Abstract
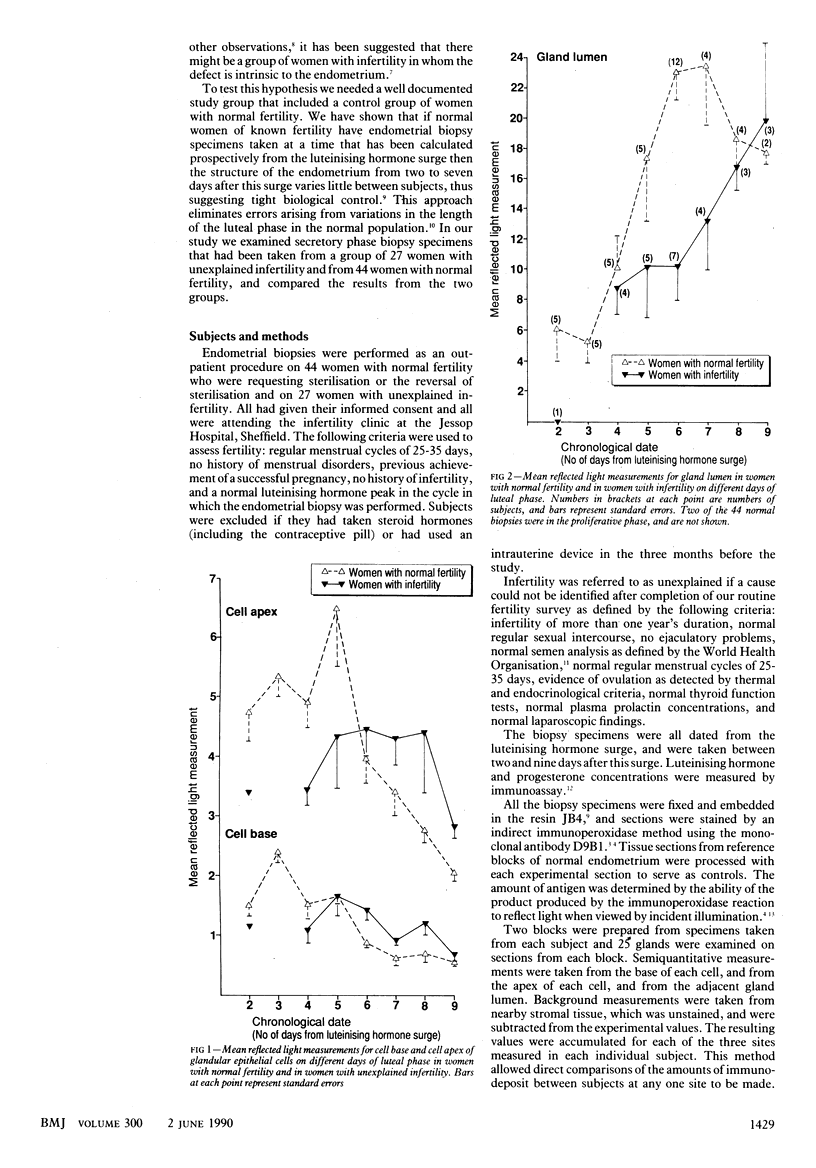
OBJECTIVE--To study a group of women with unexplained infertility to see whether they have a defect that is intrinsic to the endometrium. DESIGN--Evaluation of the functional response of the endometrium by examining endometrial biopsy specimens using immunohistochemical methods in a group of women with unexplained infertility and in a control group of women with normal fertility. PATIENTS--27 Women with unexplained infertility (average age 33.2); median duration of infertility five years. A control group of 44 women with normal fertility (average age 33.8) who were requesting sterilisation or reversal of sterilisation. SETTING--Infertility clinic, Jessop Hospital for Women, Sheffield. INTERVENTION--Secretory phase endometrial biopsy specimens were taken, with informed consent, as an outpatient procedure. MAIN OUTCOME MEASURES--Immunohistochemistry with monoclonal antibody D9B1, was used to assess the production and secretion of an oligosaccharide epitope produced by endometrial gland cells between two and seven days after the luteinising hormone surge. A reflected light measuring system was used to assess the amount of epitope within the gland cells, and in the gland lumen. RESULTS--In the control group of women, mean reflected light measurements at the cell base and cell apex peaked at three and five days after the luteinising hormone surge respectively, and in the gland lumen the epitope accumulated rapidly from three days, reaching a peak at seven days. In the women with infertility the peaks of epitope at the cell base and cell apex were lower, broader, and delayed in onset, and the build up of epitope in the gland lumen was retarded. The synthesis and secretion of the epitope in the women with infertility was therefore significantly reduced and delayed, even in the presence of normal concentrations of circulating progesterone. CONCLUSIONS--The results suggest that a primary dysfunction of the endometrium might be associated with hitherto unexplained infertility.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Seif M. W. A monoclonal antibody to a cell surface determinant in human endometrial epithelium: stage-specific expression in the menstrual cycle. Am J Obstet Gynecol. 1987 Jan;156(1):250–253. doi: 10.1016/0002-9378(87)90247-x. [DOI] [PubMed] [Google Scholar]
- Dockery P., Li T. C., Rogers A. W., Cooke I. D., Lenton E. A., Warren M. A. An examination of the variation in timed endometrial biopsies. Hum Reprod. 1988 Aug;3(6):715–720. doi: 10.1093/oxfordjournals.humrep.a136771. [DOI] [PubMed] [Google Scholar]
- Hoadley M. E., Seif M. W., Aplin J. D. Menstrual-cycle-dependent expression of keratan sulphate in human endometrium. Biochem J. 1990 Mar 15;266(3):757–763. doi: 10.1042/bj2660757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton E. A., Gelsthorp C. H., Harper R. Measurement of progesterone in saliva: assessment of the normal fertile range using spontaneous conception cycles. Clin Endocrinol (Oxf) 1988 Jun;28(6):637–646. doi: 10.1111/j.1365-2265.1988.tb03855.x. [DOI] [PubMed] [Google Scholar]
- Li T. C., Lenton E. A., Dockery P., Rogers A. W., Cooke I. D. The relation between daily salivary progesterone profile and endometrial development in the luteal phase of fertile and infertile women. Br J Obstet Gynaecol. 1989 Apr;96(4):445–453. doi: 10.1111/j.1471-0528.1989.tb02421.x. [DOI] [PubMed] [Google Scholar]
- Li T. C., Rogers A. W., Lenton E. A., Dockery P., Cooke I. A comparison between two methods of chronological dating of human endometrial biopsies during the luteal phase, and their correlation with histologic dating. Fertil Steril. 1987 Dec;48(6):928–932. doi: 10.1016/s0015-0282(16)59585-5. [DOI] [PubMed] [Google Scholar]
- Seif M. W., Aplin J. D., Awad H., Wells D. The effect of the intrauterine contraceptive device on endometrial secretory function: a possible mode of action. Contraception. 1989 Jul;40(1):81–89. doi: 10.1016/0010-7824(89)90030-9. [DOI] [PubMed] [Google Scholar]
- Seif M. W., Aplin J. D., Buckley C. H. Luteal phase defect: the possibility of an immunohistochemical diagnosis. Fertil Steril. 1989 Feb;51(2):273–279. doi: 10.1016/s0015-0282(16)60490-9. [DOI] [PubMed] [Google Scholar]
- Seif M. W., Aplin J. D., Foden L. J., Tindall V. R. A novel approach for monitoring the endometrial cycle and detecting ovulation. Am J Obstet Gynecol. 1989 Feb;160(2):357–362. doi: 10.1016/0002-9378(89)90444-4. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Seif M. W., Rogers A. W., Li T. C., Dockery P., Cooke I. D., Aplin J. D. The endometrial cycle: the expression of a secretory component correlated with the luteinizing hormone peak. Hum Reprod. 1989 Apr;4(3):236–242. doi: 10.1093/oxfordjournals.humrep.a136878. [DOI] [PubMed] [Google Scholar]
- Templeton A. A., Penney G. C. The incidence, characteristics, and prognosis of patients whose infertility is unexplained. Fertil Steril. 1982 Feb;37(2):175–182. doi: 10.1016/s0015-0282(16)46035-8. [DOI] [PubMed] [Google Scholar]


