Abstract
Francisella tularensis is a facultative intracellular bacterium capable of inducing apoptosis in murine macrophages. Here we analyzed the pathway leading to apoptosis in the murine macrophage-like cell line J774A.1 after infection with F. tularensis strain LVS (named LVS for live vaccine strain). We obtained evidence that the infection affected the mitochondria of the macrophages, since it induced release of the mitochondrial molecule cytochrome c into the cytosol and changed the potential over the mitochondrial membrane. Moreover, activation of caspase 9 and the executioner caspase 3 was also observed in the LVS-infected J774A.1 macrophages. The activated caspase 3 degraded poly(ADP-ribose) polymerase (PARP). All of these events were observed within 9 to 12 h after the initiation of infection, and maximum degradation of a synthetic caspase 3 substrate occurred at 18 h. The internucleosomal fragmentation and PARP degradation resulting from activation of this apoptotic pathway was prevented by the caspase 3 inhibitor Z-DEVD-fmk. No involvement of caspase 1, caspase 8, Bcl-2, or Bid was observed. Thus, the F. tularensis infection induces macrophage apoptosis through a pathway partly resembling the intrinsic apoptotic pathway.
Apoptosis is an evolutionarily conserved form of cell death (41) that depends upon the successive activation of a family of cysteine proteases (caspases) and occurs when a cell receives any of a variety of death signals (2, 14). Two main apoptotic pathways have been identified: an intrinsic (mitochondrial) pathway and an extrinsic (death receptor-mediated) pathway (2, 21). The intrinsic pathway engages the mitochondria to integrate different proapoptotic signals resulting from, for example, developmental programs, environmental stimuli, or senescence (11). It requires the release of cytochrome c from the mitochondrial intermembrane space to the cytosol (18, 25), and this release is a key event in the formation of the apoptosome consisting of cytochrome c, the apoptotic protease-activating factor 1, and procaspase 9 (2). This assembled complex leads to the autoactivation of procaspase 9, which in turn may activate effector caspases, such as caspase 3, 6, and/or 7 (36).
The extrinsic pathway is initiated via death receptor ligation and the subsequent formation of the death-inducing signaling complex that ultimately activates caspase 8 (21, 28). Depending on the cellular context, caspase 8 can directly activate caspase 3 to initiate degradation of a wide variety of substrates without mitochondrial involvement (2, 32), or it can cleave Bid, one of the proapoptotic Bcl-2 family members, to its truncated form which facilitates release of cytochrome c from mitochondria (12, 24). Regardless of how apoptosis is induced, the terminal events are usually similar, i.e., chromatin fragmentation, poly(ADP-ribose) polymerase (PARP) cleavage, and nuclear hypercondensation (33).
Numerous studies have shown that facultative intracellular bacteria may induce apoptosis in many types of host cells (for recent reviews, see references 9, 19, 29, and 42). The time span between the initiation of infection and onset of apoptosis varies for each pathogen, which probably reflects the diversity of the pathogenicity mechanisms that are involved in a given type of infection. For example, Salmonella has developed unique means to induce rapid or delayed macrophage cell death, with each method involving pathways leading to caspase activation (15, 17, 29, 31, 40). Legionella pneumophila and Yersinia, on the other hand, induce macrophage apoptosis from an extracellular location by activating caspase 8 and caspase 3 (4, 8, 30).
Francisella tularensis is a highly virulent, facultative intracellular bacterium and is the etiological agent of the zoonotic disease tularemia (7, 38). Previous studies have demonstrated that the bacterium survives in intracellular vacuoles and is capable of preventing the fusion of phagosomes and lysosomes (1), but little is known about how the bacterium survives and ultimately kills host cells (34). In a recent study, we have shown that although there is little or no intracellular F. tularensis multiplication during the first 12 h of infection, rapid bacterial proliferation ensues thereafter (23). Concomitantly with this late rapid bacterial multiplication, signs of apoptosis can be detected in the infected J774A.1 macrophage-like cells (23). Likewise, the F. tularensis infection leads to similar cytopathogenic effects in murine peritoneal exudate cells and RAW264.7 macrophages (unpublished data). In the present study, we explored the molecular mechanisms leading to host cell death.
MATERIALS AND METHODS
Bacterial strain and growth condition.
The F. tularensis LVS strain was supplied by the U.S. Army Medical Research Institute of Infectious Diseases (Fort Detrick, Frederick, Md.) and stored at −70°C. For each experiment, LVS bacteria from a fresh culture on modified Thayer-Martin agar (6) were cultivated overnight at 37°C in liquid Chamberlain medium (3), pelleted by centrifugation, resuspended in Ham's F-10 medium, and added to J774A.1 macrophage cell cultures. The number of bacterial CFU was determined retrospectively by counting the colonies on agar plates. F. tularensis bacteria were inactivated by formalin treatment (10% for 40 min) and added to the cell monolayer after the monolayer was washing with cell culture medium three times. The bactericidal effect of the fixation was verified by plating.
Infection of J774A.1 macrophages with F. tularensis.
The murine macrophage cell line J774A.1 (American Type Culture Collection, Manassas, Va.) was cultivated in Ham's F-10 medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum and 100 U of penicillin ml−1 at 37°C with 5% CO2. One day before infection with bacteria, cells were released by scraping and seeded into new wells using antibiotic-free medium. Preliminary analyses indicated that a multiplicity of infection (MOI) of 500 was needed to ensure that the majority of cells were infected (detectable F. tularensis in >90% of the cells), whereas only 15 to 20% of the cells contained bacteria at an MOI of 50. After overnight incubation, cultures of J774A.1 cells were established, the cell medium was removed, and Ham's F-10 medium containing F. tularensis bacteria was added to cell cultures at a designated MOI of 500 (time zero). After 2 h of incubation with F. tularensis bacteria, the cells were washed and incubated in Ham's F-10 medium with 10 μg of gentamicin (Gibco-BRL) ml−1. Under the experimental conditions used, this concentration of gentamicin has been found not to affect the intracellular replication of F. tularensis. In some experiments, cells were treated with 1 μg of cytochalasin D (Sigma, Madison, Wis.) ml−1 for 30 min prior to infection, during the 2-h incubation with F. tularensis, and for another hour after washes. The infected cells were incubated until the time points specified.
Subcellular fractionation.
Cells were scraped, collected, and resuspended in phosphate-buffered saline (PBS). The washed cell pellet was resuspended in reticulocyte standard buffer (RSB) (100 mM Tris-HCl [pH 7.5], 10 mM NaCl, 1.5 mM MgCl2) containing mini-complete protease inhibitor cocktail (Roche Molecular Biochemicals). The cells were then passed 10 times through a 26-gauge needle, and the cytoplasm was separated from the nuclei by centrifugation at 4,000 × g at 4°C for 10 min (10).
Measurements of mitochondrial permeability transition.
Following infection, approximately 106 J774A.1 cells were mixed with 5 μg of Mitosensor reagent in buffer (ApoAlert mitochondrial membrane sensor kit; Clontech Laboratories, Palo Alto, Calif.) by vortexing and then incubated at 37°C for 30 min. Cells were then washed and resuspended in incubation buffer and immediately analyzed by flow cytometry using a Becton-Dickinson FacScan machine. The Mitosensor reagent is taken up in the mitochondria of healthy cells and exhibits red fluorescence, whereas it remains in the cytoplasm of apoptotic cells in a monomeric form and exhibits green fluorescence.
Immunoblot analysis.
Uninfected or infected J774A.1 macrophages were collected, pelleted by centrifugation at 2,000 rpm (centrifuge model Z200A; Hermle Labortechnik GmbH, Wechingen, Germany) for 6 min, washed once, and resuspended in cold PBS buffer containing protease inhibitors (aprotinin [15 μM], leupeptin [20 μM], and pepstatin [15 μM]; Sigma). Equivalent amounts of proteins from whole-cell lysates of the same preparation batch were mixed with an equal volume of 2× Laemmli sample buffer, boiled, and resolved by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. After electrophoresis, the gels were blotted onto a 0.2-μm-pore-size polyvinylidene difluoride membrane with a semidry blotter (C.B.S. Scientific Co.). The membrane was blocked in PBS containing Tween and 5% milk powder overnight at room temperature and probed with the following antibodies separately: murine polyclonal antiserum raised against cytochrome c (BD PharMingen), Bcl-2, and Bcl-X (Transduction Laboratory); rabbit antiserum against poly(ADP-ribose) polymerase (PARP) (Roche Molecular Biochemicals), caspase 1 (Santa Cruz Biotechnology, Santa Cruz, Calif.), or caspase 9 (Cell Signaling Technology, Beverly, Mass.); or goat antisera against caspase 3 and Bid (Santa Cruz Biotechnology). Primary antibody binding was detected with horseradish peroxidase-conjugated donkey anti-goat immunoglobulin G (IgG) (caspase 3 and Bid), donkey anti-rabbit IgG (PARP, caspase 1, and caspase 9), sheep anti-mouse IgG (cytochrome c, Bcl-2, and Bcl-X) and visualized by using the enhanced chemiluminescence ECL Plus kit according to the manufacturer's instructions (Amersham Pharmacia Biotech). After blotting, gels were visualized by Coomassie R-250 blue staining to check the blotting efficiency and indirectly check the loading amount of protein samples. Films and dried polyacrylamide gels were scanned and imported into Adobe Photoshop.
Caspase activity assay.
Infected and uninfected J774A.1 macrophages were collected, washed with ice-cold PBS, and resuspended in 50 μl of lysis buffer (colorimetric CaspACE assay system; Promega). Complete lysis was performed by three rounds of freeze-thawing, and the supernatant fraction was collected after centrifugation at 13,000 rpm (Biofuge Heraeus; Kendro Products GmbH, Hanau, Germany) for 20 min. Caspase 3 and 8 activity was measured according to the instructions of the manufacturer by using acetyl-Asp(methyl ester [OMe])-Glu(OMe)-Val-Asp(OMe)-p-nitroaniline (Ac-DEVD-pNA) and Ac-IETD-pNA (colorimetric CaspACE assay system; Promega), respectively.
Caspase 3 inhibition assay.
Cells were treated with a 100 μM concentration of the caspase 3 inhibitor Z-DEVD-fmk or Z-IETD-fmk (Kamiya Biochemical Company, Seattle, Wash.) for 90 min before incubation with bacteria, and the inhibitor was kept in the cell medium after the addition of bacteria and after washing of cells.
DNA fragmentation.
J774A.1 macrophages (5 × 106 to 1 × 107) in 100-mm-diameter petri dishes were infected, and DNA was extracted by a previously described protocol for extraction of eukaryotic DNA (22) with some modifications. The collected cells together with their supernatants were treated with 1 ml of lysis buffer (1% NP-40 in 20 mM EDTA-50 mM Tris-HCl [pH 7.5]) at 37°C for 1 h. The lysates were first concentrated with 2-butanol and then extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol) before precipitation with ethanol plus 1/10 volume of 3 M sodium acetate (pH 4.6) and 1/100 volume of 1 M magnesium chloride. The precipitates were dried and solubilized in sterile water. Electrophoresis was performed in a 1.5% agarose gel, and the bands were visualized by UV light after ethidium bromide staining and then photographed.
Growth kinetics and infectivity of F. tularensis in J774A.1 macrophages.
F. tularensis infections were performed in triplicate wells in 24-well plates containing 2 × 105 J774A.1 cells per well. The number of F. tularensis in the monolayers was determined by lysis of cells with PBS-buffered 0.1% sodium deoxycholate solution for 2 min and plating of 10-fold dilutions on agar plates. To determine the number of intracellular bacteria at various MOIs, an immunofluorescence technique has previously been applied (39). At an MOI of 500, it was shown that a mean of 95% of J774 cells contained intracellular bacteria, and by plating, it was determined that, on average, each cell contained 2.1 bacteria after washing.
RESULTS AND DISCUSSION
F. tularensis-induced apoptosis of J774A.1 macrophages involves release of mitochondrial cytochrome c and perturbation of the mitochondrial membrane potential but does not involve Bid or caspase 8.
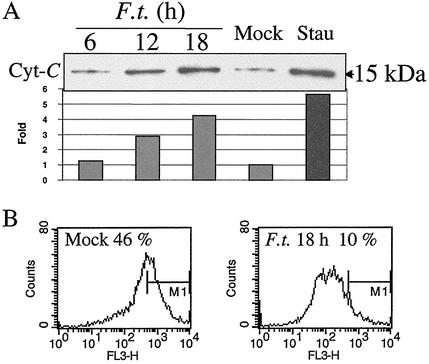
We previously demonstrated that F. tularensis induces apoptosis in murine macrophages in a time- and infection dose-dependent manner (23). To further dissect the molecular mechanisms of F. tularensis-mediated apoptosis in J774A.1 macrophages, we examined the possible involvement of early events of the intrinsic or extrinsic apoptotic pathways by time course experiments. Since the release of cytochrome c from the mitochondria to the cytosol is an early step in the intrinsic apoptotic pathway (43), we measured the levels of cytochrome c in the cytosolic fractions of macrophages by Western blot analysis. The level of released cytochrome c in F. tularensis-infected J774A.1 macrophages was similar to the level in uninfected cells at 6 h postinfection, increased gradually after 12 h, and reached its peak at 18 h (fourfold higher), which was similar to the level in cells treated with an inducer of apoptosis, staurosporine (Fig. 1A).
FIG. 1.
Release of cytochrome c and perturbation of the mitochondrial membrane potential in F. tularensis-infected J774A.1 macrophage cells. (A) Cytochrome c (Cyt-C) was enriched in cytoplasmic preparations from F. tularensis-infected J774A.1 macrophages. J774A.1 macrophages were infected for 2 h with F. tularensis LVS (F.t.) at an MOI of 500, and at indicated time points, the cytoplasmic portions were analyzed for cytochrome c by immunoblotting. Positive-control cells were treated with 1 μg of staurosporine (Stau) ml−1 for 12 h, and a sample of uninfected cells (Mock) were prepared at 18 h. Relative band intensity was analyzed using a Gel-Pro analyzer (version 3.1; Media Cybernetics L.P., Silver Spring, Md.), and the mock band level was set at 1.0. (B) Mitochondrial membrane potential was disturbed in infected macrophage cells. Eighteen hours after infection, cells were collected and stained with Mitosensor dye to measure mitochondrial membrane potential. Fluorescence-activated cell sorting analysis showed that only 10% of infected cells exhibited intense red fluorescence compared to uninfected cells (46%). FL3-H, red fluorescence intensity; M1, quantification marker of strongly fluorescent cells.
Another effect of mitochondrial involvement in induction of apoptosis is the perturbation of the mitochondrial transmembrane potential (27). We measured this potential by incubating samples with a Mitosensor dye which will be taken up and exhibit red fluorescence by cells with a normal mitochondrial membrane potential. No significant change of the membrane potential was observed in infected cells at 6 or 12 h after infection (data not shown), whereas the potential was significantly decreased at 18 h (Fig. 1B). At this time point, only 10% of F. tularensis-infected J774A.1 macrophages were Mitosensor dye positive compared to 46% in uninfected cells.
Caspase 8 has a central role in the death receptor-mediated extrinsic apoptotic pathway (2); therefore, we measured caspase 8 activity in J774A.1 macrophages by using a caspase 8-specific substrate, Ac-IETD-pNA. No increase of substrate degradation was observed at 6, 12, or 18 h after infection compared to the positive-control cells treated with tumor necrosis factor alpha and cycloheximide, and in fact, levels were lower than those of uninfected macrophages (data not shown).
Bid bridges the extrinsic pathway with its intrinsic, mitochondrial counterpart when caspase 8 activation is insufficient (24). There was no truncated form of Bid present in samples from F. tularensis-infected J774A.1 cells at 6, 12, or 18 h after infection (data not shown). Recent studies suggest that Bcl-2 family members may play a role in cytochrome c release (11, 20). To this end, we checked the expression of Bcl-2 and Bcl-x, but the levels of these two proteins in F. tularensis-infected J774A.1 macrophages did not differ from those of uninfected controls by Western blot analysis (data not shown).
Collectively, these results demonstrate that mitochondria are involved in F. tularensis-induced apoptosis, as evidenced by the release of cytochrome c and a change in the transmembrane potential. The immediate upstream signal(s) for inducing mitochondrial changes remains to be identified, but it does not involve Bid, Bcl-2, Bcl-x, or caspase 8. Therefore, we find no evidence that the death receptor-mediated extrinsic apoptotic pathway is involved in F. tularensis-induced apoptosis.
F. tularensis-induced apoptosis in J774A.1 macrophages results in activation of caspase 9 and 3 and degradation of PARP.
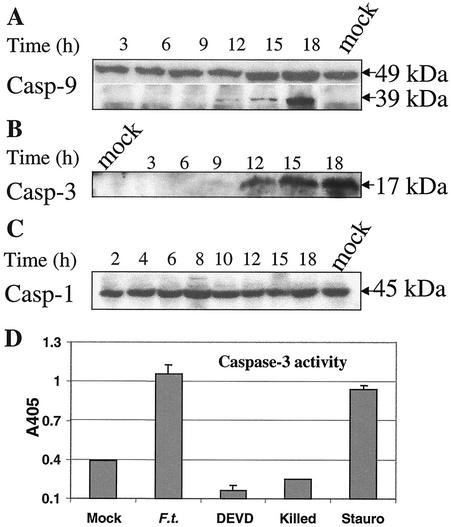
The release of mitochondrial cytochrome c into the cytosol appears to be a critical event in recruiting the apoptotic protease-activating factor 1 and procaspase 9 that, together with cytochrome c, form the apoptosome complex; formation of the apoptosome complex in turn results in activation of caspase 9 (26). Thus, we monitored the cleavage of procaspase 9 by Western blot analysis. The 39-kDa cleaved product of procaspase 9 was first observed at 12 h after infection, and the amount of this cleaved fragment increased at 15 and 18 h (Fig. 2A).
FIG. 2.
(A to C) Kinetic analysis of caspase activation from proteolysis in J774A.1 macrophages after Francisella infection. Cells were infected and collected, and cell extracts were prepared at the indicated time points (in hours; indicated over the blots) and analyzed by Western blot analysis. (A) Involvement of caspase 9 (Casp 9) in the F. tularensis-induced apoptotic pathway of J774A.1 macrophages. The 39-kDa fragment represents the cleaved product. (B) Cleavage of procaspase 3 into active caspase 3 in F. tularensis-infected J774A.1 macrophages. Cell lysates were analyzed for cleavage of procaspase 3 using immunoblots with anti-caspase 3 antibodies. (C) No detectable cleavage of caspase 1 in infected J774A.1 macrophages. (D) Caspase 3 activity in F. tularensis-infected cells. Positive-control cells were treated with 1 μg of staurosporine (Stauro) ml−1 for 12 h. The other samples were processed at 18 h. The cells were treated as follows: not infected (Mock), infected with F. tularensis (F.t.), infected plus treated with Ac-DEVD-DEVD (fluoromethyl ketone [fmk]), and treated with killed F. tularensis (Killed). Values are the means ± standard deviations (error bars) from three samples (error bars of mock-infected cells and cells treated with killed F. tularensis not visible).
A hierarchy of caspase activation events occurs subsequent to caspase 9 activation (5, 26, 35, 36). Accordingly, we analyzed the involvement of a key executioner caspase, caspase 3, in the apoptotic pathway. The cleaved product of the 32-kDa procaspase 3, a 17-kDa protein, was faintly detected at 9 h postinfection using Western blot analysis and accumulated thereafter in samples of F. tularensis-infected J774A.1 macrophages (Fig. 2B).
We also examined the possibility of any involvement of the proinflammatory caspase 1, since it is critical for the host cell death observed in experimental Shigella or Salmonella infection models (15, 16, 29). However, no cleavage of procaspase 1 was observed in the F. tularensis-infected J774A.1 cells (Fig. 2C).
To further corroborate the involvement of caspase 3 and examine the level of its activity, we monitored cleavage of a synthetic caspase 3 substrate, Ac-DEVD-pNA, in F. tularensis-infected cells. Cell lysates were prepared at different time points and incubated with the substrate. Time course experiments revealed that the increase of caspase 3 activity in infected J774A.1 macrophages was detectable at 9 h after infection and became more pronounced thereafter, whereas the activity remained at the basal level before this time point (data not shown). Its activity in infected cells at a late time point (18 h) was as high as that of the positive-control cells treated with the known apoptosis inducer staurosporine (Fig. 2D). Activation of caspase 3 occurred only after internalization of viable F. tularensis bacteria, since phagocytosis of killed F. tularensis did not activate caspase 3 (Fig. 2D) and cytochalasin D treatment decreased the level of its activity after infection (data not shown).
Activation of caspases often leads to degradation of various substrates (14, 37), and PARP is a substrate crucial for cell death (13). The cleaved 89-kDa fragment of PARP became evident at 12 h and increased thereafter (data not shown).
Taken together, a critical role for caspase 3 was identified in F. tularensis-induced macrophage apoptosis. Its importance was further corroborated by the demonstration that the caspase 3 inhibitor Ac-DEVD-fmk prevented the elevation of caspase 3 activity (Fig. 2D) and inhibited DNA fragmentation and PARP degradation in the infected cells (data not shown). The addition of the inhibitor did not affect the release of cytochrome c as determined by Western blot analysis (data not shown). This indicates that caspase 3 activation is an event subsequent to the release of cytochrome c.
Host cell apoptosis results from infections caused by several bacterial pathogens, but the molecular pathways and kinetics of F. tularensis-induced apoptosis appear to be distinct from those of other pathogens. For example, the lack of involvement of caspase 1 distinguishes it from the macrophage cell death pathway induced by Shigella or Salmonella infection (15, 16). Macrophage apoptosis induced by Yersinia enterocolitica results in a downstream cascade similar to that induced by F. tularensis, but it involves the truncation of Bid and can be inhibited by use of the pan-caspase inhibitor VAD-fmk, but not DEVD-fmk (4). Unlike F. tularensis, L. pneumophila induces macrophage apoptosis by an extracellular mechanism that leads to activation of caspase 8 and caspase 3 (8, 30).
We previously demonstrated that F. tularensis induces apoptosis in murine macrophages in a time- and infection dose-dependent manner (23). In the present study, we show that the initial activation of apoptosis occurred 9 to 12 h after the uptake of bacteria. This rather long delay suggests that apoptosis is triggered by intracellularly located bacteria and that direct binding of F. tularensis or other effects mediated by extracellular bacteria are not the initiating event for apoptosis induction. The early apoptotic events include release of cytochrome c into the cytosol, perturbation of the mitochondrial potential, and subsequent activation of caspase 9 and caspase 3. This indicates that the pathway of F. tularensis-induced apoptosis involves the mitochondria and activation of a downstream caspase cascade, particularly the activation of the executioner caspase 3, thereby resembling the intrinsic apoptotic pathway.
Acknowledgments
This study was supported in part by grants from the Stiftelsen J. C. Kempes Minnes Foundation, the Defense Advanced Project Agency (DARPA), the Swedish Medical Research Council, and the Medical Faculty, Umeå University, Umeå, Sweden.
Editor: B. B. Finlay
REFERENCES
- 1.Anthony, L. D., R. D. Burke, and F. E. Nano. 1991. Growth of Francisella spp. in rodent macrophages. Infect. Immun. 59:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain, R. E. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 5.Deveraux, Q. L., R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300-304. [DOI] [PubMed] [Google Scholar]
- 6.Eigelsbach, H. T., and C. M. Down. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87:415-425. [PubMed] [Google Scholar]
- 7.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, L. Y., and Y. Abu Kwaik. 1999. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect. Immun. 67:4886-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, L. Y., and Y. A. Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 10.Grandgirard, D., E. Studer, L. Monney, T. Belser, I. Fellay, C. Borner, and M. R. Michel. 1998. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 17:1268-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 12.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 13.Ha, H. C., and S. H. Snyder. 1999. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 96:13978-13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 15.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 17.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 19.Knodler, L. A., and B. B. Finlay. 2001. Salmonella and apoptosis: to live or let die? Microbes Infect. 3:1321-1326. [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer, S. J., M. C. Wei, M. Saito, S. Weiler, K. J. Oh, and P. H. Schlesinger. 2000. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7:1166-1173. [DOI] [PubMed] [Google Scholar]
- 21.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 22.Lai, X. H., I. Arencibia, A. Johansson, S. N. Wai, J. Oscarsson, S. Kalfas, K. G. Sundqvist, Y. Mizunoe, A. Sjostedt, and B. E. Uhlin. 2000. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect. Immun. 68:4363-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai, X. H., I. Golovliov, and A. Sjostedt. 2001. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect. Immun. 69:4691-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 25.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 26.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 27.Marzo, I., C. Brenner, N. Zamzami, S. A. Susin, G. Beutner, D. Brdiczka, R. Remy, Z. H. Xie, J. C. Reed, and G. Kroemer. 1998. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 187:1261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monack, D. M., W. W. Navarre, and S. Falkow. 2001. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 3:1201-1212. [DOI] [PubMed] [Google Scholar]
- 30.Neumeister, B., M. Faigle, K. Lauber, H. Northoff, and S. Wesselborg. 2002. Legionella pneumophila induces apoptosis via the mitochondrial death pathway. Microbiology 148:3639-3650. [DOI] [PubMed] [Google Scholar]
- 31.Santos, R. L., R. M. Tsolis, A. J. Baumler, R. Smith III, and L. G. Adams. 2001. Salmonella enterica serovar Typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms. Infect. Immun. 69:2293-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459-470. [DOI] [PubMed] [Google Scholar]
- 34.Sjöstedt, A. 2003. Virulence determinants and protective antigens of Francisella tularensis. Curr. Opin. Microbiol. 6:1-6. [DOI] [PubMed] [Google Scholar]
- 35.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, and E. S. Alnemri. 1998. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1:949-957. [DOI] [PubMed] [Google Scholar]
- 37.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 38.Tarnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 39.Telepnev, M., I. Golovliov, T. Grundstrom, A. Tarnvik, and A. Sjostedt. 2003. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell. Microbiol. 5:41-51. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden, A. W., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaux, D. L., and A. Strasser. 1996. The molecular biology of apoptosis. Proc. Natl. Acad. Sci. USA 93:2239-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 43.Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. I. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129-1132. [DOI] [PubMed] [Google Scholar]