Abstract
The G protein β subunit Gβ5 deviates significantly from the other four members of Gβ-subunit family in amino acid sequence and subcellular localization. To detect the protein targets of Gβ5 in vivo, we have isolated a native Gβ5 protein complex from the retinal cytosolic fraction and identified the protein tightly associated with Gβ5 as the regulator of G protein signaling (RGS) protein, RGS7. Here we show that complexes of Gβ5 with RGS proteins can be formed in vitro from the recombinant proteins. The reconstituted Gβ5-RGS dimers are similar to the native retinal complex in their behavior on gel-filtration and cation-exchange chromatographies and can be immunoprecipitated with either anti-Gβ5 or anti-RGS7 antibodies. The specific Gβ5-RGS7 interaction is determined by a distinct domain in RGS that has a striking homology to Gγ subunits. Deletion of this domain prevents the RGS7-Gβ5 binding, although the interaction with Gα is retained. Substitution of the Gγ-like domain of RGS7 with a portion of Gγ1 changes its binding specificity from Gβ5 to Gβ1. The interaction of Gβ5 with RGS7 blocked the binding of RGS7 to the Gα subunit Gαo, indicating that Gβ5 is a specific RGS inhibitor.
Signal transduction through heterotrimeric (Gαβγ) G proteins is governed by the cycle of GTP binding and hydrolysis by the Gα subunit (Gα). An activated receptor catalyzes the exchange of GDP bound to Gα initially for GTP, leading to the dissociation of Gα from the tightly associated Gβγ-subunit complex. In this active state, the G protein modulates the activity of second messenger-generating effector enzymes and ion channels until GTP hydrolysis returns the cascade to its resting state. It has been known for a number of years that the rate of intrinsic GTPase activity of Gα in vitro is much slower than the rate of termination of some physiological responses. Therefore, it has been proposed that additional factors accelerate GTPase activity in vivo. One class of G protein GTPase-activating proteins (GAPs) are G protein effectors such as cGMP phosphodiesterase (1) and phospholipase C (2). Most of the G protein effector molecules, however, do not posses GAP activity. In the past 2 years, a new class of GAPs for G proteins, termed regulators of G protein signaling (RGS), has emerged (for reviews, see refs. 3–5). Thus far, about 20 RGS proteins have been discovered in mammals. RGS vary dramatically in size (from 23 to 160 kDa) and sequence, but they all have a common “RGS domain” (≈120 aa), which is responsible for the binding to the Gα subunits and is sufficient for the GAP activity of RGS (6, 7). The function of the other domains in the RGS proteins remains largely unexplored. However, it had been shown that RGS12 contains a PDZ domain (8), and protein p115 RhoGEF, which has a GAP activity for Gα subunits Gα12 and Gα13, is also a guanine nucleotide-exchange factor for a small G protein, Rho (9). These findings indicate that, in addition to their interaction with Gα subunits, RGS proteins might interact with other molecules.
While investigating the native complexes of G protein β subunit Gβ5, we discovered that Gβ5 can be copurified in a tight complex with a protein identified as RGS7 (10). Gβ5 is significantly different from the previously known four Gβ subunits in structure and subcellular localization (11, 12). Whereas the Gβ subunits Gβ1–4 are more than 90% identical, Gβ5 has only about 50% identity. Previously known Gβγs stably associate with the membrane through the prenylated Gγ subunits, but more than 90% of Gβ5 in the retina is soluble. In the brain, Gβ5 is about equally distributed between the membrane and cytosol. Despite these intriguing differences, Gβ5 behaved similarly to other Gβ subunits in several functional tests in vitro. In COS cells, Gβ5 dimerized with Gγ subunits, stimulated PLCβ2, and interacted with Gαi2 (12). However, Gβ5 is different from Gβ1 in its ability to interact with effectors. Gβ5γ2 stimulated PLCβ2, but not mitogen-activated protein kinase, whereas Gβ1γ2 stimulated both (13). The effects of Gβ5γ2 and Gβ1γ2 on the two forms of adenylate cyclase, AC I and AC II, are also different (14). Purified recombinant Gβ5γ2 complex can bind to the Gαq but not to Gαi (15). Based on its unusual features, we hypothesized that Gβ5 might have a unique role.
Here we show that Gβ5-RGS7 dimers can be reconstituted in vitro from the expressed proteins. We have identified a structural domain in RGS7 that has a striking homology to G protein γ subunits and showed, by mutagenesis, that it is responsible for specific binding of Gβ5. Similar domains also could be found in the sequences of RGS6 (GenBank accession no. AF073920), RGS9 (16), and EGL-10, an RGS protein from Caenorhabditis elegans (17). While this manuscript was in submission, Snow et al. (18) described the structural features of such domains in detail and also demonstrated that the Gγ-like domain of the recently cloned RGS protein RGS11 can bind specifically to Gβ5 in vitro. The complex of Gβ5 with a portion of RGS11 molecule possesses significant GAP activity toward Gα subunit Gαo (18). In contrast, we found that Gβ5 prevents the protein–protein binding between Gαo and full-size RGS7, indicating that the role of Gβ5 might be in the inhibition of RGS-Gα interaction.
MATERIALS AND METHODS
RGS and Gβ5 Expression.
The cDNA clones of RGS7 and RGS9 were kindly provided by T. Wensel (Baylor College, Houston, TX). The coding regions of the RGS proteins, Gβ5 and Gγ2, were amplified by PCR and subcloned under the control of T3 RNA-polymerase promoter into the pBluescript KS(+) vector for expression in rabbit reticulocyte lysate.
Analysis of RGS-Gβ5 Complex Formation.
Proteins were synthesized in the presence of [35S]methionine (NEN or Amersham) by using the TNT rabbit reticulocyte lysate system (Promega) according to the manufacturer’s instructions. Reaction mixtures, containing Gβ and RGS, then were mixed and incubated for 1 h at 37°C before subjecting them to chromatography. The amount of the 35S-labeled Gβ5 and RGS7 proteins in the fractions was measured quantitatively by image analysis of the exposed film using nih image software.
Gel filtration.
Fifty microliters of translation mixture was resolved on a 37-ml Superdex 200 column equilibrated with 20 mM Tris⋅HCl, pH 7.5/100 mM NaCl/1 mM EDTA/0.5% sodium cholate. To ensure reproducibility of the position of the peaks, all chromatographies were done on the same column, and the volume of each fraction was measured by a micropipette. Because of the slight deviation of the flow rate, the fraction numbers, but not the elution volume, could be different among the experiments. The elution volumes of Blue Dextran, hemoglobin present in the lysates, and the nonincorporated [35S]methionine, which served as internal controls in each experiment, did not vary by more than the average volume of a single collected fraction in a total of more than 20 experiments. The collected fractions were analyzed on SDS/PAGE followed by radioautography. The column was calibrated at the beginning and the end of the experimental series by using protein standards for gel filtration (Sigma).
Cation-exchange chromatography on Sepharose S.
The lysates containing [35S]Met-labeled Gβ5, RGS7, or their mixture were diluted 1:5 in 20 mM Tris⋅HCl, pH 8.0/1 mM EDTA/1 mM PMSF/0.5% sodium cholate (final volume, 100 μl) and then incubated, batchwise, with 50 μl of the chromatography resin. The beads then were washed and eluted with same buffer with the addition of 400 mM NaCl, and the proteins were analyzed by SDS/PAGE. In contrast to the native Gβ5-RGS dimers that adsorb on Sepharose S quantitatively (10), a significant amount of the reconstituted complex as well as monomeric RGS7 or RGS9 was constantly left in the unbound fraction. This could be attributed to some components of the reticulocyte lysate that prevented the interaction of the proteins with the matrix or the partial denaturation of the synthesized RGS proteins, which could lead to the masking of these positively charged domains. Because Gβ5 could bind to Sepharose S only in the presence of an RGS protein, this type of chromatography served as a rapid assay of Gβ5-RGS interaction.
Immunoprecipitation.
Polyclonal antisera were raised against a synthetic peptide corresponding to amino acids 454–468 in RGS7. This sequence is unique for RGS7. Western blots using this antiserum reveal a single major band in the crude extracts of brain and retina; the specificity of this antiserum currently is under investigation and will be described in detail elsewhere. For immunoprecipitation, the antibodies were adsorbed on protein A-Sepharose and the lysates were added to the beads. After a 1-hr incubation at room temperature (with mixing) and washes with PBS, the beads were eluted with SDS and the proteins were resolved by SDS/PAGE and detected by radioautography. For immunoprecipitation of Gβ5, we used the polyclonal antibody CT215 (11). For control, we used serum obtained from rabbits before their immunization with the RGS7 peptide.
Interaction of RGS Proteins with Gα.
(His)6-tagged Gα subunit Gαo was expressed in Escherichia coli and purified on the Ni2+ beads (Qiagen) as described previously. One hundred micrograms of nearly homogeneous protein was immobilized on 100 μl of the beads. Fifty microliters of reticulocyte lysate containing RGS7, Gβ5, or their mixture was added, batchwise, to 20 μl of the Gαo beads and incubated for 1 hr with constant gentle mixing at 4°C. The unbound material was collected, the beads were washed and eluted by 300 mM imidazole or 1% SDS to remove the proteins retained on the affinity matrix, and the samples were analyzed by SDS/PAGE followed by radioautography.
RESULTS
Interaction of Gβ5 and RGS Proteins in Vitro.
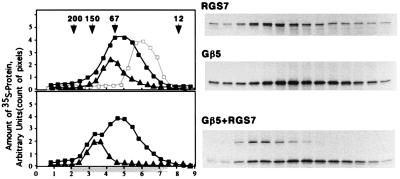
Functionally active full-size RGS7 and RGS9 proteins, Gβ5, and Gβ5L were expressed in the rabbit reticulocyte in vitro translation system. Gβ5-RGS7 complex formation was demonstrated by two types of conventional chromatography, gel filtration (Fig. 1) and cation exchange (Fig. 2), as well as immunoprecipitation with either anti-Gβ5 or anti-RGS7 antibodies (Fig. 3). The apparent molecular weight of the reconstituted Gβ5-RGS7 dimer was similar to that of the native complex (10). Interestingly, although the size of the Gβ5-RGS7 dimer was larger than that of Gβ5, the apparent molecular weight of Gβ5 decreased in the presence of Gγ2. Similar results were reported previously for Gβ1 and apparently are explained by the more compact structure of Gβγ dimer compared with the monomeric Gβ subunit (19, 20). In the presence of RGS7, Gβ5 bound to the cation exchanger Sepharose S whereas Gβ5 alone adsorbed only in trace amounts. This explains why native Gβ5 complexes could be purified on Sepharose S. According to its amino acid sequence, Gβ5, as well as the other Gβ subunits, has a net negative charge and should not bind to cation exchangers. Indeed, other Gβ subunits (i.e., Gβ1) do not bind to Sepharose S (10). In contrast, RGS6, 7, and 9 have distinct positively charged domains (pI > 9.5), and, thus, binding of native Gβ5 to this matrix could be rationalized by its association with an RGS. In accord with the apparent absence of Gγ in the purified Gβ5-RGS complexes (J.L.C. and V.Z.S., unpublished results), the addition of Gγ was not required for the Gβ5-RGS7 interaction in vitro. Furthermore, the interaction between Gβ5 and RGS7 still could occur in the presence of Gγ2. Gβ5-RGS7 binding occurred even if RGS was added to the mixture after the Gβ5γ2 dimer had been formed. This might indicate that the RGS is capable of displacing Gγ from its complex with Gβ5. In contrast to Gβ5, Gβ1 did not associate with RGS7 (Figs. 2B and 4), indicating that the interaction with the RGS proteins is specific for Gβ5. The in vitro synthesized Gβ1 was not denatured because it could bind to Gγ2 (not shown) or to the chimeric mutant of RGS7, where the Gγ-like domain was replaced with a portion of the Gγ subunit Gγ1 (see below).
Figure 1.
Gβ5-RGS7 interaction in vitro. (Upper) Overlay plot of three experiments resolving monomeric Gβ5 (■), Gβ5 with excess Gγ2 (□, gray line), and monomeric RGS7 (▴) on a Superdex 200 gel-filtration column as described in Materials and Methods. The G protein γ subunit Gγ2 was synthesized in the presence of nonradioactive methionine. The Gβ5γ2 complex has a lower apparent molecular weight than Gβ5 apparently because of a more compact structure (20). (Lower) Experiment with the mixture of Gβ5 with RGS7 (squares, position of Gβ5; triangles, RGS7). x axis: Elution volume (ml), starting (zero) at the beginning of elution of the blue dextran. Highlighted area below the axis denotes the fractions resolved by SDS/PAGE and radioautography, shown to the right. y axis: Arbitrary units based on the strength of the bands on the gel determined by the amount of pixels per band. The fractions were analyzed by SDS/PAGE followed by radioautography, and the amount of 35S-labeled Gβ5 or RGS7 was measured by image analysis of the exposed film using the nih image software. Each experiment was done at least two times, each with an independent in vitro translation.
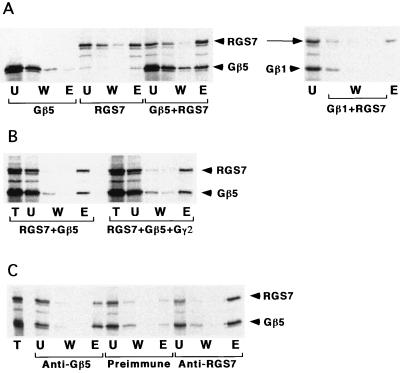
Figure 2.
Analysis of RGS7-Gβ complex formation in vitro by cation-exchange chromatography and immunoprecipitation. (A) Chromatography on Sepharose S. The lysates containing [35S]Met-labeled Gβ5, Gβ1, RGS7, or their mixture were incubated batchwise with the chromatography resin. The unbound material was collected, the beads then were washed and eluted by 300 mM of NaCl, and proteins from the fractions were analyzed by SDS/PAGE. T, total lysate loaded; U, unbound material; W, washes; E, the eluate. (B) The Gβ5γ2 complex was obtained by mixing the 35S-labeled Gβ5 and the excess of unlabeled Gγ2 under the same conditions as in Fig. 1. 35S-RGS7-containing lysate then was added to the mixture, and binding to Sepharose S was tested as in A. (C) Immunoprecipitation. The antibodies indicated were adsorbed on protein A-Sepharose, and the lysates were added to the beads. After incubation, the beads were washed and eluted by SDS, and the obtained fractions were processed as in A. T, total mixture added; W, washes; E, eluate.
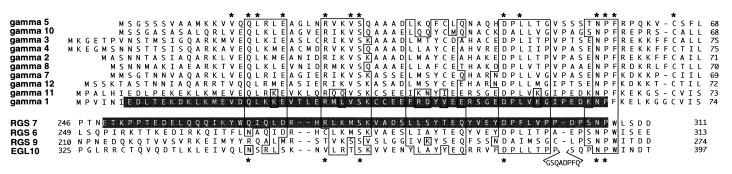
Figure 3.
Structural homology between Gγ subunits and RGS proteins. Alignment of full-length sequences of Gγ subunits and the indicated portions of RGS proteins. Asterisks above the Gγ sequences designate the residues that are identical throughout the entire Gγ class and the residues found at corresponding positions in at least one RGS protein. Boxed are the regions of homology based on the nature of amino acids, i.e., basic (K, R) acidic (E, D), hydrophobic (L, I, V, F, W, M), polar (S, T), and amides (Q, N). EGL-10 has an 8-residue insert shown below its sequence. The sequence highlighted in RGS7 was deleted in the RGS7Δ mutant (see text and Fig. 4) or swapped for the stretch of Gγ1 amino acids highlighted in Gγ1.
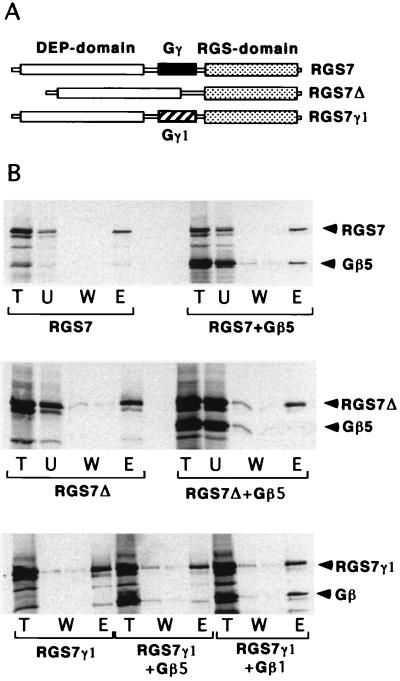
Figure 4.
Functional activity of Gγ-like domain in RGS7. (A) Structural domains in wild-type RGS7 and its mutants. The thinner bar represents the length of the protein. The stippled box is the RGS core domain; the solid box denotes the Gγ-like domain. The open box is the “DEP,” which also is found in RGS6, 7, and 9 and EGL-10; its sequence is homologous to pleckstrin (29) but the function is unknown. In the RGS7Δ mutant, the Gγ-like domain is deleted, and in the RGS7γ1 mutant, it is replaced with a portion of Gγ1 (hatched box). (B) Interaction of the mutants with Gβ5 and Gβ1. The experiments were carried out by using the 35S-labeled proteins and testing the Gβ5-RGS interaction using Sepharose S as described in the Fig. 2 legend. Gβ5 binds the wild-type RGS7 (Top), but not the RGS7Δ mutant (Middle). (Bottom) RGS7γ1 mutant binds Gβ1 instead of Gβ5.
Gγ-Like Domain in the RGS Proteins.
The interaction of Gβ5 with RGS proteins suggested that they might contain a structure resembling Gγ. Indeed, alignment of RGS and Gγ sequences revealed that RGS6, 7, and 9 as well as EGL-10, an RGS protein from C. elegans (17), contain a domain that has a striking homology to Gγ subunits (Fig. 3). This structure, termed the GGL domain, recently was described and examined by computer modeling in more detail (18). Compared with the Gα and Gβ subunit families, the Gγ subunits display a relatively low level of structural homology. As denoted by the asterisks in Fig. 3, there are only 13 residues that are identical in all the Gγ family members. According to the tertiary structure of the Gβγ complexes, these amino acids are involved in the interaction with Gβ (18, 19). Six of these key residues are present at the corresponding positions in at least one of the RGS proteins. The Gγ-like domains of the RGS proteins are clearly different from the Gγ subunits, but the Gγ subunits Gγ1 and Gγ11 also significantly deviate from the rest of the family, and, therefore, these differences do not appear to be critical for Gβ binding.
The functional activity of the Gγ-like domain of RGS7 was demonstrated by the analysis of RGS7 mutants (Fig. 4). We prepared RGS7Δ, a construct with a deleted Gγ-like domain (residues 249–305) and an RGS7-Gγ1 chimera, in which the Gγ-like domain in RGS7 was replaced with the corresponding amino acids of Gγ1 (residues 7–63; see Fig. 3). Neither of these mutants could bind to Gβ5, as follows from their inability to promote adsorption of Gβ5 on Sepharose S (Fig. 4). RGS7Δ could bind specifically to Gαo (Fig. 5A) and, thus, is likely to be folded correctly. Most importantly, although the RGS7-Gγ1 chimera did not bind to Gβ5, it instead could bind Gβ1 (Fig. 4B Bottom). Thus, the Gγ-like domain in RGS7 indeed is responsible for Gβ binding and is sufficient to confer the specificity for Gβ5. When we replaced a small portion of RGS7 located roughly in the middle of the domain (amino acids 279–283, VADSL) with the corresponding sequence of Gγ1 (residues 36–40, CCEEF), the mutant RGS7 retained its specific binding to Gβ5 (data not shown). This indicates that the key residues are located in a different region or that Gβ5-binding specificity is determined by a relatively large portion of the Gγ-like domain.
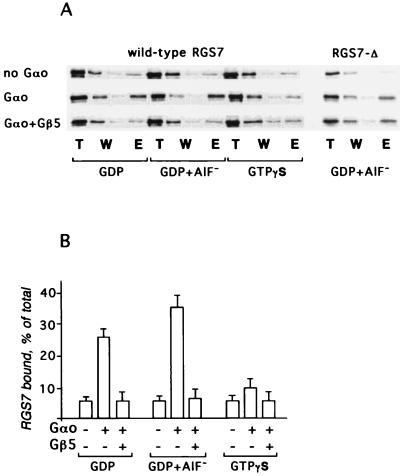
Figure 5.
Gβ5 inhibits the interaction between RGS7 and Gαo. His-tagged Gαo (30) was immobilized on the Ni2+-NTA beads, and the 35S-labeled RGS7 or its mixture with Gβ5 was applied, batchwise, to the suspension in the presence of 0.1 mM GDP/0.1 mM GDP plus 10 mM NaF and 100 μM AlCl3 or guanosine 5′-[γ-thio]triphosphate. The beads were washed and eluted with sample buffer for SDS/PAGE. The resin without Gαo was used for control of a nonspecific adsorption. (A) Radioautograms of the fractions from the chromatography resolved by SDS/PAGE followed by radioautography. (B) The amount of the protein in the bands was quantified by the image analysis of the exposed film. The bar graph shows the amount of RGS7 eluted from immobilized (His)6-Gαo or control Ni2+ beads without Gαo. Data were collected from three independent experiments.
Gβ5 Inhibits the Interaction Between RGS7 and Gαo.
To study the function of the Gβ5-RGS interaction, we examined the effect of Gβ5 on the nucleotide-dependent binding of RGS7 to Gαo (Fig. 5). As expected (21), the interaction of monomeric RGS7 with Gαo was stronger in the presence of GDP + AlF4− than in the presence of GDP or guanosine 5′-[γ-thio]triphosphate. Monomeric Gβ5 did not bind to Gαo (not shown), but strongly inhibited the association of RGS7 with the Gα subunit regardless of the nucleotide present in the assay. The effect of Gβ5 was quite dramatic, particularly in light of the fact that in all the experiments, the Gβ5/RGS7 ratio did not exceed 4:1. Gβ5 did not affect the binding of Gαo with the RGS7Δ mutant, also indicating that the inhibition of Gαo-RGS7 binding is a result of the interaction of Gβ5 with RGS rather than Gα (Fig. 5A). When expressed in bacteria, the full-size RGS7 and RGS9 are insoluble and, thus, are not available in quantities sufficient for the analysis of the Gβ5 effects in direct assays of RGS GAP activity. Our experiments using analytical amounts of 35S-labeled RGS7 for the assay of RGS-Gα binding strongly indicate that the role of Gβ5-RGS complex formation is in the inhibition of RGS function.
DISCUSSION
Interaction with Gβ5 Identifies the Gγ-Like Domain in RGS Proteins.
Our finding of a Gβ5-RGS7 complex in the retinal cytosol was quite unexpected (10), but it could be explained in light of the known ability of Gβγ complexes to associate directly with a wide array of proteins that are structurally unrelated (22–24). In contrast, the interaction of RGS7 and Gβ5 in the absence of a Gγ was hard to rationalize, because the other Gβ subunits exist only as tightly associated dimers with Gγ subunits. Previous experiments with transiently transfected cells and recombinant proteins had shown that a Gγ subunit was necessary for activities of Gβ5 such as stimulation of phospholipase C, inhibition of adenylate cyclase, and interaction with Gα subunits (11–15). Because Gβ5-Gγ dimerization is necessary for the interaction of Gβ5 with its putative effectors (11–14), we speculate that RGS proteins bearing the Gγ-like domains, through the competition with Gγ, can terminate not only Gα- but also Gβ5γ-mediated signaling. The reconstitution of the Gβ5-RGS7 dimer in vitro and the identification of the Gγ-like domain in the RGS proteins show that a G protein β subunit can exist without a Gγ. The presence of a functional Gγ-like domain in RGS proteins suggests that other molecules with similar domains may exist and be able to interact with specific Gβ or the Gβ-like WD-repeat proteins (25).
Gβ5 Is an Inhibitor of RGS-Gα Interaction.
RGS has been found to act upon all the Gα subunits except Gαs, the Gα stimulating adenylate cyclase. In vitro, RGS proteins are very powerful GAPs, and many of them, for example, RGS1, RGS4, RGS7, and RGS10, appear to be nonspecific for Gα. This raises the question of how signals transduced by G proteins can reach the appropriate effectors in the presence of RGS. It has been postulated that RGS proteins should be negatively regulated (5), but such mechanisms have not been uncovered. In this report we demonstrate that the nucleotide-dependent interaction of RGS7 with Gα subunit Gαo is abolished in the presence of Gβ5. The inhibition of the RGS-Gα interaction occurs because of the binding of Gβ5 to RGS and not to Gα. For RGS6, 7, 9, and 11, this mechanism could explain why, despite their high potency as GAPs, the signals still could be passed onto the appropriate effectors. In the presence of Gβ5, the RGS-Gα interaction can be attenuated, allowing the G protein to function longer. More experimentation is needed to explore whether the interaction of Gβ5 with RGS proteins, in turn, can be regulated by specific signals, for instance, those affecting the status of Gβ5.
Interestingly, our results with RGS7 (Fig. 5) apparently differ with the findings of Snow et al. (18), who showed that the Gβ5-RGS11 complex has GAP activity and, hence, interacts with Gαo. It is possible that RGS7 and RGS11 are regulated by distinct mechanisms or that the disagreement in the results could be a result of differences in the functional assays that were used. However, it seems more likely that the main reason for the apparent difference might be that we studied the full-size RGS7 whereas Snow et al. used a mutant that lacked the DEP domain (RGS11ΔD). RGS11ΔD in the absence of Gβ5 or the full-length RGS11 were not studied. We thus can speculate that the inhibition of RGS-Gα binding (and, hence, the RGS-GAP activity) by Gβ5 requires the presence of the DEP domain.
The majority of our reconstitution experiments were carried out with Gβ5, RGS7, and Gαo, but similar results also were observed with Gβ5L and RGS9 (data not shown). In light of the findings with RGS11 (18), the Gβ5-RGS interaction appears to be a common phenomenon. Because Gβ5 and the RGS proteins 6, 7, 9 (26–28), and 11 (18) are expressed predominantly in the central nervous system, this mechanism is likely to be specific for signaling in neurons. The Gγ-like domain also is present in EGL-10, which is found in C. elegans together with a Gβ5 subunit (GenBank accession no. Q206636), suggesting that mechanisms based on the Gβ5-RGS interaction arose early in evolution.
Acknowledgments
We thank Dr. T. Wensel (Baylor College) for providing us with the RGS7 and RGS9 cDNA clones. We also thank Dr. M. Ali (Alfa Diagnostics International, San Antonio, TX) for contributing in antipeptide antibody production. This research was supported by grants from the Stanley Glaser Foundation and the Pharmaceutical Research and Manufacturers of America Foundation, and by the Fight For Sight Research to Prevent Blindness America Foundation (to V.Z.S.). J.L.C. is a recipient of an American Heart Association (AHA) Florida Affiliate Inc. Predoctoral Fellowship. D.K.S. is supported by an AHA Florida Affiliate Inc. Postdoctoral Fellowship. V.Z.S. is an AHA Florida Affiliate Inc. Initial Investigator.
ABBREVIATIONS
- RGS
regulator of G protein signaling
- GAP
GTPase-activating protein
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF073920).
References
- 1.Arshavsky V Y, Bownds M D. Nature (London) 1992;357:416–417. doi: 10.1038/357416a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berstein G, Blank J L, Jhon D Y, Exton J H, Rhee S G, Ross E M. Cell. 1992;70:411–418. doi: 10.1016/0092-8674(92)90165-9. [DOI] [PubMed] [Google Scholar]
- 3.Dohlman H G, Thorner J. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 4.Arshavsky V Y, Pugh E N., Jr Neuron. 1998;20:11–14. doi: 10.1016/s0896-6273(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 5.Berman D M, Gilman A G. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 6.Popov S, Yu K, Kozasa T, Wilkie T M. Proc Natl Acad Sci USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faurobert E, Hurley J B. Proc Natl Acad Sci USA. 1997;94:2945–2950. doi: 10.1073/pnas.94.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snow B E, Hall R A, Krumins A M, Brothers G M, Bouchard D, Brothers C A, Chung S, Mangion J, Gilman A G, Lefkowitz R J, Siderovski D P. J Biol Chem. 1998;273:17749–17755. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- 9.Kozasa T, Jiang X, Hart M J, Sternweis P M, Singer W D, Gilman A G, Bollag G, Sternweis P C. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera J L, de Freitas F, Satpaev D K, Slepak V Z. Biochim Biophys Res Commun. 1998;249:898–902. doi: 10.1006/bbrc.1998.9218. [DOI] [PubMed] [Google Scholar]
- 11.Watson J A, Katz A, Simon M I. J Biol Chem. 1994;269:22150–22156. [PubMed] [Google Scholar]
- 12.Watson J A, Aragay A M, Slepak V Z, Simon M I. J Biol Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Coso O A, Lee C, Gutkind J S, Simonds W F. J Biol Chem. 1996;271:33575–33579. doi: 10.1074/jbc.271.52.33575. [DOI] [PubMed] [Google Scholar]
- 14.Bayewitch M L, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds W F, Vogel Z. J Biol Chem. 1998;273:2273–2276. doi: 10.1074/jbc.273.4.2273. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher J E, Lindorfer M A, DeFilippo J M, Yasuda H, Guilmard M, Garrison J C. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 16.He W, Cowan C W, Wensel T G. Neuron. 1998;20:95–102. doi: 10.1016/s0896-6273(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 17.Koelle M R, Horvitz H R. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 18.Snow B E, Krumins A M, Brothers G M, Lee S F, Wall M A, Chung S, Mangion J, Arya S, Gilman A G, Siderovski D P. Proc Natl Acad Sci USA. 1998;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt C J, Neer E J. J Biol Chem. 1991;266:4538–4544. [PubMed] [Google Scholar]
- 20.Schmidt C J, Thomas T C, Levine M A, Neer E J. J Biol Chem. 1992;267:13807–13810. [PubMed] [Google Scholar]
- 21.Berman D M, Kozasa T, Gilman A G. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 22.Clapham D E, Neer E J. Nature (London) 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 23.Neer E, Smith T F. Cell. 1996;84:175–178. doi: 10.1016/s0092-8674(00)80969-1. [DOI] [PubMed] [Google Scholar]
- 24.Ford C E, Skiba N P, Bae H, Daaka Y, Reuveny E, Shekter L R, Rosal R, Weng G, Yang C S, Iyengar R, et al. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 25.Neer E J, Schmidt C J, Nambudripad R, Smith T F. Nature (London) 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 26.Gold S J, Ni Y G, Dohlman H G, Nestler E J. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepperl D J, Shah-Basu S, VanLeeuwen D, Granneman J G, MacKenzie R G. Biochem Biophys Res Commun. 1998;243:52–55. doi: 10.1006/bbrc.1997.8056. [DOI] [PubMed] [Google Scholar]
- 28.Thomas E A, Danielson P E, Sutcliffe J G. J Neurosci Res. 1998;52:118–124. doi: 10.1002/(SICI)1097-4547(19980401)52:1<118::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Ponting C P, Bork P. Trends Biochem Sci. 1996;21:245–246. [PubMed] [Google Scholar]
- 30.Yu B, Slepak V Z, Simon M I. J Biol Chem. 1997;272:18015–18021. doi: 10.1074/jbc.272.29.18015. [DOI] [PubMed] [Google Scholar]