Abstract
Injection of neonatal bone marrow cells from mice lacking the gene encoding suppressor of cytokine signaling 1 (SOCS1) into irradiated syngeneic 129/Sv or C57BL/6 mice led to a decreased survival, more rapidly occurring in 129/Sv than in C57BL/6 mice. Moribund mice did not exhibit the acute or chronic diseases developed by Socs1-/- mice but developed a pathology characteristic of graft-versus-host disease with typical chronic inflammatory lesions in the liver, skin, lungs, and gut. The results indicate that cells derived from the Socs1-/- bone marrow are autoaggressive but did not identify the cell types involved. Failure of the engrafted Socs1-/- marrow cells to reproduce the tissue damage typical of Socs1-/- disease indicates that loss of SOCS1 from target tissues may also be required for the development of the Socs1-/- diseases, such as fatty degeneration of the liver, polymyositis, or corneal inflammation.
The suppressor of cytokine signaling 1 (SOCS1) is an inducible cellular protein that is able to suppress or truncate signaling from a number of cytokine receptors, including those for IFN-γ, IL-6, leukemia inhibitory factor (LIF), oncostatin M (OSM), IL-11, IL-2, stem cell factor (SCF), granulocyte/macrophage colony-stimulating factor (GM-CSF), and prolactin (1).
Mice with homozygous deletion of the Socs1 gene die in neonatal life with fatty degeneration and necrosis of the liver and inflammatory infiltrates of T lymphocytes, macrophages, and eosinophils in the liver, skin, lung, pancreas, and gut (2, 3). This syndrome is wholly dependent on IFN-γ and can be prevented either by the administration of antibodies to IFN-γ (4) or by generating Socs1-/- mice that also lack the gene for IFN-γ (3, 5). Mice that lack SOCS1 and are heterozygous for IFN-γ die as young adults with a syndrome dominated by extensive T lymphocyte, macrophage, and eosinophil infiltration of all striated muscles, the myocardium, and the cornea (6).
Although T and B lymphocyte numbers are subnormal in Socs1-/- mice, the T lymphocytes, particularly CD8 cells, exhibit membrane markers of activation, and there is some evidence implicating T lymphocytes as the initiators of Socs1-/- disease. Thus Socs1-/- rag-2-/- mice do not die prematurely (3), and Socs1-/- natural killer (NK) T cells have been reported to be more numerous than normal in the liver, and to be cytotoxic for syngeneic liver cells (7).
Several possibilities raised by these observations are: (i) Socs1-/- hematopoietic cells, possibly T lymphocytes, may be the primary initiators of disease development; (ii) various organs lacking SOCS1 such as the liver, muscle, heart and lung might be in an abnormally susceptible state to damage by normal-enough hematopoietic cells; or (iii) both elements may be necessary for disease development.
In an early study (3), transplantation of Socs1-/- marrow cells into syngeneic recipients was reported to be lethal and to have recapitulated the Socs1-/- disease. However, no detailed data were presented to document the pathology of such mice and to establish which, if any, of the various hematological and pathological abnormalities shown by neonatal or young adult Socs1-/- mice developed in such recipients.
The present study was undertaken to establish in detail the consequences of transplanting neonatal Socs1-/- marrow cells to irradiated adult syngeneic recipients. The data confirmed the lethal consequences of such transplantation but revealed that the mice died with graft-versus-host disease and that neither the neonatal nor the young adult Socs1-/- syndromes were reproduced in the irradiated recipients.
Materials and Methods
Mice. The Socs1 locus was targeted in embryonic stem cells derived from the 129/Sv.C3-+c+p strain (2, 8) and chimeras derived from these cells were bred with 129/Sv.Ems-+Ter? mice from the Walter and Eliza Hall Institute breeding facility (9) to generate and maintain stocks of 129/Sv Socs1-/-, Socs1+/-, and Socs1+/+ mice. All 129/Sv transplant studies used 129/Sv.Ems-+Ter? mice as recipients. Socs1-/- mice on a C57BL/6 genetic background were generated by backcrossing 129/Sv Socs1+/- mice to C57BL/6 mice for 10 generations. In all cases, Socs1-/- mice were derived from matings of Socs1+/- mice and healthy littermate Socs1+/- or Socs1+/+ mice were used as controls.
Bone Marrow Transplantation. Socs1-/- mice were identified by characteristic growth retardation at 1–2 weeks of age and killed for collection of femoral bone marrow cells. Bone marrow cells were simultaneously collected from healthy littermates as controls. The genotype of all donor mice was subsequently verified by Southern blot analysis of genomic DNA extracted from tail biopsies (2). Several hours before transplantation, recipient mice were irradiated with 11Gy of γ-irradiation divided into two equal doses given 3 h apart from a 137Cs source (Atomic Energy, Ottawa). Each recipient received 106 bone marrow cells and, typically, four to six recipients were transplanted with cells from each donor. Transplanted mice were maintained on oral antibiotic in the drinking water for 4 weeks (1.1 g/liter neomycin sulfate; Sigma).
For 129/Sv transplants, the degree of donor engraftment was assessed by Southern blot analysis of genomic DNA extracted from the bone marrow and spleen of recipient mice using a probe that distinguished the targeted Socs1 locus (present in Socs1-/- and Socs1+/- donor cells) from the endogenous Socs1 gene (2). The relative signals were quantitated by autoradiographic densitometry and used to calculate the proportion of donor cells in each tissue. In all recipient mice analyzed (three Socs1-/- and nine Socs1+/- recipients), donor cell engraftment represented >90% of the bone marrow and spleen. For C57BL/6 transplants, Ly5.1 congenic recipients were used, allowing flow cytometric analysis of donor (Ly5.2) engraftment. Analysis of blood, thymus, bone marrow, and spleen revealed donor contribution of 76–93% in recipients of Socs1-/- marrow and 92–99% in Socs1+/+ or Socs1+/- recipients.
Analysis of Mice. Mice were analyzed when clinically ill. Peripheral blood white cell and platelet counts were determined manually by using hemocytometers. Single-cell suspensions from femoral bone marrow, spleen, and peritoneal cells were prepared and differential cell counts were performed on smears or cytocentrifuge preparations stained with May–Grunwald–Giemsa. Clonal cultures of hematopoietic cells were performed as described (10). Briefly, cultures of 2.5 × 104 bone marrow cells or 5 × 104 spleen cells in 0.3% agar in DMEM supplemented with 20% newborn calf serum were stimulated by various cytokines and incubated for 7 days at 37°C in a fully humidified atmosphere of 10% CO2 in air. For the cultures, the final concentrations of growth factors used in separate cultures were: 10 ng/ml GM-CSF, 10 ng/ml G-CSF, 10 ng/ml M-CSF, 10 ng/ml IL-3, or 100 ng/ml SCF. After removal of the hematopoietic tissues, the brain, thymus, thyroid, heart, lung, salivary glands, sternum, femur, tibia, skin, liver, spleen, pancreas, kidneys, small bowel, bladder, skeletal muscle, and uterus or testes were fixed in 10% buffered formalin, the tissues blocked in paraffin, and sections stained with hematoxylin and eosin.
Flow Cytometry. Single-cell suspensions of bone marrow, lymph node, spleen, and thymus cells were prepared. Erythrocytes were lysed and the cells were stained with rat mAbs for specific cell surface markers, then analyzed by flow cytometry as described (6).
Results
The design for the initial study involved the i.v. injection of 106 bone marrow cells from 2-week-old Socs1-/- mice or phenotypically normal littermate Socs1+/+ or Socs1+/- mice that were maintained on a 129/Sv genetic background into lethally irradiated 129/Sv strain recipients. The genotype of donor mice was confirmed by Southern blotting and histological examination of the donor tissues. Because the embryonic stem cells used to generate the Socs1-/- mice were derived from a different substrain to that used in the subsequent breeding of the mice, an additional control group involved the transplantation of 106 neonatal 129/Sv recipient strain bone marrow cells.
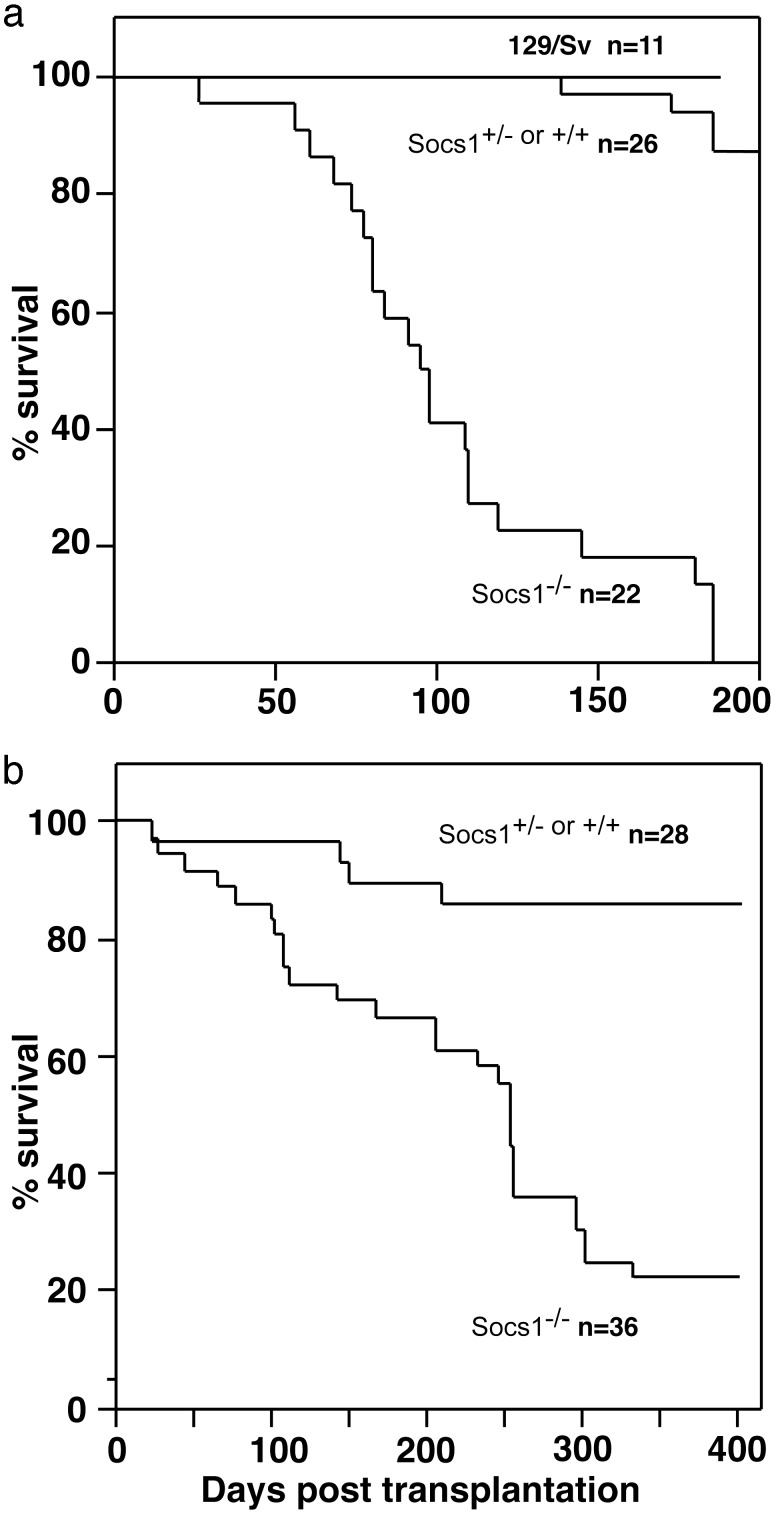
As shown in Fig. 1a, 129/Sv strain mice injected with Socs1-/- marrow cells began to become moribund at 40 days posttransplantation and were all dead by 180 days posttransplantation. This result contrasted with the behavior of recipients of cells from Socs1+/+ or Socs1+/- littermates, of which only 15% had died by 200 days posttransplantation and the recipients of control 129/Sv strain marrow cells, none of which died during this period.
Fig. 1.
Survival curves of 129/Sv recipients of Socs1-/-, Socs1+/+,or Socs1+/- or control 129/Sv bone marrow transplants (a) and C57BL/6 recipients of Socs1-/-, Socs1+/+, or Socs1+/- bone marrow transplants (b). Note the more protracted survival of C57BL/6 mice compared with that of 129/Sv mice when injected with Socs1-/- marrow cells. n, number of mice surveyed in each group.
When recipients of Socs1-/- cells became ill, 12 were examined hematologically in parallel with 18 recipients of Socs1+/+ or Socs1+/- cells. Marrow and spleen cells from 8–10 mice of each type were also analyzed for their content of clonogenic progenitor cells in agar cultures stimulated by GM-CSF, G-CSF, M-CSF, IL-3, or SCF.
In comparison with recipients of control Socs1+/+ or Socs1+/- marrow cells, recipients of Socs1-/- cells exhibited significantly higher blood neutrophil levels (3,350 ± 3,330 per μl versus 1,420 ± 1,920 per μl), somewhat lower platelet levels (4.7 ± 1.9 × 105 per μl versus 5.7 ± 1.5 × 105 per μl), and significantly lower hematocrit levels (42 ± 4% versus 48 ± 2%). Recipients of Socs1-/- cells also had a significantly smaller thymus (20 ± 7 mg), and a significant increase in spleen weight (221 ± 153 mg), compared with control mice (37 ± 10 mg and 95 ± 23 mg, respectively). The spleen of recipients of Socs1-/- cells had a significantly lower content of lymphocytes (18 ± 14% versus 74 ± 14%), and a significant elevation of erythroid cells (44 ± 20% versus 14 ± 9%) and neutrophils (15 ± 6% versus 3 ± 2%) in comparison with control recipients. In the bone marrow, there was a slightly lower content of lymphocytes in recipients of Socs1-/- cells than in control recipients (7 ± 2% versus 14 ± 6%), but no other differences of significance. No significant differences were observed between recipients of littermate Socs1+/+ or Socs1+/- cells and Socs1+/+ cells of the recipient 129/Sv substrain.
In culture, marrow cells from sick recipients of Socs1-/- bone marrow cells exhibited absolute dependency on stimulation by extrinsic growth factors and responded normally to these in terms of absolute and relative numbers of colony subtypes. The size and maturation of these colonies were also normal (data not shown). In contrast, spleen populations from recipients of Socs1-/- marrow cells had a 7- to 10-fold higher content of colony-forming progenitor cells than recipients of Socs1+/+ or Socs1+/- marrow cells (Table 1).
Table 1. Colony formation by spleen cells from 129/Sv recipients of Socs1-/- bone marrow transplants.
|
Mean no. of colonies
|
|||||||
|---|---|---|---|---|---|---|---|
| Marrow donor | Stimulus | Blast | G | GM | M | Eo | Meg |
| Socs1-/- (n = 10) | GM-CSF | 12±13 | 3±2 | 15±12 | 0.7±1.0 | ||
| IL-3 | 3±4 | 18±14 | 3±3 | 11±7 | 0.1±0.3 | 9±6 | |
| Socs1+/+ or Socs1+/- (n = 8) | GM-CSF | 1±1 | 0.5±0.5 | 1.8±2.4 | 0±0 | ||
| IL-3 | 0.3±0.7 | 1.1±1.4 | 0.5±0.5 | 2.9±2.1 | 0.1±0.4 | 1.1±1.0 | |
Data represent mean numbers of colonies ± SD developing in 7-day cultures of 50,000 spleen cells. Final concentrations of GM-CSF or IL-3 in the cultures were 10 ng/ml. Blast, blast colonies; G, granulocyte; GM, granulocyte/macrophage; M, macrophage; Eo, eosinophil; Meg, megakaryocyte colonies
Flow cytometric analysis of cell surface marker expression on cells from transplant recipients revealed a relative reduction of CD4+ CD8+ double-positive thymocytes with a concomitant increase in the percentage of CD4+ and CD8+ single-positive thymic T cells in sick recipients of Socs1-/- marrow, an observation consistent with the thymic atrophy evident in these mice (see below). Moreover, as has been observed previously in mice lacking SOCS1 (2, 11), there was a significant increase in these mice in the proportion of T lymphocytes expressing high levels of the activation marker CD44 in cells from the thymus, spleen, and lymph node.
Pathology of 129/Sv Recipients of Socs1-/- Cells. When 129/Sv recipients of Socs1-/- marrow cells became clinically ill, at least one randomly chosen control recipient of Socs1+/+ or Socs1+/- cells was analyzed in parallel. The pathological findings in the sick recipients of Socs1-/- cells together with data from control mice are shown in Table 2. It needs to be emphasized that these control data are from matching randomly chosen mice of the same age, and they were apparently healthy mice so the data cannot be compared in their entirety with the data from mice injected with Socs1-/- marrow cells.
Table 2. Pathology in 129/Sv and C57BL/6 recipients of Socs1-/- bone marrow transplants.
|
Percent frequency
|
||||||
|---|---|---|---|---|---|---|
|
129/Sv
|
C57BL/6
|
|||||
| Tissue | Parameter | Socs1-/- (n = 13) | Socs1+/+ or Socs1+/- (n = 20) | Control 129/Sv (n = 7) | Socs1-/- (n = 16) | Socs1+/+ or Socs1+/- (n=24) |
| Liver | Necrosis | 84 | 20 | 29 | 0 | 0 |
| Cuffing of bile ducts | 100 | 40 | 43 | 94 | 13 | |
| Focal granulomas | 100 | 55 | 43 | 93 | 4 | |
| Infiltration, eosinophils | 92 | 45 | 14 | 40 | 4 | |
| Infiltration, plasma cells | 77 | 10 | 0 | 36 | 0 | |
| Excess Kupffer cells | 100 | 25 | 43 | 20 | 4 | |
| Lung | Pneumonia, extensive | 54 | 5 | 14 | 13 | 0 |
| Pneumonia, small foci | 17 | 35 | 29 | 19 | 4 | |
| Cuffing of bronchi or alveolar thickening | 66 | 10 | 29 | 81 | 4 | |
| Foci, eosinophils | 46 | 25 | 29 | 19 | 0 | |
| Foci, plasma cells | 69 | 10 | 0 | 19 | 4 | |
| Foci, lymphocytes | 77 | 15 | 0 | 91 | 79 | |
| Thymus | Atrophy of cortex | 100 | 5 | 0 | 100 | 0 |
| Spleen | Lymphoid follicles | 54 | 100 | 100 | 93 | 100 |
| Naked germinal centers | 33 | 0 | 0 | 0 | 0 | |
| Excess plasma cells | 77 | 5 | 0 | 7 | 0 | |
| Excess hematopoiesis | 85 | 5 | 14 | 40 | 4 | |
| Excess erythropoiesis | 62 | 5 | 57 | 60 | 9 | |
| Excess eosinophils | 50 | 0 | 0 | 13 | 0 | |
| Lymph node | Excess plasma cells | 100 | 7 | 0 | 55 | 9 |
| Bladder | Granuloma | 64 | 0 | 0 | 15 | 19 |
| Kidney | Foci, lymphocytes | 69 | 20 | 0 | 57 | 38 |
| Foci, plasma cells | 62 | 20 | 0 | 21 | 4 | |
| Foci, eosinophils | 15 | 0 | 0 | 0 | 0 | |
| Glomerular tuft thickening | 23 | 5 | 0 | 7 | 0 | |
| Pancreas | Infiltration by macrophages/lymphocytes | 33 | 7 | 0 | 64 | 20 |
| Gut | Granuloma | 67 | 5 | 0 | 50 | 5 |
| Heart | Infiltration | 31 | 11 | 0 | 15 | 0 |
| Salivary gland | Lymphoid foci | 42 | 17 | 14 | 53 | 74 |
| Skin | Epithelial thickening | 31 | 0 | 0 | 13 | 0 |
| Keratin and macrophages | 15 | 0 | 0 | 0 | 0 | |
| Marrow | Excess granulocytes | 100 | 55 | 29 | 81 | 33 |
| Prominent eosinophils | 92 | 40 | 0 | 67 | 0 | |
| Lymphoid leukemia | 0 | 0 | 0 | 6 | 4 | |
| Reticulum cell sarcoma | 0 | 0 | 0 | 6 | 0 | |
n, number of mice examined of each type. Two additional 129/Sv mice grafted with Socs1-/- cells died with failure of engraftment, and their data (hematocrit 10% and 13%, platelets 12, 150, and 9,070 per μl) have been excluded from the table
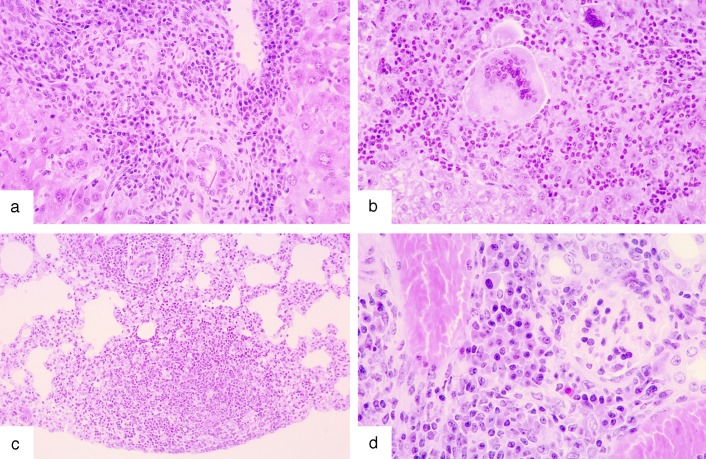
The 129/Sv recipients of Socs1-/- marrow cells showed atrophy of the thymus and most showed liver damage, including cuffing of the bile ducts with fibroblasts, lymphocytes, and macrophages, focal areas of necrosis, and characteristic multiple, quite large, chronic granulomas composed of lymphocytes, plasma cells, eosinophils, and giant multinucleate macrophages (Fig. 2 a and b). Two thirds of the lungs exhibited either thickening of alveolar walls by lymphocytes, plasma cells, and eosinophils, or patchy areas of pneumonia involving the same cell types (Fig. 2c). Two thirds of the mice showed granulomas in the gut involving lymphocytes, macrophages, and eosinophils and, in approximately two thirds of the kidneys, focal infiltrates of the same cells were present (Fig. 2d). All lymph nodes showed excess numbers of plasma cells in their hilar regions and the enlarged spleens contained excess hematopoietic populations in the red pulp involving erythroid, eosinophil, and plasma cells. Infiltrates of lymphocytes, plasma cells and macrophages were also observed in the bladder, pancreas, salivary gland, and heart of one third of the mice, and all bone marrows showed a dominant population of granulocytes, with eosinophils being prominent. In one third of the mice, the skin showed epithelial thickening, often with keratin deposition and mononuclear infiltration of the dermis. Not observed in any of these mice was fatty degeneration of the liver or inflammatory infiltrates in skeletal muscle or the cornea: disease states characteristic of neonatal Socs1-/- mice or young adult Socs1-/- Ifng+/- mice.
Fig. 2.
Characteristic tissue lesions in 129/Sv mice with graft-versus-host disease after the injection of syngeneic Socs1-/- marrow cells. (a) Liver, with cuffing of portal tract vessels by lymphocytes, macrophages, and plasma cells is shown. (b) Liver, with a focal granuloma of neutrophils, lymphocytes, and plasma cells together with multinucleate giant cells is shown. (c) Lung, with thickening of alveolar walls by lymphocytes and macrophages and a small area of pneumonia is shown. (d) Kidney, with focal infiltrate of plasma cells adjacent to a glomerulus, is shown.
The liver, lung, gut, and skin lesions of recipients of Socs1-/- cells resembled lesions that have been described in mice with graft-versus-host disease after allogeneic marrow cell transplantation (12, 13). Early studies (14) demonstrated that granuloma formation of the type encountered in the present mice does not occur in mice with graft-versus-host disease if the mice are germ free, and therefore this particular lesion is in part dependent on microbial infection.
Somewhat surprisingly, some of the apparently healthy, randomly killed, 129/Sv mice injected with Socs1+/+ or Socs1+/- cells or with control 129/Sv marrow cells showed similar lesions to those in mice injected with Socs1-/- cells. Table 2, however, shows certain differences. In control mice, the thymus was not atrophic, indicating no major ill health in the mice. Further, although some cuffing of liver vessels and focal granulomas were present in control mice, the lesions tended to be less frequent and less extensive. A similar reduction in disease severity was observed in the lungs of control mice. Control mice also rarely exhibited excess plasma cells in the lymph nodes or excess hematopoiesis in the spleen and had fewer foci of lymphocytes and plasma cells in the bladder, kidney, pancreas, gut, and heart, and no lesions in the skin.
The pathological data therefore indicated that 129/Sv mice injected with Socs1-/- marrow cells died with typical graft-versus-host disease. The odd feature of the results was that, although the control mice were clinically healthy, they did exhibit a surprisingly high frequency of lesions that were qualitatively similar in the liver and lung to those in mice injected with Socs1-/- cells, although lesions in other organs were much less evident.
Pathology of C57BL/6 Recipients of Socs1-/- Cells. Socs1-/- mice were generated on a C57BL/6 genetic background by backcrossing for 10 generations. The pathology of C57BL/6 Socs1-/- mice was similar to that described for 129/Sv Socs1-/- mice (2), again with all mice dying before weaning (data not shown).
In three separate experiments, irradiated adult C57BL/6 mice were injected with Socs1-/- or Socs1+/+ cells from neonatal donors. The data from the three experiments have been pooled in Fig. 1b, which shows the fate of 36 mice injected with Socs1-/- cells and 28 with Socs1+/+ or Socs1+/- cells. The identity of donor mice was confirmed by analysis of genomic DNA and histological examination.
C57BL/6 recipients of Socs1-/- marrow cells died at an accelerated rate compared with control mice, although more slowly than corresponding 129/Sv strain mice. The pathology of a representative sample of marrow recipients, killed when sick, is shown in Table 2. The overall disease pattern in recipient C57BL/6 strain mice was similar to that in recipient 129/Sv strain mice, but of a less severe nature. The majority of C57BL/6 mice injected with Socs1-/- cells showed cuffing of portal tracts and foci of infiltrating cells in the liver, including lymphoid cells and macrophages and, less commonly, eosinophils or plasma cells. Where inflammatory foci had developed within liver, these did not contain giant multinucleate cells.
Pneumonia was less frequent in C57BL/6 mice than in 129/Sv strain mice, but had the same general histological appearance, except for an absence of multinucleate cells. Most commonly, the lesions seen in the C57BL/6 lungs were cuffing of the bronchi and thickening of the alveolar walls by infiltrating lymphocytes and macrophages together with focal aggregates of lymphoid cells.
The thymus in C57BL/6 recipients of Socs1-/- cells was again uniformly atrophic but lymphocyte-containing follicles were present in the spleen, although the follicles were usually small in size and with no germinal centers. The spleens again commonly exhibited excessive numbers of nucleated erythroid cells.
The kidneys of C57BL/6 recipients were relatively normal except for small foci of lymphoid and sometimes plasma cells, and similar infiltrates were common in the pancreas and salivary glands. Granulomatous tissue containing lymphocytes, macrophages, and plasma cells was present in the gut of half of the recipients of Socs1-/- cells, although skin infiltrates or epithelial lesions were uncommon. In most mice, the heart appeared normal, and in most mice the marrow again was dominated by granulocytic populations, often with prominent eosinophils.
In no C57BL/6 recipients of Socs1-/- cells were inflammatory lesions observed in skeletal muscle or the cornea.
In summary, the C57BL/6 recipients of Socs1-/- marrow cells died prematurely with liver and lung infiltrates, granulomas in the gut and thymus atrophy, with other lesions being less frequent. This disease pattern is typical enough of graft-versus-host disease where the pathological changes are proceeding relatively slowly as was the case with the C57BL/6 recipients.
As shown in Fig. 1b, most C57BL/6 recipients of Socs1+/+ or Socs1+/- marrow cells survived beyond the death of most recipients of Socs1-/- cells. To terminate this protracted control experiment, the surviving mice were killed when aged between 350 and 450 days. Data from a histological analysis of these mice are shown in Table 2. In common with most C57BL/6 mice of this age, foci of lymphocytes were present in 50% of the livers and, as shown in the table, were even more common in the lungs and salivary glands. Although an analysis of mice killed arbitrarily does not present a picture of what disease states the mice would eventually have developed, the data do make clear that the mice had not developed inflammatory disease in the liver, lung, skin, and gut as had the recipients of Socs1-/- cells. These control C57BL/6 recipients of Socs1+/+ or Socs1+/- cells provided a more clear cut distinction from the disease developing in recipients of Socs1-/- cells than was the case for the 129/Sv mice.
Discussion
Hematopoietic and lymphoid cells are prominent in the diseased tissues of dying neonatal mice that lack SOCS1 (2), and in the inflammatory muscle and corneal lesions of Socs1-/- mice whose death is delayed by heterozygosity of IFN-γ, the administration of antibodies to IFN-γ or exclusion of IL-12 (4, 6, 15). These cells may merely represent inflammatory cells attracted to damaged Socs1-/- tissue, but it is much more likely that their presence is the initiating cause of the fatal tissue damage in these mice.
To test this latter proposition, Socs1-/- marrow cells were grafted into lethally irradiated syngeneic Socs1+/+ recipients. If adequate engraftment of Socs1-/- marrow cells occurred, tissue damage and/or death might occur if the cells are autoaggressive. If no damage develops, either the hematopoietic cells are not intrinsically autoaggressive or tissue damage also needs the presence of abnormalities in target tissues as a consequence of loss of SOCS1. Highly relevant in this context is the observation that tissues such as the liver in Socs1-/- mice overexpress MHC class I antigens (5) and simple overexpression of MHC class I by itself has been shown to incite autoaggression by normal T lymphocytes, resulting in severe polymyositis (16).
In an early study (3), Socs1-/- marrow cells were transplanted into irradiated recipients and resulted in premature death, which was reported to have recapitulated the original Socs1-/- disease. The present experiments using Socs1-/- marrow cells from two strains: 129/Sv and C57BL/6, have confirmed that this procedure does indeed result in premature death of irradiated syngeneic recipients. However, the disease developed by the recipients is a protracted graft-versus-host disease causing death relatively slowly. The recipients develop characteristic inflammatory lesions of graft-versus-host disease in the liver, lungs, gut, and less commonly, in the skin and other organs (12, 13). Missing particularly from the C57BL/6 recipient mice were the characteristic features of neonatal Socs1-/- disease; the fatty infiltration of hepatocytes and the uniform epithelial hyperplasia and infiltration of the skin (2). More striking was the complete absence in both recipient strains of the characteristic lesions of the delayed onset of Socs1-/- disease; the profound polymyositis, myocarditis, and corneal inflammatory lesions (6).
Marrow transplantation clearly documented the abnormal autoaggressive behavior of Socs1-/- hematopoietic cells, but not their capacity to induce Socs1-/- disease alone. In view of the graft-versus-host disease developing in the recipient mice, it is of interest that these lesions were more severe and more rapid in onset in 129/Sv strain mice than in C57BL/6 mice. Strain 129/Sv mice have been the subject of a number of studies suggesting possibly a susceptibility to hypermutation, possible contamination of the inbred 129/Sv strain, or genetic differences between 129/Sv substrains and those used to produce embryonic stem cells (17–19). Any one of these factors, if true, might result in antigenic differences between Socs1-/- 129/Sv cells and recipient 129/Sv mice that might provoke more severe graft-versus-host disease. Alternatively, 129/Sv strain mice might simply be more susceptible to tissue damage than C57BL/6 mice.
To document autoaggression by Socs1-/- marrow cells does not identify the cell type(s) or cytokines responsible for tissue damage. Socs1-/- T lymphocytes are aberrant in their maturation state, display membrane markers of activation, and hyperrespond to stimulation by IL-2 (2, 3, 20). Conversely, IFN-γ levels are elevated in Socs1-/- mice (3) and the macrophages in Socs1-/- mice are hyperresponsive to activation by IFN-γ (5) and might themselves produce agents like tumor necrosis factor that could damage tissues. Whereas intrinsic abnormalities in Socs1-/- T lymphocytes would be the most conventional manner for explaining the present induction of graft-versus-host disease in recipients of Socs1-/- cells, the situation might not allow such a simple explanation. Mice in which deletion of the Socs1 gene has been restricted to T lymphocytes do develop an abnormal pattern of maturation of T lymphocytes, but they do not develop tissue damage (21). It would seem that aberrant T lymphocytes and/or NKT cells may be insufficient alone to cause tissue damage. Their action must be supplemented by equally aberrant Socs1-/- macrophages or other marrow-derived cells to produce graft-versus-host disease, and to produce the special lesions of Socs1-/- mice, the target tissues themselves must be in a suitably abnormal state as a consequence of loss of SOCS1 to invite attack or be susceptible to attack.
Socs1-/- marrow cells exhibit autoaggression in inducing graft-versus-host disease and Socs1-/- mice exhibit increased susceptibility to experimentally induced arthritis (22). These observations raise the possibility that various related inflammatory or autoimmune diseases in humans might on occasion be based on deletion or inactivation of Socs1, whose protein product normally acts to restrict or terminate activating signals from a wide variety of cytokines.
Acknowledgments
We thank Janelle Lochland and Sally Cane for excellent technical assistance and Catherine Tilbrook for animal husbandry. This work was supported by Australian National Health and Medical Research Council Program Grant 257500, National Institutes of Health Grant CA22556, Cancer Council of Victoria Carden Fellowship (to D.M.) and Australian Medical Research and Development Operations Pty. Ltd.
Abbreviations: SOCS1, suppressor of cytokine signaling 1; CSF, colony-stimulating factor.
References
- 1.Krebs, D. L. & Hilton, D. J. (2001) Stem Cells (Dayton) 19 378-387. [DOI] [PubMed] [Google Scholar]
- 2.Starr, R., Metcalf, D., Elefanty, A. G., Brysha, M., Willson, T. A., Nicola, N. A., Hilton, D. J. & Alexander, W. S. (1998) Proc. Natl. Acad. Sci. USA 95 14395-14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marine, J. C., Topham, D. J., McKay, C., Wang, D., Parganas, E., Stravopodis, D., Yoshimura, A. & Ihle, J. N. (1999) Cell 98 609-616. [DOI] [PubMed] [Google Scholar]
- 4.Bullen, D. V., Darwiche, R., Metcalf, D., Handman, E. & Alexander, W. S. (2001) Immunology 104 92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander, W. S., Starr, R., Fenner, J. E., Scott, C. L., Handman, E., Sprigg, N. S., Corbin, J. E., Cornish, A. L., Darwiche, R., Owczarek, C. M., et al. (1999) Cell 98 597-608. [DOI] [PubMed] [Google Scholar]
- 6.Metcalf, D., Di Rago, L., Mifsud, S., Hartley, L. & Alexander, W. S. (2000) Proc. Natl. Acad. Sci. USA 97 9174-9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka, T., Tsutsui, H., Fujimoto, M., Kawazoe, Y., Kohzaki, H., Morita, Y., Nakagawa, R., Narazaki, M., Adachi, K., Yoshimoto, T., et al. (2001) Immunity 14 535-545. [DOI] [PubMed] [Google Scholar]
- 8.Szabo, P. & Mann, J. R. (1994) Development (Cambridge, U.K.) 120 1651-1660. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran, L. M. & Metcalf, D. (1999) J. Immunol. 163 5836-5842. [PubMed] [Google Scholar]
- 10.Metcalf, D., Alexander, W. S., Elefanty, A. G., Nicola, N. A., Hilton, D. J., Starr, R., Mifsud, S. & Di Rago, L. (1999) Leukemia 13 926-934. [DOI] [PubMed] [Google Scholar]
- 11.Cornish, A. L., Davey, G. M., Metcalf, D., Purton, J. F., Corbin, J. E., Greenhalgh, C. J., Darwiche, R., Wu, L., Nicola, N. A., Godfrey, D. I., et al. (2003) J. Immunol. 170 878-886. [DOI] [PubMed] [Google Scholar]
- 12.de Vries, M. J. & Vos, O. (1959) J. Natl. Cancer Inst. 23 1403-1439. [Google Scholar]
- 13.Rappaport, H., Khalil, A., Halle-Pannenko, O., Pritchard, L., Dantchev, D. & Mathe, G. (1979) Am. J. Pathol. 96 121-142. [PMC free article] [PubMed] [Google Scholar]
- 14.van Bekkum, D. W., de Vries, M. J. & van der Waay, D. (1967) J. Natl. Cancer Inst. 38 223-231. [PubMed] [Google Scholar]
- 15.Eyles, J. L., Metcalf, D., Grusby, M. J., Hilton, D. J. & Starr, R. (2002) J. Biol. Chem. 277 43735-43740. [DOI] [PubMed] [Google Scholar]
- 16.Nagaraju, K., Raben, N., Loeffler, L., Parker, T., Rochon, P. J., Lee, E., Danning, C., Wada, R., Thompson, C., Bahtiyar, G., et al. (2000) Proc. Natl. Acad. Sci. USA 97 9209-9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Threadgill, D. W., Yee, D., Matin, A., Nadeau, J. H. & Magnuson, T. (1997) Mamm. Genome 8 390-393. [DOI] [PubMed] [Google Scholar]
- 18.Simpson, E. M., Linder, C. C., Sargent, E. E., Davisson, M. T., Mobraaten, L. E. & Sharp, J. J. (1997) Nat. Genet. 16 19-27. [DOI] [PubMed] [Google Scholar]
- 19.Sechler, J. M., Yip, J. C. & Rosenberg, A. S. (1997) J. Immunol. 159 5766-5768. [PubMed] [Google Scholar]
- 20.Cornish, A. L., Chong, M. M., Davey, G. M., Darwiche, R., Nicola, N. A., Hilton, D. J., Kay, T. W., Starr, R. & Alexander, W. S. (2003) J. Biol. Chem., in press. [DOI] [PubMed]
- 21.Chong, M. M., Cornish, A. L., Darwiche, R., Stanley, E. G., Purton, J. F., Godfrey, D. I., Hilton, D. J., Starr, R., Alexander, W. S. & Kay, T. W. (2003) Immunity 18 475-487. [DOI] [PubMed] [Google Scholar]
- 22.Egan, P. J., Lawlor, K. E., Alexander, W. S. & Wicks, I. P. (2003) J. Clin. Invest. 111 915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]