Abstract
Functional signaling of endothelin 3 (ET3) and its receptor B (ETRB) has been shown to be required for the development of neural crest (NC)-derived pigment cells in mouse, but the precise role of ET3 is not completely understood. Using the avian embryo as a model, we previously reported that ET3 promotes the survival and proliferation of unipotent melanocyte and bipotent glia-melanocyte precursors in trunk NC cultures. Here we investigated whether, at later stages, embryonic pigment cells respond to ET3. Such a possibility is supported by the previous finding that, in vivo, avian melanocytes express endothelin receptor B2 (ETRB2) during migration and after their differentiation in the skin. We found that in vitro ET3 exerts a dose-dependent stimulation of proliferation and melanogenesis in NC cells that had homed to the epidermis of embryonic quail dorsal skin. Moreover, in clonal cultures of skin-derived pigment cells, ET3 induces rapid cell divisions of clonogenic melanocytes that generate a mixed progeny of melanocytes and cells devoid of pigment granules and expressing glial markers in more than 40% of the colonies. It can therefore be concluded that ET3 is strongly mitogenic to embryonic pigment cells and able to alter their differentiation program, leading them to recapitulate the glial-melanocyte bipotentiality of their NC ancestors.
Keywords: epidermis, quail embryo, clonal cultures, transdifferentiation, Schwann cell myelin protein
The pigment cells of the body are one of the numerous derivatives of the neural crest (NC), a pluripotent structure of the vertebrate embryo that forms from the lateral ridges of the dorsal neural primordium. In addition to the melanocytes, the NC is the source of the neurons and glia of the peripheral nervous system, of endocrine cells and, at the cephalic level, of mesenchymal cells (the mesectoderm) that differentiate into connective tissues and smooth muscles and form most of the head skeleton (see refs. 1 and 2 for reviews). These multiple cell types arise from multipotent precursors, whose existence was demonstrated in avian and rodent NC at the premigratory and migratory stages (3–9). The population of migratory NC cells also includes lineage-restricted precursors, which increase in number as development proceeds (5, 10–15). Finally, the phenotype of the various NC derivatives is controlled by exposure of the cells to environmental signals during their migration and at their homing sites. A number of these signals have been characterized in mice by analyzing spontaneous mutations or by gene targeting (see refs. 16–18 for reviews).
Thus the spontaneous or experimental inactivation of the murine genes encoding endothelin 3 (ET3) and its receptor (endothelin receptor B) (ETRB) causes defects of body pigmentation and posterior gut innervation (19, 20). To understand the role for ET3/ETRB signaling in the development of NC-derived cells, we have studied these ligand/receptor system in avian NC ontogeny. Avian ETRB and ET3 genes were shown to have complementary expression patterns in the gut during NC cell colonization: the enteric NC precursors express ETRB whereas the gut mesenchyme expresses ET3 (21, 22). In the skin, ET3 is expressed by the dermal mesenchyme in the mouse (23) and by the ectoderm from embryonic day 3 (E3) onward, and later on, the epidermis in avian embryos (22). In mammals, ETRB is detected along the mediolateral pathway of NC cell migration, consistent with its expression by presumptive melanocytes (24). In avian embryos, ETRB starts to be expressed by premigratory NC cells, and, later on, the NC cells that migrate dorsoventrally and their neural derivatives still express this receptor (21) whereas those taking the mediolateral pathway down-regulate the ETRB gene and switch to express another ETRB subtype, ETRB2 (25). ETRB2 gene, which is not present in NC cells migrating dorsoventrally, remains activated in melanoblasts and differentiated melanocytes in the feather buds. These data argue for a paracrine action exerted in the skin by ET3 on NC cells through ETRB in mouse and ETRB2 in birds. Moreover, ET3 turned out to be a potent mitogen to cultured quail NC cells. It promotes their differentiation into melanocytes (26) and stimulates ETRB2 expression while increasing the survival and proliferation rates of committed melanocytic precursors. In previous cloning analysis of NC cell differentiation, we could identify bipotential glial-melanocytic (GM) precursors in the migrating NC cell population (5, 7, 27) that are able to respond to ET3 by enhanced survival and proliferation as well as by a spectacular increase in melanogenesis (28).
The ET3/ETRB2 interactions therefore have a crucial role in the early development of NC-derived melanocyte precursors. Moreover, because melanocytes continue to strongly express ETRB2 while differentiating in the epidermal environment expressing ET3 (25), the question was raised as to whether their terminal differentiation within the epidermis could also be controlled by ET3. To further investigate the role of ET3 on the differentiation of NC derivatives, we have tested the effect of ET3 on pigment cells isolated in vitro from the quail embryonic epidermis. We found that ET3 exerts a strong effect on the proliferation of already differentiated melanocytes. Moreover, some cells loose their melanocytic properties and exhibit glial markers, thus showing that they are the site of a transdifferentiation process already described in retinal pigmented epithelial cells (29).
Materials and Methods
Cultures of Embryonic Epidermis.
E7.5 quail dorsal skin was excised by microsurgery. At this stage, some feather buds contain differentiated pigment cells in the thoraco-lumbar area. The epidermis was isolated from the dermis after incubating skin explants in pancreatin (GIBCO/BRL) (1:4 in DMEM) for 30 min at 37°C. Pieces of epidermis were then transferred to DMEM plus 15% FCS for 15 min, were rinsed in PBS, and were dissociated to single cells after incubation in trypsin (GIBCO/BRL) (0.25% in PBS, 10 min, 37°C). Epidermal cells were collected by centrifugation and were counted and plated at the center of culture dishes at the density of 5 × 104 cells per 50 μl in culture medium supplemented with 10% FCS, 2% chicken embryo extract, and growth factors (28). The day after, cells were fed with medium in absence or presence of ET3 (Sigma), at concentrations ranging from 0.01 to 100 nM. Cultures were maintained for 9 days at 37°C in an atmosphere of 5% CO2 and 95% air.
Isolation of Epidermal Pigment Cells by Flow Cytometry.
Epidermal cells were prepared as described above and were grown in culture medium enriched with 7% chicken embryo extract to enhance melanogenesis. After 2 days, pigment cells accounted for 15–20% of the cells in the cultures. Cells were detached by trypsin treatment and were collected by centrifugation. The pellet was resuspended in PBS (106 cells/ml) and was filtered on a nylon membrane (25 μm-pore diameter) before cell sorting by using a fluorescence-activated cell sorter (FACS Star-plus; Beckton Dickinson). A 95–98% pure fraction of pigment cells was isolated according to morphological parameters (see Results). All of the other fractions tested contained both unpigmented and pigmented cells in variable proportions (not shown). Purified pigment cells were collected and plated in culture medium with or without ET3 as described for non-sorted cells.
Clonal Cultures of Pigment Cells.
Cloning of pigment cells was performed by micromanipulation, starting from 2-day cultured epidermal cell suspensions. The epidermal cell suspension (5 × 103 cells/ml) was inspected under bright-field microscopy to distinguish between cells with or without pigment granules. Individual pigment cells were then picked up with a micropipette under microscopic control and were seeded in 70-μl drops of culture medium previously prepared in culture dishes. After 3 h, the position of each adherent pigment cell was indicated by a circular mark on the underside of the dish. Culture medium alone or containing 50 nM ET3 was added and changed every 3 days thereafter.
Immunocytochemistry and Culture Analysis.
Cultures were inspected at regular intervals to examine cell morphology, growth, and pigmentation. The total number of cells in pigment clonal cultures was counted under phase-contrast microscopy at day 1 (d1), d2, d3, d5, d9, and d13. Mass and clonal cultures were fixed at d9 and d13, respectively, as described (28). Pigment cells were recorded microscopically, and unpigmented cells were phenotypically analyzed by immunocytochemistry. Glial-specific proteins were identified by using the Schwann cell myelin protein (SMP) mAb (30, 31) and 1E8 mAb that recognizes the myelin protein Po expressed by Schwann cells and their precursors from E3 in chick (32) and quail (E.D., unpublished work). The cultures were labeled with the anti-SMP mAb followed by detection with fluorescein-Tyramide using the “direct tyramide signal amplification kit” (NEN), and then were stained with 1E8 mAb followed by Texas red-conjugated secondary antibody. After documenting glial cells, the colonies devoid of pigment cells were stained with the melanoblast/cyte early marker (MelEM) mAb (33) to assess the presence of melanoblasts, as described (28). Fluorescence was observed with a Microphot-FXA microscope (Nikon). Secondary antibodies were from Southern Biotechnology Associates, and 1E8 hybridoma cells were from Developmental Studies Hybridoma Bank at the University of Iowa.
The frequencies of the various clone types were analyzed by χ2 test [statistica for Macintosh (Statsoft, Tulsa, OK)], and differences between ET3-treated and control cultures were considered to be statistically significant when P < 0.05.
Results
Effect of ET3 on Cultures of E7.5 Epidermis.
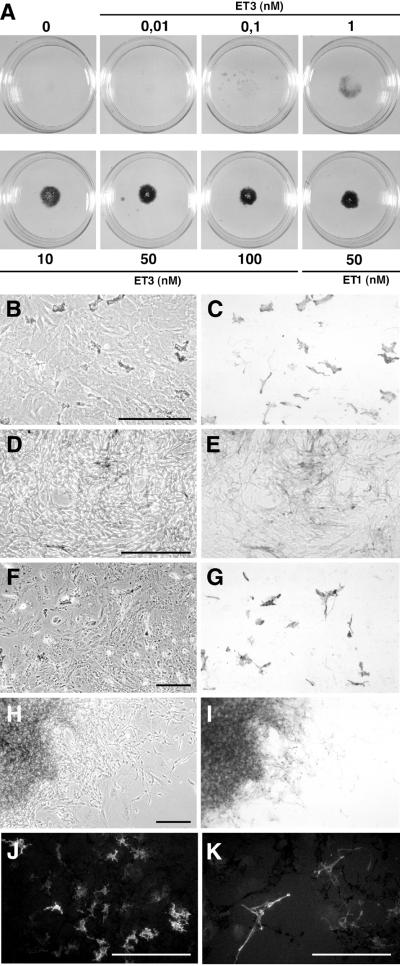
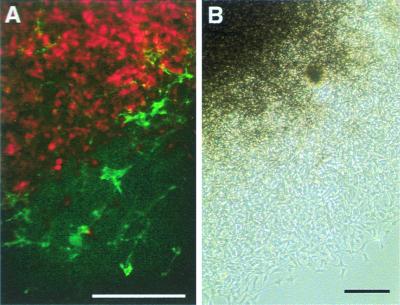
To investigate how ET3 influences the in vitro behavior of embryonic cutaneous melanocytes, cultures of epidermal cells isolated from the dorsal skin of E7.5 quails were treated with ET3, which is known to favor cell proliferation and melanogenesis in quail NC-derived cells (26, 28). At explantation time, the epidermis includes MelEM+ melanoblasts (33), the majority of which are unpigmented but will later differentiate into pigment cells. In control medium, pigmentation can be seen 24–48 h after explantation. Epidermal cells were cultured in the presence of various doses of ET3 ranging from 0.01 to 100 nM. ET3 induced dose-dependent enhancement of melanogenesis in d9 cultures (Fig. 1A). In medium devoid of ET3 or containing 0.01 nM ET3, subsets of melanocytes formed evenly distributed pigmented zones (Fig. 1 B, C, F, and G). ET3 at doses from 0.1 to 1 nM increased the number of pigment cells moderately whereas higher doses (10 to 100 nM) enhanced cell proliferation and pigmentation dramatically. Most cells exposed to 10–100 nM ET3 adopted an elongated spindle-like shape (Fig. 1 D and E), and the cultures became highly pigmented because of aggregates of densely packed melanocytes visible from d7 onward (Fig. 1 H and I). Analysis of cell phenotypes by immunochemistry showed that cells that were not carrying pigment granules were either stained by the MelEM mAb (not shown) or, for the cultures exposed to high doses of ET3, expressed the glial proteins SMP and Po (Fig. 1 J and K). Cells immunoreactive for SMP were never detected in cultures analyzed 3 h after plating and were only rarely found in a few cultures grown in control medium (not shown). Therefore, in vitro ET3 stimulates the differentiation of glial-like cells from NC cells that had homed to the epidermis. At this time, it was important to find out whether ET3 promoted differentiation of glial precursors that would have migrated to the skin and remained undifferentiated or if it induced expression of glial traits by NC cells already engaged in the melanogenic differentiation pathway.
Figure 1.
Mass cultures of epidermal cells. (A) Macroscopic views of the cultures. (B–K) Microscopic views of d3 (B–E), d7 (F–I), and d9 (J and K) cultures grown in control (B, C, F, and G) or 50 nM ET3-supplemented (D, E, and H–K) medium. Control cultures contained a subpopulation of flat pigment cells (B, C, F, and G), whereas in the presence of ET3, cells proliferated and became elongated (D and E) to yield pigment cell aggregates surrounded by unpigmented elongated cells (H and I). Treated cultures contained Po- (J) and SMP- (K) immunoreactive cells. (B, D, F, and H) Phase-contrast and corresponding (C, E, G, and I) bright-field. (J and K) UV light. (Bars = 200 μm.)
Pigment Cells Isolated from Epidermis Respond to ET3 in Vitro.
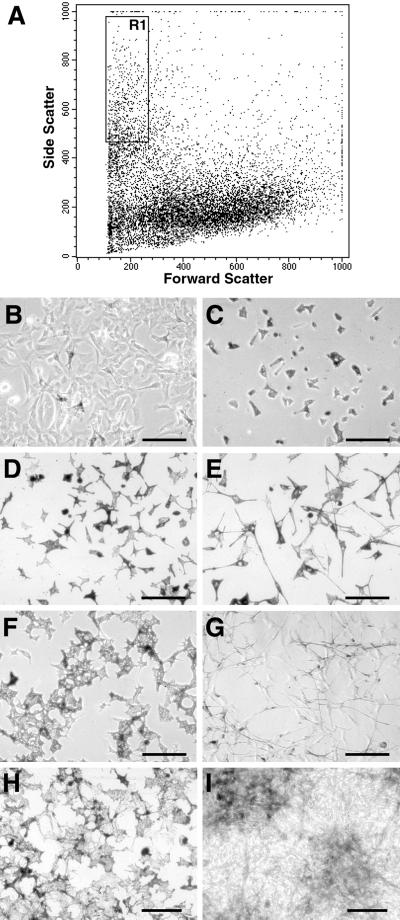
To identify ET3-responsive cells, we sorted out pigment cells from epidermal cells and submitted them to ET3. For this purpose, we used d2-epidermal cell cultures in which 15–20% of melanocytes were recognizable by their pigment granules. In the absence of melanocyte-specific surface antigens that would allow us to select immunolabeled pigment cells, we have isolated pigment cells according to physical parameters by FACS. A 95–98% pure fraction of pigment cells was sorted out from epidermal cells by specific forward and side scatter gating channels (Fig. 2A). This pigment cell fraction (Fig. 2C) was collected and cultured as described above for unsorted epidermal cells (Fig. 2B). In the presence of 50 nM ET3, pigment cells started elongating processes and dividing after 20 h (Fig. 2E); after 3 days, treated cultures contained elongated bipolar unpigmented cells (Fig. 2G), and, at d5, an increase of the pigment cell population was evident as compared with control cultures (Fig. 2 H and I). Staining with antibodies recognizing glial Po and SMP proteins showed that both glial markers are expressed by a subpopulation of cells differentiating at d5 in the presence of ET3 whereas no staining was found in control cultures (not shown).
Figure 2.
Purification of pigment cells from epidermal cells by FACS. (A) FACS analysis of epidermal-derived cells. (B–I) Microscopic views of cultures of unsorted epidermal cells in control medium (B) and sorted pigment cells (C–I) in control medium (C, D, F, and H) or in the presence of 50 nM ET3 (E, G, and I). A pure fraction of pigment cells (R1) was sorted out from epidermal cells by forward and side scatter imaging (A) with a pigment cell recovery ratio of 5–7% of total cells. Shown are unsorted cells (B) and isolated pigment cells (C) 4 h after plating in control medium. As compared with controls, ET3 induced morphological changes of pigment cells as soon as after 20 h (D and E), increased cell number and decreased pigmentation at d3 (F and G), and formation of densely packed melanocytes at d5 (H and I). (B and C) Phase-contrast. (D–I) Bright-field. (Bars = 100 μm.)
These data therefore show that ET3 causes morphological changes, increased proliferative activity, and altered the differentiation program of isolated pigment cells. These effects of ET3 do not require the presence of unpigmented epidermal cells and therefore are the result of a direct action of ET3 on pigment cells. This was further confirmed by clonal cultures of differentiated pigment cells.
ET3 Modifies Growth and Differentiation Properties of Pigment Cells in Clonal Cultures.
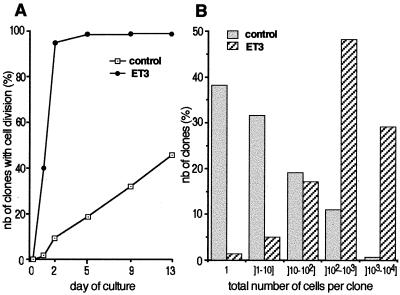
A clonal culture assay was used to characterize the response of individual epidermal melanocytes to ET3. Cultures were inspected daily to assess the survival, morphology, and proliferation of clonogenic cells. Table 1 shows that the cloning efficiency of d1 culture was about 70% in the presence or absence of ET3. In d13 cultures, more than 78% of d1 clones survived in both conditions, therefore indicating that neither short-term (d1) nor long-term (d13) clone survival is significantly affected by the addition of ET3 (Table 1). By contrast, ET3 has a prominent effect on cell proliferation. The time course of the onset and the rate of cell division were compared in control and ET3-treated cultures (Fig. 3). In control medium, 9.5 and 18% of pigment cells started to proliferate after d2 and d5, respectively, and a majority of cells were still quiescent at d13. The onset of cell division was highly promoted in ET3-treated cultures, in which 40% of pigment cells initiated cell division as soon as d1 onwards; from d2, 95% of clonogenic pigment cells had divided (Fig. 3A). ET3 treatment also increased the rate of pigment cell division, resulting in d13 colonies with total cell numbers much higher than in control cultures (Fig. 3B).
Table 1.
Survival of pigment cells in clonal cultures
| Control | + ET3 | |
|---|---|---|
| Number of plated cells | 265 | 270 |
| Number of d1 clones | 183 | 208 |
| Percent of plated cells | 69% | 77% (ns) |
| Number of d13 clones | 142 | 166 |
| Percent of plated cells | 53.6% | 61.5% (ns) |
| Percent of d1 clones | 77.6% | 80% (ns) |
The number of clone-forming cells in control and ET3-treated cultures was determined at d1 and d13 in four experiments. Results are expressed as percent of total plated cells. Clone survival is given by the number of clones at d13 expressed as percent of d1 clones. ns, not significant.
Figure 3.
Proliferative effect of ET3 on pigment cells in clonal cultures. (A) Time course of the onset of cell division: the proportion of clonal cultures in which the founder pigment cell has divided was determined as a function of the duration of the culture period and compared between control and ET3-supplemented medium. (B) Histogram of the distribution of d13-pigment cell colonies according to their total number of cells. About 70% of control clones contained 1–10 cells only whereas 76% of ET3-treated colonies (versus 15% of control clones; P < 0,0001) contained more than 100 cells.
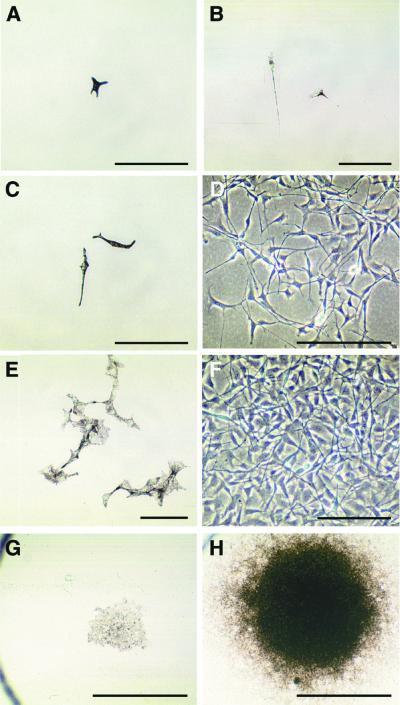
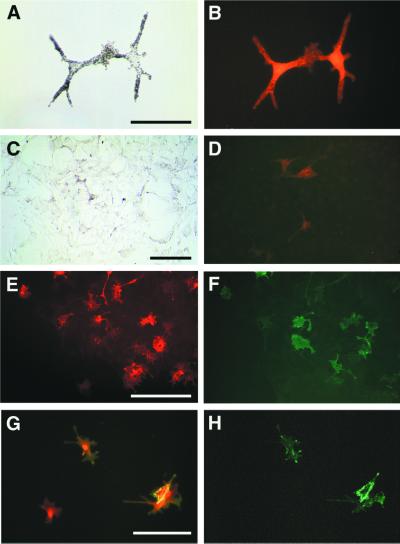
While stimulating pigment cell proliferation, ET3 induced changes in their morphology and pigmentation (Fig. 4). Individual pigment cells retained a flat fibroblastic-like morphology and cytoplasmic melanin granules when growing in control medium (Fig. 4 A, C, E, and G). By contrast, ET3-treated cells adopted a bipolar shape with straight elongated processes, showed progressive decrease in melanin, and became transiently unpigmented after d5 to d8 of culture (Fig. 4 B, D, and F). Later, ET3-treated cells recovered high pigmentation, particularly in the center of colonies of high cell density (Fig. 4H).
Figure 4.
Growth of single pigment cell cultures. Microscopic views of pigment cell colonies grown in the presence (right panels) or absence (left panels) of 50 nM ET3. (A) A pigment founder cell in d1 control culture. (B) At d1 in presence of ET3, the clonogenic cell has divided once. (C and D) At d5, untreated and treated cultures show striking differences in pigmentation and proliferative activity. (E and F) At d9, small clones of pigment cells formed in control medium (E) whereas proliferating ET3-treated cells became unpigmented (F). (G and H) Low magnification of d13-colonies showing one of the largest clones recorded in control cultures (G) and a typical large dense clone of highly pigmented cells in treated cultures. (A–C, E, G, and H) Bright-field. (D and F) Phase-contrast. [Bars = 100 μm (A–F) and 1 mm (G and H).]
Thus, at d13, nearly all (untreated and treated) clones contained pigment cells (Table 2), although unpigmented cells in addition to melanocytes were found in most treated cultures. We analyzed the clones for expression of the glial marker SMP which in vivo labels Schwann cells from E5 in quail peripheral nerves (30). SMP-immunoreactive cells were found in 41% of ET3-treated cultures whereas they were detected in only 8.4% of control colonies. In all cases, SMP+ cells were included in colonies containing melanocytes and unpigmented melanoblasts expressing the MelEM antigen (Table 2; Fig. 5). Some cells in treated colonies coexpressed MelEM and SMP (Fig. 6 G and H). In a series of experiments, we investigated the expression of Po and SMP glial markers in d13 clones. ET3 significantly increased the number of clones comprising Po+ cells as compared with controls (54% versus 6% of total clones; Table 2). Po+ cells were identified in clones containing melanocytes (Fig. 6 C and D) or containing melanocytes and also SMP+ cells (Fig. 6 E and F). It is striking that in these experiments the total number of clones containing Po+ cells was higher than clones containing SMP+ cells and nearly all colonies with SMP+ cells included also Po+ cells (Table 2). In double-labeled colonies, the great majority of SMP+ cells were also Po+ (Fig. 6 E and F), consistent with expression of Po before SMP in vivo and in vitro. Therefore, when exposed to ET3, pigment cells generate a heterogeneous progeny in which numerous cells with the glial phenotype emerge, a very rare event in untreated pigment cell cultures.
Table 2.
Phenotypic analysis of pigment cell colonies
| Control | + ET3 | P | |
|---|---|---|---|
| Total number of clones | 142 | 165 | |
| Total number of pigm+ clones | 141 (99.3%) | 159 (96.4%) | ns |
| Total number of SMP+ clones | 12 (8.4%) | 68 (41.2%) | 0,0001 |
| Total number of clones | 77 | 89 | |
| Number of Mel+ Po−SMP− clones | 72 (93.5%) | 40 (44.9%) | 0,001 |
| Number of Mel+ Po+SMP− clones | 1 (1.3%) | 23 (25.8%) | 0,005 |
| Number of Mel+ Po−SMP+ clones | 0 (0%) | 1 (1.1%) | ns |
| Number of Mel+ Po+SMP+ clones | 4 (5.2%) | 25 (28.1%) | 0,005 |
Cell phenotypes were analysed at d13 in control and ET3-treated colonies. The number of clones containing pigment cells (pigm+) and SMP+ cells was evaluated in four experiments and was expressed as percent of total clones. In two experiments, melanocytes (Mel), SMP+, and Po+ cells were recorded, and four different types of clones were defined according to the cell types they contain. P values are from statistical analysis of control and ET3-treated cultures. ns, not significant.
Figure 5.
Phenotypic analysis of d13-clones. (A) Fluorescence labeling with both MelEM (red) and SMP (green) mAb in part of a clone grown in presence of ET3. (B) Corresponding phase-contrast. The core of the clone contains densely packed MelEM+ melanocytic cells whereas most SMP+ cells differentiate in the periphery (Bars = 200 μm.)
Figure 6.
Expression of lineage markers by d13-colonies. (A and B) Control clone including two melanocytes [bright-field (A) and corresponding fluorescence labeling with MelEM (B)]. (C–H) ET3-treated colonies. (C and D) Part of a clone containing melanocytes [bright-field (C)] and cells immunoreactive for Po (D). (E and F) Double labeling showing both Po+ (E) and SMP+ (F) cells in part of a clone. (G and H) Double labeling showing SMP+ cells (H and green in G) that coexpress MelEM (red in G). [Bars = 50 μm (A and G), 100 μm (C), and 200 μm (E).]
Discussion
Our earlier experiments carried out in vitro have shown that ET3 exerts a potent proliferative effect on the quail NC cells as they start migrating. Secondary to this increase of cell proliferation, ET3 has a spectacular effect on melanogenesis (26). Moreover, soon after in vitro transplantation and under ET3 exposure, NC cells massively switch the expression of their ET3 receptor from the ETRB to ETRB2 gene (28).
Clonal analysis of avian NC cell differentiation has led to the demonstration that the migrating trunk NC contains a variety of intermediate pluripotent progenitors among which cells whose capacity is restricted to yielding glial cells and melanocytes (GM progenitors), glial cells and neurons (GN progenitors), glial cells, melanocytes, and neurons (GMN progenitors). In addition, the NC population includes monopotent committed precursors: that yield either melanocytes (M) or glial cells (G) (27, 28). ET3 was shown to stimulate both the survival and proliferation of some but not all of these cells: only GM, G, and M types of progenitors were positively affected by ET3 addition whereas the clonal efficiency and the proliferation of GMN clonogenic cells were unchanged (28).
A very striking feature of the avian pigment cell development is that the NC cells that take the mediolateral migration pathway leading them to the skin are the only subset of crest cells expressing the ETRB2 receptor in vivo (25). It seems, therefore, that these cells that are exposed to the ET3 produced at this stage by the ectoderm are led to switch from ETRB to ETRB2 expression, hence becoming rapidly specified to take the melanocytic differentiation pathway.
The fact that ET3 acts on GM, G, and M precursors raised the question as to whether premelanocytes or melanocytes that have already homed to the epidermis (“epidermal melanocytes”) could be reversed to the GM precursor state if they would receive a strong stimulation to grow by ET3. We show here that epidermal melanocytes respond to ET3 by high proliferation rate, increase in melanogenesis, and gliogenesis. Moreover, addition of ET3 to clonal cultures of already pigmented melanocytes makes them divide actively, and, in these circumstances, the progeny of the founder cell contained not only melanocytes but also cells coexpressing melanin and glial markers and unpigmented cells with the glial phenotype.
The phenotypic analysis of colonies treated with ET3 shows, therefore, that pigment cells generate a subpopulation of glial descendants during ET3-induced proliferation. The following sequence can be proposed: Proliferative pigment cells producing MelEM antigen and melanin first decrease melanin content during cell division and then acquire glial markers, first Po and, later, SMP, which occasionally are coexpressed with the MelEM antigen, the latter being down-regulated during further differentiation toward the glial cell phenotype.
This sequence of events, including loss of differentiation properties concomitant with active proliferation, followed by acquisition of new proteins specific to a different cell phenotype, can be representative of the transdifferentiation processes described in invertebrates and vertebrates (see ref. 29 for a review). It requires sequential silencing and activation of differentiation-specific genes and proceeds via an intermediate, partly dedifferentiated phenotype. The conversion in vivo and in vitro of chick retinal epithelium pigment cells into lens or neural retina is one of the most spectacular example of transdifferentiation (29, 34–36), which is regulated by growth factors, extracellular matrix components, and cell-cell interactions.
Our results show that NC-derived pigment cells are able to dedifferentiate/transdifferentiate under stimulation by ET3. Although the mechanisms regulating this process need further investigations, the present in vitro clonal study demonstrates that embryonic melanocytes can resume the bipotentiality of their NC precursors and provides further evidence for the plasticity of NC derivatives.
Acknowledgments
We thank Francis Beaujean, Sophie Gournet, and Michèle Scaglia for their help in preparing the illustrations and bibliography. This work was supported by the Centre National de la Recherche Scientifique and a grant from the Association pour la Recherche contre le Cancer.
Abbreviations
- ET3
endothelin 3
- ETR
endothelin receptor
- NC
neural crest
- d1
day 1
- SMP
Schwann cell myelin protein
- MelEM
melanoblast/cyte early marker
- E3
embryonic day 3
- FACS
fluorescence-activated cell sorter
- GM
glial-melanocytic
References
- 1.Le Douarin N. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 2.Le Douarin N M, Kalcheim C. The Neural Crest (Second Edition) New York: Cambridge Univ. Press; 1999. [Google Scholar]
- 3.Sieber-Blum M, Cohen A M. Dev Biol. 1980;80:96–106. doi: 10.1016/0012-1606(80)90501-1. [DOI] [PubMed] [Google Scholar]
- 4.Baroffio A, Dupin E, Le Douarin N M. Proc Natl Acad Sci USA. 1988;85:5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baroffio A, Dupin E, Le Douarin N M. Development (Cambridge, UK) 1991;112:301–305. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- 6.Bronner-Fraser M, Fraser S E. Nature (London) 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- 7.Dupin E, Baroffio A, Dulac C, Cameron-Curry P, Le Douarin N M. Proc Natl Acad Sci USA. 1990;87:1119–1123. doi: 10.1073/pnas.87.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stemple D L, Anderson D J. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 9.Morrison S J, White P M, Zock C, Anderson D J. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 10.Sieber-Blum M, Ito K, Richardson M K, Langtimm C J, Duff R S. J Neurobiol. 1993;24:173–184. doi: 10.1002/neu.480240205. [DOI] [PubMed] [Google Scholar]
- 11.Sextier-Sainte-Claire Deville F, Ziller C, Le Douarin N M. Dev Brain Res. 1992;66:1–10. doi: 10.1016/0165-3806(92)90134-i. [DOI] [PubMed] [Google Scholar]
- 12.Sextier-Sainte-Claire Deville F, Ziller C, Le Douarin N M. Dev Biol. 1994;163:141–151. doi: 10.1006/dbio.1994.1130. [DOI] [PubMed] [Google Scholar]
- 13.Lo L, Anderson D J. Neuron. 1995;15:527–539. doi: 10.1016/0896-6273(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 14.Henion P D, Weston J A. Development (Cambridge, UK) 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood A L, Turner E E, Anderson D J. Development (Cambridge, UK) 1999;126:3545–3559. doi: 10.1242/dev.126.16.3545. [DOI] [PubMed] [Google Scholar]
- 16.Bennett D C. In: International Review of Cytology: A Survey of Cell Biology. Jeon K W, Jarvik J, Friedlander M, editors. Vol. 146. San Diego: Academic; 1993. pp. 191–260. [Google Scholar]
- 17.Le Douarin N M, Dupin E, Ziller C. Curr Opin Genet Dev. 1994;4:685–695. doi: 10.1016/0959-437x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- 18.Joyner A L, Guillemot F. Curr Opin Neurobiol. 1994;4:37–42. doi: 10.1016/0959-4388(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 19.Baynash A, Hosoda K, Giaid A, Richardson J A, Emoto N, Hammer R E, Yanagisawa M. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 20.Hosoda K, Hammer R E, Richardson J A, Baynash A, Cheung J C, Giaid A, Yanagisawa M. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 21.Nataf V, Lecoin L, Eichmann A, Le Douarin N M. Proc Natl Acad Sci USA. 1996;93:9645–9650. doi: 10.1073/pnas.93.18.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nataf V, Amemiya A, Yanagisawa M, Le Douarin N M. Mech Dev. 1998;73:217–220. doi: 10.1016/s0925-4773(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 23.Yanagisawa H, Yanagisawa M, Kapur R P, Richardson J A, Williams S C, Clouthier D E, de Wit D, Emoto N, Hammer R E. Development (Cambridge, UK) 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- 24.Gariepy C E, Williams S C, Richardson J A, Hammer R E, Yanagisawa M. J Clin Invest. 1998;102:1092–1101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecoin L, Sakurai T, Ngo M T, Abe Y, Yanagisawa M, Le Douarin N M. Proc Natl Acad Sci USA. 1998;95:3024–3029. doi: 10.1073/pnas.95.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahav R, Ziller C, Dupin E, Le Douarin N M. Proc Natl Acad Sci USA. 1996;93:3892–3897. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupin E, Le Douarin N M. Dev Biol. 1995;168:529–548. doi: 10.1006/dbio.1995.1100. [DOI] [PubMed] [Google Scholar]
- 28.Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, Le Douarin N M. Proc Natl Acad Sci USA. 1998;95:14214–14219. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eguchi G, Kodama R. Curr Opin Cell Biol. 1993;5:1023–1028. doi: 10.1016/0955-0674(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 30.Dulac C, Cameron-Curry P, Ziller C, Le Douarin N M. Neuron. 1988;1:211–220. doi: 10.1016/0896-6273(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 31.Dulac C, Tropak M B, Cameron-Curry P, Rossier J, Marshak D R, Roder J, Le Douarin N M. Neuron. 1992;8:323–334. doi: 10.1016/0896-6273(92)90298-r. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya A, Frank E, Ratner N, Brackenbury R. Neuron. 1991;7:831–844. doi: 10.1016/0896-6273(91)90285-8. [DOI] [PubMed] [Google Scholar]
- 33.Nataf V, Mercier P, Ziller C, Le Douarin N M. Exp Cell Res. 1993;207:171–182. doi: 10.1006/excr.1993.1177. [DOI] [PubMed] [Google Scholar]
- 34.Eguchi G, Okada T S. Proc Natl Acad Sci USA. 1973;70:1495–1499. doi: 10.1073/pnas.70.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moscona A. Science. 1957;125:598–599. doi: 10.1126/science.125.3248.598. [DOI] [PubMed] [Google Scholar]
- 36.Okada, T. S., Itoh, Y., Watanabe, K. & Eguchi, G. Dev. Biol.45, 318–329. [DOI] [PubMed]