Abstract
Degenerative diseases represent a severe problem because of the very limited repair capability of the nervous system. To test the potential of using stem cells in the adult central nervous system as “brain-marrow” for repair purposes, several issues need to be clarified. We are exploring the possibility of influencing, in vivo, proliferation, migration, and phenotype lineage of stem cells in the brain of adult animals with selective neural lesions by exogenous administration (alone or in combination) of hormones, cytokines, and neurotrophins. Lesion of the cholinergic system in the basal forebrain was induced in rats by the immunotoxin 192IgG-saporin. Alzet osmotic minipumps for chronic release (over a period of 14 days) of mitogens [epidermal growth factor (EGF) or basic fibroblast growth factor (bFGF)] were implanted in animals with behavioral and biochemical cholinergic defect and connected to an intracerebroventricular catheter. After 14 days of delivery, these pumps were replaced by others delivering nerve growth factor (NGF) for an additional 14 days. At the same time, retinoic acid was added to the rats' food pellets for one month. Whereas the lesion decreased proliferative activity, EGF and bFGF both increased the number of proliferating cells in the subventricular zone in lesioned and nonlesioned animals. These results are indicated by the widespread distribution of BrdUrd-positive nuclei in the forebrain, including in the cholinergic area. Performance in the water maze test was improved in these animals and choline acetyltransferase activity in the hippocampus was increased. These results suggest that pharmacological control of endogenous neural stem cells can provide an additional opportunity for brain repair. These studies also offer useful information for improving integration of transplanted cells into the mature brain.
Stem cells obtained from an adult brain can generate all cell types when adequately stimulated in vitro. However, neural stem cells have an extremely limited ability, if any, to activate themselves in the case of brain damage. Until now, the increase in DNA synthesis, cell proliferation, and differentiation into mature phenotypes has been described in very few experimental models of neurodegenerative diseases, e.g., during strokes, mechanical brain and spinal cord injury, and in experimental allergic encephalomyelitis (EAE). In ischemic lesions, a transient and regional specific increase has been demonstrated in cell proliferation in the subventricular zone (SVZ) (1–3), cerebral cortex (1), and hippocampus, as measured by bromodeoxyuridine (BrdUrd) uptake, where newly generated cells differentiate into a neuronal phenotype (2, 4, 5). Traumatic brain (6) and spinal cord (7) injury also induce cell proliferation in the SVZ and hippocampus of the adult rat, producing new astrocytes for scar building, as well as new cells in regions distant from the lesion (8). However, the efficiency of this cell replacement is limited, because the majority of newly generated neurons die shortly after generation (3).
Endogenous neural stem cells seem to be unable to react in different experimental conditions involving lesions to selective neural populations. In a 6-hydroxydopamine lesion, a widely used model for Parkinson's disease, no spontaneous proliferation of forebrain stem cells was observed (9). Moreover, proliferation and migration in the SVZ is disrupted by amyloid-β peptide, as indicated by in vivo studies in mice with mutant amyloid-β precursor protein and after intraventricular infusion of amyloid-β peptide (10).
Accordingly, the possibility that endogenous precursors play a part in brain repair seems to be extremely limited. We are exploring the possibility of influencing, in vivo, proliferation, migration, and phenotype lineage of stem cells in the brain of adult animals with a selective lesion of cholinergic neurons in the basal forebrain induced by 192IgG-saporin. We achieve this influence through exogenous administration of molecules involved in cholinergic lineage and differentiation during development. We report how combined treatment with mitogens [epidermal growth factor (EGF) or basic fibroblast growth factor (bFGF)], retinoic acid, and NGF, which are all involved in cholinergic lineage, accelerates the proliferation in the SVZ in both lesioned and nonlesioned animals. This acceleration is indicated by the widespread distribution of BrdUrd-positive nuclei in the forebrain, including the cholinergic area. Performance in the water maze test, which was impaired in lesioned animals, was improved in treated animals. This molecular mixture also increased choline acetyltransferase (ChAT) activity in the cerebral cortex and hippocampus of lesioned animals.
Materials and Methods
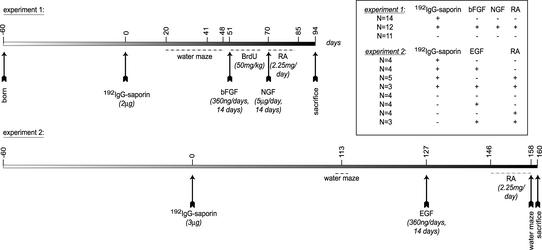
Animals, Lesion, and Treatments. Male Sprague–Dawley rats (150–175 g, Charles River Italia, Calco, Varese) were used for this study. Lesion of the cholinergic system was induced by intracerebroventricular (i.c.v.) injection of 192IgG-saporin (Advanced Targeting Systems, San Diego) at 2 or 3 μg/4.5 μl. Control, sham-operated animals were injected with the same amount of saporin alone. After behavioral testing, animals from both the sham-operated and lesioned groups were then implanted with an Alzet minipump (model 2002, Durect, Cupertino, CA) connected to an i.c.v. cannula (Alzet brain infusion kit). EGF (Promega) or bFGF (Promega) was delivered through the pump at the final dose of 360 ng/day for 14 days. After this treatment, the pump was changed in some of the animals for the purpose of NGF delivery, 5 μg/day for 14 days, dissolved in sterile PBS. NGF was prepared from the salivary gland of adult male mice by following the method described by Bocchini and Angeletti (11) and purified as a molecular weight 26,000 protein. To assess the biological activity of NGF, a sensitive cell culture bioassay using dissociated nerve cells of the superior cervical ganglia or embryonic chick sensory ganglia was used (12). At the same time, a diet enriched with retinoic acid, 2.25 mg per animal per day, was administered. This dosage is not toxic (13) and a 1:1 ratio between plasma level and brain concentration has been reported (ref. 14; see Fig. 1 for experimental timetable). Control animals were implanted with the Alzet minipump connected to an i.c.v. cannula delivering artificial cerebrospinal fluid (csf). All animal protocols mentioned herein were carried out according to the European Community Council Directives of 24 November 1986 (86/609/EEC) and approved by our intramural committee and the Ministero Istruzione Università Ricerca, in compliance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Fig. 1.
Design of the study and composition of the experimental groups in the two sets of experiments. Time 0 refers to the day of the i.c.v. injection of 192IgG-saporin; an osmotic minipump connected to an i.c.v. catheter was implanted for EGF and bFGF administration and then replaced for NGF delivery. A diet enriched with retinoic acid (RA) was then supplied. Animals were tested for behavioral performance at the indicated time intervals and all animals were then killed for biochemical, molecular, and morphological analysis.
Behavioral Test. Tasks were run in a circular pool (185 cm diameter, with a visible or hidden platform and targets on the walls), and the results were recorded and analyzed by using SMART software (PanLab, Barcelona). Latency, speed, and strategy used to reach the platform were analyzed according to the following schema: first day, three blocks (two trials each, 90 sec each trial) with the visible platform. Each trial started from random points of the pool and animals had 90 sec to reach the platform. The trial was ended when the animal remained on the platform for 15 sec. On the second day the same scheme was repeated with the hidden platform. These tests, developed to investigate fine alterations in the cholinergic system (15), can also evaluate learning and short-term memory.
Western Blotting Procedure. For Western blot experiments, five animals per group were used. Tissue homogenates were prepared by using a 10 mM Hepes/1 mM DTT (pH 7.5) lysing buffer, containing a protease inhibitor mixture (Sigma). Aliquots of protein were separated in SDS/15% polyacrylamide gels and electroblotted to nitrocellulose membranes. Filters were incubated with blocking solution (Pierce) and then with primary (rabbit polyclonal anti-Ki67, NovoCastra, Newcastle, U.K.) and then secondary antibodies. Proteins were detected by using an ECL (enhanced chemiluminescence) kit (Pierce) and exposed to radiographic film. Densitometric analysis was performed by using the AIS imaging system (Imaging Research, St. Catherines, ON, Canada).
ChAT Activity. Tissue homogenates prepared for Western blotting were used. ChAT activity was determined as described by Fonnum (16) with some modifications. Supernatants were suitably diluted and incubated at 37°C for 15 min with a reaction mix composed of 0.4 M NaCl, 1 mM EDTA, 0.4 mM eserine hemisulfate (Sigma), 0.15 M choline chloride (Sigma), 1.6 mM acetyl-CoA (Sigma), and [3H]acetyl-CoA (Amersham Biosciences) to a final specific activity of 181 GBq/mmol. The reaction was then stopped by transferring the samples to an ice bath and adding 1.5 ml of ice-cold stop solution (3.12 mM Na2HPO4/2.5 mM NaH2PO4/0.312 mM EDTA containing 6.4 mM sodium tetraphenylborate in acetonitrile), and radioactive acetylcholine was extracted by using a scintillation liquid for organic solutions and quantified with a β counter. Buffer modifications of Fonnum's original technique did not affect the assay findings.
BrdUrd Uptake and Immunohistochemistry. The proliferation rate was investigated by BrdUrd uptake. BrdUrd (50 mg/kg) was administered i.p. in experiment 1 at 55, 57, 59, 62, 64, and 66 days from the lesion. Animals were killed at the end of the experiment and sections of each brain were processed for indirect immunofluorescence. For BrdUrd visualization the monoclonal anti-BrdUrd (Roche Molecular Biochemicals) was used. For cholinergic neuron visualization, anti-ChAT (Chemicon), anti-trkA (residues 763–777, Santa Cruz Biotechnology), and anti-p75 (clone IgG192, generously supplied by E. M. Johnson, Washington University, St. Louis) were used. For glial cell visualization, anti-CD11b (OX42, Sera-Lab, Crawley Down, U.K.) and anti-GFAP (glial fibrillary acidic protein; Chemicon) were used, as was anti-embryonic NCAM antiserum [PSA-NCAM (polysialylated form of the neural cell adhesion molecule), BD PharMingen]. For double experiments with BrdUrd, the immunocytochemical procedure was applied after BrdUrd visualization. Confocal laser scan microscopy [Olympus FV500, Ar/HeNe (G) lasers and appropriate filters for green and red fluorescence] was used to analyze double-labeling experiments. Immunocytochemistry was used to verify the efficacy of the 192IgG-saporin lesion before mitogen administration, to investigate the effect of mitogens on proliferation in SVZ, and to examine the forebrain at the end of the experiments.
Esterase Activity. Esterase activity has been investigated according to the histochemical technique of Hedreen et al. (17).
Statistical Analysis. In the descriptive analysis data are expressed as mean ± SEM. Statistical analysis was carried out by using one-way ANOVA and Dunnett's test to compare the different experimental groups; behavioral data were analyzed by two-way ANOVA. Student's t test was also used when appropriate. The probability level was set at 5% (two-tailed; PRISM software package for Macintosh, GraphPad, San Diego).
Results
Results are based on two sets of experiments (Fig. 1). With the first set we aimed to study cholinergic function in animals lesioned with the immunotoxin 192IgG-saporin, treated or not with a mitogen (bFGF), a differentiating agent (retinoic acid), and a survival factor (NGF), while also keeping in view the possible involvement of endogenous stem cells. In the second set, we analyzed the effect of single treatments on cholinergic parameters. In both sets of experiments, proliferation and differentiation of endogenous stem cells in the basal forebrain and olfactory bulb were also analyzed.
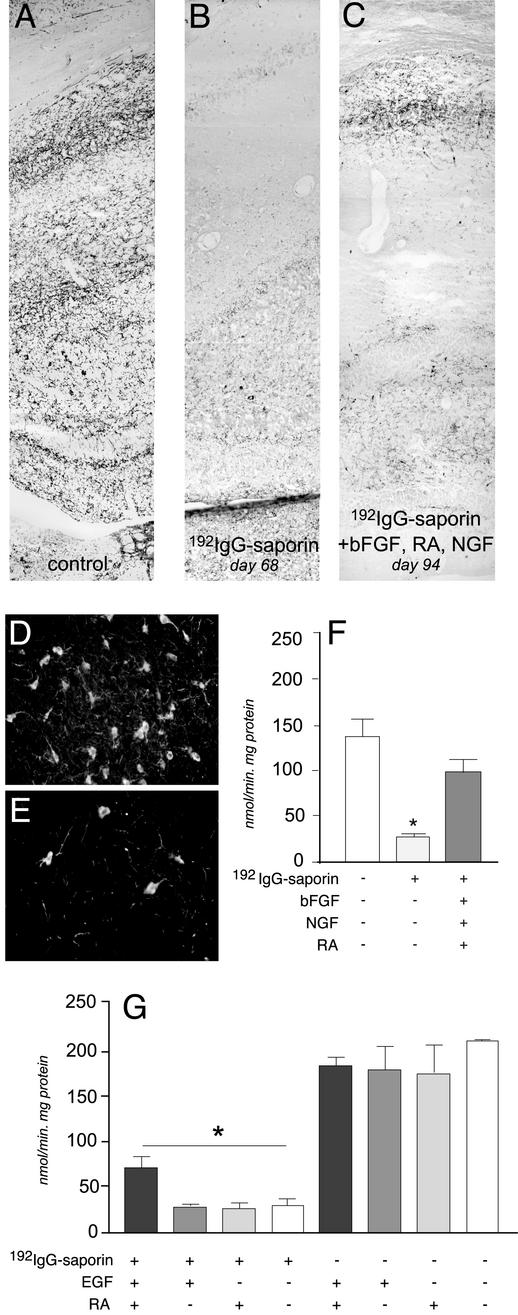
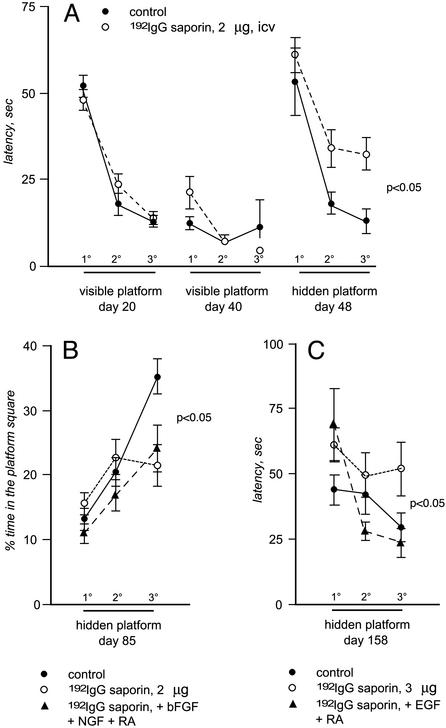
In the first set of experiments, animals were i.c.v. injected with 2 μg of 192IgG-saporin. This dose and route of administration induces a progressive decline in cholinergic innervation of the cerebral cortex and hippocampus, and leads to a disappearance of ChAT-, p75-, and trkA-positive neurons in the basal forebrain (Fig. 3 D and E), resulting in a progressive impairment of performance in the water maze test. We monitored behavioral performance in lesioned animals by using visible and hidden platform to identify any early impairment in learning and memory performance. At 48 days after the 192IgG-saporin injection, lesioned animals' performance in the water maze test was impaired, as illustrated by the latency value in the test using the hidden platform (Fig. 2A). Control animals, which were injected with saporin alone, were able to improve performance by progressive reduction of latency in the three blocks of trials, whereas self-improvement capability was significantly reduced in 192IgG-saporin-lesioned animals. Cholinergic innervation of the cerebral cortex and hippocampus was dramatically reduced, as indicated by the esterase staining (Fig. 3 A and B) and ChAT activity assay (Fig. 3F). At this time we implanted the animals (saporin and 192IgG-saporin-injected animals) with an i.c.v. catheter connected to an Alzet minipump s.c. allocated for the delivery of bFGF over a period of 14 days. In this time interval, the 192IgG-saporin-induced cholinergic deafferentation of the hippocampus did not progress any further. After that, the Alzet minipump was changed for the purpose of NGF delivery over a period of 14 days, and retinoic acid was added to the animals' diet. The water maze test with the hidden platform was then repeated (Fig. 2B). In control, nonlesioned animals, a significant improvement in the performance was observed between the trials performed with the hidden platform. This capability, which was severely impaired in 192IgG-saporin-lesioned animals, was restored by the introduction of bFGF, NGF, and retinoic acid. In addition, ChAT activity and cholinergic innervation (Fig. 3 B and C) in the hippocampus was positively affected by this treatment, as the ChAT activity of the 192IgG-saporin-lesioned animals treated with the mixture did not differ significantly from that of the control animals.
Fig. 3.
Cholinergic denervation in the hippocampus of 192IgG-lesioned animals and effects of combined treatments. Histochemical visualization of acetylcholinesterase in the hippocampal cortex of control rats (A) and 68 days after injection (B) is shown. The immunolesion induces an almost complete disappearance of esterase-reactive fibers in the hippocampus by day 68, and the deafferentation does not further worsen at 85 days (data not shown). Degeneration of cholinergic neurons in the basal forebrain is also shown (D, control, sham-operated; E, lesioned). (C) Esterase-reactive fibers were more abundant in lesioned animals after bFGF, retinoic acid (RA), and NGF administration (94 days). ChAT activity also drops in the hippocampus of lesioned animals, and combined treatment with mitogens, retinoic acid, and NGF recovers this deficit (F). In addition, the mitogens combined with retinoic acid partially recover the ChAT deficit (G), whereas single treatments do not modify ChAT activity in either lesioned or control animals. Statistical analysis was carried out by using ANOVA and Dunnett's test (P < 0.05).
Fig. 2.
The graphs report performance in the water maze task. In A, development of behavioral impairment in animals lesioned according to the paradigm used for the first set of experiments, e.g., the less severe, is illustrated by repeated tests with both visible and hidden platform. Each point represents the average of the latency over the two trials within each block. On day 20 after the injection, animals were first taught to escape to a marked, submerged platform in the water maze (visible portion of the acquisition). On the following day, the platform was in the same location but unmarked and therefore hidden from the rats' view (hidden portion of the acquisition). Three consecutive two-trial blocks of 90-sec maximum duration were run each day. Animals were then tested weekly within the hidden portion of acquisition. At 48 days after 192IgG-saporin injection, the animals showed a significant impairment in terms of latency in reaching the submerged platform (A). Repeated overall ANOVA measures between treated and untreated animals indicated a significant group effect [F(1,30) = 8.56; P < 0.005]. The lesion paradigm used in the second set of experiments produced an even more pronounced impairment in water maze performance (data not shown). Animals were then tested after combined treatments. In the first set of experiments, in which a group of lesioned animals was treated with bFGF, NGF, and retinoic acid, overall repeated ANOVA measures indicated a significant group effect [F(2,84) = 3.58; P < 0.05] at day 85 after lesion, which was evident for time spent in the platform square and not for latency (B). In the second set of experiments, in which a group of lesioned animals was treated with EGF and retinoic acid, overall repeated ANOVA measures indicated a significant group effect [F(2,80) = 3.67; P < 0.05] at day 158 after lesion (C).
With these results in mind, we have included control groups for single treatment in the second set of experiments. Animals were also more severely lesioned using a higher dose of 192IgG-saporin (3 μg) and a longer postlesion time. These adjustments led to impaired performance in the water maze test with both visible and hidden platform (data not shown). The mitogen used was EGF. Retinoic acid administration after the administration of EGF significantly improved the animals' performance in the water maze test despite the absence of NGF (Fig. 2C). None of the single treatments modified ChAT activity in control or sham-operated animals (Fig. 3G). ChAT activity was severely decreased in the hippocampus of lesioned animals, and only combined treatment with mitogens and retinoic acid induced a slight, but significant, recovery.
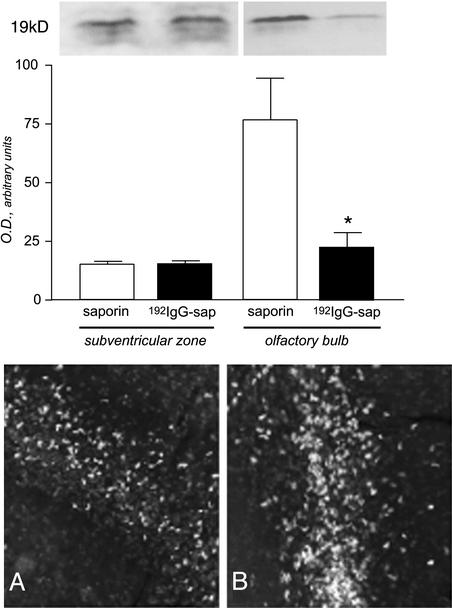
We then explored the possibility that endogenous stem cells participate in behavioral and biochemical improvement of cholinergic function after in vivo delivery of mitogens, differentiating agents, and survival agents. Degeneration of cholinergic neurons in the basal forebrain by 192IgG-saporin is associated with a reduction in the expression of the proliferation-associated nuclear antigen Ki67, a protein that is expressed by cycling cells during the late G1, S, G2 and M stages (Fig. 4). Reduction is evident in the olfactory bulb but not in the forebrain area, including the SVZ. Administration of either EGF or bFGF increases the number of BrdUrd-uptaking cells in the forebrain, as illustrated by the widespread distribution of positive cells in the tissue surrounding the SVZ (Fig. 4B) compared with animals implanted with the Alzet minipump delivering artificial csf (Fig. 4A). This effect, which has already been described in nonlesioned animals (13), was also observed in animals having a selective degeneration of cholinergic neurons, which induces a reduction of proliferative activity in the forebrain of the adult brain.
Fig. 4.
Effect of lesion and mitogen treatment on proliferative indices in the forebrain. Western blot analysis (Upper) indicates that expression of the proliferation-associated antigen Ki67 is reduced in the olfactory bulb of 192IgG-lesioned animals, and that mitogen injections expand the distribution of BrdUrd-uptaking cells in the SVZ in lesioned animals (A, control, saporin-injected; B, lesioned, 192IgG-injected + EGF).
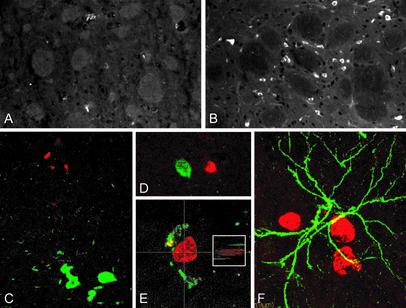
To test whether increased proliferation obtained in lesioned animals by administration of mitogens is followed by gliogenesis and/or neurogenesis, BrdUrd was administered to lesioned and control animals in the form of multiple injections during the time of mitogen delivery. These animals were then processed at the end of the experiments for immunocytochemical visualization of various antigens that are expressed by neural and glial precursors. In both experiments, a large number of small cells expressing the polysialylated (PSA) embryonic form of the neural adhesion molecule (NCAM) were observed in the area of the caudate nucleus adjacent to the SVZ in lesioned animals treated with the molecular mixture (Fig. 5; A, lesioned, nontreated; B, lesioned, treated). At least some of these cells were newly generated during the time of mitogen delivery, as indicated by the double labeling with BrdUrd (Fig. 5E). In the basal forebrain, a considerable number of BrdUrd-positive nuclei were observed in lesioned animals treated with bFGF, NGF, and retinoic acid in the vicinity of the very few ChAT- (Fig. 5C) and trkA- (Fig. 5D) immunoreactive neurons still detectable in the area. No double labeling with glial markers (OX42 for activated microglia and GFAP for astrocytes) or cholinergic markers (ChAT, p75, and trkA) was observed. No BrdUrd-positive nuclei were observed in nonlesioned animals or in lesioned, untreated animals.
Fig. 5.
Combined treatment in lesioned animals induces appearance of numerous small PSA-NCAM-positive cells in the basal ganglia close to the SVZ (B), whereas the same treatment in sham-operated animals is ineffective on this protein expression regulation (A). Numerous BrdUrd-uptaking cells are also observed in the basal forebrain area in lesioned animals, close to the remaining ChAT- (C) and trkA- (D) positive cells. Single cells having both nuclear labeling for BrdUrd (red) and PSA-NCAM (green) immunoreactivity were also found in the forebrain of lesioned animals receiving multiple treatments (E). To confirm the coexistence of BrdUrd and PSA-NCAM immunoreactivity, a small Inset shows the lateral projection of this double-labeling, as obtained by laser scan. No double-labeling of BrdUrd (red) with GFAP (green) was found (F).
Discussion
Association between “stem cell” and “brain repair” has raised high hopes in recent years. On one side, important developments in stem-cell biology, cell culture, and cloning strategies revealed a research area that could lead to unexpected clinical applications. On the other hand, the extremely limited self-repairing capacity of adult nervous tissue raises the need for new strategies of intervention in neurodegenerative diseases. The limited ability of an endogenous precursor to participate in repair attempts could be because of the intrinsic properties of adult neural stem cells, the unfavorable microenvironment of the adult brain, or the injury itself. In this study we demonstrated that the selective lesion of the cholinergic system causes a reduction in the proliferation rate in the forebrain, that mitogen administration is effective in increasing proliferation in the SVZ in a lesioned brain, that administration of molecules involved in cholinergic differentiation in a precise time sequence can reduce behavioral and biochemical cholinergic deficit, and that proliferation and differentiation of endogenous stem cells in the forebrain can most likely contribute to these effects.
Lesion of the cholinergic system in the basal forebrain was induced by i.c.v. administration of 192IgG-saporin. Saporin, when conjugated with the monoclonal antibody to rat p75 (low affinity) NGF receptor, is internalized by neurons expressing high levels of p75 receptor, affecting protein synthesis and causing cell death of cholinergic neurons in the basal forebrain and partially in the cerebellum (18). This leads to impairment in learning and memory skills and a progressive decline in acetylcholine content in the cerebral cortex and hippocampus, as it is depleted over a period of time (19). Concomitant administration of EGF or bFGF, retinoic acid, and NGF reduces both behavioral and biochemical indices of cholinergic deficit. These positive effects are observed in animals receiving multiple treatments, but not in animals treated with single types of molecules. Mitogens were delivered to force proliferation of endogenous stem cells, as the immunolesion of the cholinergic system itself inhibits the proliferation rate in both the forebrain and the dentate gyrus of the hippocampus.¶ NGF is the best characterized trophic factor for cholinergic neurons in the adult brain and also induces maturation of precursors toward cholinergic differentiation by blocking cell proliferation. Retinoic acid is a well known differentiating agent for the acquisition of neuronal fate and cholinergic phenotype in many different cell types and cell lines (21), including septal cell lines (22). Retinoic acid and NGF interact during development to induce neuron differentiation and maturation (23), but also to regulate cell cycle and differentiation in NGF-sensitive cells (24). Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures (25). Therefore, we speculate that sequential administration of retinoic acid and NGF could help acquisition of a neuronal fate and transmitter phenotype in vivo. Retinoic acid also potentiates the protective effect of NGF against apoptosis (26), and NGF acts via retinoic acid synthesis to stimulate neurite outgrowth (27). Both EGF and bFGF have previously been tested in vivo in normal animals, and both are able to increase proliferation in the SVZ (28, 29). Furthermore, our data indicate that this is also possible in animals with a lesion that reduces spontaneous proliferation.
Combined treatments allow visualization of a large number of PSA-NCAM-positive cells in the forebrain, some of which are BrdUrd-positive. We can clearly say that endogenous stem cells in the forebrain are sensitive to the molecular mixture we used, because of the regulation of proliferation rate by mitogens, and the appearance of PSA-NCAM-positive cells in the basal ganglia and of BrdUrd-positive cells in the basal forebrain. The appearance of PSA-NCAM-positive cells in the adult brain after lesion has always been associated with repair attempts, even after cortical lesions (30). Thus, the main question still open after this study is whether the positive effects (including promotion of the sprouting from neurons that survive and repair of the cholinergic system) are because of the protection of cholinergic neurons not having yet degenerated after administration of the immunotoxin. In fact, NGF alone can slightly augment cortical and hippocampal ChAT activity after p75 receptor-mediated deafferentation, but only in low-lesioned animals (15). In our experimental conditions, we found behavioral improvement and ChAT activity recovery also in lesioned animals treated with mitogens and retinoic acid, but not with NGF.
To come to the obvious question, we have not found any “newly generated cholinergic neuron” in the basal forebrain, in term of ChAT, p75, and trkA immunostaining. BrdUrd cells were OX42 and GFAP negative, and we cannot speculate at the moment on phenotype and maturation of these cells. However, ChAT activity, a more sensitive assay than ChAT immunostaining, was increased in treated animals. Moreover, BrdUrd-positive cells expressing PSA-NCAM (a marker for progenitor cells) and generated in the time-window of mitogen administration are found in the area where neurons degenerate, suggesting that signals useful for migration can be generated by damaged neurons. An attractive role of damaged neurons vs. immature cells has already been proposed for grafted cells in models of Parkinson's disease (31).
Actually, we believe that the search for newly generated neurons in the right place is a far frontier in the field of neurodegenerative disease. There is good evidence to suggest that the mature CNS is “nonpermissive” for neural remodeling different from that related to “functional plasticity.” In particular, the mature CNS is an inhospitable environment for axonal regrowth, though such growth is highly efficient in the hospitable environment of the peripheral nervous system (32). Moreover, experimental lesion ultimately alters the neurobiological context, so that one cannot exclude that the molecular signals received by neural stem cells in a lesioned brain differ from those received by stem cells in an intact brain (33). The idea of forcing endogenous stem cells, using the molecular signals to which precursors are sensitive during development, is focused on the attempt to modify the microenvironment in a direction that is more permissive for progenitor activation. One can also speculate that, apart from a direct participation of newly generated cells in neural net reconstruction, the migration of nondifferentiated cells in lesioned areas can modify the microenvironment from a molecular composition freezing the mature structure to a molecular composition more permissive in terms of repair and renewal, perhaps through the secretion of trophic substances (31). This has already been successfully proposed in experimental allergic encephalomyelitis (EAE), a demyelinating disease in which oligodendrocytes degenerate. In EAE, the administration of molecules for the purpose of oligodendrocyte maturation, together with the concomitant increase in NGF content, affects neural stem cell proliferation and induces the maturation of oligodendrocyte precursors into myelinating oligodendrocytes (34). In animals with a selective lesion of the dopaminergic nigrostriatal system, the brain infusion of transforming growth factor (TGF)-α induces a massive proliferation of forebrain stem cells, followed by migration of both glial and neural progenitors toward the TGF-α injection site. This is then followed by an increase in the number of differentiated neurons observed in the striatum and an improvement in behavioral test for activation of dopaminergic nigrostriatal pathway (9). Two points in this study correlate with our results: the lesion itself is not able to “activate” long-lasting endogenous precursors; TGF-α is effective in lesioned animals only, and not in nonlesioned animals. Moreover, axonal regeneration can be improved in the spinal cord by concomitant treatment with embryonic tissue transplant and neurotrophin delivery, suggesting that behavioral recovery can be achieved by providing a permissive environment on the injury site (32). Finally, both trophic and tropic effects have been attributed to the implant of EGF-responsive NGF-secreting cells in a model of Huntington's disease (20).
In conclusion, the present results confirm that endogenous stem cells are not automatically activated by all kinds of brain lesion, as such lesions may even produce the opposite effect. Our working hypothesis is that there is a possible window in which exogenous substances may be administered in an effort to involve endogenous stem cells in protecting the brain from the progression of degenerative diseases, as well as in repair work of the brain. The administration of multiple molecules, therefore, rather than of a single drug, could be a better pharmacological approach.
Acknowledgments
This article is dedicated to Prof. Rita Levi-Montalcini with our deepest esteem, affection, and thanks for her continuous interest in our work, encouragement, intellectual contributions, and invaluable suggestions. Technical assistance of Nadia De Sordi (Department of Veterinary Morphophysiology and Animal Production) is gratefully acknowledged. This work was supported by the Fondazione Carisbo, Bologna, Italy (L.C. and L.A.), by Telethon project 1336 (L.C.) and by the University of Bologna (ex60%, L.C.).
Abbreviations: EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; NGF, nerve growth factor; i.c.v., intracerebroventricular; SVZ, subventricular zone; ChAT, choline acetyltransferase; GFAP, glial fibrillary acidic protein; PSA-NCAM, polysialylated form of the neural cell adhesion molecule.
Footnotes
Mohapel, P., Leanza, G. & Lindvall, O., Society for Neuroscience 32nd Annual Meeting, November 2–7, 2002, Orlando, FL, program no. 23.9.2002 (CD-ROM).
References
- 1.Zhang, R. L., Zhang, Z. G., Zhang, L. & Chopp, M. (2001) Neuroscience 105, 33-41. [DOI] [PubMed] [Google Scholar]
- 2.Jin, K., Minami, M., Lan, J. Q., Mao, X. O., Batteur, S., Simon, R. P. & Greenberg, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 4710-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 8, 963-970. [DOI] [PubMed] [Google Scholar]
- 4.Yagita, Y., Kitagawa, K., Ohtsuki, T., Takasawa, K.-I., Miyata, T., Okano, H., Hori, M. & Matsumoto, M. (2001) Stroke 32, 1890-1896. [DOI] [PubMed] [Google Scholar]
- 5.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429-441. [DOI] [PubMed] [Google Scholar]
- 6.Chirumamilla, S., Sun, D., Bullock, M. R. & Colello, R. J. (2002) J. Neurotrauma 19, 693-703. [DOI] [PubMed] [Google Scholar]
- 7.Namiki, J. & Tator, C. H. (1999) J. Neuropathol. Exp. Neurol. 58, 489-498. [DOI] [PubMed] [Google Scholar]
- 8.Kernie, S. G., Erwin, T. M. & Parada, L. F. (2001) J. Neurosci. Res. 66, 317-326. [DOI] [PubMed] [Google Scholar]
- 9.Fallon, J., Reid, S., Kinyamu, R., Opole, I., Baratta, J., Korc, M., Endo, T. L., Duong, A., Nguyen, G., Karkehabadhi, M., Twardzik, D. & Loughlin, S. (2000) Proc. Natl. Acad. Sci. USA 97, 14686-14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haughey, N. J., Liu, D., Nath, A., Borchard, A. C. & Mattson, M. P. (2002) Neuromolecular Med. 1, 125-1135. [DOI] [PubMed] [Google Scholar]
- 11.Bocchini, V. & Angeletti, P. U. (1969) Proc. Natl. Acad. Sci. USA 64, 787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aloe, L. (1989) Proc. Natl. Acad. Sci. USA 86, 5636-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White, J. C., Shankar, V. N., Highland, M., Epstein, M. L., DeLuca, H. F. & Clagett-Dame, M. (1998) Proc. Natl. Acad. Sci. USA 95, 13459-13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Doze, F., Debruyne, D., Albessard, F., Barre, L. & Defer, G. L. (2000) Drug Metab. Dispos. 28, 205-208. [PubMed] [Google Scholar]
- 15.Winkler, J., Ramirez, G. A., Thal, L. J. & Waite, J. J. (2000) J. Neurosci. 20, 834-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonnum, F. (1975) J. Neurochem. 24, 407-409. [DOI] [PubMed] [Google Scholar]
- 17.Hedreen, J. C., Bacon, S. J. & Price, D. L. (1985) J. Histochem. Cytochem. 33, 134-140. [DOI] [PubMed] [Google Scholar]
- 18.Schliebs, R., Rossner, S. & Bigl, V. (1996) Prog. Brain Res. 109, 253-264. [DOI] [PubMed] [Google Scholar]
- 19.Waite, J. J., Chen, A. D., Wardlow, M. L., Wiley, R. G., Lappi, D. A. & Thal, L. J. (1995) Neuroscience 65, 463-476. [DOI] [PubMed] [Google Scholar]
- 20.Kordower, J. H., Chen, E.-Y., Winkler, C., Fricker, R., Charles, V., Messing, A., Mufson, E. J., Wong, S. C., Rosenstein, J. M., Bjorklund, A., et al. (1997) J. Comp. Neurol. 387, 96-113. [DOI] [PubMed] [Google Scholar]
- 21.Hill, D. P. & Robertson, K. A. (1997) Dev. Brain Res. 102, 53-67. [DOI] [PubMed] [Google Scholar]
- 22.Berse, B. & Blusztajn, J. K. (1995) J. Biol. Chem. 270, 22101-22104. [DOI] [PubMed] [Google Scholar]
- 23.Holst, A., Lefcort, F. & Rohrer, H. (1997) Eur. J. Neurosci. 9, 2169-2177. [DOI] [PubMed] [Google Scholar]
- 24.Paez Pereda, M., Missale, C., Grubler, Y., Arzt, E., Schaaf, L. & Stalla, G. K. (2000) Mol. Cell Endocrinol. 167, 99-106. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi, J., Palmer, T. D. & Gage, F. H. (1999) J. Neurobiol. 38, 65-81. [PubMed] [Google Scholar]
- 26.Vuillaume, I., Schraen-Maschke, S., Formstecher, P. & Sablonniere, B. (2001) Biochem. Biophys. Res. Commun. 289, 647-652. [DOI] [PubMed] [Google Scholar]
- 27.Corcoran, J. & Maden, M. (1999) Nat. Neurosci. 2, 307-308. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. (1997) J. Neurosci. 17, 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens, D. J., Seaberg, R. M. & van der Kooy, D. (2002) Eur. J. Neurosci. 16, 1045-1057. [DOI] [PubMed] [Google Scholar]
- 30.Szele, F. G. & Chesselet, M. F. (1996) J. Comp. Neurol. 368, 439-454. [DOI] [PubMed] [Google Scholar]
- 31.Bjorklund, A. & Lindvall, O. (2000) Nat. Neurosci. 3, 537-544. [DOI] [PubMed] [Google Scholar]
- 32.Coumans, J. V., Lin, T. T., Dai, H. N., MacArthur, L., McAtee, M., Nash, C. & Bregman, B. S. (2001) J. Neurosci. 21, 9334-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder, E. Y. & Park, K. I. (2002) Nat. Med. 8, 928-930. [DOI] [PubMed] [Google Scholar]
- 34.Calzà, L., Fernandez, M., Giuliani, A., Aloe, L. & Giardino, L. (2002) Proc. Natl. Acad. Sci. USA 99, 3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]