Abstract
Oxidative stress has been implicated in cognitive impairment in both old experimental animals and aged humans. This implication has led to the notion that antioxidant defense mechanisms in the brain are not sufficient to prevent age-related increase in oxidative damage and that dietary intake of a variety of antioxidants might be beneficial for preserving brain function. Here we report a dramatic loss of learning and memory function from 8 to 11 months of age in mice, associated with marked increases in several markers of brain oxidative stress. Chronic systemic administration of two synthetic catalytic scavengers of reactive oxygen species, Eukarion experimental compounds EUK-189 and EUK-207, from 8 to 11 months almost completely reversed cognitive deficits and increase in oxidative stress taking place during this time period in brain. In particular, increase in protein oxidation was completely prevented, whereas increase in lipid peroxidation was decreased by ≈50%. In addition, we observed a significant negative correlation between contextual fear learning and levels of protein oxidation in brain. These results further support the role of reactive oxygen species in age-related learning impairment and suggest potential clinical applications for synthetic catalytic scavengers of reactive oxygen species.
Aging in humans, as well as in experimental animals, is associated with a slow deterioration of cognitive performance and, in particular, of learning and memory (1–5). In humans, recent studies (6) have indicated the importance of impaired learning and memory processes, because 12% of humans with mild cognitive impairment will develop Alzheimer's disease as opposed to 2% of the general population. Oxidative damage has long been proposed to be critically involved in several pathological manifestations of aging (7, 8). Numerous studies (9–12) have indeed reported increases in protein oxidation and lipid peroxidation in various regions of aged mammalian brains. These findings have led to the notion that antioxidant defense mechanisms in the brain are not sufficient to prevent age-related increase in oxidative damage and that dietary intake of a variety of antioxidants might be beneficial for preserving brain function. In agreement with this idea, synthetic catalytic molecules that exhibit both superoxide dismutase (SOD) and catalase activity significantly increase the mean and maximum lifespan in Caenorhabditis elegans (13) and alleviate lethal oxidative pathologies in mice with genetically deleted Mn-SOD (14). The present studies tested, in wild-type mice, the effects of such molecules on age-related learning and memory impairment, and on markers of oxidative damage in the brain. We found that C57BL/6N Sim mice exhibit a dramatic decrease in learning and memory function between 8 and 11 months of age, accompanied by a marked increase in brain oxidative damage. Chronic treatment of mice with two recently developed SOD/catalase mimetics over a 3-month period almost completely reversed the age-related decline in cognitive function and increase in brain oxidative stress. These results further support the role of reactive oxygen species in age-related learning impairment and suggest potential clinical applications for synthetic catalytic scavengers of reactive oxygen species.
Materials and Methods
Materials. EUK-189 and EUK-207 were obtained from Eukarion (Bedford, MA). The mAb to oxo8dG/oxo8G was purchased from QED Bioscience (San Diego). Alzet 2004 miniosmotic pumps were obtained from Durect (Cupertino, CA). All other chemicals used were purchased from Sigma, unless indicated otherwise.
Mice and Treatments. The subjects were 105 C57BL/6N Sim (C57) female mice purchased from Simonsen Laboratories (Santa Clara, CA) at 8 months of age. Before experiments, mice were housed four per cage on sawdust bedding in the same room with a 12-h light/dark cycle and behavioral testing was performed during the light phase of the cycle. Mice were allowed free access to food and water, and their weights ranged from 22 to 32 g. Before surgery, mice were randomly assigned into one of the five following groups (16–18 mice per group): vehicle control and EUK-treated groups (low- or high-dose EUK-189 or EUK-207). An additional group of 17 8-month-old female mice was used for the untreated 8-month-old control group. Before implantation, Alzet 2004 miniosmotic pumps were primed for at least 40 h in 5% mannitol at 37°C, then loaded with either EUK-189 or EUK-207 at 1.5 mM (low concentration) or at 15 mM (high concentration) in 5% mannitol, or 5% mannitol alone (as vehicle control group). The minipumps were implanted s.c. in the 8-month-old mice according to the manufacturer's recommendations. Briefly, mice were weighed and anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg) by i.p. injection. Mice were placed into dorsal recumbency for surgery. A small 1-cm incision was made to the hip area of the mice and a small pocket was formed by spreading the s.c. connective tissues apart. The pump was placed into the prepared pocket, and the wound was then closed with 9-mm autoclips (Stoelting). Pumps delivered the drugs at 0.25 μl/h for a 28-day period, and the calculated drug infusion rates were ≈9 nmol/day for the 1.5 mM low doses of EUK and 0.09 μmol/day for the 15 mM high doses of EUK. During a 3-month treatment, pumps were replaced twice with new ones at the original sites at the end of each 28-day period of implantation. In ≈10% of the mice, the new pumps were placed on the other side of of hip area due to skin damage at the original site of implantation.
Behavioral Testing. Fear conditioning. Experiments were run in a conditioning chamber consisting of a Plexiglas cage (29 × 29 × 29 cm) with a grid floor composed of 26 stainless steel rods (0.48 cm in diameter; Coulbourn Instruments, Allentown, PA). The apparatus was located in a soundproof room. A personal computer controlled the experimental events and a video camera monitoring system was used to record freezing behavior, which is used as an index of the fear-conditioning tests (contextual and tone conditioning), for later evaluation. Each chamber was wiped with 5% ammonium hydroxide solution before training and each mouse was placed in the chamber individually. On day 1 of training, mice were put in the chamber and, after 3 min, they received three tone and foot-shock pairings (tone, 20 sec, 85 dB, 2 kHz; foot shock, 1 sec, 0.5 mA; intertrial interval, 1 min apart). One min after the final foot shock, mice were returned to their home cages. On day 2 (testing for conditioning to context), mice were placed into the experimental chamber for 8 min. Neither foot shock nor tone was given during this test. On day 3 (testing for conditioning to tone), mice were placed in an observation chamber that was completely different from the one used during conditioning in its visual and tactile properties. After 1 min in the new chamber, the same tone was presented for an 8-min test.
Behavioral analysis. Fear conditioning was measured as the percentage of time mice exhibited a freezing response. Freezing is defined as the absence of all visible movement of the body and vibrissae, aside from movement necessitated by respiration. The freezing was scored every minute and the data were converted into percentage of freezing time for every min of observation.
Mice were also tested for auditory (startle threshold) and visual (visually guided behavior) functions and for nociception (onset latency to tail flick on 51°C hot plate).
Assays for Lipid Peroxidation and Protein Oxidation. For biochemical studies, mice were killed by decapitation. Their brains were rapidly removed, dissected on a chilled platform, immediately frozen on dry ice, and stored at -70°C until assayed. Lipid peroxidation and protein oxidation were measured in brain homogenates from 11-month-old vehicle controls, EUK-189- or EUK-207-treated C57 mice, and 8-month-old untreated control mice.
The levels of lipid peroxidation were quantified by the thiobarbituric acid-reactive substances (TBARS) assay as previously described (15) with minor modifications. The tissues were homogenized in 2.5% SDS containing 6.25 μM deferoxamine and 12.5 μM probucol (to prevent further oxidation). Four hundred microliters of homogenates was added to an aqueous solution consisting of 375 μl of 20% acetic acid solution (pH 3.5) and 225 μl of 1.33% thiobarbituric acid, and the mixture was heated at 95°C for 1 h. One ml of a 15:1 1-butanol/pyridine solution was added, and TBARS were extracted into the organic layer by centrifugation at 4,000 × g for 10 min. The amounts of TBARS were determined by spectrophotometry at 532 nm and were calculated as nmol malondialdehyde equivalent per mg of protein according to a standard curve prepared from malonaldehyde bis(dimethyl acetal).
Protein carbonyl content, as an index of protein oxidation, was measured by a modification of a described technique (16). Briefly, sample tissues were homogenized in 50 mM phosphate buffer at pH 7.4 (10% wt/vol) and centrifuged at 11,000 × g for 15 min to sediment insoluble materials. The resulting supernatants containing 2–5 mg of soluble proteins were used for reaction with 2,4-dinitrophenylhydrazine (DNPH). For each sample, the supernatants were divided into two equal volumes. Four volumes of 10 mM DNPH in 2 M HCl were added to one of the sample pair, and four volumes of 2 M HCl alone were added to the other one (for reagent blank assay). Samples were then incubated for 1 h at room temperature in the dark with continuous stirring and were precipitated with an equal volume of 20% trichloroacetic acid (TCA). After 10 min on ice, samples were centrifuged at 3,000 × g for 5 min and supernatants were discarded. Protein pellets were washed in 10% TCA once and in ethanol/ethyl acetate (1:1) three times to remove free DNPH and additional lipid contaminants. Final protein precipitates were dissolved in 6 M guanidine hydrochloride solution. The differences (Δ) in absorbance between the DNPH-treated and the HCl-treated samples were determined by spectrophotometry at 375 nm, and the amount of carbonyl contents (C) was calculated by using a molar extinction coefficient (ε) of 22,000-1 cm-1 [C in nmol/ml = A375 (Δ) × 106/ε]. Data were expressed as nmol carbonyl per mg of soluble extracted protein.
Immunohistochemistry for Oxidized Nucleic Acids. Mice were anesthetized and fixed by transcardial perfusion using 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were cryoprotected with sucrose and sectioned at 25 μm on a microtome. Immunocytochemistry was performed by using the avidin–biotin–horseradish peroxidase complex (ABC) method with reagents and instructions of the VECTASTAIN Elite ABC kit from Vector Laboratories as described (17). Free-floating tissue sections were incubated in 10% normal horse serum diluted in PBS for 1 h at room temperature, and then in the presence of a mAb to oxo8dG/oxo8G (1:1,000) in 5% horse serum diluted in PBS overnight at 4°C. Sections were washed in PBS, incubated in biotinylated anti-mouse IgG (1:200 in PBS with 5% normal horse serum) for 2–3 h, washed in PBS, and incubated in the avidin–biotin complex solution for 45 min. After the diaminobenzidine reaction, sections were mounted onto gelatin-coated slides, air-dried, dehydrated in a series of graded ethanol, and coverslipped with Permount. Images were obtained with a Zeiss microscope (Axioskop 2 mot plus; ×10 objective, 0.75 optovar). Images were digitized by an AxioCam Hrc digital photo camera (Zeiss), and the automated image-scanning program KS 400 (Zeiss) was used to capture and save digitized images.
Results
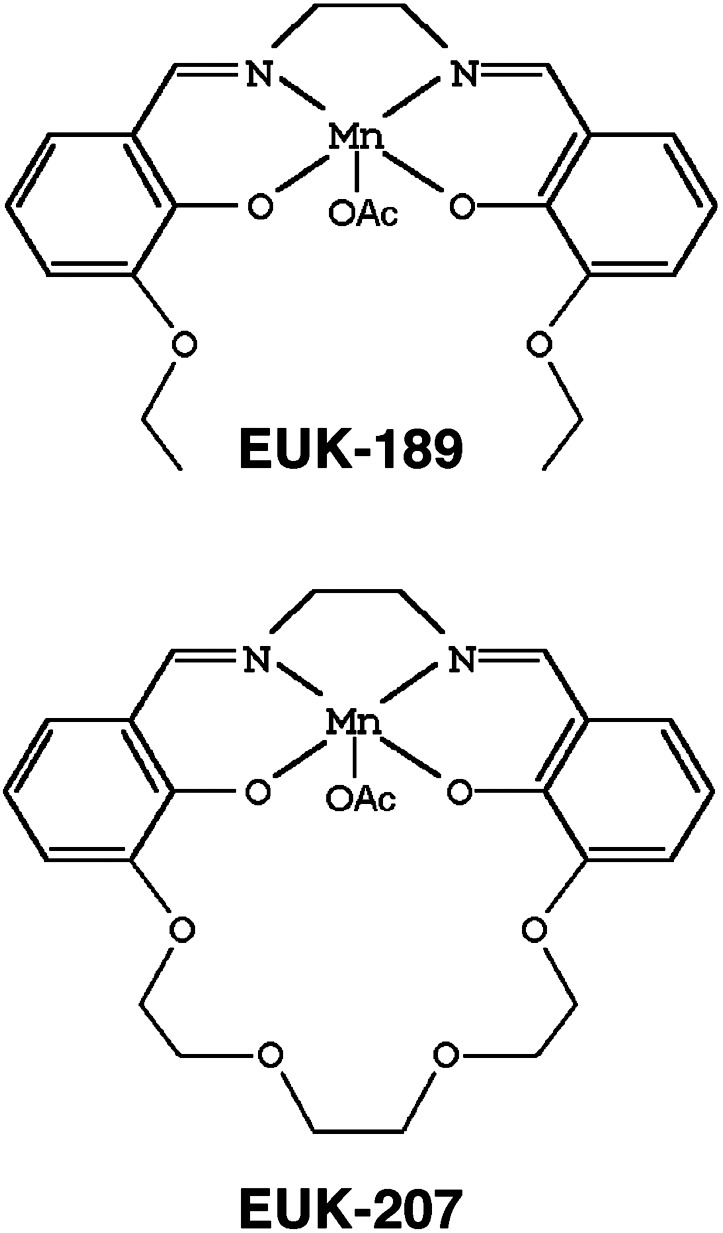
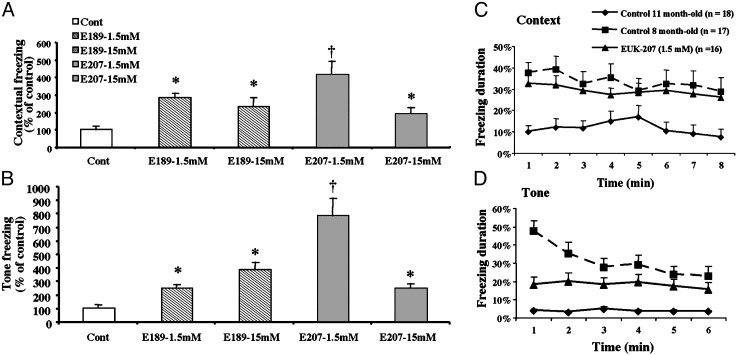
Effects of SOD/Catalase Mimetics on Fear-Conditioning Learning. We used two synthetic catalytic SOD/catalase mimetics in this study. EUK-189 is very similar to EUK-134, one molecule previously used in the C. elegans studies, having equivalent catalytic properties but increased lipophilicity and neuroprotective activity (14, 18). The second compound, EUK-207, is a cyclic analog of the class exemplified by EUK-134 and EUK-189 (Fig. 1). Compared with these compounds, EUK-207 exhibits similar catalytic activities but greater biological stability, as reflected, for example, by its longer plasma half-life. EUK-207 is, however, significantly less lipophilic than EUK-189. This is our first report of the biological activity of EUK-207. However, unpublished experiments similar to those reported by Pong et al. (18), showed that EUK-207 has potency comparable with that of EUK-189 in preventing neuronal apoptosis in vitro (K. Pong, S.R.D., and M.B., unpublished work). Based on this and preliminary observations with other bridged analogs as model compounds (C. Marcus, E. Malfroy, B.M., and S.R.D., unpublished work), we believe that lipophilicity and the added stability of the bridged structure are two independent factors that enhance efficacy. Both compounds were administered chronically by using s.c. implanted Alzet minipumps, delivering ≈9 nmol/day (low dose) or 0.09 μmol/ day (high dose) to female mice beginning at 8 months of age. The lower dose was equivalent to ≈0.14–0.2 and 0.15–0.22 mg/kg per day for EUK-189 and EUK-207, respectively. Control mice were implanted with pumps containing only vehicle, 5% mannitol in water. After 3 months of continuous treatment, mice were subjected to a fear-conditioning paradigm in which a tone and a context were associated with a mild foot shock (19, 20). The next day, mice were reexposed to the same context, and the amount of freezing during an 8-min period was recorded. On day 3, mice were exposed to the tone in a different context and the amount of freezing was recorded. Control 11-month-old mice (treated with vehicle only) exhibited low levels of freezing with both tone and context presentation, suggesting that these middle-aged mice already had impaired learning and memory (Fig. 2). Both EUK-189 and EUK-207 treatment dramatically increased tone- and context-evoked freezing (Fig. 2 A and B); the effects were significant at both doses. The effects were particularly large for the low dose of EUK-207, which produced a 4- to 8-fold enhancement in freezing duration. When comparing the performance of EUK-207-treated 11-month-old mice with that of 8-month-old control mice, the drug appeared to almost completely reverse the age-related deficit (Fig. 2 C and D). These data make the further point that there is a dramatic decrease in learning between 8 and 11 months of age in this strain of mice, whether the mice were tested with the context or the tone paradigm. It is also important to note that no significant visual and auditory defects were observed for all groups, and no differences were found in nociception (tail-flick latency). Although we did not directly test for shock sensitivity, these results suggest that the age-related differences in learning and memory are not related to changes in nociception.
Fig. 1.
Structures of EUK-189 and EUK-207.
Fig. 2.
Effects of chronic treatment with EUK-189 or EUK-207 on context and tone conditioning. Eight-month-old C57 mice were treated for 3 months with a low (1.5 mM) or a high (15 mM) concentration of EUK-189 or EUK-207 delivered i.p. continuously through Alzet minipumps. At the end of 3 months, mice were conditioned to context and tone as described in Materials and Methods. Results were calculated as percent time the mouse expressed freezing behavior during the 8-min observation period for context (A) and 6 min for tone (B). The data were then expressed as the percent of the corresponding values in vehicle control mice. Shown are means ± SEM of 16–18 mice. *, P < 0.01; †, P < 0.005 (Student's t test). Control 8-month-old mice were subjected to the same training and testing procedures, and the data were calculated as percent time the mouse expressed freezing behavior for the 8-min observation period for context (C) and 6 min for tone (D). Results are means ± SEM of 16–18 mice. Repeated-measures ANOVA indicated that the effect of age was highly significant (P < 0.001), as was the effect of EUK-207 (P < 0.01 vs. 11-month-old vehicle control). EUK-207 treatment completely reversed the aging effect on context, but only partially on tone.
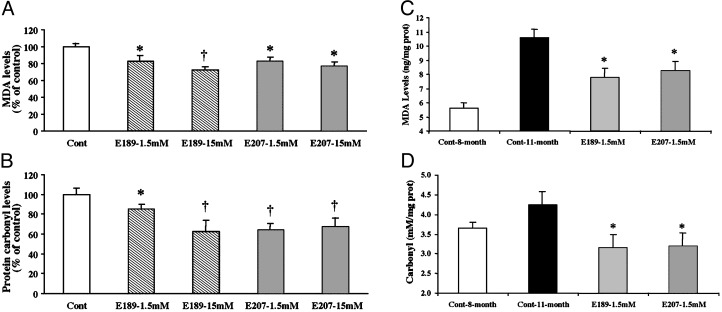
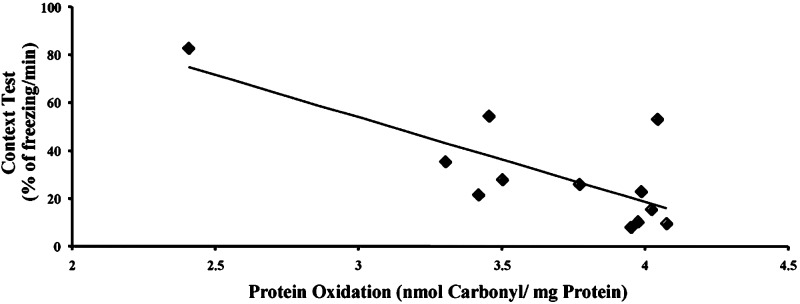
Effects of SOD/Catalase Mimetics on Brain Oxidative Stress. To determine the effects of the treatment on brain levels of oxidative load, we measured an index of lipid peroxidation (levels of equivalent malondialdehyde) as well as levels of protein oxidation in the whole brain (Fig. 3). Chronic treatment with either EUK-189 or EUK-207 produced decreases in lipid peroxidation and protein oxidation. The effects of the low doses of mimetics were generally more significant than the effects of the high doses, and represented a 20–25% reduction in the levels of oxidative stress (Fig. 3 A and B). A similar pattern of results was observed in heart, although the effects tended to be larger, with a 50% reduction in protein oxidation (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Levels of lipid peroxidation and protein oxidation in the brain were also significantly increased from 8 to 11 months (Fig. 3 C and D). Chronic treatment with both EUK-189 and EUK-207 completely reversed the age-related increase in protein oxidation and reduced by ≈50% the age-related increase in lipid peroxidation. Interestingly, the performance of the 8-month-old control mice in the contextual fear-conditioning paradigm was negatively correlated with the brain levels of protein carbonyls (Fig. 4). To further analyze the effects of chronic treatment with the lower concentration of EUK-207 on oxidative stress, we analyzed the distribution of oxidized nucleic acids by immunohistochemistry at the end of the treatment. Significant decreases in the levels of oxidized nucleic acids were observed in several brain structures, in particular in hippocampus and amygdala (Fig. 5). In hippocampus, marked accumulation of staining was present in the cell bodies of the pyramidal cells of field CA3, whereas the labeling was scattered in the amygdala. High-magnification examination of the staining (Fig. 5 Insets) indicated that it was present in the cytoplasm of the cells rather than in the nucleus, suggesting that it represents oxidized RNA, as was reported (12). Treatment with EUK-207 completely eliminated staining in both hippocampus and amygdala.
Fig. 3.
Effects of chronic treatment with EUK-189 or EUK-207 on lipid peroxidation and protein oxidation in brain homogenates. At the end of the behavioral experiments, the mice were killed and their brains (minus cerebellum) were frozen. Levels of lipid peroxidation and protein oxidation (protein carbonyls) were determined as described in Materials and Methods. Results were expressed as percentage of vehicle control value (mean ± SEM, n = 12; A and B). *, P < 0.05; †, P < 0.01 (Student's t test). Levels of lipid peroxidation and protein carbonyls were also determined in brain homogenates from the 8-month-old control C57 mice. The levels of lipid peroxidation were expressed as nmol malondialdehyde equivalent per mg of protein (C) and the levels of protein oxidation as nmol carbonyl per mg of soluble extracted protein (D), and are means ± SEM of 12 mice [C: *, P < 0.05 as compared with either 8- or 11 month-old control mice; D: *, results not significantly different from 8-month-old control mice; †, P < 0.005 as compared with 11-month-old vehicle mice (Student's t test)].
Fig. 4.
Correlation between performance in the contextual fear-conditioning task and brain levels of protein carbonyls. Individual data for contextual fear conditioning and brain protein carbonyl contents for the 8-month-old control mice were plotted and exhibited a significant negative correlation (Pearson's correlation coefficient, γ =-0.76, P < 0.005). The levels of protein oxidation were expressed as nmol of carbonyl per mg of soluble extracted protein, and contextual fear conditioning was scored as percentage of freezing duration per min (mean ± SEM of 12 mice). Note that deleting the two outlying points still resulted in a significant correlation, γ = -0.74, P < 0.005.
Fig. 5.
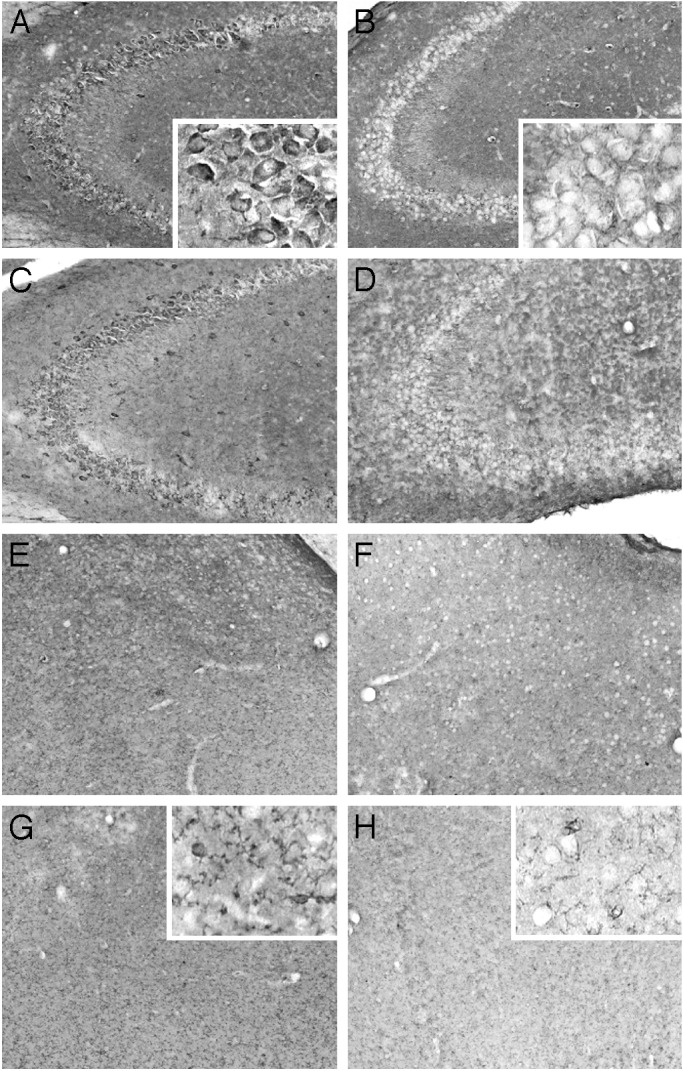
EUK-207 treatment decreases levels of oxidized nucleic acids in hippocampus and amygdala. Eleven-month-old control mice (A, C, E, and G) and EUK-207 (1.5 mM)-treated mice (B, D, F, and H) were perfused with 4% paraformaldehyde. Brains were sectioned at 25 μm and sections were processed for immunohistochemistry by using a mAb to oxo8dG/oxo8G as described in Materials and Methods. Note the clear decrease in oxo8dG/oxo8G immunoreactivity in the pyramidal cells of CA3 of treated mice (B and D) compared with control mice (A and C), and decrease in diffuse staining in amygdala (F and H vs. E and G). High-magnification Insets in A, B, G, and H illustrate the cytoplasmic cellular localization of the oxidized nucleic acids. Similar results were observed in five mice.
Discussion
Deficits in cognitive function have been reported in animals beyond late middle age, such as rats (12, 21, 22), gerbils (23), mice (24), monkeys (25), and aged humans (26, 27). These findings provided evidence that this age-related deleterious process occurs much earlier and that some cognitive abilities exhibit a dramatic decline between 8 and 11 months of age, consistent with one human study (28). From our survey of the literature, it appears that very few reports of animal studies have described changes in learning and memory during this period. One report compared learning and memory in the Morris water maze in C57BL/6Nia mice at 3, 14, and 25 months of age (29). Although there was no statistical difference between 3 and 14 months, there was a very clear trend. It was not statistically significant because there were only 5 mice per group, as opposed to 16–18 mice per group in our study. In another report (30, 31), spatial water maze deficits were observed as early as 10 months of age in C57 male mice, although they were more evident at later ages, and these age-related losses of cognitive function were associated with oxidative protein damage in the brain (30). Our results also indicate that this early-onset decline in learning and memory is associated with a very significant increase in two parameters of oxidative stress in the brain, levels of lipid peroxidation and of protein oxidation. These results support the hypothesis that oxidative stress contributes to age-related impairment in learning and memory. Previous studies (11) have shown that oxidative stress increases almost linearly with age, by ≈40%, from 2–3 to 20–21 months of age in mice. Several studies (12, 23, 24, 32) have also reported that chronic or subchronic treatment with a variety of antioxidants partially reversed age-related increase in markers of oxidative stress and decline in learning and memory in old mice. Our results further expand these studies by showing that a complete reversal in protein oxidation and a 50% reduction in age-related increase in lipid peroxidation can be attained during a 3-month chronic treatment period with a low concentration of a SOD/catalase mimetic in relatively young (8–11 months of age) mice. The same treatment dramatically decreased the levels of oxidized nucleic acids throughout the brain, particularly in the hippocampus and the amygdala, two structures critically involved in learning and memory. This treatment resulted in an almost complete reversal of age-related learning and memory deficit, suggesting that increased protein oxidation might be more critical for decline in cognitive function than lipid peroxidation. In agreement with this idea, we observed a significant negative correlation between contextual fear learning and levels of protein oxidation in brain.
Our results also indicate that there might be an optimal level of antioxidant required for maintaining the balance between prooxidant and antioxidant factors, as high doses of the SOD/ catalase mimetics generally provided less protection than did low doses. Interestingly, the same doses that provided the most protection against oxidative stress also provided the largest effects on learning and memory, further supporting the critical role of oxidative stress in age-related cognitive decline.
Context and tone conditioning are critically dependent on the functional integrity of both hippocampus and amygdala (33–36). Treatment with the SOD/catalase mimetics were effective in improving both types of learning, suggesting that age-related deficits in these two forms of learning are at least in part due to oxidative damage in both hippocampus and amygdala. Although there are suggestions that different brain structures are differentially susceptible to oxidative stress, our results would indicate that SOD/catalase mimetics are equally effective in preventing oxidative stress in hippocampus and amygdala, as indicated by the decrease in oxidized nucleic acids in both structures.
In conclusion, our data indicate that the dramatic decline in learning and memory taking place in early middle-aged mice is associated with a large increase in brain oxidative stress, and that chronic treatment with low doses of SOD/catalase mimetics almost completely reversed the behavioral and the biochemical changes. They further support a role for oxidative stress in age-related cognitive impairment and suggest a potential therapeutic use of the SOD/catalase mimetics in mild cognitive impairment associated with aging.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Aging Grant R41 AG19531 to the University of Southern California (to M.B.) and Eukarion, Inc.
Abbreviation: SOD, superoxide dismutase.
References
- 1.Grady, C. L. & Craik, F. I. (2000) Curr. Opin. Neurobiol. 10 224-231. [DOI] [PubMed] [Google Scholar]
- 2.Dean, R. L., III, Scozzafava, J., Goas, J. A., Regan, B., Beer, B. & Bartus, R. T. (1981) Exp. Aging Res. 7 427-451. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher, M. & Nicolle, M. M. (1993) Behav. Brain Res. 57 155-162. [DOI] [PubMed] [Google Scholar]
- 4.Ingram, D. K., Spangler, E. L., Iijima, S., Ikari, H., Kuo, H., Greig, N. H. & London, E. D. (1994) Life Sci. 55 2037-2049. [DOI] [PubMed] [Google Scholar]
- 5.Burke, D. M. & Mackay, D. G. (1997) Philos. Trans. R. Soc. London B 352 1845-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., Ritchie, K., Rossor, M., Thal, L. & Winblad, B. (2001) Arch. Neurol. (Chicago) 59 1985-1992. [DOI] [PubMed] [Google Scholar]
- 7.Harman, D. (1981) Proc. Natl. Acad. Sci. USA 78 7124-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floyd, R. A. (1991) Science 254 1597. [DOI] [PubMed] [Google Scholar]
- 9.Oliver, C. N., Ahn, B. W., Moerman, E. J., Goldstein, J. & Stadtman, E. R. (1987) J. Biol. Chem. 262 5488-5491. [PubMed] [Google Scholar]
- 10.Smith, C. D., Carney, J. M., Starke-Reed, P. E., Oliver, C. N., Stadtman, E. R., Floyd, R. A. & Markesbery, W. R. (1991) Proc. Natl. Acad. Sci. USA 88 10540-10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leutner, S., Eckert, A. & Muller, W. E. (2001) J. Neural Transm. 108 955-967. [DOI] [PubMed] [Google Scholar]
- 12.Liu, J., Atamna, H., Kuratsune, H. & Ames, B. N. (2002) Ann. N.Y. Acad. Sci. 959 133-166. [DOI] [PubMed] [Google Scholar]
- 13.Melov, S., Ravenscroft, J., Malik, S., Gill, M. S., Walker, D. W., Clayton, P. E., Wallace, D. C., Malfroy, B., Doctrow, S. R. & Lithgow, G. J. (2000) Science 289 1567-1569. [DOI] [PubMed] [Google Scholar]
- 14.Melov, S., Doctrow, S. R., Schneider, J. A., Haberson, J., Patel, M., Coskun, P. E., Huffman, K., Wallace, D. C. & Malfroy, B. (2001) J. Neurosci. 21 8348-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce, A. J. & Baudry, M. (1995) Free Radical Biol. Med. 18 993-1002. [DOI] [PubMed] [Google Scholar]
- 16.Dubey, A., Forster, M. J., Lal, H. & Sohal, R. S. (1996) Arch. Biochem. Biophys. 333 189-197. [DOI] [PubMed] [Google Scholar]
- 17.Rong, Y., Doctrow, S. R., Tocco, G. & Baudry, M. (1999) Proc. Natl. Acad. Sci. USA 96 9897-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pong, K., Doctrow, S. R., Huffman, K., Adinolfi, C. A. & Baudry, M. (2001) Exp. Neurol. 171 84-97. [DOI] [PubMed] [Google Scholar]
- 19.Chen, C., Kim, J. J., Thompson, R. F. & Tonegawa, S. (1996) Behav. Neurosci. 110 1177-1180. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, R., Vouimba, R. M., Baudry, M. & Thompson, R. F. (1999) Nature 402 294-296. [DOI] [PubMed] [Google Scholar]
- 21.Landfield, P. W. (1988) Neurobiol. Aging 9 571-579. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher, M. & Pelleymounter, M. A. (1988) Neurobiol. Aging 9 549-556. [DOI] [PubMed] [Google Scholar]
- 23.Carney, J. M., Starke-Reed, P. E., Oliver, C. N., Landum, R. W., Cheng, M. S., Wu, J. F. & Floyd, R. A. (1991) Proc. Natl. Acad. Sci. USA 88 3633-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredriksson, A. & Archer, T. (1996) Behav. Pharmacol. 7 245-253. [PubMed] [Google Scholar]
- 25.Walker, L. C., Kitt, C. A., Struble, R. G., Wagster, M. V., Price, D. L. & Cork, L. C. (1988) Neurobiol. Aging 9 657-666. [DOI] [PubMed] [Google Scholar]
- 26.Ciocon, J. O. & Potter, J. F. (1988) Geriatrics 43 43-48. [PubMed] [Google Scholar]
- 27.Albert, M. S. (2002) Ann. Neurol. 51 282-284. [PubMed] [Google Scholar]
- 28.Woodruff-Pak, D. S. & Thompson, R. F. (1988) Psychol. Aging 3 219-229. [DOI] [PubMed] [Google Scholar]
- 29.Fordyce, D. E. & Wehner, J. M. (1993) Neurobiol. Aging 14 309-317. [DOI] [PubMed] [Google Scholar]
- 30.Forster, M. J., Dubey, A., Dawson, K. M., Stutts, W. A., Lal, H. & Sohal, R. S. (1996) Proc. Natl. Acad. Sci. USA 93 4765-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnusson, K. R. (1997) Mech. Ageing Dev. 95 187-202. [DOI] [PubMed] [Google Scholar]
- 32.Floyd, R. A., Hensley, K., Forster, M. J., Kelleher-Anderson, J. A. & Wood, P. L. (2002) Ann. N.Y. Acad. Sci. 959 321-329. [DOI] [PubMed] [Google Scholar]
- 33.Fanselow, M. S. (2000) Behav. Brain Res. 110 73-81. [DOI] [PubMed] [Google Scholar]
- 34.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23 155-184. [DOI] [PubMed] [Google Scholar]
- 35.Vazdarjanova, A. & McGaugh, J. L. (1999) J. Neurosci. 19 6615-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoniadis, E. A. & McDonald, R. J. (2000) Behav. Brain Res. 108 1-19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.