Abstract
Development of the endocrine pancreas includes a series of early events wherein precursor cells cluster, that is migrate to form cell aggregates, which subsequently differentiate into islets of Langerhans. We show that PANC-1 cells, a human pancreatic cell line, differentiates into hormone-producing islet-like cell aggregates after exposure to a defined serum-free medium. These cells were used to provide the following evidence that fibroblast growth factor (FGF)2 is a paracrine chemoattractant during PANC-1 cell clustering: (i) FGF2 is secreted and remains bound to the extracellular matrix from where it may diffuse to form chemoattractive gradients; (ii) a subset of cells expresses FGF receptors (FGFRs) -1, -2, -3, and -4; (iii) inhibition of FGFR tyrosine kinase inhibits cell clustering; and (iv) FGF2 neutralizing antibody inhibits clustering. In addition, adult human islet-derived precursor cells, which cluster and differentiate in a manner similar to PANC-1 cells, also secrete FGF2 and express FGFRs. We conclude that FGF2, acting as a paracrine chemoattractant, stimulates clustering of precursor cells, an early step leading to islet-like cell aggregate formation. Similar processes may occur during development of the islet of Langerhans in humans.
Keywords: migration, paracrine, aggregation, differentiation
Most of our knowledge of early pancreatic development is derived from studies in rodents and chickens, starting with the seminal experiments of isolated and recombined mouse pancreatic tissues that suggested the importance of epithelial–mesenchymal interactions as determinants of pancreatic fate (1, 2). During embryogenesis, the pancreas develops from a distinct dorsal and ventral anlage within the endoderm that is induced by factors from the developing notochord and mesenchyme (3, 4). As development proceeds, pancreatic precursor cells invade the surrounding mesenchyme and form aggregates that develop into islets of Langerhans (5, 6). Several extracellular regulatory factors released from the mesenchyme and notochord (3, 7) are thought to play vital roles in the initial process of cell migration into the mesenchyme that leads to aggregate formation, a key event in the normal development of the endocrine pancreas (8).
Early development of the dorsal pancreas in chickens is tightly regulated by fibroblast growth factor (FGF)2 released from the notochord (3, 7). During embryogenesis in mice, FGFs are widely expressed in the region of the mesenchyme surrounding pancreatic precursor cells and are involved in the early stages of development of the endocrine pancreas (9–16). Thus, in chicken and mice, mesenchyme-derived FGFs are thought to act via a paracrine mechanism to stimulate migration of pancreatic precursor cells into the mesenchyme and thereby initiate the process of pancreas development. However, recent studies suggest that the mesenchyme may not be required for formation of the endocrine pancreas, although it is necessary in development of the exocrine counterpart (13, 17, 18). These studies have led to the suggestion that the default pathway for the pancreatic epithelial primordium may be islet formation (17). It follows, therefore, that signals arising from endocrine pancreatic precursor cells themselves may be sufficient to promote cell clustering and differentiation during formation of the islets of Langerhans.
We find that human pancreatic PANC-1 cells, as well as adult human islet-derived precursor cells (hIPCs), form hormone-expressing islet-like cell aggregates (ICAs) when exposed to a defined serum-free media (SFM). The PANC-1 cell system is a useful model to study early stages in development of the islet of Langerhans. Of several growth and differentiation factors tested, FGF2 was found to be the most effective chemoattractant in the clustering of PANC-1 cells. Expression analysis of FGF2 and its receptor suggests that FGF2 signaling may also participate in clustering of hIPCs. These results suggest that FGF2, not derived from the surrounding mesenchyme but secreted by endocrine precursor cells, may be an important factor in the pathway leading to islet formation from pancreatic precursor cells.
Materials and Methods
Materials. Guinea pig anti-human insulin (Linco Research Immunoassay, St. Charles, MO), mouse antiporcine glucagon (Sigma), rabbit anti-human somatostatin (DAKO), mouse anti-human FGF receptor (FGFR) (Santa Cruz Biotechnology), and mouse anti-human FGF2 (Chemicon) antibodies were visualized with secondary antibodies from Jackson ImmunoResearch or Molecular Probes. Recombinant human FGF10 and activin-A were obtained from R & D Systems. Rabbit anti-human FGF2 neutralizing antibody was obtained from Abcam Limited (Cambridge, U.K.). All other reagents were from Sigma.
Cell Culture. PANC-1 cells (American Type Culture Collection, Manassas, VA) were started as passage 1 (passages 3–6 were used for all experiments). Cells were cultured in serum-containing media (SCM), a mixture of DMEM (Cellgro, Mediatech, Herndon, VA) containing 10% FBS. To induce ICA formation, SCM was removed, cells were exposed for 60–120 s to 0.05% trypsin (CellgroMediatech) at 25°C, to loosen but not detach the cells from their extracellular matrix (ECM) and then cultured in SFM. SFM was DME/F12 medium containing 17.5 mM glucose, 1% BSA (no. 152401, ICN) and insulin— transferrin—selenium (GIBCO). This brief exposure to trypsin did not dislodge any cells (pilot studies) and was seen to be critical for efficient generation of ICAs. hIPCs were obtained from islets isolated from five human cadavers (3–58 yr old) in the Transplantation Unit of National Institutes of Health and cultured in CMRL-1066 (GIBCO) supplemented with 10% FBS (C. Wei, A.A.H., B.M.-S., E.G.-R., B.M.R., and M.C.G., unpublished results). The hIPCs formed ICAs in a manner similar to the PANC-1 cells when exposed to the SFM.
Confocal Microscopy. Cells were fixed with fresh 4% paraformaldehyde, permeabilized with chilled 50% methanol, blocked with 4% serum, and labeled with primary and secondary antibodies, followed by washing. Fluoroprobes were visualized by using a Zeiss LSM 510 confocal laser-scanning microscope equipped with an argon as well as helium–neon lasers. Image stacks were collected in confocal mode with 1-μm-thick optical sections and stored for further analysis.
Time-Lapse Microscopy. PANC-1 cells in SCM were exposed to trypsin as described above, incubated in SFM supplemented with 25 mM Hepes, pH ≈7.2, kept at 37°C with a chamber heater (Bioptechs, Butler, PA), and visualized with a Zeiss Axiovert 200 inverted microscope equipped with a Hamamatsu Photonics (Hamamatsu City, Japan) digital charge-coupled device camera. Images from the same field were captured every 3 min for up to 18 h by using metamorph software (Ver. 4.6r8, Universal Imaging, Media, MD). Stacks of images for the entire time lapse were analyzed to track the cells over time, and data on total distance traveled, migrating cell velocity, and distance from origin were obtained by using the metamorph “track-point” function. All analyses were carried out on cells that started in the field of observation and remained in the same field for the entire duration of the time lapse. Data represent analyses performed on at least 30 different cells from three independent experiments.
FGFR Tyrosine Kinase Inhibition. To confirm whether signaling via FGFR was involved in cell migration, we used a tyrosine kinase inhibitor that is known to inhibit platelet-derived growth factor as well as FGFR tyrosine kinases (AG-1296; Biomol, Plymouth Meeting, PA). AG-1296 was dissolved in DMSO and used at concentrations of 0.1, 1.0, 10, 100, and 200 μM. Cells were exposed to AG-1296 or equivalent volumes of carrier (DMSO) alone for 1 h in SCM, briefly exposed to trypsin as described above, and cultured in SFM containing the respective concentrations of the inhibitor/carrier for the next 12 h. Culture dishes were scanned for estimation of the surface area occupied by cells before and after this 12-h incubation. Estimations of cell surface area occupied were made as described under Morphometric Analysis, below.
Migration Assays. PANC-1 cells were seeded in SCM on 8-μm pore size Transwell membranes (BD Labware, Bedford, MA) at a density of 30 × 103 cells/well and incubated 2.5 h to allow the cells to adhere. Cells were then briefly exposed to trypsin as described above and incubated in basal media (0.1 ml of SFM supplemented with 0.05% gelatin and 25 mM Hepes, pH ≈7.2). Growth factors, when added, were dissolved in basal media and placed in the lower and/or upper chamber(s). Activin A (20 ng/ml), epidermal growth factor (100 ng/ml), FGF1 (100 ng/ ml), FGF2 (100 ng/ml), FGF10 (50 ng/ml), and hepatocyte growth factor (50 ng/ml) were tested. Cells were allowed to migrate at 37°C for 4.5 h. After incubation, cells on top of the membranes were removed with sterile cotton swabs. Membranes were fixed with fresh 4% paraformaldehyde for 15 min at room temperature, stained with propidium iodide (Molecular Probes) for 20 min at 37°C, washed briefly, carefully removed, and mounted in Vectashield medium (Vector Laboratories). Labeled nuclei of cells trapped in the membrane were counted on a fluorescence microscope equipped with cell-counting grids (Klarmann Rulings, Litchfield, NH). In a separate experiment, heparin beads (Sigma) were washed three times and soaked in FGF2 or PBS (control) at room temperature for 2 h. PANC-1 cell monolayers were exposed to trypsin as described above and then incubated in SFM. PBS- or FGF2-coated beads were immediately placed in these culture dishes, incubated at 37°C for the next 6 or 24 h, and visualized by time-lapse photography (described above) or processed for immunocytochemical detection of FGF2.
Morphometric Analysis. To estimate the cell-occupied surface area of the culture dish, photomicrographs from at least 20 different fields and three separate experiments of ≈80% confluent PANC-1 cells grown in SCM were captured. Cells were then treated with trypsin as described above and incubated in SFM for the next 12 h. After incubation, similar fields from the same culture dishes were captured, and images obtained were processed to binary images by using identical threshold and binary image processing parameters. Areas occupied by cells, before and after the 12-h incubation in SFM, were determined by using the 3d-for-lsm software (Zeiss). As cells clustered, the rounded and multilayered cells in ICAs occupied less surface area, thereby exposing more of the culture dish. Cell-occupied surface areas of culture dishes after 12 h in SFM are presented as percentage of the surface area occupied at the start of the experiment (taken as 100%).
FGF2 Neutralization. PANC-1 cells in SCM were dislodged from their culture dishes, washed with PBS, resuspended in SFM, and incubated at 4°C until later use. The original culture dish was washed once with PBS and incubated for 1 h at 37°C in SFM with FGF2 antibody at 0.5 mg/ml to neutralize FGF2 previously deposited by the cells in the ECM. After incubation, PANC-1 cells were returned to these dishes in the continuing presence of the antibody and observed by using time-lapse microscopy every 3 min for the next 10 h. Image stacks collected were analyzed to calculate the distance traveled by cells from their origin and the total distance traveled. Control cells in SCM or SFM were returned to dishes in which ECM had been incubated in SFM without FGF2 antibody for 1 h.
Results
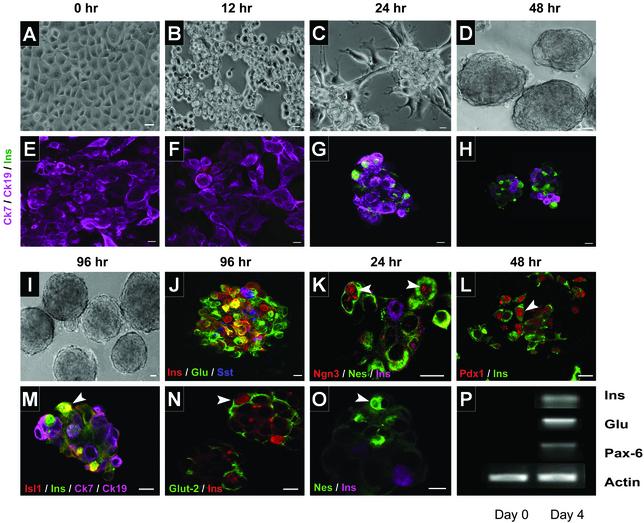
Human Pancreatic Precursor Cells Spontaneously Form ICAs After Exposure to SFM. PANC-1 cells in SCM proliferate as apparently homogeneous, epithelioid, flattened, angular cells in adherent monolayers (Fig. 1A) that do not express markers of mature endocrine cells (Fig. 1E). After brief exposure to trypsin to loosen but not detach cells from the substratum and subsequent incubation in SFM, PANC-1 cells form 3D clusters of rounded cells within 24 h that mature to hormone-expressing ICAs within the next 4 days (Fig. 1 F–J). These ICAs resemble mature human islets of Langerhans and contain cells that express insulin, glucagon, and somatostatin (Fig. 1J). hIPCs undergo a similar process under these conditions, which will be described separately (C. Wei, A.A.H., B.M.-S., E.G.-R., B.M.R., and M.C.G., unpublished results). RNA isolated from 4-day ICAs showed that transcripts for insulin, glucagon, and Pax-6 were expressed (Fig. 1P). In contrast, PANC-1 cells exposed briefly to trypsin but subsequently cultured in SCM do not form ICAs (not shown). Immunocytochemical analyses showed that ICAs contained cells that expressed Ngn-3 (Fig. 1K), Pdx-1 (Fig. 1L), and Isl-1 (Fig. 1M), transcription factors known to play an important role in mouse islet development (19). Insulin-expressing cells in ICAs coexpressed Isl-1 (Fig. 1M) and Glut-2 (Fig. 1N). During the differentiation process, ICAs expressed the intermediate filament protein nestin (Fig. 1 K and O), which has been suggested as a marker of endocrine cell differentiation. ICAs were also shown to secrete insulin in response to 20 mM glucose as well as depolarizing concentrations of KCl (data not shown), confirming a functional similarity to normal human islets. Thus, PANC-1 cells cultured in defined SFM cluster and differentiate into hormone-producing ICAs in a manner similar to primary cultures of hIPCs derived in our laboratory (C. Wei, A.A.H., B.M.-S., E.G.-R., B.M.R., and M.C.G., unpublished results), or similar precursor cells derived from adult pancreas (20, 21).
Fig. 1.
PANC-1 cells differentiate into ICAs. PANC-1 cells grow as flattened monolayers in SCM (A) and do not express insulin (E). After exposure to SFM, cells form ICAs (B–D) that show insulin-expressing cells after 2 days (F–H). These ICAs became more compact (I) by 4 days of exposure to SFM and express the major islet hormones (J) and Glut-2 (N). During aggregate formation, ICAs expressed transcription factors Ngn-3 (K), Pdx-1 (L), and Isl-1 (M). Most cells expressing Pdx-1 also expressed insulin, whereas all Isl-1- and Glut-2-expressing cells costained for insulin (L–N). At 24 h, developing ICAs also express the intermediate filament protein nestin (O). At day 4 of exposure to SFM, cells showed expression of the transcripts for insulin, glucagon, and Pax-6 (P). Arrowheads (K–O) represent cells expressing the respective transcription factors/proteins probed. E, F, H, K, L, and O are 1-μm confocal slices. G, J, M, and N are reconstructed stacks of multiple 1-μm slices. Individual probes are shown by the color of their fluorescence. Yellow is seen in cells that coexpress green and red fluoroprobes. (Bar = 10 μm.)
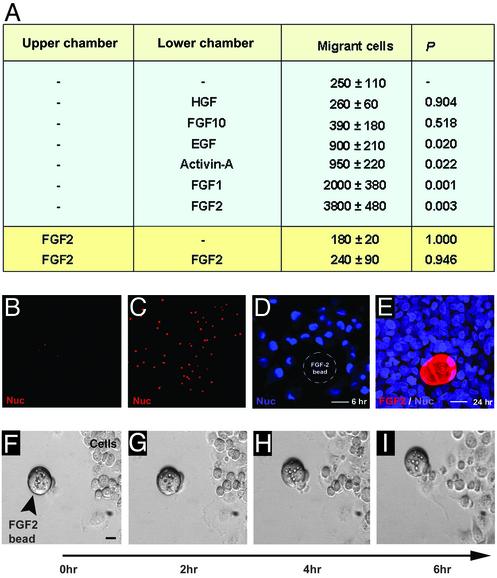
FGF2 Induces Directed Cellular Migration. PANC-1 cells formed ICAs in 1–2 days of exposure to SFM (Fig. 1 A–D). In a typical experiment, PANC-1 cells, which occupied 84 ± 1.3% of the total surface area of the culture dish in SCM, aggregated to occupy only 39 ± 1.7% (n = 6) of the surface of the dishes after 12 h in SFM. This decrease in surface occupancy apparently resulted from migration of cells to form ICAs, which, being multilayered and comprised of rounded cells, occupied less surface area. We observed that PANC-1 cells incubated in SFM migrated longer distances (150 ± 16 vs. 23 ± 3.6 μm, P < 0.0001) and traveled farther from their points of origin (37 ± 4.8 vs. 2.8 ± 0.9 μm, P < 0.0001) than cells incubated in SCM. Cells in SFM migrated six times more rapidly than cells in SCM (1.8 ± 0.2 vs. 0.3 ± 0.05 nm/s, P < 0.0001), and almost all cells in SFM were found within ICAs after 12–24 h. Time-lapse photography confirmed that cells formed ICAs by migrating to each other (see Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org) or toward newly forming aggregates (Movie 2, which is published as supporting information on the PNAS web site) and not via rapid proliferation of a sub-population of nonmigrating cells. Cells exposed to SCM did not show significant migration (Movie 3, which is published as supporting information on the PNAS web site).
To identify extracellular signaling molecules that could serve as attractants for PANC-1 cell aggregation, we monitored the effects of several growth and differentiation factors on cell migration. FGFs and activins were studied because, during pancreatic development in rodents, notochord–mesenchymal interactions produce a milieu in which FGFs and activins appear to induce migration of precursor pancreatic cells from the foregut endoderm to invade the mesenchyme and form aggregates that mature into pancreatic islets (22). In addition, we screened hepatocyte growth factor (HGF) because it causes differentiation of endodermal precursors into liver cells (23) and epidermal growth factor (EGF) because it stimulates proliferation of PANC-1 cells (24). HGF and FGF10 did not stimulate PANC-1 cell migration (Fig. 2A). EGF and activin-A caused modest (2- to 3-fold) stimulation of migration, whereas FGF1 and FGF2 stimulated migration 8- and 16-fold, respectively (Fig. 2 A). FGF2 induced chemotactic movement in PANC-1 cells rather than chemokinesis (nondirectional/random movement), because addition of FGF2 to the upper chamber alone or both did not increase migration (Fig. 2 A). These observations were confirmed directly in experiments in which PANC-1 cells in SFM were shown to migrate toward FGF2-coated heparin beads by 6 h (Fig. 2D) and then aggregate around these beads by 24 h (Fig. 2E). These findings were confirmed by using time-lapse microscopy (Fig. 2 F–I). PANC-1 cells migrated toward FGF2-coated heparin beads (see Movie 4, which is published as supporting information on the PNAS web site) but did not migrate toward or aggregate around uncoated beads (not shown). Thus, FGF2 is an effective chemoattractant inducing directed migration and aggregation of human pancreatic precursor cells to form ICAs.
Fig. 2.
FGF2 mediates directed migration of PANC-1 cells. The number of migrating PANC-1 cells ± SEM was determined after exposure to the indicated growth and differentiation factors (A). Membranes containing migrating cells are shown from representative experiments in which the lower chamber contained no added factors (B) or 100 ng/ml FGF2 (C). Cells migrate toward an FGF2-coated bead after 6 h (D) and form clusters around it after 24 h (E) of exposure to SFM. The position of the bead is outlined (D), because it was not retained during processing. Snapshots from time-lapse microscopy (Movie 4) show PANC-1 cells migrating toward an FGF2-coated heparin bead (F–I). (Bar = 10 μm.)
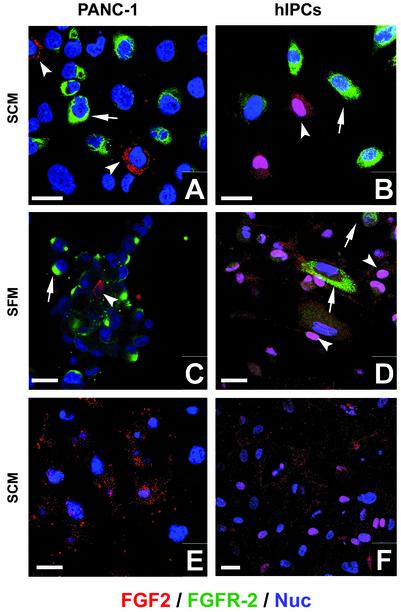
FGF2 Is Secreted and FGFRs Are Expressed by PANC-1 Cells and hIPCs. Because FGF2 was seen to be an effective chemoattractant and is also known to be an important regulator of rodent islet development in vivo, we determined whether PANC-1 cells or hIPCs express FGF2 and FGFRs. FGF2 and its high-affinity receptor FGFR-2 are expressed in PANC-1 cells as well as hIPCs cultured in SCM (Fig. 3 A and B) or after 12 h of exposure to SFM (Fig. 3 C and D). Interestingly, expression of FGF2 and the FGFRs was heterogeneous, with some cells being strongly positive, some moderately positive, and some negative. The most intriguing finding in both these cell types was that cells that were seen to be highly positive for FGF2 showed no or little immunopositivity for FGFR-2. In general, we did not observe immunopositivity for both FGF2 and FGFR-2 in the same cell (Fig. 3). We also detected similar expression of FGFR-1, -3, and -4 in PANC-1 cells and hIPCs (not shown) that generally did not colocalize with FGF2 expression. Western blotting of PANC-1 cell lysates prepared at 0 h or 1 day after exposure to SFM confirmed expression of both the low (18 kDa) as well as the high molecular mass (22 and 24 kDa) forms of FGF2 (Fig. 5, which is published as supporting information on the PNAS web site). Interestingly, the 18-kDa form of FGF2 has been implicated in increased cell migration in NIH 3T3 cells transected with cDNAs for different molecular forms of FGF2 (25). PANC-1 cells and hIPCs were capable of secreting FGF2. Confocal images taken at the ECM plane after immunostaining of PANC-1 cells (Fig. 3E) or hIPCs (Fig. 3F) revealed the presence of secreted FGF2 adhering to the matrix surrounding the cells. Thus, a subset of PANC-1 cells as well as hIPCs express and secrete FGF2, which can serve as a chemoattractant (Fig. 2) for FGFR-expressing precursor cells. PANC-1 cells are known to express FGF2 mRNA transcripts (26), and we confirmed this on microarrays and by real-time RT-PCR (not shown).
Fig. 3.
FGF2 and FGFR-2 are expressed by PANC-1 cells and hIPCs. (A–D) Expression of FGFR-2 (arrows) and FGF2 (arrowheads) observed by immunostaining of PANC-1 cells (A) or hIPCs (B) cultured in SCM and PANC-1 cells (C) or hIPCs (D) after 12 h in SFM. (E–F) Confocal section within a plane including PANC-1 cells (E) or hIPCs (F) and their ECM. Cells expressing FGF2 (red) in the nuclei (blue) appear pink. (Bar = 10 μm.)
Secreted FGF2 Stimulates ICA Formation of PANC-1 Cells. Because FGF2 and FGF1 were found to be highly effective in stimulating cell migration and because FGFRs signal via protein tyrosine kinases, we measured the effect of AG-1296, an inhibitor of protein tyrosine kinases, on ICA formation. Control cells aggregated to occupy only 37.5 ± 2.1% of the original occupied surface area. In contrast, AG-1296 effectively reduced cell aggregation in a dose-dependent manner to an extent that at the highest concentrations of the inhibitor almost 90% of the original occupied surface area remained occupied with cells (92.4 ± 1.8%). Thus, inhibition of tyrosine kinase signaling during the active phases of cell migration inhibited aggregate formation in a dose-dependent manner with an IC50 of ≈1 μM.
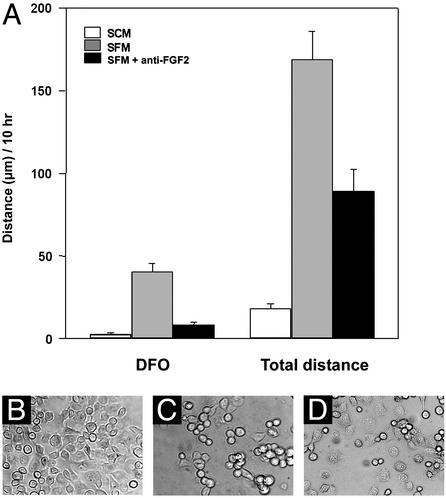
To confirm that cell clustering was influenced by FGF2 either released from cells or bound to the ECM, we performed a neutralization study (Fig. 4). PANC-1 cells growing in SCM were removed from their culture dishes by using trypsin, and the remaining ECM was washed and incubated with an FGF2-neutralizing antibody or with a control polyclonal antiglucagon antibody for 1 h. PANC-1 cells in SFM were then transferred to these antibody-coated culture dishes in the continuing presence of antibody and observed by time-lapse photography (Fig. 4). Cells returned to culture dishes that were not exposed to antibody and subsequently incubated in SCM or SFM served as controls. FGF2 antibody reduced distance traveled by cells from their origin by almost 5-fold (40 ± 4.9 vs. 8.1 ± 1.9 μm) as well as the total distance traveled by ≈2-fold. In contrast, an identical concentration of antiglucagon antibody did not affect cell migration (not shown). Thus, FGF2 antibody specifically reduced cell aggregation as illustrated by photomicrographs showing the final positions of cells after the 10-h incubation in SCM (Fig. 4B), SFM (Fig. 4C), and SFM containing FGF2 antibody (Fig. 4D). This finding supports the idea that FGF2 in the cell milieu plays an important role in modulating the rate and direction of cell migration during formation of ICAs.
Fig. 4.
FGF2-neutralizing antibody inhibits PANC-1 cell migration. Cells and culture dishes were prepared as described in Materials and Methods. After brief trypsin exposure (see Materials and Methods), cells were incubated in SCM, SFM, or SFM in the presence of FGF2 antibody and observed for 10 h by time-lapse microscopy. Results are mean ± SEM of distance from the origin (DFO) to the final position of each cell as well as the sum of distances traveled by each cell measured at 3-min intervals (A). Data were obtained from three independent experiments with at least 15 cells analyzed in each experiment. Representative photomicrographs after 10 h incubation in SCM (B), SFM (C), and SFM with FGF2 antibody (D) illustrate that FGF2 antibody inhibited cell aggregation.
Discussion
We envision that factors secreted by precursor cells stimulate clustering, a prerequisite to formation of mature hormone-expressing ICAs. To develop an experimental model that would allow us to assess autocrine or paracrine factors that influence cell clustering, we show that an undifferentiated human pancreatic cell line, PANC-1 cells, can be induced to differentiate into hormone-expressing ICAs. PANC-1 cells had been shown previously to undergo differentiation into insulin-expressing cells after stable expression of Pdx-1 and exposure to glucagon-like peptide 1 (27). In our study, PANC-1 cells formed ICAs when incubated in SFM. The differentiation process in PANC-1 cells appears to be similar to the in vitro differentiation of precursor or stem cells described by others (20, 21, 28).
It is noteworthy that PANC-1 cells were originally cloned from a single cell. PANC-1 cells, therefore, provide a model in which an effect of a secreted growth or development factor would occur via an autocrine or paracrine mechanism involving cells of the same type rather than via the previously established paracrine mechanism for rodent islet development in which a growth or developmental factor is secreted by mesenchymal cells and acts on epithelial precursors (8). After exposure to SFM, PANC-1 cells migrate toward each other to form clusters that subsequently differentiate into cells expressing Pdx-1, Pax-6, Isl-1, Glut-2, and insulin, markers of mature β cells; glucagon, a marker of α cells; and somatostatin, a marker of δ cells. Approximately 7% of cells within ICAs produced more than one hormone, in particular, insulin and glucagon, at 4 days of exposure to SFM. Cells that express more than one hormone have been reported in the developing pancreas (4, 29).
Because these cells expressed several transcription factors known to be important in mouse islet development (Fig. 1 K–P), we decided to use the system to study early events that might be involved in formation of the islet of Langerhans. In particular, we focused on the process of cell clustering, that is, the process in which cells migrate toward each other to form an aggregate. We began by testing a number of growth and differentiation factors that have been implicated in endocrine pancreas development in rodents (30). Although several factors exhibited chemoattractant properties for PANC-1 cells, we found that FGF2 was most effective. FGF2 is known to bind to several related receptors that signal via tyrosine kinase transduction pathways, and we found that PANC-1 cells express FGFRs (subtypes 1–4). Induction of precursor cell migration via FGFRs has been suggested to occur during pancreas development in rats (13, 31) and is also documented in other cell systems (32). Most of the FGFs act as extracellular regulatory molecules by binding to these cell surface receptors. Our observations of FGF2 immunopositivity in PANC-1 cells, hIPCs, and their ECMs (Fig. 3) point to the potential importance of FGF2 in early stages of clustering of pancreatic precursor cells. In support of a role for extracellular FGF2 in PANC-1 cell clustering, we showed that inhibition of tyrosine kinase signaling and, more specifically, antibody neutralization of extracellular FGF2 inhibited migration. Our finding of local reservoirs of FGF2 within the ECM is consistent with the idea that FGF2 signaling may be spatially regulated and that FGF2 bound in the ECM could be released to form local gradients.
Two mechanisms are thought to be involved in the release of FGFs from the cell matrix: (i) enzymatic cleavage and (ii) dissociation and binding to a carrier protein that can then deliver FGF to its receptors (9). The former process is known to be mediated directly, via degradation of matrix, or indirectly, via activation of plasmin, by the urokinase-type plasminogen activator (uPA) (33, 34). uPA-mediated release of FGF2 from binding sites in ECM has also been shown in vivo during angiogenesis (35), and up-regulation of uPA by FGF2 has been demonstrated (36). It is noteworthy that we found that both soluble extracellular and cell-associated uPA activities were increased during the first 5 h after exposure to SFM and had decreased after 24 h (not shown). Microarray analysis showed that uPA mRNA was 3- to 4-fold down-regulated in PANC-1 cells after 1 day in SFM (not shown). On the basis of these findings, we propose that certain cells within this population of pancreatic precursor cells synthesize and secrete higher levels of FGF2 that is retained bound to the ECM in an inactive state. FGF2 released from the ECM is then available to serve as a chemotactic factor to stimulate migration of these cells into clusters. It is also possible that FGF2 released directly from cells could form gradients and contribute to the clustering process.
We interpret our findings in PANC-1 cells as follows. PANC-1 cells proliferating in SCM are undifferentiated and express markers of “ductal” cells, such as cytokeratin-7 and -19, but do not express islet hormones (Fig. 1E). Although FGF2 was present in PANC-1 cells in SCM, we think that “inhibitory factors” present in serum restrict cell migration. Brief exposure to trypsin followed by culture in SFM was critical to optimize the process of migration and aggregate formation. On exposure to SFM, the cells start clustering. Clustering is stimulated by FGF2 via two paracrine mechanisms, direct secretion from cells and release from ECM. This process provides directionality of migration for FGFR-expressing cells toward cells that continue to secrete FGF2 and to areas within the ECM where “reservoirs” of FGF2 have been deposited. After cells have formed clusters, morphogenesis ensues in response to signals generated in part by cell–cell contact. Further studies in our laboratory using hIPCs (Fig. 3) and cells isolated from human fetal pancreata (not shown) suggest that similar mechanisms occur in these precursors and thus may occur during embryonic development of human islet cells.
On the basis of our findings in the PANC-1 model system and observations from primary adult human pancreatic islet-derived precursor cells (as well as human fetal cells), we conclude that FGF2 can act in an autocrine/paracrine manner to stimulate the initial process of cell aggregation and cluster formation, an important step in the development and differentiation of endocrine pancreas. Considering that mesenchymal factors probably do not contribute to islet formation (13, 17, 18), we propose that endoderm-derived chemoattractants (like FGF2) play an important role in cell aggregation and differentiation to ICAs. Although previous reports have provided evidence for the chemotactic properties of FGF2 in other systems (limb, lung, and exocrine pancreas development), we have shown that endoderm-derived FGF2 is a chemotactic factor that acts on these human endodermal pancreatic precursor cells to initiate islet formation. Our findings support a mechanism for FGF2 action in human islet development that involves an autocrine or paracrine action among cells derived only from the endoderm. Such local FGF2 effects within the developing pancreatic epithelium could play an important role in the formation and development of islet of Langerhans in humans.
Supplementary Material
Acknowledgments
We thank Dr. David Harlan, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, for providing adult human cadaveric pancreatic islets.
Abbreviations: FGF, fibroblast growth factor; FGFR, FGF receptor; ICA, islet-like cell aggregate; hIPC, adult human islet-derived precursor cells; SFM, serum-free media; SCM, serum-containing media; ECM, extracellular matrix; uPA, urokinase-type plasminogen activator.
References
- 1.Golosow, N. & Grobstein, C. (1962) Dev. Biol. 4, 242-255. [DOI] [PubMed] [Google Scholar]
- 2.Wessells, N. K. & Cohen, J. H. (1967) Dev. Biol. 15, 237-270. [DOI] [PubMed] [Google Scholar]
- 3.Kim, S. K., Hebrok, M. & Melton, D. A. (997) Development Supplement (Cambridge, U.K.) 124, 4243-4252. [DOI] [PubMed] [Google Scholar]
- 4.Slack, J. M. (1995) Development (Cambridge, U.K.) 121, 1569-1580. [DOI] [PubMed] [Google Scholar]
- 5.Apelqvist, A., Ahlgren, U. & Edlund, H. (1997) Curr. Biol. 7, 801-804. [DOI] [PubMed] [Google Scholar]
- 6.Grapin-Botton, A., Majithia, A. R. & Melton, D. A. (2001) Genes Dev. 15, 444-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebrok, M., Kim, S. K. & Melton, D. A. (1998) Genes Dev. 12, 1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund, H. (2001) Diabetologia 44, 1071-1079. [DOI] [PubMed] [Google Scholar]
- 9.Powers, C. J., McLeskey, S. W. & Wellstein, A. (2000) Endocr. Relat. Cancer 7, 165-197. [DOI] [PubMed] [Google Scholar]
- 10.Szebenyi, G. & Fallon, J. F. (1999) Int. Rev. Cytol. 185, 45-106. [DOI] [PubMed] [Google Scholar]
- 11.Kato, S. & Sekine, K. (1999) Cell Mol. Biol. 45, 631-638. [PubMed] [Google Scholar]
- 12.LeBras, S., Czernichow, P. & Scharfmann, R. (1998) Diabetologia 41, 1474-1481. [DOI] [PubMed] [Google Scholar]
- 13.Miralles, F., Czernichow, P., Ozaki, K., Itoh, N. & Scharfmann, R. (1999) Proc. Natl. Acad. Sci. USA 96, 6267-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celli, G., LaRochelle, W. J., Mackem, S., Sharp, R. & Merlino, G. (1998) EMBO J. 17, 1642-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohuchi, H., Hori, Y., Yamasaki, M., Harada, H., Sekine, K., Kato, S. & Itoh, N. (2000) Biochem. Biophys. Res. Commun. 277, 643-649. [DOI] [PubMed] [Google Scholar]
- 16.Bhushan, A., Itoh, N., Kato, S., Thiery, J. P., Czernichow, P., Bellusci, S. & Scharfmann, R. (2001) Development (Cambridge, U.K.) 128, 5109-5117. [DOI] [PubMed] [Google Scholar]
- 17.Scharfmann, R. (2000) Diabetologia 43, 1083-1092. [DOI] [PubMed] [Google Scholar]
- 18.Gittes, G. K., Galante, P. E., Hanahan, D., Rutter, W. J. & Debas, H. T. (1996) Development (Cambridge, U.K.) 122, 439-447. [DOI] [PubMed] [Google Scholar]
- 19.Schwitzgebel, V. M., Scheel, D. W., Conners, J. R., Kalamaras, J., Lee, J. E., Anderson, D. J., Sussel, L., Johnson, J. D. & German, M. S. (2000) Development (Cambridge, U.K.) 127, 3533-3542. [DOI] [PubMed] [Google Scholar]
- 20.Bonner-Weir, S., Taneja, M., Weir, G. C., Tatarkiewicz, K., Song, K. H., Sharma, A. & O'Neil, J. J. (2000) Proc. Natl. Acad. Sci. USA 97, 7999-8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soria, B. (2001) Differentiation 68, 205-219. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. K. & Hebrok, M. (2001) Genes Dev. 15, 111-127. [DOI] [PubMed] [Google Scholar]
- 23.Zaret, K. S. (2001) Curr. Opin. Gen. Dev. 11, 568-574. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, L. O., Cluck, M. W., Lovas, S., Otvos, F., Murphy, R. F., Schally, A. V., Permert, J., Larsson, J., Knezetic, J. A. & Adrian, T. E. (2001) Br. J. Cancer 84, 926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikfalvi, A., Klein, S., Pintucci, G., Quarto, N., Mignatti, P. & Rifkin, D. B. (1995) J. Cell Biol. 129, 233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohr, M., Schmidt, C., Ringel, J., Kluth, M., Muller, P., Nizze, H. & Jesnowski, R. (2001) Cancer Res. 61, 550-555. [PubMed] [Google Scholar]
- 27.Hui, H., Wright, C. & Perfetti, R. (2001) Diabetes 50, 785-796. [DOI] [PubMed] [Google Scholar]
- 28.Lumelsky, N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389-1394. [DOI] [PubMed] [Google Scholar]
- 29.De Krijger, R. R., Aanstoot, H. J., Kranenburg, G., Reinhard, M., Visser, W. J. & Bruining, G. J. (1992) Dev. Biol. 153, 368-375. [DOI] [PubMed] [Google Scholar]
- 30.Edlund, H. (2002) Nat. Rev. Genet. 3, 524-532. [DOI] [PubMed] [Google Scholar]
- 31.Elghazi, L., Cras-Meneur, C., Czernichow, P. & Scharfmann, R. (2002) Proc. Natl. Acad. Sci. USA 99, 3884-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura, T., Mochizuki, Y., Kanetake, H. & Kanda, S. (2001) Biochem. Biophys. Res. Commun. 289, 801-806. [DOI] [PubMed] [Google Scholar]
- 33.Whitelock, J. M., Murdoch, A. D., Iozzo, R. V. & Underwood, P. A. (1996) J. Biol. Chem. 271, 10079-10086. [DOI] [PubMed] [Google Scholar]
- 34.Rifkin, D. B., Mazzieri, R., Munger, J. S., Noguera, I. & Sung, J. (1999) APMIS 107, 80-85. [DOI] [PubMed] [Google Scholar]
- 35.Ribatti, D., Leali, D., Vacca, A., Giuliani, R., Gualandris, A., Roncali, L., Nolli, M. L. & Presta, M. (1999) J. Cell Sci. 112, 4213-4221. [DOI] [PubMed] [Google Scholar]
- 36.Presta, M., Moscatelli, D., Joseph-Silverstein, J. & Rifkin, D. B. (1986) Mol. Cell. Biol. 6, 4060-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.