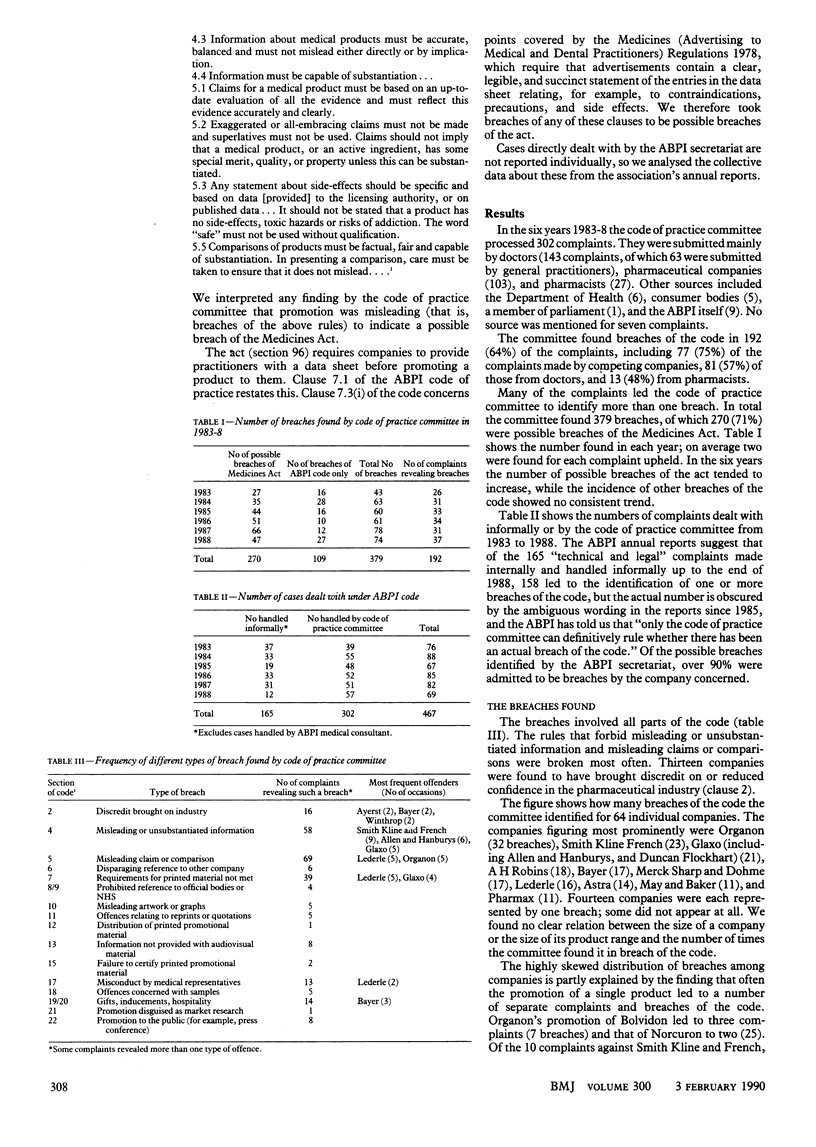
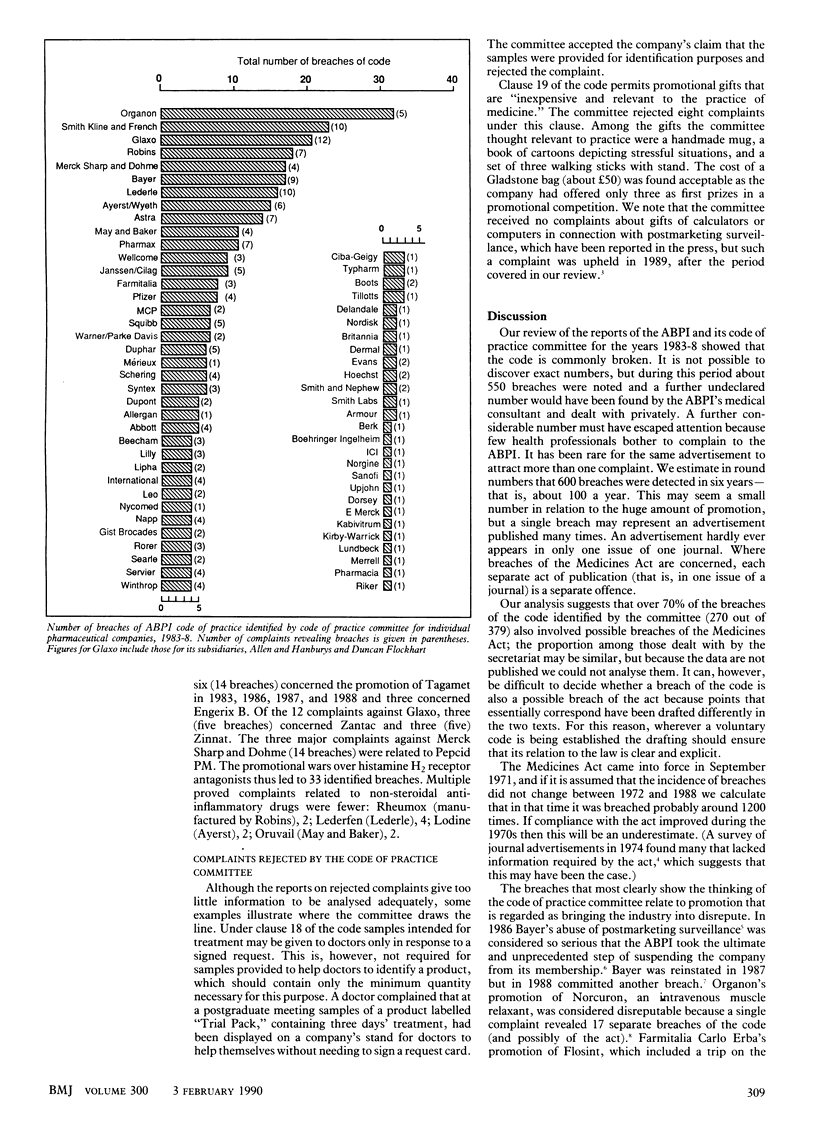
Abstract
Since 1958 the Association of the British Pharmaceutical Industry (ABPI) has attempted to regulate the promotion of prescription medicines through its code of practice. This regulation is described and analysed for the six years 1983-8 using the reports on 302 complaints considered by its code of practice committee and annual reports. The complaints came mainly from doctors (143, 48%) and competing companies (103, 33%). The committee found a total of 379 breaches of the code in 192 (63%) of the complaints. Additional breaches were detected by informational scrutiny of advertisements by the ABPI secretariat. Analysis showed that 270 (71%) of these breaches involved possible breaches of the Medicines Act. The rules that forbid misleading or unsubstantiated information and misleading claims or comparisons were broken most often. The committee found the most frequent offenders to be Organon (32 breaches), Smith Kline and French (23), Glaxo (21), A H Robins (18), Bayer (17), Merck Sharp and Dohme (17), and Lederle (16). Often the promotion of one product led to several breaches. The promotional wars over histamine H2 receptor antagonists accounted for 33 breaches. It is estimated that in 1983-8 about 100 breaches of the code were detected a year. In the 18 years 1972-88 the Medicines Act was breached probably over 1200 times. Health ministers, by not enforcing the regulations controlling promotion, have abrogated their responsibility to the ABPI, but the evidence suggests that the code has failed to deter promotional excesses. The ABPI's wish to secure compliance with the code seems weaker than its wish to pre-empt outside criticism and action: its self regulation seems to be a service to itself rather than to the public. It is suggested that the code of practice committee should become publicly accountable, that the majority of its members should represent the health professions and the public, and that effective sanctions are needed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier J., Herxheimer A. Medicines Act passes crucial test. Lancet. 1988 Jun 11;1(8598):1349–1349. doi: 10.1016/s0140-6736(88)92172-1. [DOI] [PubMed] [Google Scholar]
- Hayward R. A., Shapiro M. F., Oye R. K. Laboratory testing on cerebrospinal fluid. A reappraisal. Lancet. 1987 Jan 3;1(8523):1–4. doi: 10.1016/s0140-6736(87)90698-2. [DOI] [PubMed] [Google Scholar]
- Stimson G. V. Information contained in drug advertisements. Br Med J. 1975 Nov 29;4(5995):508–509. doi: 10.1136/bmj.4.5995.508. [DOI] [PMC free article] [PubMed] [Google Scholar]


