Abstract
We show that the MutY protein competes with the MutS-dependent mismatch repair system to process at least some A · C mispairs in vivo, converting them to G · C pairs. In the presence of an increased dCTP pool resulting from the loss of nucleotide diphosphate kinase, the frequency of A · T→G · C transitions at a hot spot in the rpoB gene is 30-fold lower in a MutY-deficient derivative than in the wild type.
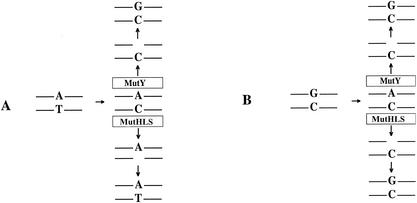
The MutY protein, the product of the mutY gene in Escherichia coli (18), is a glycosylase that plays an important role in the repair of oxidatively damaged DNA (11, 12). Loss of function in both chromosomal copies of the human gene encoding MutY leads to increased susceptibility to colon cancer in humans (1, 9). MutY removes A residues that are mispaired with 7,8-dihydro-8-oxoguanine (oxoG) (11, 13), a frequent oxidation product of DNA (4). This removal allows repair polymerases to restore a C across from the 8-oxoG (25), allowing the MutM protein to remove the 8-oxoG (11, 13; for reviews, see references 12 and 15). Subsequent repair synthesis restores the original G · C base pair. MutY also removes the A from A · G mispairs (2), from A · 8-oxoA mispairs (13), and, to a lesser extent, from A · C mispairs (13, 21). Because the MutY protein does not discriminate between old and new strands, the ability to remove A from A · C mispairs may potentially immortalize mutations stemming from A · C mispairs in which A is the correct base, even though these mispairs may be substrates for correction by the MutSHL-dependent mismatch repair (MMR) system (for a review, see reference 17). In these cases, the original A · T base pair would be converted to a G · C base pair. In this sense, the MutY protein competes with the MMR system for the processing of A · C mispairs. Figure 1 portrays the different outcomes of A · C mispairs arising from replication errors at an A · T base pair.
FIG. 1.
Competition between the MutY protein and the MutHLS MMR system. (A) Misreplication at an A · T base pair leads to an A · C mispair that is corrected by the MutHLS MMR system but converted to a G · C pair by MutY. (B) Misreplication at a G · C base pair leads to an A · C mispair that is converted back to a G · C pair by both MutY and the MMR system.
The E. coli rpoB/Rifr system.
We decided to study the contribution of the MutY protein to A · T→G · C mutations that can arise via an A · C mispair by using the previously described E. coli rpoB/Rifr system to monitor mutations (7). We have extended the work of others (8, 19, 22-24) to generate a system that can analyze 69 different base substitutions in the rpoB gene (7). Recent work (20; E. Wolff, M. Kim, and J. H. Miller, unpublished data) has added several sites to this collection, so that as many as 73 different base substitutions can be monitored by analyzing E. coli Rifr mutants at 37°C (Table 1). Of these 73 mutations, one particular A · T→G · C mutation, at bp 1547, is a hot spot in a wild-type background and a very strong hot spot in a mutS background (7, 16). Although it is not clear what proportion of the A · T→G · C transition mutations at this site result from A · C rather than T · G mispairs, comparing the rates of mutations at this hot spot in wild-type and mutY backgrounds seems to be a straightforward way to look for effects of the presence or absence of MutY on transitions. However, G · C→T · A mutations are elevated in a mutY background, significantly increasing the Rifr mutant frequencies (17) (Table 2). Only a small percentage of the Rifr mutants would be caused by other mutations. Thus, 13 of 15 mutations in rpoB obtained in a mutY strain result from G · C→T · A transversions (Table 1). Therefore, we sought to increase the level of A · C mispairs by employing a strain with a defect in the ndk gene (16), which encodes the enzyme nucleotide diphosphate kinase. Nucleotide diphosphate kinase is involved in maintaining nucleotide triphosphate levels, and ndk mutant strains have 20-fold-higher levels of dCTP and 7-fold-higher levels of dGTP, as well as higher levels of spontaneous mutations (10), than do strains without a defect in the ndk gene. We have analyzed the mutator effect of ndk strains and shown that certain base substitutions and frameshifts are considerably elevated in ndk mutS double mutants, indicating that replication errors are involved (16). Among mutations in rpoB leading to Rifr, the mutations in both ndk and ndk mutS strains predominate at the A · T→G · C hot spot at bp 1547 (16). Because the level of mutations at this hot spot in ndk strains is high enough, we can determine whether the MutY protein is involved in generating A · T→G · C mutations at the bp 1547 hot spot by comparing the levels of mutations at this site in ndk and ndk mutY strains (for methods, see references 7 and 14).
TABLE 1.
Distribution of mutation in rpoB
| Mutation | Site (bp) | Amino-acid change | No. of strains with rpoB mutation
|
|||||
|---|---|---|---|---|---|---|---|---|
| WT | mutS mutant | mutS mutY double mutant | mutY mutant | ndk mutant | ndk mutY double mutant | |||
| AT → GC | 443 | Q148R | 5 | 0 | 0 | 0 | 1 | 0 |
| 1522 | S508P | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1532 | L511P | 2 | 7 | 7 | 0 | 2 | 0 | |
| 1534 | S512P | 9 | 11 | 7 | 0 | 2 | 3 | |
| 1538 | Q513R | 0 | 3 | 0 | 0 | 1 | 0 | |
| 1547 | D516G | 23 | 48 | 17 | 1 | 70 | 2 | |
| 1552 | N518D | 1 | 3 | 4 | 0 | 0 | 0 | |
| 1577 | H526R | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1598 | L533P | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1703a | N568S | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1715 | I572T | 0 | 0 | 0 | 0 | 0 | 0 | |
| GC → AT | 1520 | G507D | 0 | 0 | 0 | 0 | 0 | 0 |
| 1535 | S512F | 4 | 0 | 0 | 0 | 0 | 0 | |
| 1546 | D516N | 3 | 9 | 1 | 0 | 0 | 0 | |
| 1565 | S522F | 3 | 0 | 0 | 0 | 0 | 0 | |
| 1576 | H526Y | 7 | 0 | 0 | 1 | 0 | 0 | |
| 1585 | R529C | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1586 | R529H | 2 | 1 | 0 | 0 | 0 | 0 | |
| 1592 | S531F | 5 | 1 | 0 | 0 | 0 | 0 | |
| 1595 | A532V | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1600 | G534S | 0 | 3 | 0 | 0 | 0 | 0 | |
| 1601 | G534D | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1610a | G537D | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1691 | P564L | 4 | 0 | 0 | 0 | 0 | 0 | |
| 1721 | S574F | 4 | 0 | 0 | 0 | 0 | 0 | |
| 2060b | R687H | 0 | 0 | 0 | 0 | 0 | 0 | |
| AT → TA | 443 | Q148L | 18 | 0 | 0 | 0 | 6 | 18 |
| 1532 | L511Q | 1 | 0 | 0 | 0 | 3 | 0 | |
| 1538 | Q513L | 7 | 0 | 0 | 0 | 0 | 0 | |
| 1547 | D516V | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1568 | E523V | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1577 | H526L | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1598 | L533H | 2 | 0 | 0 | 0 | 0 | 0 | |
| 1714 | I572F | 4 | 0 | 0 | 0 | 0 | 0 | |
| 1715 | I572N | 1 | 0 | 0 | 0 | 0 | 0 | |
| AT → CG | 437 | V146G | 0 | 0 | 0 | 0 | 0 | 0 |
| 443 | Q148P | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1525 | S509R | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1532 | L511R | 3 | 0 | 0 | 0 | 1 | 0 | |
| 1534 | S512A | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1538 | Q513P | 0 | 0 | 0 | 0 | 0 | 2 | |
| 1547 | D516A | 0 | 0 | 0 | 0 | 0 | 1 | |
| 1577 | H526P | 1 | 0 | 0 | 0 | 0 | 1 | |
| 1598 | L533R | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1687 | T563P | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1714 | I572L | 14 | 0 | 0 | 0 | 1 | 0 | |
| 1715 | I572S | 4 | 0 | 0 | 0 | 2 | 0 | |
| GC → TA | 436 | V146F | 0 | 0 | 1 | 0 | 0 | 0 |
| 442 | Q148K | 0 | 0 | 0 | 1 | 0 | 0 | |
| 444 | Q148H | 1 | 0 | 0 | 2 | 0 | 0 | |
| 1527 | S509R | 2 | 0 | 0 | 2 | 0 | 0 | |
| 1535 | S512Y | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1537 | Q513K | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1546 | D516Y | 2 | 0 | 0 | 0 | 0 | 0 | |
| 1565 | S522Y | 0 | 0 | 0 | 1 | 0 | 0 | |
| 1576 | H526N | 2 | 0 | 0 | 0 | 0 | 0 | |
| 1578 | H526Q | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1586 | R529L | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1592 | S531Y | 0 | 0 | 0 | 2 | 0 | 0 | |
| 1595 | A532E | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1600 | G534C | 3 | 0 | 0 | 2 | 0 | 0 | |
| 1601 | G534V | 1 | 0 | 0 | 1 | 0 | 0 | |
| 1708 | G570C | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1721 | S574Y | 2 | 0 | 0 | 2 | 0 | 0 | |
| 1585b | R529S | 0 | 0 | 0 | 0 | 0 | 0/PICK> | |
| GC → CG | 444 | Q148H | 0 | 0 | 0 | 0 | 0 | 0 |
| 1527 | S509R | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1574 | T525R | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1576 | H526D | 3 | 0 | 0 | 0 | 0 | 0 | |
| 1578 | H526Q | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1585 | R529G | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1601 | G534A | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1691 | P564R | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1709 | G570A | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1716 | I572M | 0 | 0 | 0 | 0 | 0 | 0 | |
| None found | 4 | 0 | 0 | 0 | 0 | 0 | ||
| Totalc | 152 | 86 | 37 | 15 | 90 | 27 | ||
Sites that have been detected in a related study (J. H. Miller, E. Wolff, and M. Kim, unpublished data).
Sites that show temperature effects (8) between 30 and 42°C and that may not yield Rifr colonies at 37°C.
TABLE 2.
rpoB frequencies and notes of mutationsa
| Strain | Mean value (95% CL)b
|
|
|---|---|---|
| f (10−8) | μ (10−8) | |
| WTc | 7.6 (5.2-8.8) | 1.5 (1.1-1.7) |
| mutY mutant | 79 (68-90) | 11 (7.4-12) |
| ndk mutant | 570 (530-630) | 64 (60-70) |
| mutS mutant | 935 (848-1,110) | 120 (110-140) |
| ndk mutY mutant | 206 (126-280) | 26 (17-34) |
The rpoB mutation frequencies (f) per cell were calculated by dividing the median number of mutants by the average number of cells in a series of cultures, and the mutation rates (μ) per replication were calculated from these values by the method of Drake (5).
CL, confidence limits (3).
WT, wild type.
Distribution of mutations in rpoB.
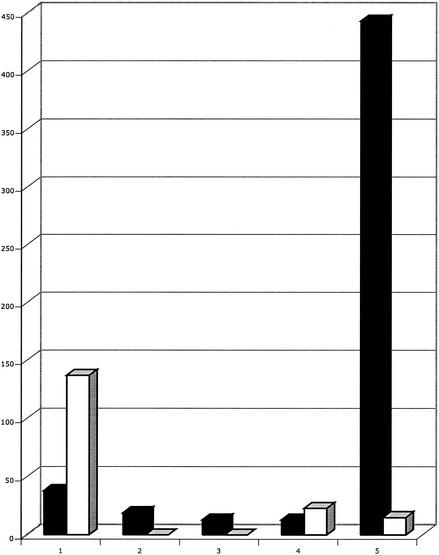
Table 1 shows the distribution of rpoB mutations in wild-type, mutS, mutY, and ndk strains and in the ndk mutY and mutS mutY double mutants. Table 2 shows some comparative mutation frequencies and mutation rates per replication. ndk strains have 43-fold-higher rates of rpoB mutations that lead to Rifr colonies than the wild type, and mutS strains have approximately a 80-fold-higher mutation rate (Table 2) than the wild type, although most of the mutations in the ndk and mutS strains are at one site (position 1547; A · T→G · C) (Table 1). The defect in mutY has no detectable effect on the mutation rate in mutS strains (data not shown) and no effect on the mutS spectrum (Table 1; note the different sample sizes). This lack of effect occurs because in the absence of MMR, which is the consequence of being a mutS mutant, there is no way to repair A · C mismatches, derived from A · T base pairs (Fig. 1A), regardless of the presence or absence of the MutY protein. However, in an ndk strain that is MMR proficient, the defect in the mutY gene lowers the frequency of mutations in rpoB 2.5- to 3-fold (Table 2) and, most importantly, virtually eliminates the position 1547 A · T → G · C hot spot from the mutational spectrum (Table 1). In the absence of this hot spot, the next most frequent mutation, A · T→T · A at position 443, now becomes the most frequent mutation and appears as a new hot spot. Figure 2 incorporates the mutation frequencies into the comparison of the ndk and ndk mutY spectra and shows that MutY-deficient derivatives of ndk strains have 30-fold-lower levels of A · T→G · C mutations at position 1547 in rpoB. (The apparent severalfold increase of A · T→T · A mutations at position 443 may not be significant, because of the small sample size of these mutations at position 443 in the distribution for the ndk mutants.) This finding demonstrates an in vivo effect of MutY on increasing transition mutations at certain A · T base pairs due to its ability to remove A from A · C mispairs and represents an example of a repair enzyme actually being involved in creating mutations under certain conditions. The effect of MutY on lowering A · T→G · C transversions in a mutT background has been described previously (6, 26), as has a 4.6-fold decrease in A · T→G · C mutations at one site in trpA (6).
FIG. 2.
Comparative mutation frequencies in ndk and ndk mutY strains. The frequencies of mutations in the rpoB gene at five different sites are shown for both ndk (white bars) and ndk mutY (black bars) backgrounds (see also Tables 1 and 2). The five sites (left to right) are as follows: 1, A · T→T · A at bp 443; 2, A · T→T · A at bp 1532; 3, A · →G · C at bp 1532; 4, A · T→G · C at bp 1534; 5, A · T→G · C at bp 1547.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (grant ES0110875).
REFERENCES
- 1.Al-Tassan, N., N. H. Chmiel, J. Maynard, N. Fleming, A. L. Livingston, G. T. Williams, A. K. Hodges, D. R. Davies, S. S. David, J. R. Sampson, and J. P. Cheadle. 2002. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nature 30:227-232. [DOI] [PubMed] [Google Scholar]
- 2.Au, K. G., L. Clark, J. H. Miller, and P. Modrich. 1989. The Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc. Natl. Acad. Sci. USA 86:8877-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon, W. J., and F. J. Massey, Jr. 1969. Introduction to statistical analysis. McGraw-Hill, New York, N.Y.
- 4.Dizdaroglu, M. 1985. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry 24:4476-4481. [DOI] [PubMed] [Google Scholar]
- 5.Drake, J. W. 1991. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl. Acad. Sci. USA 88:7160-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler, R. G., S. J. White, C. Koyama, S. C. Moore, R. L. Dunn, and R. M. Schaaper. 2003. Interactions among the Escherichia coli mutT, mutM, and mutY damage prevention pathways. DNA Repair 2:159-173. [DOI] [PubMed] [Google Scholar]
- 7.Garibyan, L., T. Huang, M. Kim, E. Wolff, A. Nguyen, T. Nguyen, A. Diep, K. Hu, A. Iverson, H. Yang, and J. H. Miller. 2003. Use of the rpoB to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair 2:593-608. [DOI] [PubMed] [Google Scholar]
- 8.Jin, D. J., and C. A. Gross. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45-58. [DOI] [PubMed] [Google Scholar]
- 9.Jones, S., P. Emmerson, J. Maynard, J. M. Best, S. Jordan, G. T. Williams, J. R. Sampson, and J. P. Cheadle. 2002. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum. Mol. Genet. 11:2961-2967. [DOI] [PubMed] [Google Scholar]
- 10.Lu, Q., X. Zhang, N. Almaula, C. K. Mathews, and M. Inouye. 1995. The gene for nucleoside diphosphate kinase functions as a mutator gene in Escherichia coli. J. Mol. Biol. 254:337-341. [DOI] [PubMed] [Google Scholar]
- 11.Michaels, M. L., C. Cruz, A. P. Grollman, and J. H. Miller. 1992. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc. Natl. Acad. Sci. USA 89:7022-7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaels, M. L., and J. H. Miller. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 174:6321-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels, M. L., J. Tchou, A. P. Grollman, and J. H. Miller. 1992. A repair system for 8-oxo-7,8-dihydrodeoxyguanine. Biochemistry 31:10964-10968. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Miller, J. H. 1998. The “GO” system in Escherichia coli, p. 97-105. In J. A. Nikoloff and M. F. Hoekstra (ed.), DNA damage and repair, vol. 1. Humana Press, Totowa, N.J.
- 16.Miller, J. H., P. Funchain, W. Clendenin, T. Huang, A. Nguyen, E. Wolff, A. Yeung, J. Chiang, L. Garibyan, M. M. Slupska, and H. Yang. 2002. Escherichia coli strains (ndk) lacking nucleoside diphosphate kinase are powerful mutators for base substitutions and frameshifts in mismatch-repair-deficient strains. Genetics 162:5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modrich, P., and R. Lahue. 1996. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 65:101-133. [DOI] [PubMed] [Google Scholar]
- 18.Nghiem, Y., M. Cabrera, C. G. Cupples, and J. H. Miller. 1988. The mutY gene: a mutator locus in Escherichia coli that generates GC → TA transversions. Proc. Natl. Acad. Sci. USA 85:2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovchinnikov, Y. A., G. S. Monastyrskaya, S. O. Guriev, N. F. Kalinina, E. D. Sverdlov, A. I. Gragerov, I. A. Bass, I. F. Kiver, E. P. Moiseyeva, V. N. Igumnov, S. Z. Mindlin, V. G. Nikiforov, and R. B. Khesin. 1983. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol. Gen. Genet. 190:344-348. [DOI] [PubMed] [Google Scholar]
- 20.Petersen-Mahrt, S. K., R. S. Harris, and M. S. Neuberger. 2002. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 418:99-103. [DOI] [PubMed] [Google Scholar]
- 21.Radicella, J. P., E. A. Clark, and M. S. Fox. 1988. Some mismatch repair activities in Escherichia coli. Proc. Natl. Acad. Sci. USA 85:9674-9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangarajan, S., G. Gudmundsson, Z. Qiu, P. L. Foster, and M. F. Goodman. 1997. Escherichia coli DNA polymerase II catalyzes chromosomal and episomal DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 94:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds, M. G. 2000. Compensatory evolution in rifampicin-resistant Esherichia coli. Genetics 156:1471-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Severinov, K., M. Soushko, A. Goldfarb, and V. Nikiforow. 1993. Rifampicin region revisited. J. Biol. Chem. 268:14820-14825. [PubMed] [Google Scholar]
- 25.Shibutani, S., M. Takeshita, and A. P. Grollman. 1991. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349:431-434. [DOI] [PubMed] [Google Scholar]
- 26.Vidmar, J. J., and C. G. Cupples. 1993. MutY repair is mutagenic in mutT− strains of Escherichia coli. Can. J. Microbiol. 39:892-894. [DOI] [PubMed] [Google Scholar]