Abstract
Recessive N-ethyl-N-nitrosourea (ENU)-induced mutations recovered at the fitness-1 (fit1) locus in mouse chromosome 7 cause hematopoietic abnormalities, growth retardation, and shortened life span, with varying severity of the defects in different alleles. Abnormal iron distribution and metabolism and frequent scoliosis have also been associated with an allele of intermediate severity (fit14R). We report that fit14R, as well as the most severe fit15R allele, are nonsense point mutations in the mouse ortholog of the human phosphatidylinositol-binding clathrin assembly protein (PICALM) gene, whose product is involved in clathrin-mediated endocytosis. A variety of leukemias and lymphomas have been associated with translocations that fuse human PICALM with the putative transcription factor gene AF10. The Picalmfit1–5R and Picalmfit1–4R mutations are splice-donor alterations resulting in transcripts that are less abundant than normal and missing exons 4 and 17, respectively. These exon deletions introduce premature termination codons predicted to truncate the proteins near the N and C termini, respectively. No mutations in the genes encoding Picalm, clathrin, or components of the adaptor protein complex 2 (AP2) have been previously described in which the suite of disorders present in the Picalmfit1 mutant mice is apparent. These mutants thus provide unique models for exploring how the endocytic function of mouse Picalm and the transport processes mediated by clathrin and the AP2 complex contribute to normal hematopoiesis, iron metabolism, and growth.
Characterization of mutations causing anemia and/or iron metabolism/transport defects has proven to be a valuable approach for studying the development and regulation of both the hematopoietic system and body-iron content (e.g., refs. 1–7). Mice hemi-or homozygous for N-ethyl-N-nitrosourea (ENU)-induced mutations at the fit1 locus have shortened life span, severe runting, microcytic hypochromic anemia, lowered white blood cell counts, and reduced erythroid and myeloid progenitor cell populations during both fetal and adult hematopoiesis (2, 8). Additional study of the most severe allele, fit15R (formerly fit14397SB), revealed reticulocytosis, extramedullary erythropoiesis in the spleen, iron deposition in the liver, and B cell deficiency (2). Detailed study of an allele of intermediate severity, fit14R (formerly fit14226SB), showed that the anemia was mildly regenerative and demonstrated functional iron deficiency, abnormal iron distribution suggestive of impaired iron transport from the liver to other tissues, biochemical evidence of liver dysfunction, increased myeloid/erythroid ratios in the bone marrow, and scoliosis and lumbar vertebral abnormalities (9–11). The fit1 mutants exhibit alterations in hematopoiesis, iron metabolism, and bone growth, unlike other animal models of inherited microcytic hypochromic anemia (11). Moreover, they may also be a unique model for the study of scoliosis (11).
We report here that both of these fit1 alleles are point mutations in the mouse phosphatidylinositol-binding clathrin assembly protein (Picalm) gene, which encodes an assembly protein involved in adaptor protein complex 2 (AP2)-dependent clathrin-mediated endocytosis in the cell (12–14). The mutations disrupt different splice-donor sites and result in transcripts that are missing exons and are predicted to truncate Picalm proteins. This finding demonstrates that Picalm and likely the clathrinmediated endocytic processes involving it play important roles in hematopoiesis, iron metabolism, and growth.
Materials and Methods
Mice. The origin and initial maintenance of mouse strains carrying the fit14R and fit15R mutations, as well as the generation of mice hemizygous for the fit1 alleles [i.e., Tyrc fit1/Del(Tyr fit1)26DVT, hereafter referred to as fit1/Del26DVT], have been described previously (2, 8, 15, 16). Tyrc-ch +/Del26DVT mice are phenotypically normal (2). The fit1 alleles analyzed in this study were induced on a BALB/cRl chromosome and are maintained as Tyrc fit1 +/Tyrc-ch + fr heterozygotes by mating them each generation to inbred FRCH/R (Tyrc-ch + fr/Tyrc-ch + fr) mice.
Bacterial Artificial Chromosome (BAC) Isolation and Analysis. BAC clone RPCI-23-68G17, derived from a C57BL/6J library (17), was purchased from ResGen/Invitrogen; BAC DNA was isolated for analysis according to the manufacturer's instructions. SSLP markers D7Mit32, D7Mit352, and D7Mit300; STS markers M-09035 and M-02716; and markers for the D7Cwr11D, D7Mlk1 and D7Mlk2 loci, all of which map within the deletions and yeast artificial chromosome (YAC) contigs spanning the fit1 interval (15, 18, 19), were mapped against the BAC by PCR analyses, by using specific primers. SSLP primers were purchased from ResGen/Invitrogen, and the sequences of all other primers used in this study are available (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). BAC DNA was physically sheared and subcloned into plasmids, which were then sequenced (4,028 sequence reads).
RT-PCR Analysis of Picalm cDNA. Total RNA was isolated from various tissues of mice as described (20). First-strand cDNA was synthesized from ≈3 μg of total RNA, by using both oligo-dT and random decamer priming, with the RETROscript Kit (Ambion, Austin, TX), following the manufacturer's instructions. The resulting cDNAs were amplified by PCR with the use of specific primers (see Fig. 3A and Table 1) and, to reduce the incorporation of Taq-induced errors, a 9:1 mixture of Taq (AmpliTaq, Perkin–Elmer) and PfuTurbo (Stratagene) polymerases. The final PCR reaction contained RT cDNA (≈750 ng), specific primers (0.2 μM each), dNTPs (200–250 μM each), and MgCl2 (1.5–1.75 mM) in a total volume of 25–50 μl. In general, 30–35 PCR cycles of denaturation at 94°C for 30 s, annealing at 52–59°C for 30 s, and extension at 72°C for 30–40 s were performed. Some amplifications were performed by including 10 touchdown-PCR cycles before the general cycling conditions.
RT-PCR products either were sequenced directly with the same primers used for the PCR after ExoSAP-IT (United States Biochemical) treatment or were cloned (TOPO-TA Cloning Kit, Invitrogen Life Technologies) and then sequenced with vector primers. DNA sequencing was performed in both forward and reverse directions with the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems), according to the manufacturer's instructions. After ethanol precipitation, the reaction products were analyzed with an ABI 377 sequencing system (Applied Biosystems).
Mutation Analysis. RT-PCR primers were designed, on the basis of cDNA sequence assembled from all of the ESTs, to amplify overlapping ≈500-bp fragments that together spanned the entire Picalm ORF and were predicted, on the basis of the EST assembly, not to be alternatively spliced. Normal and size-altered RT-PCR products obtained from liver RNAs derived from wild-type and mutant mice (three individuals of each) were cloned and sequenced. Nucleotide substitutions in genomic DNA responsible for cDNA alterations were identified by amplifying fragments containing the affected exons and splice junction sequences from 30–60 ng of genomic DNA template, using the same general conditions as described above for RT-PCR, and sequencing the PCR products. To characterize the fit15R mutation, primers 181 and 182, corresponding to sequences in introns 3 and 4, were used to amplify a 388-bp product containing exon 4 and its adjacent splice-site sequences from fit15R/Del26DVT DNA. For the fit14R allele, primers 214 and 215, designed from introns 16 and 17, were used to amplify a 415-bp product containing exon 17 and its flanking splice sites from fit14R/Del26DVT DNA.
Probe Hybridizations. A mixture of the 147/148 and 149/150 RT-PCR products (probe A) was column purified, labeled with ALL-IN-ONE Random Prime DNA Labeling Mix (Sigma), and hybridized to the Northern blot.
Bioinformatic Analyses. blast (genome survey sequence database) analysis (www.ncbi.nlm.nih.gov/BLAST) of the Y6CD3 sequence identified end-sequence from BAC RPCI-23–68G17. The sequence trace data from the BAC were analyzed, assembled, and edited with the phred, phrap, and consed programs (www.phrap.org). Murine ESTs with homology to human and rat PICALM cDNAs (GenBank accession nos. NM_007166, XM_165625, AF041374, and AF041373) were also identified by blast analysis. artemis comparison tool (www.sanger.ac.uk/software/ACT), consed, and blast were used to determine the correct order and orientation of the BAC sequence contigs and the exon–intron boundaries of Picalm by alignment of the BAC sequences with the mouse consensus cDNA sequence made from EST assemblies. Sequence editing of PCR products (and clones of PCR products) and the assembly of the PCR sequences and ESTs (see Table 2, which is published as supporting information on the PNAS web site, for GenBank accession numbers and sources of these ESTs) into contiguous cDNA sequences, were performed with factura and autoassembler software (Applied Biosystems), respectively. Identities and similarities between the mouse and human cDNA and amino acid sequences were determined, and sequence alignments were performed, by analyses with blast and macvector (clustalw alignment) software.
Results
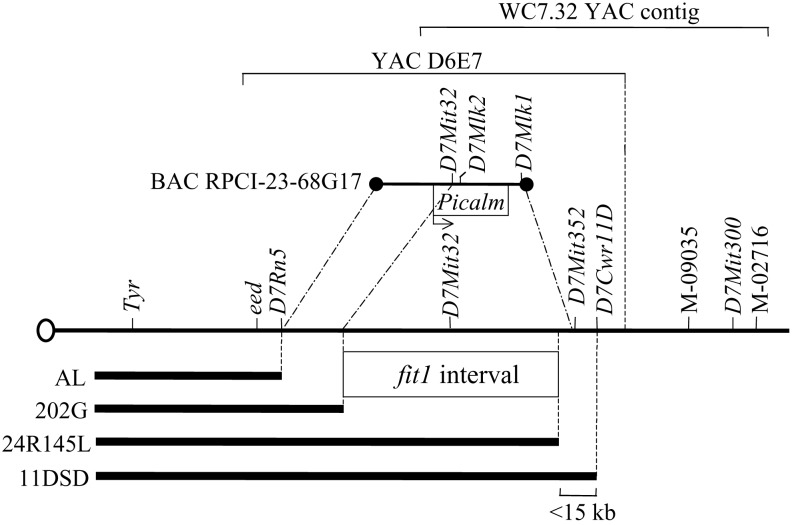
Identification of the Mouse Picalm Gene at the fit1 Locus. We previously (15) used a series of Tyr deletions to define a minimum deletion interval, between the eed and D7Cwr11D loci, that contains fit1, and we identified a YAC contig containing this DNA segment (Fig. 1; ref. 18). In the search for fit1 candidates, two unique-sequence DNA clones that mapped within the minimal deletion interval, Y6CD3 and C58 (from the D7Mlk1 and D7Mlk2 loci, respectively; data not shown), were isolated from YAC clone D6E7 and sequenced. Database searching indicated that part of the Y6CD3 sequence was complementary to end sequence from the BAC clone RPCI-23-68G17. PCR typing determined that the BAC also contains the D7Mlk2 and D7Mit32 markers (data not shown), both of which map within the deletion interval containing fit1 (Fig. 1). The BAC was sequenced, and blast analysis of the sequence revealed the presence of the mouse ortholog of the human PICALM (formerly CALM) gene (12). PICALM maps to HSA chromosome 11q14 and appears to be a ubiquitously expressed paralog of the SNAP91 gene, which encodes the synaptic clathrin-assembly protein AP180 (12, 22). A number of leukemias (both acute lymphocytic and acute myeloid) and lymphomas have been reported in which the human PICALM gene forms fusion transcripts with the putative transcription factor AF10 in HSA chromosome 10 by virtue of t(10, 11) translocations (e.g., refs. 23–25). AP180 and PICALM function in the recruitment of clathrin and AP2 to cell membranes at sites of coated-pit formation and clathrin-vesicle assembly (13, 14, 26, 27).
Fig. 1.
Genetic and physical map of a segment of mouse chromosome 7 containing the fit1 locus. Chromosome 7 is indicated by the line with the oval (depicting the centromere) at its left end. Relative locations of genetic loci (italicized) and STS markers (M-09035 and M-02716) are shown above the chromosome. No physical distance is implied by placement of any of the markers. Bold lines below the chromosome represent Del(Tyr) deletions, and their allele names (21) are shown (Left). The minimal deletion interval containing the fit1 locus (15) is indicated. A YAC contig spanning part of the region is shown above the chromosome [390-kb YAC clone D6E7 (18); WC7.32 contig is described at www.informatics.jax.org/searches/contig.cgi?1251 (660 kb from D7Mit32 to M-02716)]. D7Rn5 and D7Cwr11D are loci derived from the indicated deletion breakpoints. The RPCI-23-68G17 BAC clone (194 kb) containing the Picalm gene (with the transcriptional orientation shown) and several markers is shown above the chromosome. The lines projecting from the ends of the BAC to the chromosome indicate that the BAC is located between, but does not include, the D7Rn5 and D7Mit352 loci. The portion of the BAC between its distal end and the dashed/dotted line extending from the D7Mit32 locus indicates the minimum part of the BAC known to be located within the fit1 interval.
The location of the mouse Picalm gene, and the fact that clathrin/AP2-mediated endocytosis of the transferrin/transferrin–receptor complex from the plasma membrane is the main mechanism of iron uptake into most cells (28, 29), suggested that Picalm was a good candidate for fit1, the only locus associated with severe anemia that was identified in this mutagenized chromosomal region (2, 8). Thus, we first assembled a composite mouse Picalm cDNA sequence of ≈3.6 kb (Fig. 2A and data not shown) from numerous murine ESTs and partial cDNAs that were identified by homology to human and rat PICALM cDNAs (12, 30). blast analysis of the murine ESTs in GenBank also indicated the potential for several alternatively spliced Picalm cDNA isoforms (Fig. 2 A).
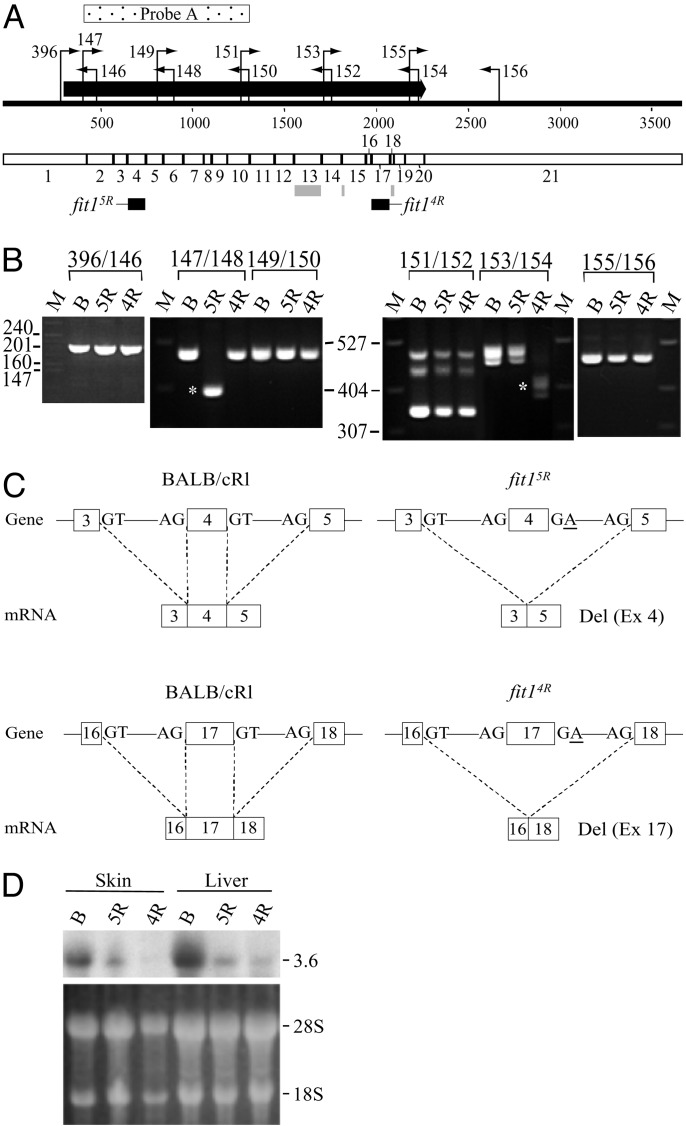
Fig. 2.
Mouse Picalm mRNA map and effects of fit1 mutations on the gene. (A) Map of a composite 3,660-bp mouse Picalm mRNA predicted from the murine ESTs and partial cDNAs homologous to the human and rat PICALM cDNAs. The portion of the mRNA encoding the protein (ORF) is indicated by the thick black arrow above the map. The locations of the primers used in RT-PCR analyses are indicated above the map along with the numeric primer labels. The segment of the cDNA shown by the stippled rectangle above the map (Probe A) was used as a probe on the Northern blot (D). The boundaries of the 21 Picalm exons in the cDNA are shown below the map. Alternatively spliced exons or exon segments in the wild-type gene are designated by the gray rectangles, and the exons that are missing in mRNAs derived from the fit15R and fit14R alleles are indicated by the black boxes below the map. (B) Picalm RT-PCR products separated by agarose gel electrophoresis. The RNA samples analyzed are indicated above each lane: B, BALB/cRl (+/+) control; 5R, fit15R/Del26DVT; 4R, fit14R/Del26DVT. RT-PCR primer pairs are shown above the lane headings. The sizes of the molecular weight marker (M) bands are shown at left for the 396/146 RT-PCR products and in the middle for the other products. Abnormal-sized RT-PCR products obtained from mutant RNAs (asterisks): fit15R, 394-bp 147/148 product; fit14R, 407-, 398-, and 383-bp 153/154 products. The sequence of the 460-bp 151/152 RT-PCR product (the middle band of the three bands obtained) revealed it is derived from another gene (Fpgs) that is unrelated to Picalm except for part of the primer sequences. (C) Summary of the alterations caused by the two N-ethyl-N-nitrosourea (ENU)-induced mutations at the genomic DNA and mRNA levels. Exons are indicated by numbered boxes. The relevant wild-type splice acceptor (AG) and splice donor (GT) sites (Left Upper and Lower) and the mutated splice donor sequences (Right) are shown. The splicing events leading to the mRNA products shown are indicated by the dashed lines. In each case, BALB/cRl represents the wild-type splicing pattern. (D) Hybridized Northern blot (Upper) showing the major ≈3.6-kb Picalm transcript in control and mutant mice. The blot contains total RNAs from the skins and livers of 1-week-old mice with genotypes indicated above each lane, as defined in B. The blot was hybridized with Probe A (A). A loading control (ethidium-bromide-stained RNA after transfer to the membrane) is shown below the autoradiogram (18S and 28S ribosomal RNA bands are indicated). If the level of expression from each mutant fit1 allele was the same as that from the wild-type (BALB/cR1) allele, then the signal intensity of the transcript detected in the 5R and 4R lanes (RNAs from hemizygous mutants) is expected to be only 50% of the signal intensity of the wild-type transcript in the B lane.
RT-PCR Analysis and Sequencing of the Wild-Type Mouse Picalm cDNA. PCR primers based on the consensus composite cDNA sequence predicted from the murine ESTs were used to amplify six overlapping segments of the Picalm cDNA (Fig. 2B) from liver and spleen RNAs derived from BALB/cRl mice, which carry the wild-type fit1 allele. RT-PCR products of expected sizes were obtained with all primer pairs, and one or more additional products were obtained with the 151/152 and 153/154 primer pairs (Fig. 2B), in agreement with the expectation of several different alternatively spliced isoforms of the Picalm mRNA. All of the RT-PCR products were cloned and sequenced, and the sequences were assembled into versions of Picalm cDNA that encode variant forms of the Picalm protein (Figs. 2 A and 3A). The largest potential cDNA assembled from these products, which includes all of the alternatively spliced segments, is a 2.4-kb cDNA sequence containing a complete ORF predicted to encode a 660-aa protein (Fig. 3A). Comparison of the comprehensive ≈3.6-kb cDNA and BAC sequences revealed that the mouse Picalm gene is comprised of 21 exons (Figs. 2 A and 3A; exon sizes and exon–intron junction sequences are shown in Table 3, which is published as supporting information on the PNAS web site).
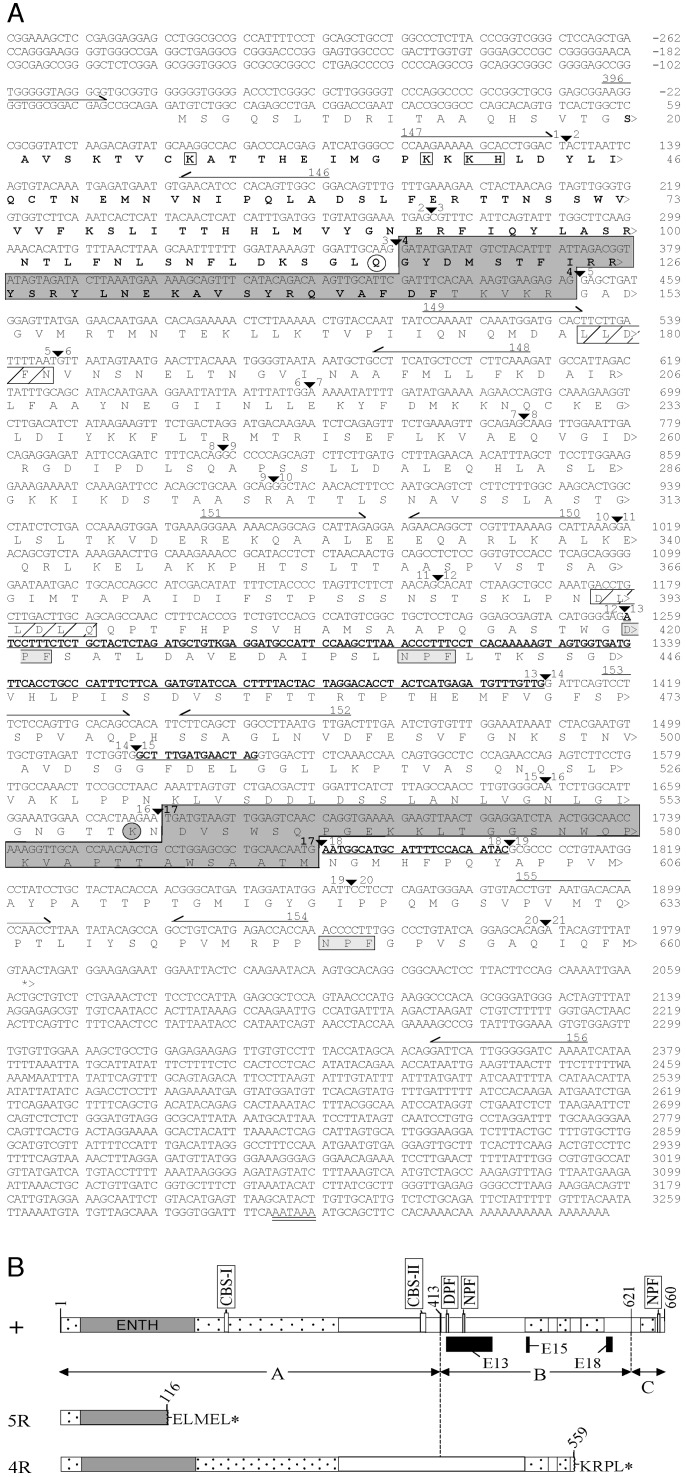
Fig. 3.
Picalm cDNA and proteins. (A) Sequences of the mouse Picalm cDNA and protein [sequence is available in the Third Party Annotation Section of the DNA Data Bank of Japan (DDBJ)/EMBL/GenBank databases under the accession no. TPA: BK001028]. The predicted amino acid sequence of the encoded protein is shown below the cDNA sequence. The cDNA sequence is numbered according to the largest potential cDNA that was assembled from the mouse ESTs/cDNAs homologous to the human and rat cDNAs and includes all of the alternatively spliced segments. The exon junctions are indicated by ▾; the locations of PCR primers in the sequences are shown by arrows above the corresponding sequence. The 2,398 bp of sequence shown between primers 396 and 156 was derived from the inbred control BALB/cRl strain used in this study (GenBank accession no. AY206701) and represents the assembly of the sequences of the different RT-PCR products obtained from BALB/cRl with all six primer pairs (Fig. 2B). The sequences located 5′ and 3′ of this segment are the consensus sequences obtained from an assembly of the mouse ESTs (Table 2) derived from these regions of the cDNA. The sequences of the exons missing in the transcripts produced by the fit15R and fit14R alleles (exons 4 and 17, respectively) are highlighted by dark gray shading, and the last normal amino acid residue of the putative truncated protein encoded by each allele is circled (unshaded and shaded for fit15R and fit14R, respectively). The sequences of the alternatively spliced exons (exons 13 and 18, and the first 15 bp of exon 15) defined in this study are shown underlined and in bold. The polyadenylation signal (AATAAA) is underlined twice. The amino acid residues of the epsin N-terminal homology domain (residues 20–145) are shown in bold, and the amino acids implicated in the binding of membrane PtdIns(4,5)P2 (14) are enclosed by open boxes. Enclosed by light-gray boxes are the amino acid sequence DPF, a motif that is known to interact with the α-adaptin appendage domain of AP2 (27, 31, 32), and two NPF motifs, which interact with protein-binding modules (Eps15 homology domains) found in several proteins that are also part of the endocytic machinery (31, 33). Putative type I and II clathrin-binding sequences (CBS-I and -II) (34) are indicated by the diagonally striped boxes. (B) Full-length and truncated Picalm proteins predicted to be encoded by the wild-type (+) and two mutant transcripts (below, fit1 mutant alleles shown to the left). The largest predicted form of the wild-type protein (660 residues) is shown here and would be produced by incorporation of all three alternatively spliced segments [indicated by the black boxes shown below the schematic of the protein and encoded by exon 13 (E13), exon 18 (E18), and the first 15 bp of exon 15 (E15)]. The number at the end of each truncated protein indicates the amino acid residue at which the normal sequence ends; the amino acid sequences shown to the right of the truncated protein are extra frameshift-induced residues added to the C-terminal end of the protein before a stop codon (asterisk) occurs. Gray-shaded area, epsin N-terminal homology (ENTH) domain; stippled areas, regions of highest homology with AP180 proteins (13). The locations of the DPF and NPF motifs, and the putative type I and II clathrin-binding sequences (CBS-I and -II) in the normal protein are shown. Three different segments of the protein (labeled as A, B, and C) are known to contain distinct sites of interaction with CHC, the major binding partner of PICALM (13). The C-terminal portion from residues 414–660 (segments B and C) has a much stronger interaction with CHC than the N-terminal segment A (residues 1–413); the last 40 residues (segment C) are essential for binding CHC with high affinity (13).
The sequence analyses also showed that the multiple RT-PCR products obtained with the 151/152 and 153/154 primer pairs (Fig. 2B) represented alternatively spliced forms of the transcript in which exons 13 and 18 and the first 15 bp of exon 15 are present in various combinations (Figs. 2 A and 3). The omission of any of these alternatively incorporated cDNA segments in the Picalm transcript (in all possible combinations) still preserves the reading frame, so that all of the possible alternative transcripts can encode the remainder of the C-terminal residues of the protein (Fig. 3A). Due to the different possible transcript forms, the normal protein could range from 597 to 660 aa in length. No ESTs or cDNAs that would encode the largest potential protein were identified in the sequence databases, whereas several that would encode a ≤655-residue protein can be found. Therefore, it is not yet known whether the complete 660-residue protein shown in Fig. 3 is actually produced in vivo. If it is, it is likely a minor product of the gene.
blast analysis of this mouse cDNA sequence identified two human cDNA (GenBank accession nos. NM_007166 and XM_165625) and many EST sequences, which were then assembled into a comprehensive human cDNA sequence that includes all alternatively spliced segments, including the same three regions identified in mouse and five additional regions that are apparently unique to human (sequence alignments shown in Fig. 4, which is published as supporting information on the PNAS web site). blast comparison of the largest potential mouse (Fig. 3A) and human PICALM cDNA sequences revealed an identity of 94.5% in the ORF and 91% overall (Fig. 4). The amino acid sequences of the mouse and human proteins predicted from these cDNAs are 98.5% similar and 97.7% identical, and both include all of the defined functional domains/motifs (amino acid sequence alignments shown in Fig. 5, which is published as supporting information on the PNAS web site), indicating Picalm is highly conserved.
Hybridization of a Picalm cDNA probe (Fig. 2 A) to a Northern blot containing BALB/cRl RNAs identified a predominant transcript of ≈3.6 kb in several tissues (Fig. 2D and data not shown), consistent with the ubiquitously expressed human gene (12). The mouse cDNA predicted from the available ESTs is also ≈3.6 kb in length (Figs. 2 A and 3A), suggesting the composite sequence represents a full-length transcript.
Two fit1 Mutations Lead to Picalm Transcripts That Are Missing Exons and Predicted to Encode Truncated Proteins. The primers used to amplify the six overlapping segments of the wild-type Picalm cDNA were also used to amplify these segments from liver RNAs derived from fit1/Del26DVT hemizygotes. Most of the RT-PCR products obtained from the mutants were the same size as from wild type; however, smaller-than-normal products, but no wildtype-sized products, were obtained from mutant fit15R/Del26DVT and fit14R/Del26DVT mice with the 147/148 and 153/154 primer pairs, respectively (Fig. 2B). Cloning and sequencing of these mutant-specific RT-PCR products revealed missing exons in the mutants (exon 4 in fit15R and exon 17 in fit14R) (Figs. 2 A and C and 3A), and that both alternatively spliced regions within the 153/154 amplicon are correctly incorporated into the size-altered fit14R-derived transcripts. The relative abundance of the different alternatively spliced 153/154 RT-PCR products obtained from the fit14R mutants also appears to be similar to that in wild-type (Fig. 2B). In each mutant, the absence of the exon in the mRNA leads to a frameshift in the ORF 3′ of the missing exon and, as a result, the incorporation of a few aberrant codons followed by a premature termination codon. Thus, the mRNAs obtained from the mutant fit15R and fit14R alleles are predicted to encode proteins truncated at residues 116 and 559, respectively (Fig. 3B). Northern blot analysis also revealed that mice hemizygous for these fit1 mutations express a Picalm transcript close in size to that in wild-type mice but at a level considerably lower than the wild-type level, which was particularly evident in the fit14R/Del26DVT mutants (Fig. 2D). Most likely, this is due to nonsense mutation-mediated mRNA decay (35) and will also lead to reduced levels of the predicted truncated proteins.
To confirm the two fit1 mutations at the genomic level, the affected exons (exons 4 and 17) and their flanking intronic sequences containing the splice acceptor and donor sites were amplified by PCR from genomic DNA of the mutant and wild-type animals. Sequencing of these PCR products revealed that, in both mutant alleles, the splice donor site immediately following the affected exon was altered from a GT to a GA, which leads to the exon deletion observed in the mRNA by splicing via the splice donor for the preceding exon (Fig. 2C).
Discussion
We have observed that two mouse fit1 mutations of differing phenotypic severity disrupt independent splice-donor sites and result in Picalm transcripts with missing exons and of lower abundance than normal. Due to the incorporation of premature termination codons downstream of the missing exons, the transcripts produced from the severe and milder alleles are predicted to specify proteins truncated near the N and C termini, respectively. This finding indicates that Picalm is the fit1 gene (now designated Picalmfit1). Because no other in vivo disorders have been associated with recessive mutations in Picalm, the Picalmfit1 mutants present unique models for exploring the role of the mouse Picalm protein in the clathrin-mediated endocytic processes that are essential for hematopoiesis, iron uptake/metabolism, and growth.
The human PICALM protein and its neuron-specific paralog, AP180, are a part of the endocytic machinery involved in the AP2-directed formation of clathrin-coated vesicles from cell membranes, but appear to participate in the formation of distinct types of coated vesicles (13, 14, 26, 27). Disruption of individual AP2-subunit genes arrests development during embryonic stages in both Drosophila melanogaster and Caenorhabditis elegans (36). A null mutation of the D. melanogaster SNAP91 ortholog (lap) is lethal at the early larval stage, although some escapers survive to the pupal and even adult stages (37). Null mutations of the C. elegans SNAP91 ortholog, unc-11, are viable with various locomotion defects (38). We have now shown that the ubiquitously expressed paralog of SNAP91 in mammals, Picalm, is not essential for early embryogenesis but is necessary for juvenile survival. The more specific hematopoietic and growth effects found later in development in the most phenotypically severe allele (Picalmfit1–5R), however, do have their beginnings in the early fetus, in which defects in growth and hematopoiesis can be detected as early as day 14.5 of gestation (2). This suggests that at least some degree of AP2/clathrin-mediated endocytosis must occur without Picalm, and that the endocytic processes involved in hematopoiesis, iron uptake/metabolism, and growth are more dependent on Picalm than those mediating other cellular functions. The reports that overexpression of either PICALM (13) or AP180 (14) in transfected cells severely impairs the uptake of transferrin and epidermal growth factor by blocking coated-pit formation and endocytosis of the receptor–ligand complexes (14) are consistent with an important in vivo role for mouse Picalm in the endocytic machinery involved in iron uptake and growth regulation.
The molecular architectures of AP180 and a related endocytic accessory protein, epsin 1, are designed for the rapid and efficient recruitment of clathrin and AP2 to sites of coated-pit formation in cellular membranes (27). Mutations in both lap and unc-11 are known to interfere with clathrin-dependent recycling of synaptic vesicles and the regulation of vesicle size and number (37, 38). Because of its strong affinity for clathrin, colocalization with a major depot of clathrin-coated vesicles, and almost complete colocalization in the cell with AP2, but not AP1, PICALM has been implicated in the recruitment of clathrin and AP2 to cellular membranes for the formation of nonsynaptic vesicles (13). Moreover, because AP180 is thought to regulate synaptic vesicle size and number (37, 38), its close paralog, PICALM, may be involved in the similar regulation (27) of nonsynaptic vesicles. Decreased serum levels and abnormal tissue distribution of iron in mice hemizygous for the Picalmfit1–4R mutation suggest that iron transport from the liver to other tissues is impaired (10). Because erythroid hemoglobin synthesis places particularly heavy demands on the transferrin cycle (39), decreased efficiency of AP2-dependent clathrin-mediated endocytosis due to a mutational defect in the predicted recruitment and vesicular-regulation functions of Picalm in the mutant mice could have a larger impact on transferrin endocytosis and subsequent hemoglobinization than on nonhematopoietic functions, a hypothesis that can now be tested rigorously.
The most phenotypically severe mutant allele, Picalmfit1–5R, has the potential to encode a protein that is truncated after amino acid residue 116 (removing 82% of the amino acid sequence). This truncated protein would lack all of the defined structural motifs known to interact with clathrin, the AP2 complex, and other endocytic proteins (13, 27, 31–34). It would also be missing the C-terminal ≈20% of the epsin N-terminal homology domain (Fig. 3), which mediates associations with cell membranes and clathrin vesicle-mediated internalization of receptor–ligand complexes via interactions with membrane phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] (14, 40). The major binding partner of human PICALM is clathrin heavy chain (CHC), although weaker interactions among PICALM and the α-adaptin subunit of AP2 and several other uncharacterized proteins have been reported (13). Three different segments of PICALM are known to contain sites of interaction with CHC (Fig. 3B), but the last 40 C-terminal residues have the highest affinity (13) despite the absence of any currently defined clathrin-binding sequences (34) in that segment (Fig. 3). Thus, similarly to AP180 (27), PICALM must interact with CHC via additional as yet undefined structural motif(s) in this C-terminal part of the protein. We have also found that the transcript produced from the milder Picalmfit1–4R allele has the potential to encode a truncated protein that is missing only the C-terminal 101-residue portion containing the highest-affinity CHC binding site(s) and one of the NPF motifs.
Generation of an antibody to the very N terminus of mouse Picalm will be necessary to address whether both truncated proteins are in fact stably produced in the mutant mice and, if they are, to assess the quantity and functional state of each mutant protein. Overexpression of full-length human PICALM or a C-terminal portion of the protein (residues 414–652) in cells coimmunoprecipitates CHC and inhibits the uptake of transferrin and epidermal growth factor, whereas overexpression of the N-terminal 1–413-residue portion does not have these effects (13). This indicates that the C-terminal segment contains the major binding sites responsible for interacting with the endocytic machinery both in vitro and in vivo. It is therefore apparent that, even if it is produced at an appreciable level, the small N-terminal portion of Picalm encoded by the mutant Picalmfit1–5R allele (residues 1–116) will also not be able to interact with clathrin and the endocytic machinery and recruit them to the appropriate sites of coated-pit formation. Although the 1–413 segment does not interact with CHC at a detectable level, the 1–613 residue portion of human PICALM is capable of interacting with CHC but with less affinity than the complete protein or the C-terminal 414–652 segment (13). This indicates that the 1–569-residue portion encoded by the milder Picalmfit1–4R allele will at best interact with CHC with lower-than-normal affinity, which is consistent with the hypomorphic phenotype observed for this mutation in comparison to the more severe, and likely null, Picalmfit1–5R phenotype.
PICALM now joins the growing list of proteins involved in endocytosis and intracellular transport that, when mutated, lead to a visible phenotype in mammals. Well-known examples are mutations in the AP3-mediated pathway, such as mocha (41, 42) and pearl (43) mice (mutated δ and β3A subunits of the AP3 complex, respectively); Hermansky–Pudlak syndrome type-2 in humans (β3A) (44); and pallid or muted mice, in which mutant pallidin or muted protein likely affects events later in vesicle-trafficking such as vesicle-docking and fusion (45, 46). Such mutations generally result in defects in intracellular protein sorting and the structure and function of lysosomes and storage organelles such as melanosomes and platelet granules. The Picalmfit1 mutations may act independently, or, more likely, implicate the involvement of clathrin/AP2-mediated transport pathways in at least the developmental events observed as abnormal in Picalmfit1 mutants (2).
Supplementary Material
Acknowledgments
We thank Dr. L. J. Hauser for help in the assembly of the BAC sequence; Drs. D. Johnson, Y. Liu, E. Michaud, and M. Garrick for suggestions on the manuscript; and the Joint Genome Institute of the Department of Energy for sequencing the BAC. This research was sponsored by the Office of Biological and Environmental Research, U.S. Department of Energy, under Contract DE-AC05-00OR22725 with UT-Battelle, LLC.
Abbreviations: PICALM, phosphatidylinositol-binding clathrin assembly protein; CHC, clathrin heavy chain; AP2, adaptor protein complex 2; BAC, bacterial artificial chromosome; YAC, yeast artificial chromosome.
Data deposition: The BALB/cRl Picalm cDNA sequence was deposited in the GenBank database (accession no. AY206701); the entire sequence shown in Fig. 3 is available in the Third Party Annotation Section of the DNA Data Bank of Japan (DDBJ)/EMBL/GenBank databases (accession no. TPA: BK001028).
References
- 1.Russell, E. S. (1979) Adv. Genet. 20 357-459. [PubMed] [Google Scholar]
- 2.Potter, M. D., Shinpock, S. G., Popp, R. A., Godfrey, V., Carpenter, D. A., Bernstein, A., Johnson, D. K. & Rinchik, E. M. (1997) Blood 90 1850-1857. [PubMed] [Google Scholar]
- 3.Fleming, M. D., Romano, M. A., Su, M. A., Garrick, L. M., Garrick, M. D. & Andrews, N. C. (1998) Proc. Natl. Acad. Sci. USA 95 1148-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler, E., Felitti, V., Gelbart, T. & Ho, N. (2001) Drug Metab. Dispos. 29 495-499. [PubMed] [Google Scholar]
- 5.Ponka, P. (2002) Semin. Hematol. 39 249-262. [DOI] [PubMed] [Google Scholar]
- 6.Takeda, A., Takatsuka, K., Connor, J. R. & Oku, N. (2002) Biometals 15 33-36. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto, K., Yoshida, K., Miyagoe, Y., Ishikawa, A., Hanaoka, K., Nomoto, S., Kaneko, K., Ikeda, S. & Takeda, S. (2002) Biochim. Biophys. Acta 1588 195-202. [DOI] [PubMed] [Google Scholar]
- 8.Rinchik, E. M., Carpenter, D. A. & Selby, P. B. (1990) Proc. Natl. Acad. Sci. USA 87 896-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultze, A. E., Schaeffer, D. O., Potter, M. D. & Johnson, D. K. (1997) Comp. Haematol. Int. 7 143-151. [Google Scholar]
- 10.Schultze, A. E., Poppenga, R. H. & Johnson, D. K. (1998) Comp. Haematol. Int. 8 72-76. [Google Scholar]
- 11.Schultze, A. E., McEntee, M. F., Daniel, G. B. & Johnson, D. K. (1999) Lab. Anim. Sci. 49 260-268. [PubMed] [Google Scholar]
- 12.Dreyling, M. H., Martinez-Climent, J. A., Zheng, M., Mao, J., Rowley, J. D. & Bohlander, S. K. (1996) Proc. Natl. Acad. Sci. USA 93 4804-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebar, F., Bohlander, S. K. & Sorkin, A. (1999) Mol. Biol. Cell 10 2687-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford, M. G., Pearse, B. M., Higgins, M. K., Vallis, Y., Owen, D. J., Gibson, A., Hopkins, C. R., Evans, P. R. & McMahon, H. T. (2001) Science 291 1051-1055. [DOI] [PubMed] [Google Scholar]
- 15.Potter, M. D., Klebig, M. L., Carpenter, D. A. & Rinchik, E. M. (1995) Mamm. Genome 6 70-75. [DOI] [PubMed] [Google Scholar]
- 16.Rinchik, E. M. & Carpenter, D. A. (1999) Genetics 152 373-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osoegawa, K., Tateno, M., Woon, P. Y., Frengen, E., Mammoser, A. G., Catanese, J. J., Hayashizaki, Y. & de Jong, P. J. (2000) Genome Res. 10 116-128. [PMC free article] [PubMed] [Google Scholar]
- 18.Holdener, B. C., Thomas, J. W., Schumacher, A., Potter, M. D., Rinchik, E. M., Sharan, S. K. & Magnuson, T. (1995) Genomics 27 447-456. [DOI] [PubMed] [Google Scholar]
- 19.Rikke, B. A., Johnson, D. K. & Johnson, T. E. (1997) Genetics 147 787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebig, M. L., Wilkinson, J. E., Geisler, J. G. & Woychik, R. P. (1995) Proc. Natl. Acad. Sci. USA 92 4728-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinchik, E. M. & Russell, L. B. (1990) in Genome Analysis, eds. Davies, K. & Tilghman, S. (Cold Spring Harbor Lab. Press, Plainview, NY), Vol. 1, pp. 121-158. [Google Scholar]
- 22.Morris, S. A., Schroder, S., Plessmann, U., Weber, K. & Ungewickell, E. (1993) EMBO J. 12 667-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narita, M., Shimizu, K., Hayashi, Y., Taki, T., Taniwaki, M., Hosoda, F., Kobayashi, H., Nakamura, H., Sadamori, N., Ohnishi, H., et al. (1999) Br. J. Haematol. 105 928-937. [DOI] [PubMed] [Google Scholar]
- 24.Bohlander, S. K., Muschinsky, V., Schrader, K., Siebert, R., Schlegelberger, B., Harder, L., Schemmel, V., Fonatsch, C., Ludwig, W. D., Hiddemann, W., et al. (2000) Leukemia 14 93-99. [DOI] [PubMed] [Google Scholar]
- 25.Carlson, K. M., Vignon, C., Bohlander, S., Martinez-Climent, J. A., Le Beau, M. M. & Rowley, J. D. (2000) Leukemia 14 100-104. [DOI] [PubMed] [Google Scholar]
- 26.McMahon, H. T. (1999) Curr. Biol. 9 R332-R335. [DOI] [PubMed] [Google Scholar]
- 27.Kalthoff, C., Alves, J., Urbanke, C., Knorr, R. & Ungewickell, E. J. (2002) J. Biol. Chem. 277 8209-8216. [DOI] [PubMed] [Google Scholar]
- 28.Conrad, M. E., Umbreit, J. N. & Moore, E. G. (1999) Am. J. Med. Sci. 318 213-229. [DOI] [PubMed] [Google Scholar]
- 29.Marsh, M. (2001) Endocytosis (Oxford Univ. Press, Oxford).
- 30.Kim, H. L. & Lee, S. C. (1999) Exp. Mol. Med. 31 191-196. [DOI] [PubMed] [Google Scholar]
- 31.Marsh, M. & McMahon, H. T. (1999) Science 285 215-220. [DOI] [PubMed] [Google Scholar]
- 32.Owen, D. J., Vallis, Y., Noble, M. E., Hunter, J. B., Dafforn, T. R., Evans, P. R. & McMahon, H. T. (1999) Cell 97 805-815. [DOI] [PubMed] [Google Scholar]
- 33.Paoluzi, S., Castagnoli, L., Lauro, I., Salcini, A. E., Coda, L., Fre', S., Confalonieri, S., Pelicci, P. G., Di Fiore, P. P. & Cesareni, G. (1998) EMBO J. 17 6541-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake, M. T. & Traub, L. M. (2001) J. Biol. Chem. 276 28700-28709. [DOI] [PubMed] [Google Scholar]
- 35.Lewis, B. P., Green, R. E. & Brenner, S. E. (2003) Proc. Natl. Acad. Sci. USA 100 189-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehm, M. & Bonifacino, J. S. (2002) Gene 286 175-186. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, B., Koh, Y. H., Beckstead, R. B., Budnik, V., Ganetzky, B. & Bellen, H. J. (1998) Neuron 21 1465-1475. [DOI] [PubMed] [Google Scholar]
- 38.Nonet, M. L., Holgado, A. M., Brewer, F., Serpe, C. J., Norbeck, B. A., Holleran, J., Wei, L., Hartwieg, E., Jorgensen, E. M. & Alfonso, A. (1999) Mol. Biol. Cell 10 2343-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponka, P. (1997) Blood 89 1-25. [PubMed] [Google Scholar]
- 40.Itoh, T., Koshiba, S., Kigawa, T., Kikuchi, A., Yokoyama, S. & Takenawa, T. (2001) Science 291 1047-1051. [DOI] [PubMed] [Google Scholar]
- 41.Kantheti, P., Qiao, X., Diaz, M. E., Peden, A. A., Meyer, G. E., Carskadon, S. L., Kapfhamer, D., Sufalko, D., Robinson, M. S., Noebels, J. L., et al. (1998) Neuron 21 111-122. [DOI] [PubMed] [Google Scholar]
- 42.Setaluri, V. (2000) Pigment Cell Res. 13 128-134. [DOI] [PubMed] [Google Scholar]
- 43.Feng, L., Seymour, A. B., Jiang, S., To, A., Peden, A. A., Novak, E. K., Zhen, L., Rusiniak, M. E., Eicher, E. M., Robinson, M. S., et al. (1999) Hum. Mol. Genet. 8 323-330. [DOI] [PubMed] [Google Scholar]
- 44.Feng, L., Novak, E. K., Hartnell, L. M., Bonifacino, J. S., Collinson, L. M. & Swank, R. T. (2002) Blood 99 1651-1658. [PubMed] [Google Scholar]
- 45.Huang, L., Kuo, Y. M. & Gitschier, J. (1999) Nat. Genet. 23 329-332. [DOI] [PubMed] [Google Scholar]
- 46.Falcon-Perez, J. M., Starcevic, M., Gautam, R. & Dell'Angelica, E. C. (2002) J. Biol. Chem. 277 28191-28199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.