Abstract
Substrate cleavage by the Neurospora Varkud satellite (VS) ribozyme involves a structural change in the stem-loop I substrate from an inactive to an active conformation. We have determined the NMR solution structure of a mutant stem-loop I that mimics the active conformation of the cleavage site internal loop. This structure shares many similarities, but also significant differences, with the previously determined structures of the inactive internal loop. The active internal loop displays different base-pairing interactions and forms a novel RNA fold composed exclusively of sheared G-A base pairs. From chemical-shift mapping we identified two Mg2+ binding sites in the active internal loop. One of the Mg2+ binding sites forms in the active but not the inactive conformation of the internal loop and is likely important for catalysis. Using the structure comparison program mc-search, we identified the active internal loop fold in other RNA structures. In Thermus thermophilus 16S rRNA, this RNA fold is directly involved in a long-range tertiary interaction. An analogous tertiary interaction may form between the active internal loop of the substrate and the catalytic domain of the VS ribozyme. The combination of NMR and bioinformatic approaches presented here has identified a novel RNA fold and provides insights into the structural basis of catalytic function in the Neurospora VS ribozyme.
RNA molecules play essential roles in many cellular processes. These include the enzymatic activity of ribozymes that are required for protein synthesis and certain RNA processing reactions (1). NMR and x-ray crystallographic studies have provided some insights into the relationship between RNA structure and catalysis; however, interpretation of structure–function relationships, even in the well studied hammerhead ribozyme (2), continues to be challenging (1). It has also been difficult to make any generalizations about the role of RNA structure in catalysis, in part because of the small number of known ribozymes and the limited amount of structural information available. We are studying the Neurospora Varkud satellite (VS) ribozyme to provide information about the role of tertiary structure and conformational changes in RNA catalysis.
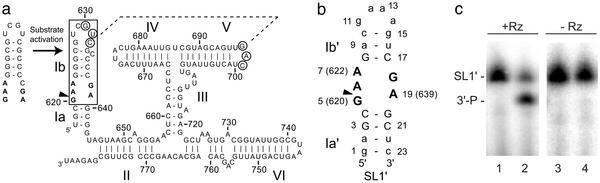
The Neurospora VS ribozyme originates from an abundant RNA satellite of 881 nt found in the mitochondria of the Varkud-1c strain of Neurospora (3). Fragments of ≈120–180 nt derived from this natural RNA sequence undergo self-cleavage at a specific phosphodiester bond to produce 5′-OH and 2′,3′-cyclic phosphate termini (Fig. 1a) (3–5). Although these products are characteristic of other small ribozymes, the VS ribozyme possesses unique primary (4), secondary (6), and tertiary structures (7–9). The secondary structure of the self-cleaving VS ribozyme is characterized by six helical domains (Fig. 1a); stem-loop I forms the substrate domain and stem-loops II–VI comprise the catalytic domain (6). When these two domains are synthesized separately, the catalytic domain can perform the same cleavage reaction, known as the trans reaction, with multiple turnover (10). There is no reported high-resolution structure of the full VS ribozyme at this time, but tertiary structure models have been proposed (8, 9).
Fig. 1.
(a) Sequence and secondary structure of the Neurospora VS ribozyme. The interaction between stem-loops I and V is indicated by a dashed line, and residues involved in this interaction are circled (7). Upon tertiary folding of the ribozyme, stem-loop I (subdivided into Ia and Ib) undergoes a structural change from an inactive to an active conformation (11). The minimal substrate domain for the trans cleavage reaction is boxed (10), and the cleavage site is indicated by the arrowhead. (b) Sequence and secondary structure of SL1′ RNA. WT and mutant nucleotides are represented by uppercase and lowercase letters, respectively. (c) SL1′ is cleaved by the VS ribozyme. 3′-32P-end-labeled SL1′ (25 nM) was incubated at 30°C in 40 mM Tris·HCl (pH 8.0), 50 mM KCl, and 100 mM MgCl2 in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of unlabeled RZ6 ribozyme (51) (37 μM) for 0 h (lanes 1 and 3) or 16 h (lanes 2 and 4) (12). Full-length SL1′ and the 3′ product (3′-P) were separated by denaturing PAGE and visualized with a PhosphorImager.
The catalytic domain of the VS ribozyme recognizes its stem-loop substrate primarily through tertiary interactions (7, 8). So far, the best-characterized tertiary interaction is a Mg2+-dependent loop–loop interaction between stem-loops I and V, which is important for catalysis (Fig. 1a) (7, 11). Before tertiary folding of the ribozyme, stem-loop I adopts an inactive conformation, termed the unshifted helix (Fig. 1a) (11, 12). On binding to stem-loop V, stem-loop I adopts an active conformation, termed the shifted helix (11, 12). In the active conformation of stem-loop I, base-pairing partners for G623, G624, and G625 are replaced by adjacent cytidines, creating a bulge at position 634 and reducing the size of the internal loop from 6 to 5 nt (Fig. 1a) (11, 12). Stem-loop I variants that can form the active conformation are cleaved by the VS ribozyme; however, those that are restricted to the inactive conformation are not (11).
We report here the NMR structure of SL1′, a stem-loop I RNA that mimics the active conformation of the VS ribozyme cleavage site internal loop (Fig. 1b). In addition, we present NMR chemical shift mapping of Mg2+ binding sites in this RNA and a bioinformatic search of the SL1′ internal loop fold in other RNAs. This work reveals that the SL1′ internal loop forms a novel RNA fold also present in 16S and 23S rRNA. Comparison between the active and inactive conformations (13, 14) of the substrate internal loop provides insights into the mechanism of substrate activation by the VS ribozyme.
Methods
Sample Preparation. Unlabeled, 15N-labeled, and 13C/15N-labeled SL1′ RNAs were synthesized in vitro by using T7 RNA polymerase (generously provided by K. Morden, Louisiana State University, Baton Rouge), a synthetic oligonucleotide template (DNA Express, Macromolecular Resources, Fort Collins, CO), and nucleoside triphosphates (15, 16). The 23-mer SL1′ was purified by denaturing gel electrophoresis, dephosphorylated at its 5′ end with calf alkaline phosphatase (Roche Molecular Biochemicals), and further purified by DEAE-Sephacel chromatography (Amersham Biosciences). The RNA (1–5 mM) was exchanged into 10 mM d11-Tris (pH 7.0), 50 mM NaCl, 0.2 mM EDTA, 0.05 mM NaN3 in 9:1 H2O/D2OorD2O. Before each set of NMR experiments the RNA was heated to 95°C for 2 min and snap-cooled in ice water.
NMR Spectroscopy. NMR data were collected at 25°C on a Varian Inova 600-MHz spectrometer equipped with a z-axis pulse-field gradient probe, either a 1H{13C/15N} triple resonance probe or a 1H-19F{15N-31P} indirect detection probe. 1H, 13C, and 15N chemical shift assignments were obtained by using the following experiments: 2D 1H-15N heteronuclear single quantum coherence (HSQC), 2D 1H-1H flip-back watergate-NOESY, 2D G-specific H(NC)-total correlation spectroscopy (TOCSY)-(C)H, 2D A-specific (H)N(C)-TOCSY-(C)H, 2D C-specific and U-specific H(NCCC)H, 2D 1H-13C constant-time HSQC, 2D 1H-15N MQ-(HC)N(C)H, 3D HCCH-COSY, 3D HCCH-TOCSY, and 3D 13C-edited heteronuclear multiple quantum correlation-NOESY (17). Distance restraints were derived from nuclear Overhauser effect (NOE) in 3D 13C/15N-edited NOESY-HSQC (80 and 240 ms) (18), 3D 13C-edited heteronuclear multiple quantum correlation-NOESY (80 and 240 ms), and 2D 1H-15N Carr Purcell Meiboom Gill-NOESY (150 ms) (19) experiments. 2D HNN-COSY (20) and 2D H(CN)N(H) (21) spectra were collected to detect 2JNN couplings across hydrogen bonds in Watson–Crick and sheared G-A base pairs, respectively. A 2D double quantum filtered COSY was collected to define δ torsion angles. Spectra were processed with NMRPIPE/NMRDRAW (22) and analyzed with NMRVIEW (23).
Structure Calculations. NOE-derived distance restraints were separated in four ranges, strong (1.8–3.3 Å), medium (1.8–3.9 Å), weak (1.8–5.5 Å), or very weak (1.8–7.0 Å). Distance ranges with lower bounds of 2.5–4.0 Å and upper bounds of 40 Å were defined for very small or absent NOESY crosspeaks between H1′,H2′, and H6/H8 protons of residue i and H5′/H5″ protons of residues i, i – 1, and i + 1. Because of strong NMR evidence for formation of Watson–Crick base pairs in helices Ia and Ib and of the sheared G-A base pair in the tetraloop, canonical distance restraints were used to define these base pairs. In the internal loop, G5 N2–A19 N7 and G18 N2–A6 N7 distances were restrained to 1.8–3.15 Å based on results from the 2D H(CN)N(H) spectrum (21). Torsion angle constraints were derived from a 2D double quantum filtered COSY (for δ) and comparative analyses of NOE data (for δ and γ).
3D structures were calculated with restrained molecular dynamics and simulated annealing in X-PLOR 3.840 (24). From a set of 50 structures with randomized torsion angles, 29 structures satisfied the experimental restraints (no distance violation >0.1 Å and no torsion angle violation >5°), and, from these, the 10 lowest energy structures were selected for analysis. An average structure was calculated from the 29 final structures and minimized against the experimental restraints. The program MOLMOL was used for visualization and structural analysis (25).
Metal Binding Studies. Chemical-shift changes induced by addition of 10 mM MgCl2 were monitored for all of the 1′, 2′, 3′, 6/8, and 2/5 proton and carbon atoms of SL1′. The chemical-shift changes (Δ in ppm ± 0.03 ppm) are calculated according to the equation Δ = [(ΔH)2 + (0.3 × ΔC)2]1/2, where ΔH and ΔC are, respectively, the 1H and 13C chemical-shift differences between the assigned 1H-13C constant-time HSQC (26) correlations of the control sample [10 mM d11-Tris (pH 7.0)/50 mM NaCl/0.05 mM NaN3] and those of the Mg2+-supplemented sample. The values of ΔH and ΔC were also tabulated for the resolved 1′, 2′, 3′, 6/8, and 2/5 protons and carbons after each addition of MgCl2 (0.25, 0.5, 0.75, 1.0, 2.0, 5.0, 10, 20, and 40 mM MgCl2). These data were used to obtain apparent Kd values when the total change at 40 mM MgCl2 (ΔT) was ≥0.1 ppm and ≥0.45 ppm for 1H and 13C chemical shifts, respectively. Apparent Kd values were calculated by assuming single independent 1:1 binding models by fitting the equation Δobs = (ΔT)/(2 × [RNA]T) × {(M + [RNA]T + Kd) – ((M + [RNA]T + Kd)2 – (4 M × [RNA]T))1/2} (27, 28), where Δobs is the observed shift at each MgCl2 concentration and [RNA]T and M are the total concentrations of RNA and metal ion, respectively. Because multiple binding sites are detected in SL1′, the apparent Kd represents an upper value of the real Kd (27, 28).
Pattern Search of RNA Structures in the Protein Data Bank (PDB). Using MC-SEARCH (P.G. and F.M., unpublished data), we searched for other occurrences of the SL1′ internal loop fold. mc-search is a computer program, derived from the automated RNA annotation program mc-annotate (29, 30), that searches RNA structure files for regions that match a user-defined pattern. The searched pattern was described only in terms of primary and secondary structures; the sequence of the residues in the internal loop was fixed to that of the SL1′ internal loop, but the sequence of the closing base pairs was not fixed (30). No additional structural interactions were included in the search pattern for the bases in the internal loop. The searched database contained high-resolution crystal structures (3.0 Å or less) of the PDB (31). The identified structures were compared and classified by using hierarchical clustering based on the rms deviation (rmsd) metric (29).
Results and Discussion
Structure Determination. To study the active form of the stem-loop I internal loop, we initially synthesized a series of mutant stem-loop I RNAs with sequences designed to form only the active conformation (11, 12). All of the RNAs tested that retain the natural sequence in the terminal loop I did not adopt a single conformation and were not suitable for further NMR analysis. Only the RNA shown in Fig. 1b gave high-quality NMR data. This stem-loop I mimic, termed SL1′, contains WT nucleotides for the active internal loop and for at least one closing base pair on each side of the internal loop (Fig. 1b). SL1′ is cleaved in the presence but not the absence of the catalytic domain of the VS ribozyme (Fig. 1c). As expected, cleavage of SL1′ is slower than for WT stem-loop I, because SL1′ cannot form the loop–loop interaction with stem-loop V (11).
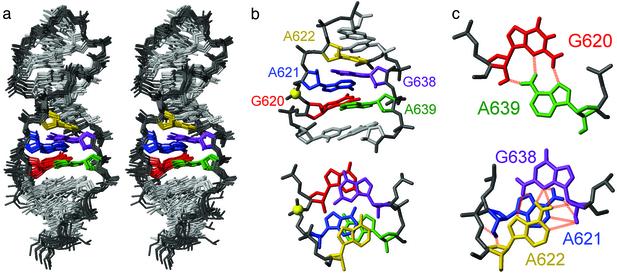
The structure of SL1′ was determined by using heteronuclear NMR methods, and 3D structures were calculated by using restrained molecular dynamics and simulated annealing. The structural statistics (Table 1) and the superposition of the 10 lowest energy structures (Fig. 2a) indicate that the structure of this RNA, particularly of the active internal loop, is well defined by the NMR data. The individual stems and the GAAA tetraloop are also individually well defined and adopt A-form helix and GNRA tetraloop conformations (32), respectively.
Table 1. Structural statistics.
| Distance restraints | |
| From standard NOESY | 1,081 |
| Internucleotide | 608 |
| Intranucleotide | 473 |
| From 2D 1H-15N Carr Purcell Meiboom Gill-NOESY | 24 |
| Hydrogen bonds from 2JNN couplings | 9 |
| Others (W-C and G11-A14) | 37 |
| rmsd from experimental restraints | |
| Distance restraints, Å (1,105) | 0.0068 ± 0.0004 |
| Dihedral restraints, ° (40) | 0.13 ± 0.02 |
| rmsd from idealized geometry | |
| Bonds, Å | 0.00452 ± 0.00003 |
| Angles, ° | 1.036 ± 0.003 |
| Impropers, ° | 0.361 ± 0.004 |
| Heavy-atom rmsd, Å | |
| Overall (residues 2-22) | 1.00 ± 0.44 |
| GAAA tetraloop (residues 10-15) | 0.21 ± 0.08 |
| Internal loop (residues 5-7 and 18-19) | 0.27 ± 0.13 |
Fig. 2.
NMR structure of SL1′.(a) Stereoview of the heavy-atom superposition of the 10 lowest energy structures. (b) Minimized average structure of the active internal loop viewed from the minor groove (Upper) and down the helix axis (Lower). The phosphorus at the cleavage site is shown as a yellow sphere. (c) Base pair geometry for internal loop residues in the minimized average structure. The 2′-OH and amino protons are shown here in addition to the heavy atoms. The dashed lines represent potential hydrogen bonds based on short distances (<3.4 Å) observed in at least one structure. Internal loop nucleotides are colored according to the scheme presented in b.
Description of the Active Conformation of the Stem-Loop I Internal Loop. The internal loop of the active stem-loop I adopts a novel structural fold, which is composed entirely of sheared G-A base pairs (Fig. 2). The geometries of all G-A base pairs in the internal loop are defined by many experimental restraints, including NOEs from exchange-broadened amino groups (Fig. 6a, which is published as supporting information on the PNAS web site, www.pnas.org) (19) and 2JNN couplings across the GN2-AN7 hydrogen bond (Fig. 6b) (21). Theoretical distance or planarity restraints were not used to define base-pairing in the internal loop. Residues G620 and A639 adopt a canonical sheared G-A base pair, with planarity of the two bases (Fig. 2c). The most striking feature of this internal loop is the sharing of G638 for the formation of two sheared G-A base pairs, G638–A621 and G638–A622 (Fig. 2). We will refer to this structural element as the shared sheared G-A base pairs. In all of the calculated structures, the base of G638 is not coplanar with the base of either A621 or A622, but rather lies between the two planes defined by these adenines (Fig. 2). We observed short interresidue distances characteristic of either direct or water-mediated hydrogen bonds for both G-A base pairs (Fig. 2c): G638 N3–A621 H62 (2.41–2.63 Å); G638 H22–A621 N7 (2.28–2.84 Å); G638 N3–A622 H62 (2.55–3.25 Å); and G638 H22–A622 N7 (2.97–3.30 Å for 9 of 10 structures). There is no evidence for slow dynamics in the internal loop; however, the possibility for fast dynamic averaging (time scale less than milliseconds) between multiple base-pairing schemes cannot be ruled out.
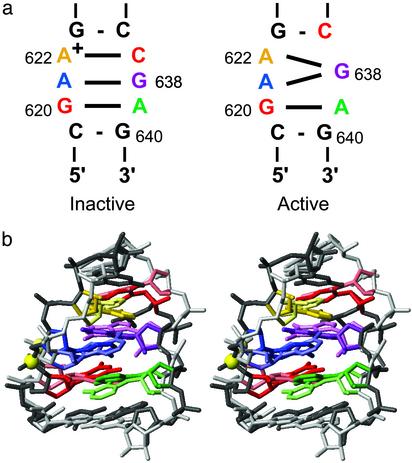
Comparison with the Inactive Conformation of the Stem-Loop I Internal Loop. Two structures of the inactive form of the stem-loop I internal loop had been previously determined by NMR spectroscopy (13, 14). In both cases, the inactive internal loop is characterized by tandem sheared G-A base pairs and a wobble A622+–C637 base pair with a pKa of ≈6.2 for the adenine imino group (Fig. 3a) (13, 14). From pH-dependent 15N chemical-shift studies, a pKa of 4.0 (± 0.1) was determined for A622 in the active internal loop (not shown). Although the deprotonation of A622+ may help to allow the transition to the active conformation, it is not sufficient because the interaction with stem-loop V is also required (11, 12).
Fig. 3.
Structural comparison of the inactive and active conformations of the VS ribozyme stem-loop I internal loop. (a) Summary of the base-pairing interactions in the inactive (Left) (13, 14) and active (Right) conformations. (b) Stereoview for comparing the inactive and active conformations. The inactive stem-loop I conformation (pastel colors) is the best representative conformer from the ensemble of NMR structures of Michiels et al. (ref. 13; PDB ID code 1E4P), and the active conformation (darker colors) is the minimized average structure described here. The superposition was obtained by minimizing the rmsd for heavy atoms of residues C619–G623 and C637–G640. Internal loop nucleotides are colored according to the scheme presented in a.
We superimposed heavy atoms of residues C619–G623 and C637–G640 of the minimized average structure of SL1′ with those of the inactive internal loop structure of Michiels et al. (13) (Fig. 3b). The heavy atom rmsd for this superposition is 2.0 Å. Overall, there are many similarities between these two structures, including the sheared G620–A639 and G638–A621 base pairs, as well as the cross-strand stacking between G620 and G638 and between A621 and A639 (Figs. 2b and 3b). The largest differences between the two structures are at one end of the internal loop, near residues A622 and C637. In the inactive form, the protonated A622 forms a wobble A622+–C637 base pair, whereas in the active form the deprotonated A622 forms a sheared G-A base pair with G638, and C637 forms a Watson–Crick G-C base pair with G623. These differences in base-pairing affect the position of the phosphate backbone for residues A621, A622, and G638 (Fig. 3b) and the positions of stem Ib residues with respect to the internal loop (not shown). Despite these seemingly subtle conformational differences, the active stem-loop I is effectively cleaved by the catalytic domain of the VS ribozyme, whereas stem-loop I mutants locked in the inactive conformation are not (11). As will be presented below, the active internal loop conformation provides two unique structural characteristics: a divalent metal-ion binding site and a tertiary interaction motif.
Interactions of SL1′ with Mg2+ Although cleavage by the VS ribozyme has been detected with several divalent metal ions (33) and monovalent salts (2 M Li2SO4) (34), Mg2+ is likely the biological cation necessary for catalysis (33). From the NMR structure of SL1′, we find that the active internal loop contains two 5′-A R-3′/5′-Y G-3′ motifs previously identified as divalent metal-ion binding sites in other RNAs (Fig. 4a) (35–37). In the crystal structure of the hammerhead ribozyme, a Mg2+ is found associated to a 5′-A G-3′/5′-C G-3′ motif, within close distance of the A 5′ phosphate and of the G N7 on the 5′-A G-3′ strand (Fig. 4a) (36). The importance of the internal loop 5′-A622 G623-3′/5′-C637 G638-3′ motif for catalysis by the VS ribozyme is supported by biochemical data. Mutational studies have indicated that the 623–637 base pair must fit the R-Y consensus (11), and phosphorothioate substitution experiments have demonstrated the importance of the 5′ phosphate of A622 for catalysis (38).
Fig. 4.
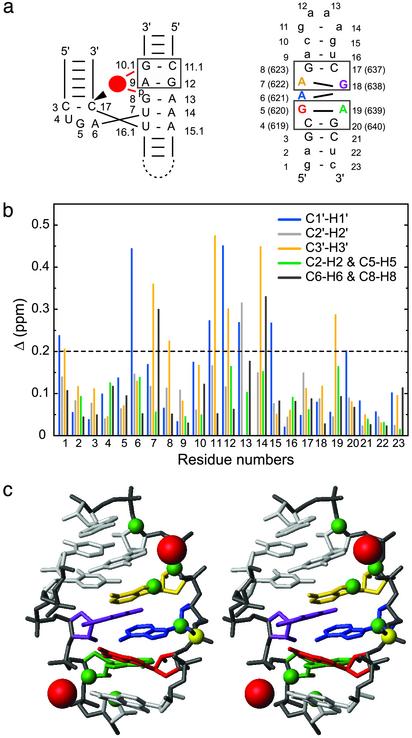
Mg2+ binding sites of SL1′.(a) Schematic comparing the 5′-A G-3′/5′-C G-3′ metal-ion binding motif (box) of the hammerhead ribozyme (Left) (36) and the two 5′-A G-3′/5′-C G-3′ motifs (boxes) in the internal loop of SL1′ (Right). For the hammerhead ribozyme, the bound Mg2+ is represented by a red circle, and the cleavage site is indicated by the arrowhead. (b) Chemical-shift changes (Δ in ppm ± 0.03 ppm; see Methods) after addition of 10 mM MgCl2 for the 1′, 2′, 3′, 6/8, and 2/5 proton and carbon atoms of all SL1′ residues. (c) Summary of Mg2+ binding data on the stereoview of the minimized average structure of SL1′ (residues C4-A9 and U16-G20). Significant chemical-shift changes after addition of 10 mM MgCl2 (Δ >0.2 ppm) were mapped as green spheres on the respective C1′, C2′, C3′, C6/C8, and C2/C5 atoms. Putative locations for the Mg2+ (red spheres) were obtained by heavy-atom superposition of the 5′-A G-3′/5′-C G-3′ motif from the x-ray structure of the Mg2+-bound hammerhead ribozyme (only Mg2+ are shown) (36) with the 5′-A639 G640-3′/5′-C619 G 620-3′ (rmsd of 1.01 Å) and the 5′-A622 G623-3′/5′-C637 G 638-3′ (rmsd of 1.56 Å) motifs from the minimized average structure of SL1′.
We used chemical-shift mapping to investigate the Mg2+ binding sites in SL1′. 2D 1H-13C constant-time HSQC spectra of SL1′ were recorded before and after addition of various concentrations of MgCl2. After addition of 10 mM MgCl2, significant changes in 1H and 13C chemical shifts (Δ > 0.2 ppm) are observed at the end of the helix, in the internal loop, and in the GAAA tetraloop (Fig. 4b). These changes are mapped onto the structure of SL1′ in Fig. 4c. Apparent Kd values for Mg2+ were calculated based on Mg2+-dependent 1H and 13C chemical-shift changes for resolved signals (Fig. 7, which is published as supporting information on the PNAS web site) (27). For the GAAA tetraloop, a Kd of 3.4 ± 0.6 mM was obtained, in agreement with published data (28, 39). In the internal loop, two regions are affected by Mg2+; we obtained a Kd of 3.6 ± 0.7 mM for residues A621, A622, and G623 and a Kd of 2.4 ± 1.1 mM for residues A639 and G640. These results suggest that there are at least two Mg2+ binding sites in the active internal loop and that each Mg2+ binding site is located near the 5′-A G-3′ strand of a 5′-A G-3′/5′-C G-3′ motif. We attempted to locate more precisely the divalent metal binding sites in SL1′ by using cobalt hexammine and MnCl2. However, because of the presence of multiple binding sites, the data were too complex and could not be interpreted unambiguously. To illustrate the possible location of the Mg2+ binding sites in the internal loop of the VS ribozyme, we superimposed the Mg2+-bound 5′-A G-3′/5′-C G-3′ motif of the hammerhead crystal structure with each of these two 5′-A G-3′/5′-C G-3′ motifs of SL1′ (Fig. 4c).
The 5′-A639 G640-3′/5′-C619 G620-3′ structural motif, present in both the inactive and active internal loops, is not part of the minimal stem-loop I substrate and is therefore not essential for catalysis (Fig. 1a). The 5′-A622 G623-3′/5′-C637 G638-3′ motif, however, is found solely in the active internal loop and is essential for activity (11, 38). Our chemical-shift mapping data indicate that a Mg2+ binds near the 5′-A622 G623-3′ site of the internal loop. Because mutations and chemical modifications of potential Mg2+ ligands at this site impaired substrate cleavage (11, 38), it is likely that this Mg2+ plays an important role in catalysis. Interestingly, modeling of a Mg2+ at the 5′-A622 G623-3′/5′-C637 G638-3′ motif positions this ion in the vicinity of the phosphate at the cleavage site (Fig. 4c). This Mg2+ may be important for substrate docking in the active site or the chemical reactions of the VS ribozyme.
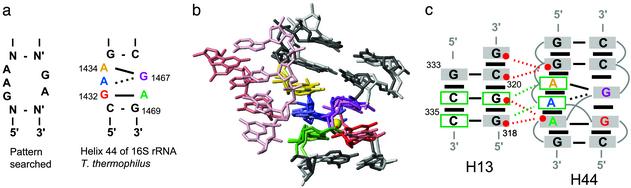
Other Occurrences of the Active Internal Loop Fold. To investigate whether the SL1′ internal loop fold exists in other RNAs, we used an automated program MC-SEARCH (P.G. and F.M., unpublished data) to search for RNA structure patterns in the PDB (31). The pattern searched contained the sequence of the SL1′ internal loop flanked by closing base pairs (Fig. 5a). MC-SEARCH found several occurrences of this pattern (Fig. 5a) in the database, consisting of multiple structures of two different helical domains of rRNA. One is from helix 44 of 16S rRNA found in the small ribosomal subunit of Thermus thermophilus (40) (Fig. 5). The other one is from helix 25 of 23S rRNA found in the large ribosomal subunit of Haloarcula marismortui (41) (Fig. 8, which is published as supporting information on the PNAS web site). Surprisingly, even though no hydrogen bonding or stacking interactions were specified in the internal loop of the pattern searched, the RNAs found are very similar in structure to the internal loop of SL1′. The sequence of helix 44 of 16S rRNA matches exactly the sequence of the internal loop of SL1′ and its closing base pairs (Fig. 5a). Heavy-atom superposition of internal loop residues of helix 44 of 16S rRNA [PDB ID code 1FJG (40)] and SL1′ yields a rmsd of 1.40 Å (Fig. 5b). The main difference between these structures is in the shared sheared G-A base pairs; in helix 44, A1433 is displaced away from G1467 such that there is only one potential hydrogen bond between its base and the base of G1467 (Fig. 5).
Fig. 5.
The internal loop structure of the active stem-loop I is a tertiary interaction motif (a) Sequence and secondary structure of the pattern searched by mc-search and of helix 44 of 16S rRNA of T. thermophilus (40) (b) A superposition between helix 44 (PDB ID code 1FJG; pastel colors) and the minimized average structure of SL1′ (darker colors) was obtained by minimizing the rmsd for heavy atoms of the five residues in the internal loop (1.40 Å). Also shown are residues in helix 13 of 16S rRNA, which form a tertiary interaction with helix 44 (c) Summary of the base-pairing and stacking interactions in helices 13 and 44 and of tertiary contacts between them. Solid and dashed black lines indicate base pairs with two hydrogen bonds (either Watson–Crick or sheared G-A) and one hydrogen bond, respectively. Black rectangles indicate base stacking. Red spheres indicate riboses involved in ribose–ribose contacts (red dashed lines). The two adenines A1433 and A1434 participate in A-minor motifs (green dashed lines).
Interestingly, the minor grooves of helix 44 of 16S rRNA and helix 25 of 23S rRNA both participate in long-range tertiary interactions termed canonical ribose zippers (Figs. 5 and 8) (42). In 16S rRNA of T. thermophilus, the internal loop of helix 44 interacts with the stem of helix 13 through multiple ribose–ribose contacts, and the two adenines, A1433 and A1434, participate in A-minor motifs with C335 and G319-C334, respectively (Fig. 5c) (43). This ribose zipper is similar to that formed by the GAAA tetraloop and tetraloop receptor, where two consecutive adenines stacked on a sheared G-A base pair participate in A-minor motifs (42, 44, 45). In addition, the base stacking pattern in helix 44 of 16S rRNA (43) and in the internal loop of SL1′ is strikingly similar to that of the GNRA fold (32).
For optimal cleavage by the VS ribozyme, the stem-loop I substrate must participate in multiple tertiary interactions with the catalytic domain (7, 8). These include the well characterized loop–loop interaction with stem-loop V (Fig. 1a) (7) and interactions with helix II and helix VI (8, 46, 47), which contains the proposed active site (38, 46, 48–50). Substrate binding to the catalytic domain of the VS ribozyme is facilitated by formation of the active stem-loop I conformation (8, 51). Here, we have shown that this active conformation functions as a tertiary ribose zipper motif in 16S and 23S rRNAs (Fig. 5). Analysis of stem-loop ribose zippers in rRNA structures indicates that adenines are favored in the loop (42); a similar preference for adenine residues at positions 621 and 622 in the stem-loop I of the VS ribozyme has been demonstrated from in vitro selection experiments (11). In the active stem-loop I conformation, A621 and A622 are well positioned to form A-minor motifs (Fig. 5b), whereas in the inactive stem-loop I, the A622+–C637 base pair (Fig. 3) would hinder such interaction. Based on these similarities in sequence and structure, we speculate that the active conformation of the VS ribozyme stem-loop I internal loop forms a ribose zipper with either helix II or VI of the catalytic domain.
Conclusion
The NMR study of the active internal loop of the VS ribozyme reveals a novel RNA fold. This fold consists exclusively of sheared G-A base pairs and forms only in the active but not the inactive conformation of the VS ribozyme substrate internal loop. The active internal loop conformation differs from the inactive one by the rearrangement of base pairs and formation of a divalent metal-ion binding site that appears important for catalysis. In addition, a bioinformatic analysis has identified the active internal loop fold as part of tertiary interaction motifs in rRNAs. Future structural studies will determine whether a similar tertiary interaction forms in the Neurospora VS ribozyme.
Supplementary Material
Acknowledgments
We thank J. G. Omichinski for discussions, K. Morden for T7 RNA polymerase, A. Majumdar for pulse sequences, and W. G. Scott for PDB coordinates of the hammerhead ground-state structure with Mg2+. This work was supported in part by a Basil O'Connor Starter Scholar Research Award from the March of Dimes Birth Defects Foundation and a National Science Foundation Career Award (to P.L.), the Canadian Institutes of Health Research (R.A.C. and F.M.), and the National Science and Engineering Research Council (F.M.). R.A.C. is a Canada Research Chair, and F.M. is a Canadian Institutes of Health Research Investigator.
Abbreviations: HSQC, heteronuclear single quantum coherence; NOE, nuclear Overhauser effect; PDB, Protein Data Bank; rmsd, rms deviation; VS, Varkud satellite.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1OW9).
References
- 1.Doudna, J. A. & Cech, T. R. (2002) Nature 418, 221–228. [DOI] [PubMed] [Google Scholar]
- 2.Murray, J. B., Dunham, C. M. & Scott, W. G. (2002) J. Mol. Biol. 315, 121–130. [DOI] [PubMed] [Google Scholar]
- 3.Saville, B. J. & Collins, R. A. (1990) Cell 61, 685–696. [DOI] [PubMed] [Google Scholar]
- 4.Guo, H. C. T., De Abreu, D. M., Tillier, E. R. M., Saville, B. J., Olive, J. E. & Collins, R. A. (1993) J. Mol. Biol. 232, 351–361. [DOI] [PubMed] [Google Scholar]
- 5.Rastogi, T. & Collins, R. A. (1998) J. Mol. Biol. 277, 215–224. [DOI] [PubMed] [Google Scholar]
- 6.Beattie, T. L., Olive, J. E. & Collins, R. A. (1995) Proc. Natl. Acad. Sci. USA 92, 4686–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastogi, T., Beattie, T. L., Olive, J. E. & Collins, R. A. (1996) EMBO J. 15, 2820–2825. [PMC free article] [PubMed] [Google Scholar]
- 8.Hiley, S. L. & Collins, R. A. (2001) EMBO J. 20, 5461–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafontaine, D. A., Norman, D. G. & Lilley, D. M. (2002) EMBO J. 21, 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, H. C. T. & Collins, R. A. (1995) EMBO J. 14, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen, A. & Collins, R. A. (2000) Mol. Cell 5, 469–478. [DOI] [PubMed] [Google Scholar]
- 12.Andersen, A. A. & Collins, R. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7730–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michiels, P. J. A., Schouten, C. H. J., Hilbers, C. W. & Heus, H. A. (2000) RNA 6, 1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flinders, J. & Dieckmann, T. (2001) J. Mol. Biol. 308, 665–679. [DOI] [PubMed] [Google Scholar]
- 15.Milligan, J. F., Groebe, D. R., Witherell, G. W. & Uhlenbeck, O. C. (1987) Nucleic Acids Res. 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikonowicz, E. P., Sirr, A., Legault, P., Jucker, F. M., Baer, L. M. & Pardi, A. (1992) Nucleic Acids Res. 20, 4507–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cromsigt, J., van Buuren, B., Schleucher, J. & Wijmenga, S. S. (2001) Methods Enzymol. 338, 371–399. [DOI] [PubMed] [Google Scholar]
- 18.Pascal, S. M., Muhandiram, D. R., Yamazaki, T., Forman-Kay, J. D. & Kay, L. E. (1994) J. Magn. Reson. 103, 197–201. [Google Scholar]
- 19.Mueller, L., Legault, P. & Pardi, A. (1995) J. Am. Chem. Soc. 117, 11043–11048. [Google Scholar]
- 20.Dingley, A. J. & Grzesiek, S. (1998) J. Am. Chem. Soc. 120, 8293–8297. [Google Scholar]
- 21.Majumdar, A., Kettani, A., Skriptin, E. & Patel, D. J. (1999) J. Biomol. NMR 15, 207–211. [DOI] [PubMed] [Google Scholar]
- 22.Delaglio, F., Grzesiek, S., Vuister, G. W., Zhu, G., Pfeifer, J. & Bax, A. (1995) J. Biomol. NMR 6, 277–293. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, B. A. & Blevins, R. A. (1994) J. Biomol. NMR 4, 603–614. [DOI] [PubMed] [Google Scholar]
- 24.Brünger, A. T. (1992) X-PLOR 3.1 Manual (Yale Univ. Press, New Haven, CT).
- 25.Koradi, R., Billeter, M. & Wüthrich, K. (1996) J. Mol. Graphics 14, 51–55. [DOI] [PubMed] [Google Scholar]
- 26.Santoro, J. & King, G. C. (1992) J. Magn. Reson. 97, 202–207. [Google Scholar]
- 27.Gonzalez, R. L., Jr., & Tinoco, I., Jr. (1999) J. Mol. Biol. 289, 1267–1282. [DOI] [PubMed] [Google Scholar]
- 28.Maderia, M., Horton, T. E. & DeRose, V. J. (2000) Biochemistry 39, 8193–8200. [DOI] [PubMed] [Google Scholar]
- 29.Gendron, P., Lemieux, S. & Major, F. (2001) J. Mol. Biol. 308, 919–936. [DOI] [PubMed] [Google Scholar]
- 30.Lemieux, S. & Major, F. (2002) Nucleic Acids Res. 30, 4250–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman, H. M., Westbrook, A., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000) Nucleic Acids Res. 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heus, H. A. & Pardi, A. (1991) Science 253, 191–194. [DOI] [PubMed] [Google Scholar]
- 33.Collins, R. A. & Olive, J. E. (1993) Biochemistry 32, 2795–2799. [DOI] [PubMed] [Google Scholar]
- 34.Murray, J. B., Seyhan, A. A., Walter, N. G., Burke, J. M. & Scott, W. G. (1998) Chem. Biol. 5, 587–595. [DOI] [PubMed] [Google Scholar]
- 35.Pley, H. W., Flaherty, K. M. & McKay, D. B. (1994) Nature 372, 68–74. [DOI] [PubMed] [Google Scholar]
- 36.Scott, W. G., Finch, J. T. & Klug, A. (1995) Cell 81, 991–1002. [DOI] [PubMed] [Google Scholar]
- 37.Hansen, M. R., Simorre, J. P., Hanson, P., Mokler, V., Bellon, L., Beigelman, L. & Pardi, A. (1999) RNA 5, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood, V. D., Beattie, T. L. & Collins, R. A. (1998) J. Mol. Biol. 282, 741–750. [DOI] [PubMed] [Google Scholar]
- 39.Rüdisser, S. & Tinoco, I., Jr. (2000) J. Mol. Biol. 295, 1211–1223. [DOI] [PubMed] [Google Scholar]
- 40.Carter, A. P., Clemons, W. M., Jr., Brodersen, D. E., Wimberley, B. T., Morgan-Warren, R. & Ramakrishnan, V. (2000) Nature 407, 340–348. [DOI] [PubMed] [Google Scholar]
- 41.Klein, D. J., Schmeing, T. M., Moore, P. B. & Steitz, T. A. (2001) EMBO J. 20, 4214–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura, M. & Holbrook, S. R. (2002) J. Mol. Biol. 320, 455–474. [DOI] [PubMed] [Google Scholar]
- 43.Nissen, P., Ippolito, J. A., Ban, N., Moore, P. B. & Steitz, T. A. (2001) Proc. Natl. Acad. Sci. USA 98, 4899–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pleij, H. W., Flaherty, K. M. & McKay, D. B. (1994) Nature 372, 111–113. [DOI] [PubMed] [Google Scholar]
- 45.Cate, J. H., Gooding, A. R., Podell, E., Zhou, K., Golden, B. L., Kundrot, C. E., Cech, T. R. & Doudna, J. A. (1996) Science 273, 1678–1685. [DOI] [PubMed] [Google Scholar]
- 46.Hiley, S. L., Sood, V. D., Fan, J. & Collins, R. A. (2002) EMBO J. 21, 4691–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins, R. A. (2002) Biochem. Soc. Trans. 30, 1122–1126. [DOI] [PubMed] [Google Scholar]
- 48.Lafontaine, D. A., Wilson, T. J., Norman, D. G. & Lilley, D. M. (2001) J. Mol. Biol. 312, 663–674. [DOI] [PubMed] [Google Scholar]
- 49.Lafontaine, D. A., Wilson, T. J., Zhao, Z.-Y. & Lilley, D. M. J. (2002) J. Mol. Biol. 323, 23–34. [DOI] [PubMed] [Google Scholar]
- 50.Sood, V. D. & Collins, R. A. (2002) J. Mol. Biol. 320, 443–454. [DOI] [PubMed] [Google Scholar]
- 51.Zamel, R. & Collins, R. A. (2002) J. Mol. Biol. 324, 903–915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.