Abstract
Neandertal femora are distinct from contemporaneous near-modern human femora. Traditionally, these contrasts in femoral shape have been explained as the result of the elevated activity levels and limited cultural abilities of Neandertals. More recently, however, researchers have realized that many of these femoral differences may be explained by the cold-adapted bodies of Neandertals vs. the warm-adapted bodies of near-modern humans. This study explicitly tests this proposed link between climate-induced body proportions and femoral shape by considering the entire hip as a unit by using geometric morphometric methods adapted to deal with articulated structures. Based on recent human patterns of variation, most contrasts in shape between the femora of Neandertals and near-modern humans seem to be secondary consequences of differences in climate-induced body proportions. These results, considered in light of hip mechanics during growth, highlight the importance of developmental and functional integration in determining skeletal form.
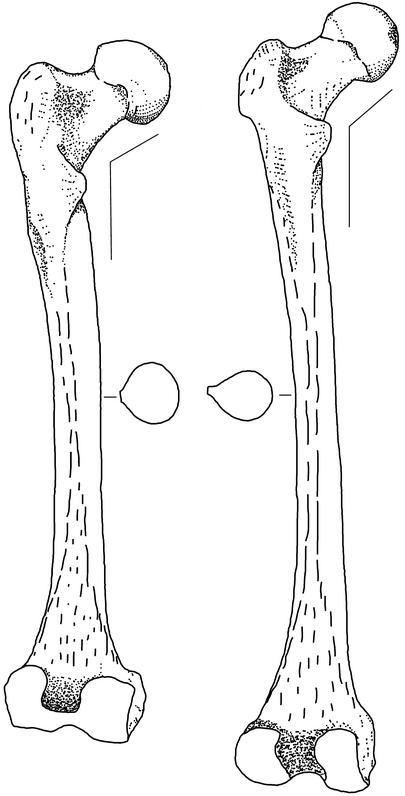
From the first detailed description of a Neandertal skeleton at the turn of the twentieth century by Marcellin Boule (1), researchers have documented a suite of features that characterize Neandertal femora. These features include large articulations relative to length, a thick, rounded shaft, and a low angle between the neck and the shaft (1–4). Traditionally, the distinctiveness of Neandertal femora has been explained as the result of elevated activity levels resulting from limited cultural sophistication (5, 6) or simpler social organization (3), in part because contemporaneous near-modern humans [fossils such as those from the sites of Qafzeh and Skhul that share many derived features with recent humans and no derived Neandertal features but variably express primitive features and are associated with Middle Paleolithic industries (7, 8)] and succeeding modern humans (associated with Upper Paleolithic industries) have femora with a contrasting set of features (refs. 3, 4, and 9; Fig. 1). The behavioral differences implied by contrasts in femoral shape are especially intriguing, because the Neandertals and near-modern humans are archaeologically virtually indistinguishable (10). More recently, however, researchers have argued that differences in femoral cross-sectional and external shaft dimensions and relative articular size between Neandertals and near-modern humans may instead result from adaptation to different climates (11–14).
Fig. 1.
Distinctive features of the Neandertal femur. (Left) The Neandertal 1 (Feldhofer Cave Neandertal) femur. (Right) The Skhul IV near-modern human femur. Relative to near-modern humans, the Neandertal femur has larger articulations (head and distal end), a thicker and rounder shaft, and a lower neck-shaft angle. Adapted from McCown and Keith (9).
Endothermic species exhibit climate-related geographical patterns in overall size and body proportions (15–17). Individuals living in cold climates tend to be larger and have shorter limbs than their warm-climate counterparts, presumably because a low ratio of surface area to mass in cold climates enhances heat retention, whereas a high ratio in warm climates facilitates heat dissipation. Researchers disagree on the mechanism, but the empirical pattern is robust for mammals (16).
Climate affects human morphology as in other animal species (18, 19); this finding is striking given that humans can use culture to buffer at least some aspects of the environment. Humans living in cold climates tend to have wide bodies, short limbs relative to their trunks, and abbreviated distal limb segments; people from warm climates show the reverse pattern (termed cold-adapted vs. warm-adapted body shapes or proportions). In the skeletal elements of the hip region (pelvis and femur), these patterns manifest themselves most strikingly in differences in absolute biiliac breadth (maximum width) of the pelvis and in ratios of biiliac breadth to femur length (19).
The Neandertal pelvis was wide relative to the length of the femur, and near-modern humans were characterized by narrower waists and longer limbs (19). Neandertal body proportions have been termed “hyperpolar” (20) or “hyperarctic” (i.e., more extreme body proportions than Inuit peoples), and they probably were an adaptation to the glacial environments of Pleistocene Europe. The near-modern contemporaries of Neandertals lived in much warmer environments, because the geographic center of their range was in Africa.
Here, I use geometric morphometric methods adapted to deal with articulated structures to explicitly test the link between climate-induced body proportions and femoral shape. From a set of anatomical landmarks, the techniques of geometric morpho-metrics allow complex changes along multivariate axes to be converted back into the original geometry of the objects and visualized. These anatomical visualizations make interpretations much easier than with traditional multivariate analysis. By linking shape variation with biomechanical knowledge, it is possible to make functional morphological interpretations.
Materials and Methods
As a baseline for comparison with fossil hominids, data were collected on a recent human sample of sacra, os coxae, and femora from 97 male individuals: 57 from warm-climate locations (10 Australian aborigines, 18 Khoi-San, 18 eastern African “Bantu,” and 11 southern African “Bantu”) and 40 from cold-climate locations (17 British, 9 Inuit, and 14 Aleutian Islanders). The fossil sample consists of two European Neandertal femora (Spy 2 right femur and Neandertal 1 left femur) and one near-modern human femur (cast made by Mario Chech of the Skhul IV right femur; ref. 21).
Using a Microscribe digitizer (Immersion Corporation, San Jose, CA), a Macintosh laptop computer, and software written by the author, the 3D coordinate locations of 39 unilateral landmarks (26 on the pelvis and 13 on the femur; see Table 1, which is published as supporting information on the PNAS web site, www.pnas.org.) were collected on matched sets of sacra, os coxae, and femora. Supplementary points were also recorded to calculate femoral head diameter and mid-shaft medio-lateral and antero-posterior thickness. Only femoral data could be collected on the fossil specimens (the sacrum and/or both os coxae were too fragmentary). For each recent human individual, the hemi-pelvis was rearticulated from separate data for the os coxa and the sacrum by using a combination of manual articulation and visual marking of points with subsequent mathematical rearticulation using these visually marked points (21).
The landmark data for the hemi-pelvis and femur were superimposed separately (unarticulated) by using generalized procrustes analysis (GPA). Then, for each individual, residuals from the mean configurations were calculated. These GPA residuals are shape variables, and they approximate coordinates in a Euclidean space tangent to Kendall's non-Euclidean shape space (a space where location, rotation, and size have been removed; ref. 22). The overall hip shape of each recent human individual (combined hemi-hip shape) was the combination of the hemi-pelvis and femur GPA residuals, the ratio of hemipelvis centroid size to femur centroid size (23), and three additional shape/robusticity variables: the ratios of femoral head diameter, mid-shaft medio-lateral thickness, and mid-shaft antero-posterior thickness to femur centroid size.
To examine shape differences, discriminant functions were calculated by using multivariate regression of the principal components of combined hemi-hip shape variables (Y) on a dummy climate variable (X) (24). The included principal components always explained >90% of the total variance (25) and did not exceed 3n – 7 components (22), where n is the number of landmarks. Using a similar approach to Adams (23), shape changes along the combined hemi-hip discriminant axis were visualized by regressing shape variables calculated from articulated and anatomically oriented hemi-hips (21) onto discriminant function scores. Trees of shape similarity were also created from UPGMA (unweighted pair group method with arithmetic mean) clustering analyses based on Euclidean distances along the same principal components as for the discriminant functions (other clustering algorithms produced similar results). In contrast to the discriminant function analyses, the geographic origins of the groups were not an input to the clustering analyses.
Clustering and discriminant function analyses that included fossil specimens were calculated by using the same methods as for the recent human combined hemi-hip analyses but from principal components of just femur shape variables (using a reduced set of 11 landmarks due to a missing superior greater trochanter on Spy 2).
All analyses were performed by using software written by the author, jmp (SAS Institute, Cary, NC), and matlab (Mathworks, Natick, MA). Based on data recollected multiple times on the same individuals (26), methodological error was found to be only a small fraction of the variation along the multivariate axes discussed below.
Results
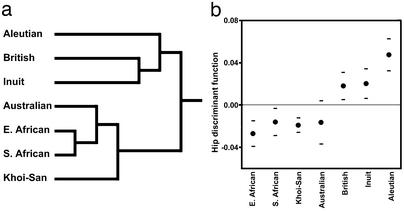
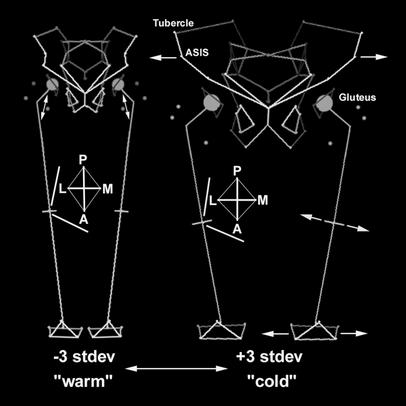
The primary split in a tree generated by a UPGMA clustering analysis of recent human hip shape corresponds to climatic zones (Fig. 2a). Using the same data, a discriminant function successfully separates recent human individuals from cold vs. warm regions; therefore, the shape changes along this axis should accurately reflect differences between cold and warm climate individuals (Fig. 2b). Individuals from cold climates tend to have relatively wide bodies as compared with individuals from warm climates, and these are the primary changes along the discriminant function axis (Fig. 3). These gross differences in body proportions also have more detailed morphological consequences. Cold-adapted individuals have femora with large femoral heads and distal ends relative to length, thick and round shafts, and low neck-shaft angles; warm-adapted individuals show the reverse pattern. These femoral changes are accompanied by pelvic changes. Individuals from cold climates tend to have wider pelvic apertures, longer pubic bones, more flared iliac blades, more laterally pointing anterior-superior iliac spines, more anteriorly located iliac tubercles, and more posteriorly rotated dorsal iliac blades relative to individuals from warm climates.
Fig. 2.
Differences in hip shape between recent human groups from warm vs. cold climates. (a) UPGMA clustering tree based on the combined hemi-hip shape variables. The major split in the tree is between individuals from warm vs. cold climates. (b) Scores along a hip shape discriminant axis that was calculated to separate groups by climate of origin. A dot signifies a group mean, and the horizontal lines are standard deviations. The horizontal, broken line is the overall sample mean. Groups from warm climates have negative scores; groups from cold climates have positive scores.
Fig. 3.
Changes along the hip discriminant function that was calculated to separate recent human groups by climate of origin. The left stick figure shows –3 (warm climate) and the right stick figure shows +3 (cold climate) SD from the sample mean along the discriminant function axis. Darker shading indicates greater depth in the stick figures. For clarity, the circles representing femoral heads have been scaled to 50% head diameter, and hemi-hip changes have been mirrored. Notice the large articulations, thicker and rounder shaft, and lower neck-shaft angle of the cold-climate femur relative to the warm-climate femur. There are pelvic differences as well.
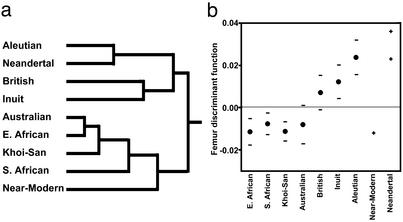
When fossil specimens are included in an analysis of femur shape (femur centroid size does not clearly separate recent humans by climatic zone), the results are as expected, given differences in body proportions between Neandertals and their near-modern human contemporaries. In a UPGMA clustering analysis (Fig. 4a) and along a discriminant function axis that separates recent human groups by climate of origin (Fig. 4b), the two European Neandertals (Neandertal 1 and Spy 2) fall with cold-climate recent humans. Interestingly, the Neandertals link with Aleutian Islanders (instead of forming an out-group within the cold-climate cluster), suggesting that Neandertals are not particularly extreme in femur shape. Holliday (18) obtained similar results for certain measures of body shape (e.g., femur length relative to skeletal trunk height), but he concluded that, in aggregate, Neandertal bodies are hyperpolar. The near-modern human (Skhul IV) is similar to warm-climate groups. Other more fragmentary fossil specimens are morphologically consistent with the specimens analyzed here, but they are not complete enough to be examined in multivariate space.
Fig. 4.
Fossil hominid femur shape. (a) UPGMA clustering tree based on femur shape variables. The Neandertals cluster with cold-climate recent humans, and the near-modern human clusters with warm-climate groups. (b) Scores along a femur discriminant function that was calculated to separate recent humans by climate of origin. For the recent humans, dots signify group means, and horizontal lines are SD. For the fossil specimens, each “+” represents an individual specimen. The horizontal, broken line is the overall sample mean. The Neandertals plot near Aleutian islanders (Spy 2 above Neandertal 1), and the near-modern human plots alongside warm-climate groups.
Discussion
Differences in relative joint size and shaft dimensions of the femur between individuals with cold-adapted and warm-adapted bodies seem to be either the direct consequence of variation in relative body weight or secondary consequences of the interaction between body proportions and the magnitude of mechanical stress (12, 14, 18, 19). However, explaining the link between body proportions and femoral neck-shaft angle is more complicated. Neck-shaft angle changes during development: newborns start out with high angles that decrease over time to reach lower adult angles (3, 27, 28). This decrease is governed by mechanical stress. During development, the growth-plate of the femoral head remains approximately perpendicular to the habitual angle of hip joint reaction force (28, 29), and when infants begin to walk, hip joint reaction force becomes more horizontal (due to the addition of abductor forces), eventually resulting in a lower neck-shaft angle. Body proportions are genetically determined, at least in part, and geographic differences in them appear very early in life (18, 19). Thus, a likely explanation for the link between body proportions and neck-shaft angle is that starting from an early age, cold-adapted vs. warm-adapted body proportions produce different angles of hip joint reaction force (relative to the femur), ultimately resulting in differences in adult neck-shaft angle. The changes in hip geometry during development in children with different body proportions need to be investigated in detail to test this hypothesis rigorously.
This study focused on external femoral features. However, contrasts between Neandertals and near-modern humans in the cross-sectional shape of the femoral shaft also seem to be the mechanical consequence of body proportions (12). Moreover, although shaft bowing is often cited as an exclusive feature of the Neandertal femur, when this feature is quantified, Neandertals are not statistically distinguishable from their contemporaries (30). Therefore, the results of this study combined with other studies (12–14, 18–20) show that most if not all clear contrasts in shape between the femora of Neandertals and near-modern humans seem to be secondary consequences of differences in climate-induced body proportions. It is unclear whether or not variation in body proportions can explain patterns in femoral robusticity and neck-shaft angles between Neandertals and their modern human successors or within the Upper Paleolithic of Europe (11); first, as discussed above, the exact mechanical link between neck-shaft angle and body proportions needs to be established.
This study also supports theoretical proposals based on recent advances in evolutionary developmental biology that anatomical regions can be understood functionally only when they are considered as units that are integrated by developmental and mechanical constraints (31). Whenever possible, hypotheses of integration should be established empirically, and, as illustrated here, geometric morphometric techniques adapted to deal with articulated structures can aid these investigations.
Supplementary Material
Acknowledgments
I am grateful to D. Hunt, I. Tattersall, K. Mowbray, G. Avery, A. Morris, Y. Rak, C. Simon, R. Orban, P. Semal, L. Humphrey, R. Kruszynski, R. Foley, M. Bellatti, H.-E. Joachim, A. Langaney, P. Mennecier, G. Spedini, G. Manzi, B. Chiarelli, and G. D'Amore for generously allowing me to study comparative and fossil material under their care. I also thank all of the graduate students and other individuals who assisted me with collections. R. Klein, C. Roseman, T. Steele, and two anonymous reviewers commented on drafts of this paper. This research was funded by grants from the L.S.B. Leakey Foundation, the Morrison Institute for Population and Resource Studies at Stanford University, the A. W. Mellon Foundation, and the Department of Anthropological Sciences at Stanford University.
Abbreviation: UPGMA, unweighted pair group method with arithmetic mean.
References
- 1.Boule, M. (1912) Ann. Paléontol. 7, 105–192. [Google Scholar]
- 2.Weidenreich, F. (1941) Palaeontol. Sinica New Ser. D 5, 1–150. [Google Scholar]
- 3.Trinkaus, E. (1993) J. Hum. Evol. 25, 393–416. [Google Scholar]
- 4.Trinkaus, E. (1983) The Shanidar Neandertals (Academic, New York).
- 5.Stringer, C. B. & Gamble, C. (1993) In Search of the Neanderthals: Solving the Puzzle of Human Origins (Thames and Hudson, New York).
- 6.Trinkaus, E. (1983) in The Mousterian Legacy: Human Biocultural Change in the Upper Pleistocene, ed. Trinkaus, E. (British Archaeological Reports International, Oxford), Vol. 164, pp. 165–200. [Google Scholar]
- 7.Klein, R. G. (1999) The Human Career: Human Biological and Cultural Origins (Univ. Chicago Press, Chicago).
- 8.Pearson, O. M. (2000) Evol. Anthropol. 9, 229–247. [Google Scholar]
- 9.McCown, T. D. & Keith, A. (1939) The Stone Age of Mount Carmel: The Fossil Human Remains from the Levalloiso-Mousterian (Clarendon Press, Oxford), Vol. II.
- 10.Klein, R. G. (2000) Evol. Anthropol. 9, 17–36. [Google Scholar]
- 11.Trinkaus, E. (1997) Proc. Natl. Acad. Sci. USA 94, 13367–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinkaus, E., Ruff, C. B. & Churchill, S. E. (1998) in Neanderthals and Modern Humans in Western Asia, eds. Akazawa, T., Bar-Yosef, O. & Aoki, K. (Plenum, New York).
- 13.Trinkaus, E. (2000) in Neanderthals on the Edge: Papers from a Conference Marking the 150th Anniversary of the Forbe's Quarry Discovery, Gibraltar, eds. Stringer, C. B., Barton, R. N. E. & Finlayson, J. (Oxbow Books, Oxford), pp. 227–236.
- 14.Pearson, O. M. (2000) Curr. Anthropol. 41, 569–607. [PubMed] [Google Scholar]
- 15.Bergmann, C. (1847) Göttinger Studien 3, 595–708. [Google Scholar]
- 16.Ashton, K. G., Tracy, M. C. & deq Ueiroz, A. (2000) Am. Nat. 156, 390–415. [DOI] [PubMed] [Google Scholar]
- 17.Allen, J. A. (1877) Radical Rev. 1, 108–140. [Google Scholar]
- 18.Holliday, T. W. (1997) J. Hum. Evol. 32, 423–447. [DOI] [PubMed] [Google Scholar]
- 19.Ruff, C. B. (1994) Yearbook Phys. Anthropol. 37, 65–107. [Google Scholar]
- 20.Holliday, T. W. (1997) Am. J. Phys. Anthropol. 104, 245–258. [DOI] [PubMed] [Google Scholar]
- 21.Weaver, T. D. (2002) in Anthropological Sciences (Stanford Univ. Press, Palo Alto, CA), pp. 221.
- 22.Dryden, I. L. & Mardia, K. V. (1998) Statistical Shape Analysis (Wiley, New York).
- 23.Adams, D. C. (1999) Evol. Ecol. Res. 1, 959–970. [Google Scholar]
- 24.Penin, X., Berge, C. & Baylac, M. (2002) Am. J. Phys. Anthropol. 118, 50–62. [DOI] [PubMed] [Google Scholar]
- 25.Hennessy, R. J. & Stringer, C. B. (2002) Am. J. Phys. Anthropol. 117, 37–48. [DOI] [PubMed] [Google Scholar]
- 26.O'Higgins, P. & Jones, N. (1998) J. Anat. 193, 251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson, J. Y. & Trinkaus, E. (1998) J. Anat. 192, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimkes, B., Posel, P., Plitz, W. & Jansson, V. (1993) J. Pediatr. Orthoped. 13, 431–436. [DOI] [PubMed] [Google Scholar]
- 29.Maquet, P. G. J. (1985) Biomechanics of the Hip: As Applied to Osteoarthritis and Related Conditions (Springer, New York).
- 30.Shackelford, L. L. & Trinkaus, E. (2002) Am. J. Phys. Anthropol. 118, 359–370. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy, C. O., Cohn, M. J. & White, T. D. (1999) Proc. Natl. Acad. Sci. USA 96, 13247–13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.