Abstract
G proteins modulate synaptic transmission. Regulators of G protein signaling (RGS) proteins accelerate the intrinsic GTPase activity of Gα subunits, and thus terminate G protein activation. Whether RGS proteins themselves are under cellular control is not well defined, particularly in native cells. In dorsal root ganglion neurons overexpressing RGS3, we find that G protein signaling is rapidly terminated (or “desensitized”) by calcium influx through voltage-gated channels. This rapid desensitization is most likely mediated by direct binding of calcium to RGS3, as deletion of an EF-hand domain in RGS3 abolishes both the desensitization (observed physiologically) and a calcium-RGS3 interaction (observed in a gel-shift assay). A naturally occurring variant of RGS3 that lacks the EF hand neither binds calcium nor produces rapid desensitization, giving rise instead to a slower calcium-dependent desensitization that is attenuated by a calmodulin antagonist. Thus, activity-evoked calcium entry in sensory neurons may provide differential control of G protein signaling, depending on the isoform of RGS3 expressed in the cells. In complex neural circuits subjected to abundant synaptic inhibition by G proteins (as occurs in dorsal spinal cord), rapid termination of inhibition by electrical activity by EF hand-containing RGS3 may ensure the faithful transmission of information from the most active sensory inputs.
The time course of cellular signaling by heterotrimeric GTP-binding (G) proteins is limited by the intrinsic GTPase activity of Gα subunits, which promotes formation of the inactive Gαβγ heterotrimer. Several mechanisms have been described to alter this time course, including the activation of a multifunctional protein family, regulators of G protein signaling (RGS) (1–3). RGS family members share a well conserved 120 aa “RGS domain” that interacts directly with Gα-GTP subunits (4) and terminates signaling by accelerating intrinsic GTPase activity (5, 6). Such GTPase-accelerating activity has been shown to regulate a variety of cellular functions, including membrane trafficking (7, 8), lymphocyte chemotaxis (9), hormone signaling (10–13), K+ channel activation (14, 15), Ca2+ channel modulation (16–20), and synaptic transmission (21). Such data argue that RGS proteins may provide a physiological mechanism by which cells transition between excited and inhibited states.
In addition to the C-terminal RGS domain, the N termini of most RGS proteins also contain other functional domains that are well conserved among members of a given RGS subfamily (2, 5). Such domains have been shown to contribute to a number of molecular properties of RGS proteins, including target selectivity (22, 23), subcellular localization (24, 25), substrate specificity (16, 26), receptor selectivity (27), and regulation of GTPase-accelerating activity (23).
Although much is known about the structure and the downstream effectors of RGS proteins, the mechanisms underlying their activation have been little explored, particularly in native cells. Studies described here have used dorsal root ganglion (DRG) neurons from embryonic chick to study the mechanisms underlying the initiation of RGS3-dependent signaling (28–33). In these neurons, the gene encoding RGS3 gives rise to several alternatively spliced transcripts, and we have cloned five variants using library screening methods (GenBank accession nos. AY124773–AY124777; P.T. and K.D., unpublished data). We report here that the physiological actions of two of these variants, RGS3s and RGS3ss, are differentially initiated by calcium influx through voltage-gated channels. We demonstrate a rapid calcium-dependent effect of RGS3s that is likely to be mediated by direct calcium binding to an EF hand domain in the N terminus; a second, slower effect of RGS3ss (which lacks the EF hand) is also calcium-dependent and appears to be mediated by calmodulin (CaM). Both mechanisms terminate G protein-dependent inhibition of the calcium channels, but with distinct time courses, and may provide positive feedback mechanisms in vivo to maintain calcium influx preferentially in the most active population of sensory neurons.
Materials and Methods
Retroviral Vector Constructs. RGS3s and RGS3ss cDNAs (GenBank accession nos. AY124775 and AY124774, respectively) were C-terminally tagged with the hemagglutinin (HA) epitope (TAC GAC GTG CCC GAC TAC GCC) by PCR. Complementary DNAs for RGS3s and RGS3ss were first subcloned in frame into the adaptor plasmid Cla12 NCO and then into the ClaI site of RCASBP retroviral variants of the B-subgroup (generous gift of D. Fekete, Purdue University, West Lafayette, IN), as described previously (34, 35). Properly oriented constructs were identified by PCR and sequencing. A mutant RGS3s cDNA lacking the EF-like domain (“ΔEF”) was generated by mutating RGS3s/RCASB with use of the QuikChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions and the following primer set: 5′-GAT GAG GAT GAA GAG AAG CGC AGC AGC ATG-3′ (sense) and 5′-CAT GCT GCT GCG CTT CTC TTC ATC CTC ATC-3′ (antisense). The deletion was confirmed by sequencing.
Virus Production and Infection. Avian-specific, replication-competent retrovirus stocks were generated by transfecting chicken embryo fibroblasts and concentrated to optimal titers for injection, as described (35). To infect proliferating DRG neuron precursors in the neural crest, retroviral vectors were microinjected into the neural tube of stage 9 White Leghorn chick embryos (Spafas) in ovo. After injection, chick embryos were maintained in a nonturning, forced-air draft, humidified egg incubator at 37°C, to allow normal growth until dissection.
Immunocytochemistry. Intact DRGs, dissected from wild-type or infected 11- to 12-day-old chick embryos, were fixed in 2% freshly prepared paraformaldehyde for 30 min, cryo-protected in 30% sucrose for 30 min, embedded in OCT (Electron Microscopy Sciences, Fort Washington, PA), and frozen in liquid N2. Frozen sections (7 μm) were cut by using a cryostat, transferred to glass slides, and air dried. For immunocytochemical detection of infected cells, sections were first incubated 30 min with 0.25% gelatin (in PBS) to block nonspecific binding, followed by 1 h with rabbit anti-HA primary antiserum (1:500 dilution), 1 h with a biotinylated goat anti-rabbit secondary antibody (1:150 dilution, The Jackson Laboratory), and 40 min with FITC-conjugated streptavidin (1:1,000, The Jackson Laboratory). Sections were rinsed in PBS, fixed with cold methanol, mounted (Vectashield, Vector Laboratories), and visualized with a Zeiss Axioskop.
Recombinant Proteins. Escherichia coli, strain BL21 (DE3), were transformed with C-terminally His-6-tagged RGS3s, RGS3ss, and ΔEF subcloned into pET11a (Novagen). Cells were grown in LB medium with ampicillin (100 mg/ml) at 37°C to an OD600 = 0.6–0.8, then induced with isopropyl-1-thio-β-D-galactopyranoside (0.5 mM) for 4 h. Cells were concentrated by centrifugation and lysed by freezing and sonication in 8 M urea buffer (with 20 mM Tris·HCl, pH 8/100 mM NaCl). The lysate was centrifuged (12,000 × g for 30 min), and the supernatant fraction was loaded onto a minicolumn containing 50 μl of Ni-nitrilotriacetic acid affinity resin (Qiagen, Hilden, Germany). Proteins were adsorbed onto the column by using buffers of decreasing urea concentration. A final wash with buffer A (50 mM Hepes, pH 8/20 mM 2-mercaptoethanol/100 mM NaCl/0.1 mM phenylmethylsulfonylfluoride) removed the residual urea. The proteins were eluted from the column with 250 μl of buffer A and either 250 mM (RGS3ss) or 150 mM (RGS3s) imidazole, then stored at -80°C.
Calcium-Induced Mobility-Shift Assay. The methods of Rosel et al. (36) were used to test for direct interactions between Ca2+ and RGS3s, RGS3ss, and ΔEF. Recombinant proteins were diluted into Laemmli sample buffer containing 2 mM Ca2+, 2 mM Ba2+, or 2 mM EGTA (with no added Ca2+) and separated electrophoretically by using standard 12.5% Tris-glycine SDS/PAGE. To fully denature some samples (as noted), protein in loading buffer was incubated for 20 min at 100°C in a water bath, before loading on gels. After electrophoresis, gels were stained with Coomassie blue.
Cell Dissociation and Electrophysiological Recording. DRGs were dissected from either wild-type or RCASB-infected 11- to 12-day-old chick embryos, as indicated, dissociated mechanically as described previously (37, 38), and maintained in a humidified, CO2 incubator at 37°C for 4–24 h before use. Extracellular recording solution contained 133 mM NaCl, 1 mM CaCl2, 0.8 mM MgCl2, 25 mM Hepes, 12.5 mM NaOH, 5 mM glucose, 10 mM tetraethylammonium chloride, 6 × 10-4 mM tetrodotoxin, 10-2 mM bicuculline, pH 7.4; where noted, the calcium was replaced by 0.75 mM barium. Standard tight-seal, whole-cell recording methods were used. Starting electrode resistances were 1–2 MΩ when filled with a solution containing 150 mM CsCl, 5 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 5 mM MgATP, 10 mM Hepes, pH 7.4. γ-Aminobutyric acid (GABA) was bath-applied to the cells at 100 μM in standard external solution by using a pressurized, large-bore “sewer pipe” system with subsecond delivery times. Removal of GABA was achieved by active superfusion with standard bath solution. All experiments were performed at room temperature (≈20°C). Data were digitized and acquired by using an ITC-16 A/D interface (Instrutech, Great Neck, NY) and a Power Macintosh G3 computer. Analyses were performed in IGORPRO (WaveMetrics, Lake Oswego, OR) and EXCEL (Microsoft). Data are reported as means ± SEMs.
Results
RGS3 Variants Can Be Expressed to High Levels in Primary DRG Neurons. We have explored the molecular mechanisms underlying the effects of two alternatively spliced variants of RGS3, RGS3s and RGS3ss (Fig. 1a). By using a retrovirus, the proteins were expressed to high levels in chick DRG neurons (the parent cell from which the clones were generated) to ensure that any RGS3-mediated mechanisms dominate in electrophysiological assays. The RGS3 cDNAs were coupled at the C-terminal end to an HA epitope tag, subcloned into a retroviral vector (34, 35), and the virus microinjected into stage 9 chicken embryo neural tubes (35). Immunolabeling of DRGs demonstrated a high rate (≈90%) of neuronal infection (Fig. 1b). The infected ganglia from stage 37 to 38 (11- to 12-day-old) embryos were dissociated into single cells and calcium channel currents measured with tight-seal, whole-cell voltage clamp recording 2–24 h after dissociation.
Fig. 1.
RGS3 proteins can be overexpressed in chick sensory neurons. (a) Schematic diagram of the primary structure of RGS3s and RGS3ss splice variants. Marked are EF-like (black), RGS (gray), and CaM-binding (cross-hatched) domains. RGS3ss lacks the first 125 aa of RGS3s, including the EF-like region. (b) Frozen sections of intact dorsal root ganglia from 12-day-old embryos, 10.5 days after infection with either empty retroviral vector (“virus control”) or retroviral vectors carrying HA-tagged RGS3s (“RGS3s”). Sections were labeled with anti-rabbit HA primary antiserum followed by biotinylated goat anti-rabbit secondary antiserum and FITC-conjugated streptavidin. The low-intensity staining of cells infected with empty virus represents background fluorescence (comparable with sections labeled with secondary antiserum in the absence of primary antiserum). (Calibration bar: 20 μm.) (c) Representative families of superimposed calcium currents evoked by 35-ms step depolarizations to potentials between -50 and 50 mV in wild-type DRG neurons (Left) or cells infected with viral vector alone (Right). (Calibration: 2 nA, 10 ms.) (d) Current–voltage relationships (means ± SEMs) for calcium currents recorded from uninfected DRG neurons (Left, circles, n = 18), vector alone-infected neurons (triangles, n = 10), or neurons expressing recombinant RGS3s (Right, diamonds, n = 12) or RGS3ss (Right, squares, n = 12).
Calcium currents recorded from cells infected with virus backbone with or without RGS3 coding sequences were no different from those recorded from uninfected control cells (Fig. 1c). However, virtually all cells dissociated from ganglia infected with retrovirus carrying RGS3s or RGS3ss exhibited a consistent 10-mV rightward shift in the voltage dependence of current activation relative to uninfected cells or cells infected with retroviral vector backbone (Fig. 1d). Although the molecular mechanism underlying this shift is unknown, it provides strong confirmation of the immunocytochemical demonstration that most DRG cells studied expressed the recombinant RGS3 proteins. The voltage shift further suggests that the RGS3 proteins were expressed at amounts sufficient to affect the majority of calcium channels.
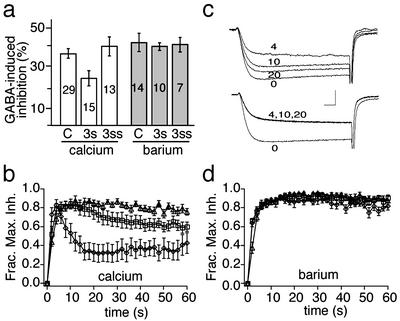
RGS3 Terminates the Inhibition of Ca2+ Channels by GABA. GABA, acting through Go protein-coupled GABAB receptors is a well known inhibitor of voltage-dependent calcium channels in chick DRG neurons (37, 39). This modulation is naturally attenuated through mechanisms involving both RGS proteins (16, 20, 22) and G protein receptor kinases (38). To determine whether the RGS3 isoforms altered GABA-induced inhibition of calcium current, a saturating concentration of GABA (100 μM) was bath-applied and current-monitored over time in cells expressing RGS3s, RGS3ss, or viral backbone alone. GABA-mediated inhibition of calcium current was reduced by 42% in cells overexpressing RGS3s but was unaffected in RGS3ss-expressing cells as compared with controls infected with retroviral backbone (Fig. 2a). Furthermore, in RGS3s-expressing cells, the inhibition of calcium current by prolonged GABA applications was accompanied by a rapid attenuation (or “desensitization”) that was not observed in RGS3ss-expressing or control cells (Fig. 2 b and c). Within ≈15 s of GABA exposure, calcium current recovered to 59% of control levels and was maintained at that level for >1 min. In the same 15-s period, the GABA responses of control, vector alone-, or RGS3ss-infected cells exhibited no discernable desensitization. At longer times (60 s), desensitization of GABA-induced inhibition in RGS3ss-expressing neurons was still significantly less than that of RGS3s-expressing cells (only 23% recovery), although slightly greater than that for cells infected with empty retroviral vector (7% recovery) (Fig. 2b).
Fig. 2.
RGS3s attenuates GABA-mediated inhibition of calcium channel currents in a calcium-dependent fashion. (a) Mean GABA-induced inhibition of calcium (white bars) or barium (gray bars) currents in cells infected with vector alone (“C”), or vector containing RGS3s (“3s”) or RGS3ss (“3ss”) coding sequence (n values noted on bars; 3s in calcium, P < 0.05 Student's two-tailed t test relative to C and 3ss). (b and d) Fraction of maximal GABA-induced inhibition of calcium (b) or barium (d) currents in cells infected with virus backbone alone (triangles, n = 17) or virus containing RGS3s (diamonds, n = 12) or RGS3ss (squares, n = 13). Error bars represent SEMs. (c) Representative calcium (Upper) or barium (Lower) currents from RGS3s-expressing neurons; numbers next to traces indicate the time (in seconds) at which the trace was acquired relative to the start of GABA application. Tail currents are truncated. [Calibration: 1.3 nA (Upper), 2 nA (Lower), 2 ms.]
RGS3-Mediated Desensitization Is Calcium-Dependent. To identify the molecular determinants underlying the kinetically distinct effects of RGS3s and RGS3ss, we compared the structure of the two splice variants. RGS3ss lacks 125 N-terminal residues present in RGS3s (Fig. 1a). Motif analysis of this region of RGS3s identified an EF-hand-like domain that appears to be contained in (and specific for) all of the longer forms of RGS3 identified to date (Fig. 3a). Given that EF hands have been shown to bind calcium with high selectivity in a large number of proteins (40), we explored the possibility that the rapid desensitization unique to RGS3s involves direct calcium binding to this site. As one test of this hypothesis, calcium was replaced by barium in the extracellular solution, because barium binds poorly to many calcium-dependent proteins, including EF hands (40). Total inhibition of barium current produced by GABA was similar to that observed for calcium current in virus backbone-infected cells. However, use of barium as the charge carrier eliminated the effect of RGS3s on the acute GABA-mediated N current inhibition (Fig. 2a) and on both fast and slow RGS3-dependent desensitization of inhibition during prolonged GABA applications (Fig. 2d). As a control for any potential nonspecific, deleterious effects of barium on the desensitization pathway, the time course of GABA-mediated inhibition was monitored first in barium saline (for 60 s) and then the saline was rapidly switched to one containing calcium. Calcium-dependent desensitization was unaltered by this pretreatment with barium. Thus, although barium is unable to activate the desensitization process, it does not poison it. These results demonstrate that calcium, entering through the calcium channel, is required for the RGS3-mediated effects.
Fig. 3.
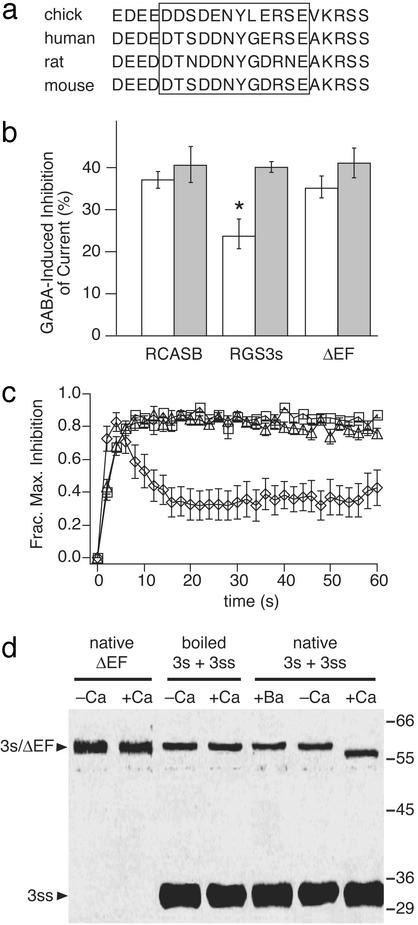
Calcium binding to EF hand promotes rapid activation of RGS3s. (a) Sequence alignment of RGS3 EF-hand domains. (b) Mean GABA-induced inhibition of calcium (white bars) or barium (gray bars) currents in cells infected with retroviral vector alone (“RCASB”), or vector containing RGS3s or ΔEF cDNA (as marked; n values between 4 and 29). *, P < 0.05, Student's two-tailed t test relative to control. (c) Fraction of maximal GABA-induced inhibition of calcium currents in cells infected with virus backbone alone (triangles, n = 17) or virus containing RGS3s (diamonds, n = 12), or ΔEF (squares, n = 15). Data from control and RGS3s replotted from Fig. 2 a and b. Error bars represent SEMs. Means do not reach 1.0 because of cell-to-cell variation in the time that maximal inhibition is achieved. (d) Coomassie blue-stained gels of recombinant RGS3s, RGS3ss, and ΔEF protein (arrowheads at left) solubilized in sample buffer containing either 2 mM calcium (+Ca) or 2 mM EGTA (with no added calcium, -Ca), as marked at top, denatured by boiling (“boiled”) or not (“native”), and separated electrophoretically. Size markers (in kilodaltons) are indicated at right.
Rapid Calcium-Dependent Desensitization Is Mediated by an EF-Hand Domain in RGS3s. To assess further the contribution of the EF hand to the rapid calcium-dependent desensitization observed in RGS3s-expressing cells, we deleted 13 residues containing the EF loop in RGS3s (Fig. 3a). The mutant RGS3s (“ΔEF”) was well expressed in the neurons, as assessed immunocytochemically (not shown). In addition, the RGS3-dependent rightward shift in the current-voltage relationship was unaltered by the EF loop deletion (not shown), suggesting that the deletion does not significantly alter the three-dimensional conformation of RGS3s. ΔEF expression eliminated both the overall reduction in GABA-induced inhibition (Fig. 3b) and the rapid component of calcium-dependent desensitization (Fig. 3c) produced by wild-type RGS3s. Again, no desensitization of GABA-induced inhibition was observed for barium currents in cells expressing ΔEF (not shown). These results suggest the possibility that calcium binds directly to the EF loop in RGS3s to promote desensitization.
A direct interaction between calcium and RGS3 was tested by using a standard gel mobility shift assay, in which the gel migration rates of many calcium-binding proteins have been shown to be enhanced when calcium is included in the sample buffer (36, 41–43). RGS3s, RGS3ss, and ΔEF were expressed in E. coli, purified as described in Materials and Methods, and electrophoretically separated in Laemmli sample buffer containing 2 mM calcium, barium, or EGTA (with no added divalent cations). Calcium, but not barium, enhanced migration of RGS3s, whereas the mobilities of RGS3ss and ΔEF were unaffected (Fig. 3d), indicating that the EF loop is critical for the calcium-induced mobility shift. Further, boiling RGS3s eliminated the mobility shift, suggesting that the protein maintains at least some of its native conformation in SDS-containing sample buffer. These results are consistent with those reported for other EF-hand-containing proteins (36, 41–43) and strongly suggest that direct calcium binding to the EF loop of RGS3s is essential for rapid RGS3s-dependent desensitization.
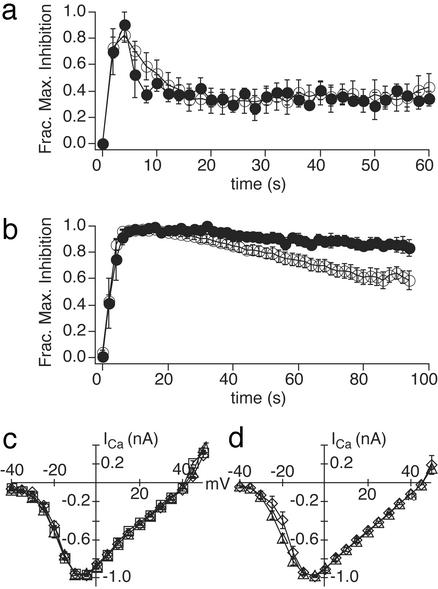
Slow Calcium-Dependent Desensitization Is Mediated by CaM. Although lacking the ability to promote rapid calcium-dependent desensitization, RGS3ss mediates a slow component of desensitization that also requires calcium influx (Fig. 2 b and d), suggesting a second mechanism for calcium-dependent RGS activation independent of calcium–EF-hand interactions. Recently, other RGS proteins (e.g., RGS2, RGS4, and GAIP) have been shown to be activated by calcium in vitro, by means of an indirect mechanism requiring CaM (44–46). The Ca/CaM complex directly binds RGS, thereby relieving a tonic inhibitory interaction with phosphatidylinositol 3,4,5-trisphosphate (PIP3) (44, 46). Because both RGS3s and RGS3ss contain a putative CaM-binding site (Fig. 1a), we tested for an involvement of CaM in calcium-dependent RGS3 action by using the CaM inhibitor, calmidazolium. The neurons were pretreated for 15–45 min with 10 μM calmidazolium before the application of GABA to allow intracellular access of the inhibitor. In cells expressing RGS3ss, the inhibitor blocked the slow component of calcium-dependent desensitization (Fig. 4b). This action of calmidazolium was selective in that the antagonist has no effect on the rapid, calcium-dependent desensitization in cells expressing wild-type RGS3s (Fig. 4a). These data are consistent with slow desensitization being mediated by calcium binding to CaM and fast desensitization produced by direct binding of calcium to the EF hand in RGS3s.
Fig. 4.
CaM antagonist calmidazolium (CMZ) selectively inhibits slow RGS3ss activation. Fraction of maximal GABA-induced inhibition of calcium current plotted as a function of time in cells expressing RGS3s (n = 12 control; n = 4 CMZ) (a) or RGS3ss (n = 9 control; n = 5 CMZ) (b). Cells were bathed in extracellular solution with (filled symbols) or without (open symbols) 10 μM CMZ for 15–45 min before GABA application. (c) Current–voltage relationships for calcium currents recorded from DRG neurons infected with virus backbone alone (triangles, n = 6) or virus containing RGS3s (diamonds, n = 4), bathed in 10 μM CMZ. (d) Current–voltage relationships for barium currents recorded from DRG neurons infected with virus backbone alone (triangles, n = 10) or virus containing RGS3s (diamonds, n = 10) or RGS3ss (squares, n = 7). All data are means ± SEMs.
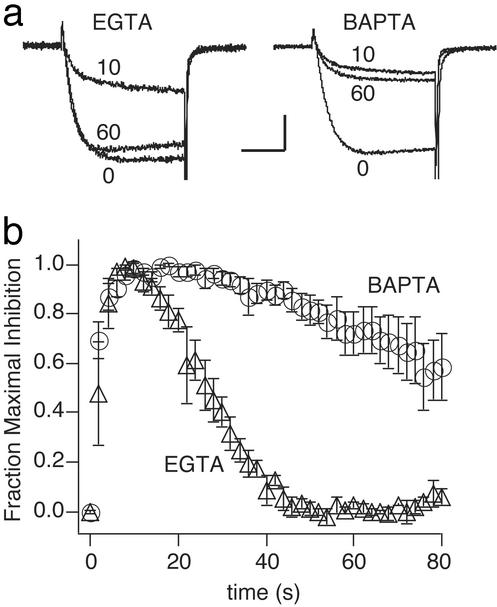
Calcium-Dependent Desensitization in Native Cells. If calcium-dependent activation of RGS3 is a physiologically significant process, then it should proceed under conditions in which the signaling molecules are expressed at natural levels. To test this hypothesis, we studied the time course of GABA-induced inhibition of calcium current in acutely dissociated, native DRG neurons. Recognizing that RGS3 concentrations in native cells will be much lower than those in our overexpression studies above, we were concerned that the high calcium buffering conditions routinely used in the overexpression studies (5 mM BAPTA) might overwhelm naturally occurring calcium-dependent desensitization in native cells. Therefore, in parallel with BAPTA, we also tested the cells under less stringent calcium-buffering conditions (0.5 mM EGTA). The rate of desensitization was dependent on the strength of intracellular calcium buffering. Under lower buffering conditions, desensitization of GABA-mediated inhibition was complete in less than 30 s; over the same period, under higher buffering conditions, the rate of desensitization was at least 4-fold slower (Fig. 5). Because 0.5 mM EGTA provides a degree of calcium buffering approximating that of native cytoplasm (47–49), these results indicate that calcium influx through voltage-dependent channels is sufficient to promote robust desensitization under natural conditions.
Fig. 5.
Low Ca2+ buffering reveals Ca2+-dependent desensitization at native levels of RGS3 expression. (a) Calcium currents recorded from chick sensory neurons dialyzed with 0.5 mM EGTA or 5 mM BAPTA (as marked); numbers next to traces indicate the time (in seconds) at which the traces were acquired relative to the start of GABA application. Tail currents are truncated. [Calibration: 200 pA (Left), 400 pA (Right), 5 ms.] (b) GABA-induced inhibition of calcium current normalized to maximum and plotted as a function of time for cells dialyzed with 0.5 mM EGTA (triangles, n = 3) or 5 mM BAPTA (circles, n = 4).
Discussion
These results demonstrate that calcium influx through plasma membrane voltage-dependent channels directly and differentially initiates desensitization of G protein signaling by two naturally occurring variants of RGS3 in sensory neurons. The rapid desensitization is most likely mediated by direct binding of calcium to RGS3s, whereas the slower activation appears to be mediated indirectly by CaM. Thus, RGS3-mediated termination of G protein signaling is initiated by cellular activity.
The molecular mechanisms underlying calcium-dependent desensitization are still undefined. Experiments to assess the possibility of calcium-dependent GTPase-accelerating activity of recombinant chick RGS3s protein, to date, have been precluded by problems with protein insolubility under nondenaturing conditions. The recent report that human RGS3 binds Gβγ (50) suggests a second possible mechanism for RGS3s-dependent desensitization, because Gβγ is known to mediate voltage-dependent (but not voltage-independent) inhibition of N channels by directly binding to the pore-forming subunit (51). Whether sequestration of Gβγ by RGS3s is calcium-dependent or whether this mechanism helps to mediate calcium-dependent desensitization by RGS3s must be tested. When the two components of inhibition were separately analyzed in our study, however, RGS3s desensitized both equally (data not shown), suggesting that if RGS3s-dependent sequestration of Gβγ is involved in the desensitization process, it is not the only means by which RGS3s terminates N current modulation.
A recent study by Popov et al. (44) may provide insight into the mechanism underlying the slow calcium-dependent activation of RGS3ss reported here. Many RGS proteins (including RGS3) contain consensus CaM-binding sites; Ca-CaM competes with PIP3 for binding RGS and consequently increases RGS activity by suppressing a resting, tonic PIP3-mediated inhibition (44). Such competition between PIP3 and Ca/CaM alters G protein-mediated activation of K+ channels in cell-free patches of membrane from cardiac myocytes exposed to bath-applied RGS4, PIP3, calcium, and Ca/CaM (45, 46). The importance of this mechanism for regulating RGS activity in vivo remains to be determined, because relief of PIP3-induced inhibition in the reconstitution experiments appears to require a high concentration of internal calcium (100 μM) for long times (tens of seconds), conditions that are unlikely to be achieved in intact cells. It is possible, however, that the calcium sensitivity would be enhanced in intact cells and that the slow Ca/CaM-dependent desensitization reported here for RGS3ss involves competition between CaM and PIP3 for binding to the RGS domain of RGS3ss.
For both direct and indirect forms of calcium-dependent regulation, the data imply that RGS3 must reside near the active calcium channel. Ca2+ influx through such channels creates steep concentration gradients, with intracellular Ca2+ ≥100 μM near the inner mouth of the channel but falling off very rapidly with distance (52–54). The high Ca2+ concentration reached within these “nanodomains” (≈50 nm of channel pore) is required to activate many Ca2+-dependent processes, such as exocytosis (55). Although high concentrations of rapid Ca2+ chelators (e.g., BAPTA) further restrict the size of the Ca2+ nanodomain, regions of unbuffered Ca2+ remain near the channel pore (49, 56, 57). In our experiments, when RGS3 was overexpressed, calcium-dependent desensitization was observed even in the presence of high concentrations of intracellular BAPTA (5 mM), arguing that the Ca2+ acceptor molecules responsible for desensitization must lie in significant numbers within the Ca2+ microdomain. However, the fact that desensitization was incomplete under these conditions [in contrast to low Ca2+ buffering conditions approximating that of native cytoplasm (47–49)] further argues that the Ca2+ acceptor is not confined to the microdomain. Although it is formally possible that the Ca2+ acceptor is some molecule other than RGS3s, elimination of fast Ca2+-dependent desensitization after deletion of the RGS3 EF loop strongly supports the conclusion that RGS3, itself, is the Ca2+ acceptor.
The idea that RGS3 resides very near the active calcium channel is further supported by our finding that RGS3 overexpression shifts the calcium channel current-voltage relationship (Fig. 1d); such changes in channel gating may be due to direct binding of RGS3 to the channel complex, as has been demonstrated for RGS12 (22). Alternatively, it is possible that RGS3 is recruited in a Ca2+-dependent fashion to the channel complex from a nearby cytoplasmic pool, as has been shown for human RGS3 in a mesangial cell line treated with calcium ionophore (28). The fact that RGS3-dependent alterations in the voltage activation range were only observed under conditions of protein overexpression makes the voltage shift of dubious physiological significance, but this remains to be directly tested. In either case, however, our results strongly suggest that RGS3 is ultimately integrated into spatially restricted signaling complexes that contain receptors, G proteins, and calcium channels (58).
In conclusion, our data suggest that signaling units, with G protein-activating and -deactivating machinery, provide precise control over the rate, amplitude, and time course of calcium influx. The presence of two, naturally occurring RGS3 isoforms, each dominated by a different form of calcium regulation, suggests that differential isoform expression is likely to provide flexibility in control over G protein signaling. Tethering of calcium channels to their downstream effector molecules [e.g., synaptotagmin I in nerve terminal (59, 60) or ryanodine receptors in muscle (61)] places the effector precisely where it will be most sensitive to regulation. Given the diversity of physiological processes that are triggered by calcium influx through voltage-dependent channels [e.g., exocytosis, muscle contraction, and gene transcription, each with a unique calcium dependence (55, 62, 63)], such signaling machines may specify coupling between calcium influx and its diverse downstream effectors. Furthermore, in complex neural networks under tonic modulation by G proteins (as occurs in the dorsal spinal cord) (64), such activity-dependent desensitization could provide a gateway for selectively enhancing the transmission of information from the most active neurons.
Acknowledgments
We thank Drs. Daniel Cox, Michael Goy, Michael Mendelsohn, and Timothy Turner for helpful comments on the manuscript. This work was supported by Public Health Service Grants NS16483 (to K.D.) and NS21725 (to M.H.J.) and Human Frontier Science Program long-term and short-term fellowships (to P.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RGS, regulators of G protein signaling; DRG, dorsal root ganglion; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; GABA, γ-aminobutyric acid; CaM, calmodulin; PIP3, phosphatidylinositol 3,4,5-trisphosphate; HA, hemagglutinin.
References
- 1.Druey, K. M., Blumer, K. J., Kang, V. H. & Kehrl, J. H. (1996) Nature 379, 742-746. [DOI] [PubMed] [Google Scholar]
- 2.Ross, E. M. & Wilkie, T. M. (2000) Annu. Rev. Biochem. 69, 795-827. [DOI] [PubMed] [Google Scholar]
- 3.Koelle, M. R. & Horvitz, H. R. (1996) Cell 84, 115-125. [DOI] [PubMed] [Google Scholar]
- 4.Tesmer, J. J., Berman, D. M., Gilman, A. G. & Sprang, S. R. (1997) Cell 89, 251-261. [DOI] [PubMed] [Google Scholar]
- 5.Berman, D. M., Wilkie, T. M. & Gilman, A. G. (1996) Cell 86, 445-452. [DOI] [PubMed] [Google Scholar]
- 6.De Vries, L., Zheng, B., Fischer, T., Elenko, E. & Farquhar, M. G. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 235-271. [DOI] [PubMed] [Google Scholar]
- 7.De Vries, L., Elenko, E., McCaffery, J. M., Fischer, T., Hubler, L., McQuistan, T., Watson, N. & Farquhar, M. G. (1998) Mol. Biol. Cell 9, 1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wylie, F., Heimann, K., Le, T. L., Brown, D., Rabnott, G. & Stow, J. L. (1999) Am. J. Physiol. 276, C497-C506. [DOI] [PubMed] [Google Scholar]
- 9.Reif, K. & Cyster, J. G. (2000) J. Immunol. 164, 4720-4729. [DOI] [PubMed] [Google Scholar]
- 10.Miles, R. R., Sluka, J. P., Santerre, R. F., Hale, L. V., Bloem, L., Boguslawski, G., Thirunavukkarasu, K., Hock, J. M. & Onyia, J. E. (2000) Endocrinology 141, 28-36. [DOI] [PubMed] [Google Scholar]
- 11.Harder, S., Lu, X., Wang, W., Buck, F., Gershengorn, M. C. & Bruhn, T. O. (2001) Endocrinology 142, 1188-1194. [DOI] [PubMed] [Google Scholar]
- 12.Neill, J. D., Duck, L. W., Sellers, J. C., Musgrove, L. C., Scheschonka, A., Druey, K. M. & Kehrl, J. H. (1997) Endocrinology 138, 843-846. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee, T. K., Eapen, A. K. & Fisher, R. A. (1997) J. Biol. Chem. 272, 15481-15487. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh, O., Kubo, Y., Miyatani, Y., Asano, T. & Nakata, H. (1997) Nature 390, 525-529. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, S., Inanobe, A., Chachin, M., Aizawa, Y. & Kurachi, Y. (2000) J. Physiol. 526, 341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diverse-Pierluissi, M. A., Fischer, T., Jordan, J. D., Schiff, M., Ortiz, D. F., Farquhar, M. G. & De Vries, L. (1999) J. Biol. Chem. 274, 14490-14494. [DOI] [PubMed] [Google Scholar]
- 17.Jeong, S. W. & Ikeda, S. R. (2000) J. Neurosci. 20, 4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melliti, K., Meza, U., Fisher, R. & Adams, B. (1999) J. Gen. Physiol. 113, 97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammermeier, P. J. & Ikeda, S. R. (1999) Neuron 22, 819-829. [DOI] [PubMed] [Google Scholar]
- 20.Tosetti, P., Turner, T., Lu, Q. & Dunlap, K. (2002) J. Biol. Chem. 277, 46001-46009. [DOI] [PubMed] [Google Scholar]
- 21.Chen, H. & Lambert, N. A. (2000) Proc. Natl. Acad. Sci. USA 97, 12810-12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiff, M. L., Siderovski, D. P., Jordan, J. D., Brothers, G., Snow, B., De Vries, L., Ortiz, D. F. & Diverse-Pierluissi, M. (2000) Nature 408, 723-727. [DOI] [PubMed] [Google Scholar]
- 23.Levay, K., Cabrera, J. L., Satpaev, D. K. & Slepak, V. Z. (1999) Proc. Natl. Acad. Sci. USA 96, 2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heximer, S. P., Lim, H., Bernard, J. L. & Blumer, K. J. (2001) J. Biol. Chem. 276, 14195-14203. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh, O., Masuho, I., Terakawa, I., Nomoto, S., Asano, T. & Kubo, Y. (2001) J. Biol. Chem. 276, 5052-5058. [DOI] [PubMed] [Google Scholar]
- 26.Skiba, N. P., Martemyanov, K. A., Elfenbein, A., Hopp, J. A., Bohm, A., Simonds, W. F. & Arshavsky, V. Y. (2001) J. Biol. Chem. 276, 37365-37372. [DOI] [PubMed] [Google Scholar]
- 27.Zeng, W., Xu, X., Popov, S., Mukhopadhyay, S., Chidiac, P., Swistok, J., Danho, W., Yagaloff, K. A., Fisher, S. L., Ross, E. M., et al. (1998) J. Biol. Chem. 273, 34687-34690. [DOI] [PubMed] [Google Scholar]
- 28.Dulin, N. O., Sorokin, A., Reed, E., Elliott, S., Kehrl, J. H. & Dunn, M. J. (1999) Mol. Cell. Biol. 19, 714-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheschonka, A., Dessauer, C. W., Sinnarajah, S., Chidiac, P., Shi, C. S. & Kehrl, J. H. (2000) Mol. Pharmacol. 58, 719-728. [DOI] [PubMed] [Google Scholar]
- 30.Lu, Q., Sun, E. E., Klein, R. S. & Flanagan, J. G. (2001) Cell 105, 69-79. [DOI] [PubMed] [Google Scholar]
- 31.Castro-Fernandez, C., Janovick, J. A., Brothers, S. P., Fisher, R. A., Ji, T. H. & Conn, P. M. (2002) Endocrinology 143, 1310-1317. [DOI] [PubMed] [Google Scholar]
- 32.Kehrl, J. H., Srikumar, D., Harrison, K., Wilson, G. L. & Shi, C. S. (2002) Genomics 79, 860-868. [DOI] [PubMed] [Google Scholar]
- 33.Wang, Q., Liu, M., Mullah, B., Siderovski, D. P. & Neubig, R. R. (2002) J. Biol. Chem. 277, 24949-24958. [DOI] [PubMed] [Google Scholar]
- 34.Morgan, B. A. & Fekete, D. M. (1996) Methods Cell Biol. 51, 185-218. [DOI] [PubMed] [Google Scholar]
- 35.Williams, B. M., Temburni, M. K., Levey, M. S., Bertrand, S., Bertrand, D. & Jacob, M. H. (1998) Nat. Neurosci. 1, 557-562. [DOI] [PubMed] [Google Scholar]
- 36.Rosel, D., Puta, F., Blahuskova, A., Smykal, P. & Folk, P. (2000) FEBS Lett. 473, 323-327. [DOI] [PubMed] [Google Scholar]
- 37.Diverse-Pierluissi, M., Goldsmith, P. K. & Dunlap, K. (1995) Neuron 14, 191-200. [DOI] [PubMed] [Google Scholar]
- 38.Diverse-Pierluissi, M., Inglese, J., Stoffel, R. H., Lefkowitz, R. J. & Dunlap, K. (1996) Neuron 16, 579-585. [DOI] [PubMed] [Google Scholar]
- 39.Dunlap, K. (1981) Br. J. Pharmacol. 74, 579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falke, J. J., Drake, S. K., Hazard, A. L. & Peersen, O. B. (1994) Q. Rev. Biophys. 27, 219-290. [DOI] [PubMed] [Google Scholar]
- 41.Lenz, S. E., Braunewell, K. H., Weise, C., Nedlina-Chittka, A. & Gundelfinger, E. D. (1996) Biochem. Biophys. Res. Commun. 225, 1078-1083. [DOI] [PubMed] [Google Scholar]
- 42.Garrigos, M., Deschamps, S., Viel, A., Lund, S., Champeil, P., Moller, J. V. & le Maire, M. (1991) Anal. Biochem. 194, 82-88. [DOI] [PubMed] [Google Scholar]
- 43.Geiser, J. R., van Tuinen, D., Brockerhoff, S. E., Neff, M. M. & Davis, T. N. (1991) Cell 65, 949-959. [DOI] [PubMed] [Google Scholar]
- 44.Popov, S. G., Krishna, U. M., Falck, J. R. & Wilkie, T. M. (2000) J. Biol. Chem. 275, 18962-18968. [DOI] [PubMed] [Google Scholar]
- 45.Ishii, M., Inanobe, A., Fujita, S., Makino, Y., Hosoya, Y. & Kurachi, Y. (2001) Circ. Res. 89, 1045-1050. [DOI] [PubMed] [Google Scholar]
- 46.Ishii, M., Inanobe, A. & Kurachi, Y. (2002) Proc. Natl. Acad. Sci. USA 99, 4325-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts, W. M. (1994) J. Neurosci. 14, 3246-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, W. M. (1993) Nature 363, 74-76. [DOI] [PubMed] [Google Scholar]
- 49.Naraghi, M. & Neher, E. (1997) J. Neurosci. 17, 6961-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi, C. S., Lee, S. B., Sinnarajah, S., Dessauer, C. W., Rhee, S. G. & Kehrl, J. H. (2001) J. Biol. Chem. 276, 24293-24300. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda, S. R. & Dunlap, K. (1999) Adv. Second Messenger Phosphoprotein Res. 33, 131-151. [DOI] [PubMed] [Google Scholar]
- 52.Chad, J. E. & Eckert, R. (1984) Biophys. J. 45, 993-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon, S. M. & Llinas, R. R. (1985) Biophys. J. 48, 485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fogelson, A. L. & Zucker, R. S. (1985) Biophys. J. 48, 1003-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Augustine, G. J. (2001) Curr. Opin. Neurobiol. 11, 320-326. [DOI] [PubMed] [Google Scholar]
- 56.Stern, M. D. (1992) Cell Calcium 13, 183-192. [DOI] [PubMed] [Google Scholar]
- 57.Issa, N. P. & Hudspeth, A. J. (1996) Proc. Natl. Acad. Sci. USA 93, 9527-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davare, M. A., Avdonin, V., Hall, D. D., Peden, E. M., Burette, A., Weinberg, R. J., Horne, M. C., Hoshi, T. & Hell, J. W. (2001) Science 293, 98-101. [DOI] [PubMed] [Google Scholar]
- 59.Charvin, N., L'Eveque, C., Walker, D., Berton, F., Raymond, C., Kataoka, M., Shoji-Kasai, Y., Takahashi, M., De Waard, M. & Seagar, M. J. (1997) EMBO J. 16, 4591-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leveque, C., el Far, O., Martin-Moutot, N., Sato, K., Kato, R., Takahashi, M. & Seagar, M. J. (1994) J. Biol. Chem. 269, 6306-6312. [PubMed] [Google Scholar]
- 61.Mouton, J., Ronjat, M., Jona, I., Villaz, M., Feltz, A. & Maulet, Y. (2001) FEBS Lett. 505, 441-444. [DOI] [PubMed] [Google Scholar]
- 62.Striessnig, J. (1999) Cell. Physiol. Biochem. 9, 242-269. [DOI] [PubMed] [Google Scholar]
- 63.Finkbeiner, S. & Greenberg, M. E. (1998) J. Neurobiol. 37, 171-189. [PubMed] [Google Scholar]
- 64.Millan, M. J. (2002) Prog. Neurobiol. 66, 355-474. [DOI] [PubMed] [Google Scholar]