Abstract
Mutations in Cu/Zn superoxide dismutase (SOD) are associated with the fatal neurodegenerative disorder amyotrophic lateral sclerosis (ALS). There is considerable evidence that mutant SOD has a gain of toxic function; however, the mechanism of this toxicity is not known. We report here that purified SOD forms aggregates in vitro under destabilizing solution conditions by a process involving a transition from small amorphous species to fibrils. The assembly process and the tinctorial and structural properties of the in vitro aggregates resemble those for aggregates observed in vivo. Furthermore, the familial ALS SOD mutations A4V, G93A, G93R, and E100G decrease protein stability, which correlates with an increase in the propensity of the mutants to form aggregates. These mutations also increase the rate of protein unfolding. Our results suggest three possible mechanisms for the increase in aggregation: (i) an increase in the equilibrium population of unfolded or of partially unfolded states, (ii) an increase in the rate of unfolding, and (iii) a decrease in the rate of folding. Our data support the hypothesis that the gain of toxic function for many different familial ALS-associated mutant SODs is a consequence of protein destabilization, which leads to an increase in the formation of cytotoxic protein aggregates.
The Cu/Zn-superoxide dismutase (SOD) is a very stable homodimeric metalloenzyme that catalyzes the dismutation of superoxide radical to hydrogen peroxide and molecular oxygen (1, 2). Each monomer of SOD forms an eight-stranded Greek key β-barrel (3), containing an independent active site that tightly binds a catalytic copper ion and a structural zinc ion. A genetic linkage between mutations in SOD and the disease amyotrophic lateral sclerosis (ALS) was first established in 1993 (4). Since this time, >90, predominantly missense, mutations have been identified at sites spread throughout the structure of the protein (5, 6). ALS is a devastating paralytic disorder caused by degeneration of motor neurons in the brain and spinal cord, leading to death, typically between 3 and 5 years of disease onset. Mutations in SOD are associated with ≈20% of cases of familial ALS (fALS) and represent the major known cause of ALS (7).
There is strong evidence for a gain of toxic function by mutant SOD; however, the molecular mechanism for this toxicity has yet to be discerned (5, 6). Many different specific mechanisms for mutant SOD toxicity have been proposed; these largely involve aberrant oxidative chemistry and/or misfolding and aggregation of mutant SOD (6). There is significant evidence indicating that fALS may be another example of a protein conformational disorder (reviewed in refs. 6 and 8–10). In such disorders, which include, for example, prion diseases, Ig light chain disorders, and transthyretin amyloidoses, naturally occurring proteins are altered or mutated, and the variant proteins misfold to form aggregates (11). In fALS, inclusions (i.e., protein aggregates) that are intensely immunopositive for SOD are observed in motor neurons and astrocytes (9). There is evidence that the inclusions are initially composed mainly of granular material and later form randomly oriented 15- to 25-nm granule-coated fibrils (9). Aggregate formation is reduced, and cell viability is increased when mutant SOD is coexpressed with protein-folding chaperones in cell culture, suggesting that aggregation and cytotoxicity are related (12). Further support for the hypothesis that SOD aggregation is a cause of fALS comes from model studies in mice, in which insoluble mutant SOD complexes are observed well before inclusion bodies and motor neuron pathology become apparent (13).
Although the effects of SOD mutations have been studied extensively in vivo, relatively little is known about the properties of purified mutant SOD, and general correlations between properties of mutant SOD and fALS disease characteristics have not been identified (14). In this article, we describe the effects on SOD folding and aggregation for four biologically and chemically diverse fALS mutations: A4V, the most common fALS mutation in North America, which has a particularly short disease duration; G93A, which has been studied extensively in fALS mouse models; G93R; and E100G. A4V introduces a larger hydrophobic residue into the SOD dimer interface. G93 is located in a tight turn; this residue is a mutational hotspot for substitution of various larger and less flexible residues such as A or R. E100G disrupts a salt bridge and introduces a smaller, more flexible residue at the end of a β-strand. Thus, these mutants may reveal diverse mechanisms, by which SOD folding and stability are altered in fALS. We use as a reference protein in which the two free cysteines at positions 6 and 111 in WT SOD are mutated to alanine and serine (AS SOD), respectively. Whereas WT SOD undergoes irreversible thermal denaturation caused by the formation of nonphysiological intermolecular disulfide bonds, this process is eliminated in AS SOD, which unfolds reversibly (2), facilitating the determination of changes in stability caused by fALS mutations. Further attractive features of AS SOD as a control protein are that cysteine 6 in human SOD is often replaced by alanine in other species (15) and that the AS protein has structure, activity, and thermal stability very similar to WT SOD (2, 3).
Materials and Methods
Preparation of Recombinant SOD. fALS SOD mutations were made as described (16) or using the Quickchange protocol (Stratagene). Recombinant proteins were expressed and purified by using a modification of the procedure of Getzoff et al. (16) in which a Poros PE column replaced the DEAE column. Protein metal content was determined by inductively coupled plasma atomic emission spectroscopy. Activity was measured by SOD inhibition of pyrogallol autooxidation (17). Protein with no metal bound (apo SOD) was generated by dialyzing the purified protein against EDTA at pH 3.8 (18).
Differential Scanning Calorimetry (DSC). DSC scans were obtained with a MicroCal VP-DSC. SOD samples were prepared in 20 mM Hepes buffer at pH 7.8 or in 20 mM Mes buffer at pH 5.4. Samples were scanned at a rate of 1°C/min.
Rates of Unfolding. Guanidinium (Gdm)Cl-induced unfolding rates were monitored by fluorescence, using a Fluorolog3–22 (Instruments SA, Edison, NJ), with excitation and emission wavelengths of 282 and 360 nm, respectively. Solutions contained various concentrations of GdmCl, 0.04–0.06 mg/ml protein, and 20 mM Hepes, pH 7.8, at 25°C. Rates slower than ≈0.01 s–1 were measured by manual mixing, whereas faster rates were measured by stopped-flow, using an SFM4/Q instrument (Molecular Kinetics, Pullman, WA) interfaced to the Fluorolog3–22. Raw kinetic data were fit by using BIOKINE software (version 2.10, Molecular Kinetics).
Trifluoroethanol (TFE)-Induced Aggregation. A solution containing 8–25% (vol/vol) TFE, 25 μM thioflavin T (ThT), 50 mM Mes, pH 5.4, or 50 mM acetate buffer, pH 5.5, was preequilibrated at 25°C. Aggregation was initiated by the addition of SOD protein to a final concentration of 0.2 mg/ml. Aggregation was followed by monitoring ThT fluorescence by using a Fluorolog3–22 with excitation and emission wavelengths of 440 and 482 nm, respectively. The criterion for occurrence of aggregation was observation of a 2% increase in ThT fluorescence within 60 min of addition of TFE.
Heat-Induced Aggregation. Protein at a final concentration of 0.2, 0.025, or 0.01 mg/ml was added to 20 mM Mes, pH 5.4 and preequilibrated at 5°C or 25°C, and then the solution was heated at ≈0.7°C/min. The temperature of onset of aggregation, Tagg, was monitored by 90° light scattering at 450 nm, with excitation and emission slit widths of 1 and 2 nm, respectively.
ThT Binding. TFE-induced aggregation was first allowed to reach a steady state, then the reaction was diluted into ThT solutions with a final concentration of 50 mM glycine, pH 9.0, at 25°C. Fluorescence spectra were scanned immediately after dilution, using an excitation wavelength of 440 nm and excitation and emission slit widths of 1 and 5 nm, respectively. Heat-induced aggregates were prepared by incubating at a temperature above Tagg until steady state was reached; aggregates were then diluted to a final concentration between 0.01 and 0.1 mg/ml protein, in ThT solutions containing a final concentration of 50 mM glycine, pH 9.0, and fluorescence spectra were measured as for the TFE-induced aggregates.
Congo Red Binding. Protein aggregates were diluted to a final concentration of 0.05–0.07 mg/ml, in 50 mM Mes, pH 5.4, or 50 mM acetate, pH 5.5, 10 μM Congo red. For TFE aggregates, final solutions also contained between 8% and 25% (vol/vol) TFE. Absorption spectra were acquired with a Cary 1Bio UV/visible spectrophotometer (Varian).
Transmission Electron Microscopy. Aggregate suspensions were incubated on 400-mesh carbon-coated formvar copper grids (Marivac, St. Laurent, Quebec) or on 300-mesh formvar copper grids prepared in house. After drawing off excess solution, grids were air-dried, then stained with 2% (wt/vol) uranyl acetate. Specimens were viewed with a Philips CM20 electron microscope at an accelerating voltage of 200 kV. Images were digitized using a Gatan 679 slow-scan CCD camera and analyzed using DIGITALMICROGRAPH (version 2.1, Gatan).
Results
Preparation of Homogeneous Recombinant Holo and Apo SOD. Control and mutant SODs were prepared in both holo (1 Cu and 1 Zn per monomer) and apo forms. In contrast to incorrect metallation of SOD prepared with other expression systems (19–21), several lines of evidence demonstrate that the holo SOD prepared here is fully and correctly metallated. First, inductively coupled plasma atomic emission spectroscopy analysis revealed that the holo SOD preparations contain 1 mol of Cu and 1 mol of Zn per mol of polypeptide. Second, the pyrogallol activity assay showed that the specific activity is comparable for all holo proteins, ≈1,800 units/mg protein. Because bound copper is essential for enzymatic activity, this indicates that the copper site is fully occupied. Third, DSC scans of the holo proteins have a single peak, consistent with the presence of only one species, i.e., the correctly metallated protein. Full metallation of the SOD proteins studied here has also been observed by others (22, 23).
Apo SOD was prepared by dialyzing protein against EDTA at low pH (18). The loss of metal was confirmed by lack of detectable activity, by no bound metal being detected by inductively coupled plasma atomic emission spectroscopy, and by a single peak in DSC scans at a much lower temperature than for the holo proteins (see below).
fALS Mutations Decrease Protein Stability. The stabilities of holo and apo SODs were measured by DSC (Fig. 1 and Table 1). At pH 7.8, the thermal unfolding was more than ≈70–80% reversible for holo SODs and ≈100% reversible for apo SODs. All of the fALS mutations reported here (A4V, G93A, G93R, and E100G) and other fALS mutations (V14M, H43R, H46R, H48Q, G93D, G93V, G93S, and R143D; data not shown) decrease the thermal stability of SOD. Destabilization is observed for both the holo and the apo proteins, but the degree of destabilization is different in the two forms. The apparent temperature of maximum specific heat capacity, Tm, is typically decreased by ≈5–15°C in all of the mutant holo proteins studied and by ≈3–11°C in the apo proteins (Table 1).
Fig. 1.
DSC of apo and holo SODs at pH 7.8 (A) and apo SODs at pH 5.4 (B). (A) Specific heat capacity per mol dimer versus temperature for E100G (dashed line) and control SOD (solid line), with reference buffer–buffer scan subtracted. Solutions contained 0.2–0.7 mg/ml protein in 20 mM Hepes, pH 7.8. Apo and holo E100G have lower stability and unfold at lower temperatures than the corresponding forms of the control AS protein. (B) Specific heat capacity per mol dimer versus temperature for (left to right) apo A4V (dashed line), apo E100G (dotted line), and apo control (solid line) with reference buffer–buffer scan and then specific heat capacity of native state subtracted. Solutions contained 0.2 mg/ml protein in 20 mM Mes, pH 5.4. The shapes of the traces are distorted compared with those at pH 7.8 due to exothermic aggregation. Arrowheads show the corresponding onset of aggregation for (left to right) apo A4V, apo E100G, and apo control, as measured by 90° light scattering.
Table 1. Thermal stability data for holo and apo SODs.
| Holo
|
Apo
|
||
|---|---|---|---|
| Protein | Tm*, °C | Tm*, °C | Tagg†, °C |
| Control | 92.7 ± 0.3 | 59.6 ± 0.2 | 47 ± 1 |
| G93A | 88.0 ± 0.5 | 49.1 ± 0.5 | 8 ± 0 |
| G93R | 77.6 ± 0.6 | 56.3 ± 0.4 | 8 ± 0 |
| E100G | 87.3 ± 0.1 | 51.8 ± 0.1 | 34 ± 5 |
| A4V | 88.1 ± 0.1 | 50.7 ± 0.0 | 27 ± 4 |
Apparent temperature of maximum specific heat capacity for 0.5 mg/ml protein, pH 7.8; n = 3 experiments.
Temperature of onset of aggregation for 0.025 mg/mL protein, pH 5.4; n = 2 experiments.
DSC scans were also acquired at pH 5.4, where holo and apo SOD unfold at slightly lower temperature and with much lower reversibility than at pH 7.8 (Fig. 1B). The decreased reversibility at lower pH is caused by increased protein aggregation, which distorts the DSC trace by causing an exotherm at increased temperature (Fig. 1B).
Rates of Unfolding Are Increased in Holo and Apo fALS SODs. Rates of GdmCl-induced unfolding, kunf, of holo and apo SODs were measured at pH 7.8 by monitoring changes in intrinsic protein fluorescence. The unfolding kinetics of the holo and apo SODs in general follow double exponential kinetics (data not shown), and both kinetic phases are increased to varying extents for the mutant proteins. The unfolding rates for holo and apo SODs were compared at 6 and 4 M GdmCl, respectively (Fig. 2). Owing to its slower unfolding, both unfolding rates can be measured at these GdmCl concentrations for AS SOD; however, for the mutants, the relatively small amplitude fast phase is largely complete within the experimental dead time, and so only the slow phase can be measured. Overall, the holo and apo mutant SODs unfold between 2- and 50-fold more rapidly than the control AS. The increased rates of unfolding suggest that folded mutant SODs may more readily access unfolded or partially folded states that have an increased propensity to aggregate.
Fig. 2.
Rates of unfolding for holo SODs in 6 M GdmCl (• and ▪) and apo SODs in 4 M GdmCl (○ and □) in 20 mM Hepes, pH 7.8, 25°C. Owing to the slower unfolding of AS SOD, both the fast (• and ○) and slow (▪ and □) unfolding phases could be measured for this protein. Because of faster unfolding of the mutants, the faster phase is largely complete in the experimental dead time, and only the slow phase was measured.
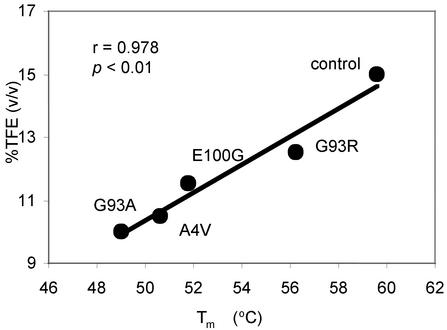
TFE-Induced Aggregation Occurs More Readily for fALS Mutant SODs. The propensity of the mutant SODs to aggregate was investigated systematically at pH 5.4 by using TFE or heat (see below) to induce aggregation; these agents have been used extensively to study aggregation of other proteins. The minimum amount of TFE required to induce SOD aggregation was determined by measuring enhancement of ThT fluorescence upon binding of this dye to SOD aggregates (24). In general, the aggregates induced by TFE were also visible by eye. The level of ThT fluorescence enhancement was typically ≈2- to 3-fold at steady state (Fig. 3A). Addition of up to 80% TFE caused no aggregation for holo AS and holo G93A. Thus, holo SODs do not readily aggregate in TFE, presumably because of the very high stability of the metallated proteins (Table 1). In contrast, apo SODs readily aggregated in TFE, and the amount of TFE required to induce aggregation was correlated with the Tm measured by DSC (Fig. 4). These results demonstrate that the decreased stability of the fALS SOD mutants is associated with an increased propensity of these proteins to aggregate.
Fig. 3.
ThT and Congo red binding properties of SOD aggregates. Spectra are shown for solutions with (○) and without (•) protein. (A) ThT fluorescence emission spectra for TFE-induced aggregates of apo G93A. (B) Congo red absorbance spectra for TFE-induced aggregates of apo G93A. (C) ThT fluorescence emission spectrum for heat-induced aggregates of apo G93R. (D) Congo red absorbance spectrum for heat-induced aggregates of apo A4V.
Fig. 4.
Correlation between propensity for aggregation and conformational stability of apo SOD. Minimum percentage of TFE (vol/vol) required to induce protein aggregation monitored by enhancement of ThT fluorescence versus Tm. Aggregation was induced by adding protein to a final concentration of 0.2 mg/ml in 50 mM acetate buffer, pH 5.5, at 25°C. The data fit to a straight line by least-squares regression have a high correlation coefficient (r = 0.978) and a low probability of chance correlation (P < 0.01).
The 2- to 3-fold ThT fluorescence enhancement for SOD aggregates is orders of magnitude smaller than the ≈1,000-fold enhancement typically observed for amyloid aggregates (25). Binding of Congo red to the TFE aggregates gives rise to a small shoulder centered at ≈565 nm (Fig. 3B); this change is also smaller and different from the large increase in absorbance at 541 nm typically seen for binding to amyloid (26). These observations suggest that the TFE aggregates are distinct from amyloid and have similar tinctorial properties to in vivo ALS aggregates (27).
Electron microscopy of the TFE-induced aggregates showed a range of morphologies, including fibrils, amorphous granular aggregates, and large densely stained regular structures (Fig. 5 A–C). The fibrils resemble the 10- to 15-nm (27) and 15- to 25-nm (9) granule-coated fibrils observed in ALS patients, and the 13-nm fibrils found in mice expressing fALS mutant SOD (28). Some of the TFE-induced fibrils have a diameter of ≈12–15 nm and consist of two or possibly three strands that are loosely coiled about one another (Fig. 5B). Thicker fibrils with a diameter of 15–25 nm consist of two aligned or loosely wound 12- to 15-nm fibrils (Fig. 5B). The fibrils are often associated with amorphous granular aggregates (Fig. 5A) or are further assembled into larger structures (Fig. 5 A and B). The dimensions of the fibrils, the association of the fibrils with amorphous granular aggregates, and the bumpy surface of the fibrils resemble the description of in vivo SOD aggregates (9, 27, 28). These in vitro studies suggest that decreased stability of fALS SOD mutants is correlated with an increased propensity of these proteins to form aggregates and that in vitro SOD aggregates resemble aggregates found in vivo.
Fig. 5.
Electron microscopy of in vitro SOD aggregates induced by TFE (A–C) and heat (D–F) showing representative aggregate morphologies. Bars are 100 nm. (A) Amorphous aggregates (right middle), as well as fibrils associated with amorphous aggregates (middle) and merging into larger heavy-staining aggregates (left). (B) Single fibril of diameter ≈12–15 nm (right arrow) apparently consisting of two narrower strands loosely wound together. Single fibrils associate in pairs (left arrow) that are aligned or loosely twisted together to form thicker fibrils with net diameter of ≈15–25 nm. (C) Large, heavily stained amorphous fractal-like aggregates. (D) Amorphous light-colored aggregate spheres or distorted spheres up to ≈100 nm in diameter, associated with thinner fibrils. (E) Higher magnification of D shows heat-induced fibrils resembling TFE-induced fibrils (B). (F) Mechanism of aggregate assembly whereby amorphous light-colored aggregate spheres of various diameters elongate into thinner fibrils. (A–E) Apo G93A. (F) Apo A4V.
Heat-Induced Aggregation Occurs More Readily for fALS Mutant SOD. In addition to monitoring the aggregation of SOD induced by TFE, we investigated aggregation induced by heat. Owing to the very high thermal stability of holo SOD (Fig. 1, Table 1), these studies were also performed on apo SOD. The onset of aggregation at increased temperature was detected by monitoring 90° light scattering (29) (Fig. 6). The fALS SOD mutants aggregate at temperatures that are much lower than the temperature that induces the aggregation of the AS control SOD; typically, this difference is 12–40°C.
Fig. 6.
Heat-induced aggregation of apo SOD monitored by 90° light scattering. From left to right, apo G93A (▵), apo A4V (•), apo E100G (□), and apo control (⋄) are shown. For clarity, absolute signals have different offsets added. Solutions contained 20 mM Mes, pH 5.4, and 0.010–0.025 mg/ml protein.
The properties of the heat aggregates of SOD were similar to the properties of the TFE aggregates. The heat aggregates cause <2-fold enhancement of ThT fluorescence (Fig. 3C) and very little change in the Congo red spectrum (Fig. 3D). The heat aggregates contained morphologically similar but apparently more abundant fibrils than the TFE aggregates (Fig. 5 D–F) and fewer amorphous granular and large, strongly stained regular structures. In addition, the heat aggregates contained abundant spherical structures with no apparent internal structure and diameters up to, typically, ≈100 nm (Fig. 5 D–F); these spheres were much less prevalent in the TFE aggregates. The exact dimensions of granular aggregates observed in fALS have not been described in detail, but it is possible that the spherical structures are similar to these granular aggregates (9). A unifying characteristic is that ALS-like aggregates are formed in vitro under different destabilizing conditions, i.e., at increased temperature or upon addition of TFE. This finding suggests that SOD may have a fundamental propensity to form these aggregates under any destabilizing conditions.
Mechanism of Aggregation. The mechanism of aggregate assembly was investigated by acquiring electron micrographs at different time points during the aggregation, which was monitored by light scattering. For the heat aggregates, we observed a remarkable morphological change whereby amorphous spherical structures were observed first; these then appeared to stretch out into thinner fibrils, and they then associated into larger structures (Fig. 5F). The morphology of the TFE aggregates largely remained constant with time, but there was also evidence for the sphere-to-fibril transition. This structural change represents a potential mechanism for formation of SOD aggregates in ALS.
Discussion
We have measured the effects of various fALS mutations on SOD folding and aggregation, for both the holo and the apo forms of the protein. Previous studies have reported that recombinant SOD is often heterogeneous in its metallation state (19–21); however, the holo and apo preparations used here are homogeneous, based on measurements of metal content, enzyme activity, and DSC. We find that the mutant SODs characterized in detail herein as well as other fALS mutants have decreased thermal stability. These data taken together with other thermal denaturation data (19) and apparent midpoints for chemical denaturation data (21) demonstrate that a decrease in protein stability is common to all fALS SOD mutants studied to date.
The mutants studied here exhibit small to moderate decreases in stability and have increased unfolding rates, with the extent of destabilization and increase in unfolding rates differing between holo and apo forms of the protein (Table 1, Fig. 2). For the apo SODs, decreased stability is correlated with an increased propensity to aggregate (Fig. 4). Aggregation probably occurs via a partly folded or unfolded state because heat aggregation occurs at temperatures close to the onset of the DSC unfolding transitions, not only for apo SODs but also for holo SODs (data not shown). Also, TFE aggregation occurs only when sufficient TFE has been added to induce unfolding (data not shown). It should be noted that various mutation-induced kinetic as well as structural changes can influence heat-induced aggregation, and so the temperature of onset of heat-induced aggregation is not always correlated with equilibrium protein stability (29).
Our results suggest several mechanisms by which fALS mutations may enhance protein aggregation: (i) increased equilibrium population of partly folded or unfolded protein; (ii) increased rates of protein unfolding; and (iii) decreased rates of protein folding. With respect to the first mechanism, decreases in Tm for SOD mutants can reasonably be expected to be correlated with decreases in thermodynamic stability (2, 30). This implies that for the mutants the relative equilibrium population of partially folded or unfolded states will be increased relative to the native state, and this may lead to enhanced aggregation. Because the stability of apo SOD is much lower than holo SOD, this mechanism may be particularly important for mutations that significantly destabilize the apo state. Consistent with this, a correlation between decreased stability in chemical denaturation experiments and mean survival time after ALS diagnosis has been reported for the apo form of five fALS mutant SODs (21). Regarding the second mechanism, the SOD mutants studied here unfold more rapidly than control, and so they may more often access aggregation-prone unfolded or partially folded states. Folding rates for mutant SODs have not been measured; however, considering the relatively small increases in unfolding rates, we expect the folding rates to be decreased to varying extents for the mutant proteins. Slower folding may lead to increased aggregation by allowing more time for aggregation to occur for newly synthesized SOD, or by increasing time required for SOD to fold when associated with a folding chaperone (see below).
We find evidence for an interesting aggregation process for SOD in which amorphous aggregates transform into fibrils. Aggregate morphologies can be very sensitive to solution conditions in vitro (31) and in vivo (32), and so in vitro morphological data must be interpreted carefully. It is noteworthy, however, that under destabilizing conditions SOD tends to form aggregates, both amorphous and fibrillar, that resemble in vivo aggregates (9, 27, 28). Moreover, the transformation of the amorphous aggregates to fibrillar structures in vitro may have similarities to structural changes of aggregates in fALS patients (9).
The mutations studied here decrease SOD stability, increase unfolding rates, and increase the propensity to aggregate. Similar effects of mutations have been observed in other protein conformational disorders such as transthyretin amyloidoses (33), Ig light chain disorders (34), and lysozyme amyloidoses (35). These similarities provide further support for the hypothesis that fALS is a protein conformational disorder.
Recent evidence suggests that the cytotoxic agents in protein conformational disorders may be small soluble aggregates that form early in the aggregation process, rather than large mature aggregates (reviewed in ref. 36). The small aggregates may interact with and overwhelm protein chaperones and degradation pathways, leading to deregulation of many cellular functions and, ultimately, to cell death. There is considerable evidence that a similar disease mechanism may occur in fALS. First, the morphology of some of the amorphous SOD aggregates resembles those of aggregates of non-disease-associated proteins shown to be toxic (37), suggesting that SOD aggregates may also be toxic. Second, the formation of high molecular weight complexes of mutant SOD occurs well before the onset of disease symptoms in mice models (13), implicating aggregate formation as an early, perhaps causative, step in ALS. Finally, mutant SODs show increased interaction with heat shock proteins (38, 39), and up-regulation of protein chaperones preserves the viability of cells expressing fALS SOD mutants (12). Thus, increased SOD aggregation may divert chaperones from other essential cellular functions.
Our data suggest that the different specific effects of SOD mutations may all lead to a common first step in fALS disease, namely, the aggregation of mutant SOD. SOD aggregation may increase because the mutant proteins have decreased stability, increased unfolding rate, and/or decreased folding rate, for holo, apo, or other mismetallated forms of SOD. This general explanation can account for the in vivo toxicity of many different fALS-associated SOD mutations and suggests that preventing or correcting toxic aggregation could provide useful strategies for prevention or treatment of ALS.
Acknowledgments
We thank Dasa Lipovšek, Joe Gaspar, and Clare Siddall for insightful discussions and assistance with manuscript preparation and the Natural Sciences and Engineering Research Council (Canada) for funding this research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ALS, amyotrophic lateral sclerosis; fALS, familial ALS; Gdm, guanidinium; SOD, superoxide dismutase; DSC, differential scanning calorimetry; TFE, trifluoroethanol; ThT, thioflavin T; apo, SOD protein with no metal bound.
References
- 1.Fridovich, I. (1986) Adv. Enzymol. Relat. Areas Mol. Biol. 58, 61–97. [DOI] [PubMed] [Google Scholar]
- 2.Lepock, J. R., Frey, H. E. & Hallewell, R. A. (1990) J. Biol. Chem. 265, 21612–21618. [PubMed] [Google Scholar]
- 3.Parge, H. E., Hallewell, R. A. & Tainer, J. A. (1992) Proc. Natl. Acad. Sci. USA 89, 6109–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362, 59–62. [DOI] [PubMed] [Google Scholar]
- 5.Rowland, L. P. & Shneider, N. A. (2001) N. Engl. J. Med. 344, 1688–1700. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland, D. W. & Rothstein, J. D. (2001) Nat. Rev. Neurosci. 2, 806–819. [DOI] [PubMed] [Google Scholar]
- 7.Orrell, R. W. (2000) Neuromuscular Disord. 10, 63–68. [DOI] [PubMed] [Google Scholar]
- 8.Julien, J. P. (2001) Cell 104, 581–591. [DOI] [PubMed] [Google Scholar]
- 9.Kato, S., Takikawa, M., Nakashima, K., Hirano, A., Cleveland, D. W., Kusaka, H., Shibata, N., Kato, M., Nakano, I. & Ohama, E. (2000) Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 163–184. [DOI] [PubMed] [Google Scholar]
- 10.Shibata, N., Hirano, A., Yamamoto, T., Kato, Y. & Kobayashi, M. (2000) Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 143–161. [DOI] [PubMed] [Google Scholar]
- 11.Soto, C. (2001) FEBS Lett. 498, 204–207. [DOI] [PubMed] [Google Scholar]
- 12.Bruening, W., Roy, J., Giasson, B., Figlewicz, D. A., Mushynski, W. E. & Durham, H. D. (1999) J. Neurochem. 72, 693–699. [DOI] [PubMed] [Google Scholar]
- 13.Johnston, J. A., Dalton, M. J., Gurney, M. E. & Kopito, R. R. (2000) Proc. Natl. Acad. Sci. USA 97, 12571–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratovitski, T., Corson, L. B., Strain, J., Wong, P., Cleveland, D. W., Culotta, V. C. & Borchelt, D. R. (1999) Hum. Mol. Genet. 8, 1451–1460. [DOI] [PubMed] [Google Scholar]
- 15.Bannister, W. H., Bannister, J. V., Barra, D., Bond, J. & Bossa, F. (1991) Free Radical Res. Commun. 1, 349–361. [DOI] [PubMed] [Google Scholar]
- 16.Getzoff, E. D., Cabelli, D. E., Fisher, C. L., Parge, H. E., Viezzoli, M. S., Banci, L. & Hallewell, R. A. (1992) Nature 358, 347–351. [DOI] [PubMed] [Google Scholar]
- 17.Marklund, S. & Marklund, G. (1974) Eur. J. Biochem. 47, 469–474. [DOI] [PubMed] [Google Scholar]
- 18.McCord, J. M. & Fridovich, I. (1969) J. Biol. Chem. 244, 6049–6055. [PubMed] [Google Scholar]
- 19.Rodriguez, J. A., Valentine, J. S., Eggers, D. K., Roe, J. A., Tiwari, A., Brown, R. H., Jr., & Hayward, L. J. (2002) J. Biol. Chem. 277, 15932–15937. [DOI] [PubMed] [Google Scholar]
- 20.Goto, J. J., Zhu, H., Sanchez, R. J., Nersissian, A., Gralla, E. B., Valentine, J. S. & Cabelli, D. E. (2000) J. Biol. Chem. 275, 1007–1014. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg, M. J., Tibell, L. & Oliveberg, M. (2002) Proc. Natl. Acad. Sci. USA 99, 16607–16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liochev, S. I., Chen, L. L., Hallewell, R. A. & Fridovich, I. (1998) Arch. Biochem. Biophys. 352, 237–239. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso, R. M., Thayer, M. M., DiDonato, M., Lo, T. P., Bruns, C. K., Getzoff, E. D. & Tainer, J. A. (2002) J. Mol. Biol. 324, 247–256. [DOI] [PubMed] [Google Scholar]
- 24.Chiti, F., Taddei, N., Bucciantini, M., White, P., Ramponi, G. & Dobson, C. M. (2001) EMBO J. 19, 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeVine, H., III (1999) Methods Enzymol. 309, 274–284. [DOI] [PubMed] [Google Scholar]
- 26.Klunk, W. E., Jacob, R. F. & Mason, R. P. (1999) Methods Enzymol. 309, 285–305. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto, K., Hirai, S., Yamazaki, T., Sun, X. Y. & Nakazato, Y. (1991) Neurosci. Lett. 129, 233–236. [DOI] [PubMed] [Google Scholar]
- 28.Stieber, A., Gonatas, J. O. & Gonatas, N. K. (2000) J. Neurol. Sci. 173, 53–62. [DOI] [PubMed] [Google Scholar]
- 29.Chrunyk, B. A. & Wetzel, R. (1993) Protein Eng. 6, 733–738. [DOI] [PubMed] [Google Scholar]
- 30.Fersht, A. (1999) Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding (Freeman, New York).
- 31.Khurana, R., Gillespie, J. R., Talapatra, A., Minert, L. J., Ionescu-Zanetti, C., Millett, I. & Fink, A. L. (2001) Biochemistry 40, 3525–3535. [DOI] [PubMed] [Google Scholar]
- 32.Buxbaum, J. & Gallo, G. (1999) Hematol. Oncol. Clin. North Am. 13, 1235–1248. [DOI] [PubMed] [Google Scholar]
- 33.Hammarstrom, P., Jiang, X., Hurshman, A. R., Powers, E. T. & Kelly, J. W. (2002) Proc. Natl. Acad. Sci. USA 99, 16427–16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetzel, R. (1997) Adv. Protein Chem. 50, 183–242. [DOI] [PubMed] [Google Scholar]
- 35.Booth, D. R., Sunde, M., Bellotti, V., Robinson, C. V., Hutchinson, W. L., Fraser, P. E., Hawkins, P. N., Dobson, C. M., Radford, S. E., Blake, C. C. & Pepys, M. B. (1997) Nature 385, 787–793. [DOI] [PubMed] [Google Scholar]
- 36.Kirkitadze, M. D., Bitan, G. & Teplow, D. B. (2002) J. Neurosci. Res. 69, 567–577. [DOI] [PubMed] [Google Scholar]
- 37.Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C. M. & Stefani, M. (2002) Nature 416, 507–511. [DOI] [PubMed] [Google Scholar]
- 38.Shinder, G. A., Lacourse, M. C., Minotti, S. & Durham, H. D. (2001) J. Biol. Chem. 276, 12791–12796. [DOI] [PubMed] [Google Scholar]
- 39.Okado-Matsumoto, A. & Fridovich, I. (2002) Proc. Natl. Acad. Sci. USA 99, 9010–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]