nucleotide 140 of purR (T → A), producing a single amino acid change (isoleucine → lysine).
Conclusions.
In the present study we showed that when VISA strains were made more vancomycin resistant by in vitro passage, the phenotypic changes that distinguished VISA from vancomycin-susceptible strains (increased cell wall thickness, decreased cell wall cross-linking, and reduced growth rate) were greatly exaggerated, providing sets of isogenic mutants that were suitable for transcriptional profiling. An analysis of the transcriptomes of two passage-generated, highly vancomycin-resistant VISA isolates compared to those of their parents revealed a striking increase in transcription (from 15- to 30-fold) for each gene in an 11-gene purine biosynthetic operon (confirmed by real-time PCR and associated with a mutation in the operon's regulator [purR]). The finding that the transcription of other genes in the purine biosynthetic pathway (e.g., purA, purB, guaC, and a xanthine/uracil permease family protein gene), unlinked to the purE-purD operon and not regulated by purR, was also increased provides some support for the contention that there was a true increase in purine biosynthesis in the vancomycin-resistant mutants.
Explanations for the increase in purine biosynthesis are speculative at this point but we can offer the following hypothesis that is consistent with microarray data. As shown in Fig. 3, metabolic alterations are directed toward increased production of AMP. AMP can be degraded via deoD, phosphorylated to produce ATP for further energy use, or reduced to dATP by nrdD and incorporated into nucleic acids. The transcription of both deoD and nrdD is decreased in vancomycin-resistant derivatives, suggesting that AMP is shunted toward ATP for use as an energy source. The biosynthesis of polymers is one of the most energetically demanding of all cellular processes, and one of the most abundant large polymers in gram-positive bacteria is peptidoglycan. The ratio of cell wall volume to total cell volume in VP-32 was increased 41% over that measured for P-100. The demands for a 41% increase in cell wall content in vancomycin-resistant cells would require a large increase in ATP generation for energy. ATP generation through substrate-level phosphorylation would likely be reduced as cells became more vancomycin resistant, because precursor metabolites would be shunted to cell wall construction. An increase in purine biosynthesis, therefore, would supply more AMP for phosphorylation by remaining pathways. Markedly slowed cellular growth could be either a symptom of the cell's energy deficiency or another factor that contributes to decreased ATP production.
An additional group of genes with increased transcription in the vancomycin-resistant passage derivatives were those involved in cell wall hydrolysis. An absolute increase in cell wall hydrolytic enzyme activity may be required to accommodate the insertion of new cell wall subunits into the growing peptidoglycan polymer.
The results of this analysis provide new insights into the demands that resistance to vancomycin places on the cell. Progressive increases in resistance to vancomycin that occur by mutation and selection are accompanied by increases in cell wall thickness and in the transcription of genes involved in purine biosynthesis and cell wall autolysis to meet the energetic and constructive demands of new cell wall biosynthesis. The costs to the cell of using these mechanisms for resistance may explain why this phenotype is relatively uncommon despite the abundance of vancomycin in the clinical environment.
Acknowledgments
The contribution of K. E. Nelson to the understanding of metabolic alterations in the vancomycin-resistant S. aureus strains is gratefully acknowledged.
This work was supported in part by NIH grants R-37AI 35705 (G. L. Archer) and U-01AI 45667 (S. Gill) and VA Merit Grant 0010 (M. W. Climo).
E. Mongodin and J. Finan contributed equally to this project.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2000. Staphylococcus aureus with reduced susceptibility to vancomycin—Illinois, 1999. JAMA 283:597-598. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2000. Staphylococcus aureus with reduced susceptibility to vancomycin—Illinois, 1999. Morb. Mortal. Wkly. Rep. 48:1165-1167. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin-United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 4.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M. N. Kim, M. C. Ploy, N. El Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui, L., H. Murakami, K. Kuwahara-Arai, H. Hanaki, and K. Hiramatsu. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finan, J. E., G. L. Archer, M. J. Pucci, and M. W. Climo. 2001. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josefsson, E., K. W. McCrea, D. Ni Eidhin, D. O'Connell, J. Cox, M. Hook, and T. J. Foster. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 144:3387-3395. [DOI] [PubMed] [Google Scholar]
- 8.McGahee, W., and F. D. Lowy. 2000. Staphylococcal infections in the intensive care unit. Semin. Respir. Infect. 15:308-313. [DOI] [PubMed] [Google Scholar]
- 9.Moreira, B., S. Boyle-Vavra, B. L. deJonge, and R. S. Daum. 1997. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1788-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeltz, R. F., V. K. Singh, J. L. Schmidt, M. A. Batten, C. S. Baranyk, M. J. Nadakavukaren, R. K. Jayaswal, and B. J. Wilkinson. 2000. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob. Agents Chemother. 44:294-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosato, A. E., W. A. Craig, and G. L. Archer. 2003. Quantitation of mecA transcription among oxacillin-resistant Staphylococcus aureus clinical isolates. J. Bacteriol. 185:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz, M. E., I. C. Guerrero, and C. U. Tuazon. 2002. Endocarditis caused by methicillin-resistant Staphylococcus aureus: treatment failure with linezolid. Clin. Infect. Dis. 35:1018-1020. [DOI] [PubMed] [Google Scholar]
- 13.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46:1492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin, B. S., A. Stein, and H. Zalkin. 1997. Interaction of Bacillus subtilis purine repressor with DNA. J. Bacteriol. 179:7394-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieradzki, K., M. G. Pinho, and A. Tomasz. 1999. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J. Biol. Chem. 274:18942-18946. [DOI] [PubMed] [Google Scholar]
- 16.Sieradzki, K., and A. Tomasz. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J. Bacteriol. 181:7566-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strandén, A. M., K. Ehlert, H. Labischinski, and B. Berger-Bächi. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng, M., and H. Zalkin. 2000. Mutations in the Bacillus subtilis purine repressor that perturb PRPP effector function in vitro and in vivo. Curr. Microbiol. 41:56-59. [DOI] [PubMed] [Google Scholar]