Abstract
Many areas of biomedical research depend on the analysis of uncommon variations in individual genes or transcripts. Here we describe a method that can quantify such variation at a scale and ease heretofore unattainable. Each DNA molecule in a collection of such molecules is converted into a single magnetic particle to which thousands of copies of DNA identical in sequence to the original are bound. This population of beads then corresponds to a one-to-one representation of the starting DNA molecules. Variation within the original population of DNA molecules can then be simply assessed by counting fluorescently labeled particles via flow cytometry. This approach is called BEAMing on the basis of four of its principal components (beads, emulsion, amplification, and magnetics). Millions of individual DNA molecules can be assessed in this fashion with standard laboratory equipment. Moreover, specific variants can be isolated by flow sorting and used for further experimentation. BEAMing can be used for the identification and quantification of rare mutations as well as to study variations in gene sequences or transcripts in specific populations or tissues.
The study of DNA sequence variation is important for many areas of research. The study of germ-line variations is essential for assessing the role of inheritance in normal and abnormal physiologic states (1). Other variations, developed somatically, are responsible for neoplasia (2). The identification of such mutations in urine, sputum, and stool can therefore be used for the detection of presymptomatic cancers (3–5). Similarly, the detection of somatic mutations in lymph nodes, blood, or bone marrow can provide data about the stage of disease, prognosis, and appropriateness of various therapies (5). Somatic mutations in nonneoplastic cells also occur and seem to accumulate as humans age or are exposed to environmental hazards (6). Such mutations occur in only a small fraction of the cells in a tissue, thereby complicating their analysis.
Central to the investigation of many of these issues is the detection and quantification of sequence variants within a population of DNA molecules. The number of molecules in each such collection is finite and therefore countable. Consider, for example, a collection of red and green balls. Counting these balls is simple in principle but subject to basic probability theory. If there is only one red ball for every 500 green balls, then it is necessary to count several thousand balls to get an accurate estimate of the proportion of red balls. If it is difficult to count enough balls to make a reliable estimate, one can elute the paint off all the balls and measure the color of the resultant paint mix.
In analogous fashion, small numbers of DNA molecules that vary by subtle changes (single base-pair substitutions or small deletions or insertions) can be counted directly by amplifying individual DNA molecules (single-molecule PCR) (7–12, 29). Such digital techniques have been shown to be extremely useful for measuring variation in genes or their transcripts, but digital technologies have been limited thus far to counting tens to thousands of molecules in the wells of microtiter plates, on microscope slides, or after electrophoresis of individual PCR products. Analog techniques, analogous to the elution of paint from the balls described above, are generally easier to implement and can assess millions of molecules simultaneously (e.g. ref. 13). However, their accuracy and sensitivity are limited by instrumental and experimental noise.
In this article we describe a digital technology called BEAMing (on the basis of four of its principal components: beads, emulsion, amplification, and magnetics), which has the power to assess millions of molecules and can be generally applied to the study of genetic variation. The technology involves conversion of single DNA molecules to single magnetic beads, each containing thousands of copies of the sequence of the original DNA molecule. The number of variant DNA molecules in the population then can be assessed by staining the beads with fluorescent probes and counting them by using flow cytometry. Beads representing specific variants can be recovered through f low sorting and used for subsequent confirmation and experimentation.
Materials and Methods
Step 1: Coupling Oligonucleotides to Beads. Superparamagnetic beads of 1.05 ± 0.1 μm in diameter, covalently bound to streptavidin, were purchased from Dynal Biotech (no. 650.01, Lake Success, NY). Beads were washed once with 1× PCR buffer (50 mM KCl/20 mM Tris·HCl, pH 8.4) and then suspended in bind-and-wash buffer (5 mM Tris·HCl/0.5 mM EDTA/1.0 M NaCl, pH 7.5). Beads were incubated in bind-and-wash buffer for 30 min at room temperature in the presence of 10 μM oligonucleotides (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). These oligonucleotides were modified with a dual biotin group at the 5′ end, with the biotin groups separated by a six-carbon linker (Integrated DNA Technologies, Coralville, IA). After binding, the beads were washed three times with 1× PCR buffer to thoroughly remove unbound oligonucleotides.
Step 2: Preparing Microemulsions. Microemulsions for PCR were prepared by slight modifications of described methods (14, 15). The oil phase was composed of 4.5% Span 80 (no. S6760, Sigma), 0.40% Tween 80 (no. S-8074, Sigma), and 0.05% Triton X-100 (no. T9284, Sigma) in mineral oil (no. M-3516, Sigma). The oil phase was freshly prepared each day. The aqueous phase consisted of 67 mM Tris·HCl (pH 8.8), 16.6 mM NH4SO4, 6.7 mM MgCl2, 10 mM 2-mercaptoethanol, 1 mM dATP, 1 mM dCTP, 1 mM dGTP, 1 mM dTTP, 0.05 μM forward primer, 25 μM reverse primer, 45 units of Platinum Taq (no. 10966-034, Invitrogen), various amounts of template DNA (see Results), and ≈108 oligonucleotide-coupled beads in a total volume of 300 μl. The forward primer was an oligonucleotide with a sequence that was identical to the 3′ 20–22 nt of that described in step 1 and was not modified with biotin.
Water-in-oil microemulsions were prepared by dropwise addition of 200 μl of the aqueous phase to 400 μl of the oil phase previously placed in a 2-ml round-bottom cryogenic vial (no. 430661, Corning). The dropwise addition was performed over ≈1 min while the mixture was being stirred at 1,400 rpm with a magnetic microstir bar (no. 58948-353, VWR Scientific) on a VWR model 565 magnetic stirrer. After the addition of the aqueous phase, the mixture continued to be stirred for a total time of 30 min. Two emulsions were made at once by placing two tubes in a rack placed at the center of the magnetic stirrer.
Step 3: PCR Cycling. The emulsions were aliquoted into five wells of a 96-well PCR plate, each containing 100 μl. PCR was carried out under the following cycling conditions: 94°C for 2 min, 40 cycles of 94°C for 15 sec, 57°C for 30 sec, and 70°C for 30 sec. The PCR products analyzed in this study ranged from 189 to 239 bp.
Step 4: Magnetic Capture of Beads. After PCR cycling, the microemulsion from five wells of a PCR plate were pooled and broken by the addition of 800 μl of NX buffer (100 mM NaCl/1% Triton X-100, 10 mM Tris·HCl, pH 7.5, and 1 mM EDTA) in a 1.5-ml tube (no. 430909, Corning). After vortexing for ≈20 sec, the beads were pelleted by centrifugation in a microcentrifuge at 8,000 rpm (5,000 × g) for 90 sec. The top oil phase and all but ≈300 μl of the aqueous phase were removed from the tube, and 600 μl of NX buffer was added. After vortexing for 20 sec and centrifugation for 90 sec, the top oil phase and all but ≈300 μl of the aqueous phase were removed. The addition of 600 μl of NX buffer, vortexing, and centrifugation were repeated once more, and the top oil portion and all but ≈300 μl of the aqueous phase were removed. The tube then was placed on a magnet (MPC-S, Dynal), and the rest of the supernatant was pipetted off carefully. The beads were washed an additional three times with 1× PCR buffer by using magnetic separation rather than centrifugation and finally resuspended in 100 μl of 1× PCR buffer.
Step 5: Sequence Differentiation. Two oligonucleotide probes were used for each reaction. One was 5′-labeled with 6-carboxyfluorescein and was specific for one allele, whereas the second was 5′-labeled with biotin and was specific for the other allele. Probes were synthesized by Integrated DNA Technologies. The 30-μl hybridization reactions contained 10 μM of each probe and 5–25 million beads in 1× PCR buffer. Reactions were performed in PCR plates on a thermal cycler by heating to 94°C for 30 sec and then cooling to 75°C at a rate of 0.5°C/sec, cooling to 45°C at 0.2°C/sec, and finally cooled to 30°C at 1°C/sec. All subsequent steps were performed at room temperature. The reactions were transferred to a 96-well Costar plate (no. 3797, Corning) and placed on a 96-well magnet. Beads were collected magnetically by exposing them to the magnet for 2 min. The supernatant was removed and the beads washed three times with 1× PCR buffer by pipetting them and collecting for 2 min. They were finally resuspended in 100 μl of B-PCR buffer (1 mg/ml BSA in 1× PCR buffer). The beads then were incubated for 10 min in a total volume of 100 μl of B-PCR buffer containing 3 μg of Alexa-488 rabbit anti-f luorescein antibody (no. A-11090, Molecular Probes) and 3 μg of Nutravidin labeled with R-phycoerythrin (no. A-2660, Molecular Probes) in B-PCR buffer. The beads were washed three times and resuspended in B-PCR buffer as described above. They then were incubated for 10 min in a total volume of 100 μl of B-PCR buffer containing 6 μg of Alexa 488-conjugated chicken anti-rabbit antibody (no. A-21441, Molecular Probes) and 3 μg of biotinylated goat anti-avidin antibody (no. BA-0300, Vector Laboratories). The beads were washed three times and resuspended in B-PCR buffer as described above. They then were incubated for 10 min in a total volume of 100 μl of B-PCR buffer containing 3 μg of an Alexa 488-conjugated goat anti-chicken antibody (no. A-11039, Molecular Probes) and 3 μg of R-phycoerythrin-labeled streptavidin (no. S-866, Molecular Probes). This solution then was washed an additional three times with 1× PCR buffer and resuspended in 20 μl of 1× PCR buffer.
Step 6: Flow Cytometry. The bead suspension was diluted to a concentration of ≈106 to 107 beads per ml in 10 mM Tris·HCl/1 mM EDTA (no. 351-010-131, Quality Biologicals, Gaithersburg, MD) and analyzed by using an LSR instrument (BD Biosciences, Franklin Lakes, NJ). The instrument was set up for standard two-color analysis by using an argon laser and optical filters that distinguished between the two fluorescent dyes. No spectral deconvolution was required because the major bead populations were well separated. In some cases, scanning was performed with FACScan or FACSCalibur instruments (BD Biosciences), yielding equivalent results. Sorting was carried out with a FACSVantage SE instrument (BD Biosciences). The flow-cytometry data were analyzed by using cellquest software (BD Biosciences).
Template Preparation and Sequence Analyses. Human genomic DNA was purified with DNeasy (no. 69504, Qiagen, Valencia, CA). RNA was purified with Quickprep (no. 27-9255-01, Amersham Pharmacia Biosciences). Reverse transcription of RNA was performed by using Superscript II reverse transcriptase (no. 18064014, Invitrogen) according to manufacturer instructions. PCR with genomic DNA or reverse transcripts as templates was performed as described (7). PCR products to be used as templates for BEAMing or sequencing were purified with QIAquick (no. 28104, Qiagen). Sequencing reactions were performed by using BigDye v3.0 reagents (Applied Biosystems) and analyzed by capillary electrophoresis (Spectrumedix 9600, State College, PA).
Results
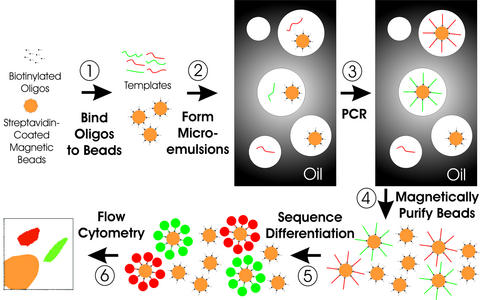
BEAMing consists of the six steps diagrammed in Fig. 1.
Fig. 1.
Schematic of BEAMing. Step 1: Magnetic beads covalently coated with streptavidin are bound to biotinylated oligonucleotides (oligos). Step 2: An aqueous mix containing all the necessary components for PCR plus primer-bound beads and template DNA are stirred together with an oil/detergent mix to create microemulsions. The aqueous compartments (white circles in the gray oil layer) contain an average of less than one template molecule and less than one bead. Red and green templates represent two template molecules, the sequences of which differ by one or many nucleotides. Step 3: The microemulsions are temperature-cycled as in a conventional PCR. If a DNA template and a bead are present together in a single aqueous compartment, the bead-bound oligonucleotides act as primers for amplification. The straight red and green lines connected to the beads represent extension products from the two different kinds of templates. Step 4: The emulsions are broken, and the beads are purified with a magnet. Step 5: After denaturation, the beads are incubated with oligonucleotides that can distinguish between the sequences of the different kinds of templates. Fluorescently labeled antibodies then are used to label the bound hybridization probes, which renders the beads containing PCR product as red or green after appropriate laser excitation. Step 6: Flow cytometry is used to count the red and green beads.
Step 1: Coupling Oligonucleotides to Beads. We used streptavidin beads because of the simplicity of coupling biotinylated oligonucleotides to them. Oligonucleotides with just a single 5′-biotin group were found to dissociate from the beads during temperature cycling, whereas oligonucleotides labeled with dual biotin groups at their 5′ end (separated by a six-carbon linker) were stable to cycling. As determined by fluoroscopic measurements of oligonucleotides doubly labeled with 6-carboxyfluorescein and biotin, ≈105 oligonucleotide molecules were bound to each bead. We found that short oligonucleotides (20 bases) did not work as well for priming as longer ones (41 bp), perhaps because of steric hindrance at the bead surface. It is likely that amino-, sulfhydryl-, or carboxyl-modified oligonucleotides covalently coupled to beads modified with corresponding reactive groups could also function as bead-bound primers for BEAMing.
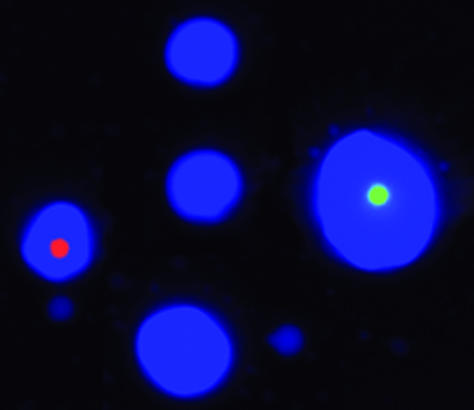
Step 2: Preparing Microemulsions. The size of the individual aqueous compartments were 5.4 ± 2.7 μm in diameter (Fig. 2). We estimated that an emulsion comprising 200 μl of aqueous solution and 400 μl of oil would contain ≈3 × 109 compartments with an average diameter of 5 μm. Approximately 108 beads were included in each emulsion such that only one in ≈30 compartments contained a bead. The optimal amount of template was experimentally determined to be ≈5 × 108 molecules, so that one in approximately six compartments contained a template molecule.
Fig. 2.
Photograph of a typical microemulsion. Microemulsions were made as described in Materials and Methods with the exception that the aqueous compartments contained cascade blue-labeled dCTP and the beads were prelabeled by binding to oligonucleotides coupled to R-phycoerythrin (red) or Alexa 488 (green). One microliter of microemulsion was deposited in 1 μlofoil on a microscope slide before photography. Of the seven aqueous compartments visible in this picture, two contain beads. Note the heterogeneous size of the aqueous compartments (beads are 1.05 μm in diameter).
Step 3: PCR Cycling. PCR priming by oligonucleotides coupled to beads was found to be very inefficient compared with the priming by the same oligonucleotides when free in solution. For this reason, a small amount of nonbiotinylated forward primer identical in sequence to the biotinylated oligonucleotide coupled to the beads was included in the reactions. This facilitated the first few rounds of amplification of the single template within each aqueous compartment. In the absence of additional primer, no detectable amplification on the beads was generated. Conversely, if too much additional primer was included, no amplification on the beads occurred because of competition with the primers in solution. An excess of the reverse primer was included in the aqueous compartment to maximize the probability that bead-bound oligonucleotides extended by polymerase would serve as templates for further amplification cycles.
Step 4: Magnetic Capture of Beads. There are several ways to break water-in-oil emulsions including extraction with organics (14). We found that simply adding nonionic detergents produced phase separations without any detectable modification of the beads or DNA molecules bound to them. By measuring the amount of DNA that could be released from the beads after restriction endonuclease digestion, we estimate that >10,000 extended PCR products were present, on average, per bead.
Step 5: Sequence Differentiation. Most fluorescence-based methods for distinguishing alleles in homogeneous or two-phase assays can be used to assess allelic variation captured on beads. These methods include single nucleotide extension, allele-specific priming, or hybridization. We generally used hybridization of fluorescein-conjugated or biotin-conjugated oligonucleotides for discrimination. As shown in Fig. 1 and Table 1, these oligonucleotides had a stem–loop structure, with the middle of the loop containing the variant nucleotide(s). This design was based on studies of molecular beacons wherein a stem–loop structure was shown to improve allelic discrimination markedly (16). The oligonucleotides we used differed from molecular beacons in that there was no need for a quenching group. Such quenching is required for homogeneous assays when unhybridized oligonucleotides cannot be removed from the reactions before assay but is not necessary for solid-phase assays such as those used with beads.
Step 6: Flow Cytometry. Optimum results in flow cytometry depend on high fluorescent signals on the beads. We generally enhanced the fluorescence emanating from the hybridization probes with secondary reagents. For example, Alexa 488-labeled antibodies were used to enhance the signals emanating from f luorescein-coupled oligonucleotide probes. Similarly, R-phycoerythrin-labeled streptavidin was used to generate a signal from biotin-labeled oligonucleotide probes. Flow cytometers equipped with two or three lasers and appropriate filters have the capacity to distinguish multiallelic loci and perform multiplex analysis of several genes simultaneously. The newest generation of flow cytometers can also analyze >70,000 events per sec. In addition to the analytical power of flow cytometry, fluorescence-activated cell sorter instruments can separate specific populations of beads for further analysis.
Characteristics of Microemulsions. Pilot experiments demonstrated that simply stirring the water-in-oil mixtures described in Materials and Methods produced very stable microemulsions of a size compatible with that of the beads. In the experiment shown in Fig. 2, the aqueous compartment contained a blue dye and 1-μm magnetic beads that were labeled by binding to fluorescently labeled oligonucleotides. The appearance of emulsions immediately after their formation is shown in Fig. 2. As expected, this appearance was unchanged after temperature cycling during PCR (15). Most aqueous compartments contained no beads, as expected from the calculations in step 2. Those compartments that did contain beads generally contained only one, although a fraction contained more, as expected from a Poisson distribution and nonuniform aqueous compartment sizes. “Heterozygous” beads containing PCR products representing both alleles are produced when two or more DNA template molecules are contained within a single aqueous compartment. Such heterozygotes can compromise the accuracy of the analyses under some circumstances (see Discussion).
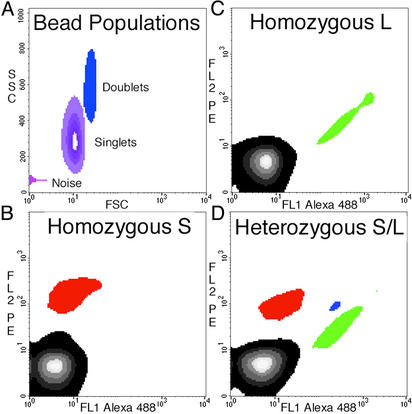
Detection of Homozygotes and Heterozygotes. Fig. 3 shows typical results obtained with human DNA samples. The MID42 marker used in this experiment was chosen from a collection of diallelic short insertion/deletion polymorphisms assembled by Weber et al. (17). These alleles are particularly simple to distinguish with hybridization probes because the two alleles at each locus differ by ≈4 bases. The probe for the longer (L) allele was labeled with fluorescein (green), and the probe for the shorter (S) allele was labeled with R-phycoerythrin (red).
Fig. 3.
Density plots of flow-cytometric data obtained from BEAMing. The locus queried in this experiment was MID42, and PCR products generated from genomic DNA were used as templates in the microemulsions. (A) Forward scatter (FSC) and side scatter (SSC) of all beads show that ≈80% of the total beads are singlets, with most of the remaining beads aggregated as doublets. The “noise” is instrumental and is observed with blank samples containing no beads. The instrument output was gated such that only singlets were analyzed for fluorescence analysis. The patterns observed from an individual homozygous for the L allele (A), homozygous for the S allele (B), and heterozygous for L and S (D) are shown in B–D, respectively. The regions containing beads hybridizing to the L and S allele probes are labeled green and red, respectively. The region containing beads that did not hybridize to any probe is black, and the region containing beads that hybridized to both probes is blue. The blue beads arose from aqueous compartments in which both types of template molecules were present. The proportion of singlet beads that hybridized to at least one of the probes was 2.9%, 4.3%, and 20.3% in B–D, respectively. The forward-scatter and side-scatter plots in A represent the same beads analyzed in D. FL1, fluorescent channel 1; FL2, fluorescent channel 2; PE, R-phycoerythrin.
Fig. 3A shows a plot of the side scatter vs. forward scatter of beads after BEAMing. In general, >75% of beads were dispersed as single particles, with the remainder aggregated in groups of two or more. Subsequent flow-cytometric analysis was confined to the singlet beads, gated as outlined in Fig. 3A.
Fig. 3 B–D show density plots of gated beads generated with various templates. In Fig. 3B, a template from an individual homozygous for the L allele was included in the emulsion. Two populations of beads were apparent. Ninety-eight percent of the beads contained no PCR product (black), and the remaining 2% fluoresced in the FL1 channel (colored green in Fig. 3). Fig. 3C represents the analysis of an individual homozygous for the S allele. Two populations of beads were again apparent, but this time the labeled population fluoresced in the FL2 channel (colored red in Fig. 3). Fig. 3D presents density plots from the analysis of an individual heterozygous at the MID42 locus. Four populations of beads are evident: the black region represents beads without any PCR product; the red region represents beads containing PCR products from the S allele; the green region represents beads containing PCR products from the L allele; and the blue region represents beads containing PCR products from both alleles. Beads containing PCR products from both alleles were derived from aqueous compartments that contained more than one template molecule. The number of such beads increased in a nonlinear fashion as more template molecules were added. At the extreme, when all aqueous compartments are saturated, virtually all beads will register as blue. Operationally, we found that the bead populations were most distinct when the number of beads containing any PCR product was <10% of the total beads analyzed.
PCR Products, Genomic DNA, or cDNA as Templates. The results shown in Fig. 3 were generated by using PCR products made from human genomic DNA samples. Because the ratio of the beads representing L alleles to those representing S alleles was 1.0 in this experiment, it was clear that the initial PCR did not preferentially amplify either allele. The use of PCR products rather than genomic DNA permitted large numbers of alleles to be amplified from even small quantities of starting DNA. In general, 10–100 pg of PCR products 200 bp in size were found to be optimal for BEAMing, producing PCR-mediated extension of primers on ≈1–10% of labeled beads.
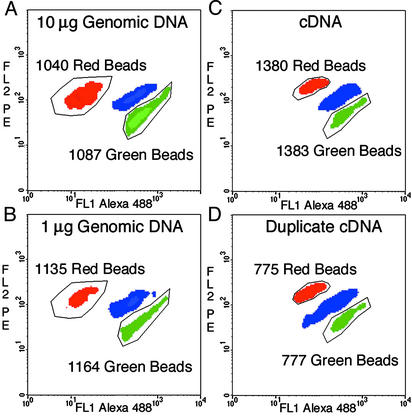
In some situations it might be useful to use genomic DNA rather than PCR products as templates for BEAMing. The data in Fig. 4 A and B show flow-cytometric data from an experiment wherein 10 or 1 μg of human genomic DNA was used as template for BEAMing at the MID42 locus. Patterns very similar to those shown in Fig. 3 were observed, although fewer beads were labeled than when PCR products were used as templates.
Fig. 4.
Density plots of BEAMing with genomic DNA or RT-PCR products as templates. The data in A and B were generated by including 10 and 1 μg of human genomic DNA, respectively, in the microemulsions, querying the MID42 locus. The data in C and D were generated by using emulsions that contained ≈50 pg of PCR products synthesized from cDNA of lymphoblastoid cells, querying the calpain-10 locus. The green and red regions correspond to the L and S alleles for MID42 and to the A and G alleles for calpain-10. The number of beads in the outlined regions containing red or green beads is shown in each case. The proportion of singlet beads that hybridized to at least one of the probes was 1.2%, 0.6%, 6.8%, and 4.2% in A–D, respectively. The outlined regions used for counting in A and B were identical, as were those used for C and D. Beads that did not hybridize to any probe were gated out and therefore not evident in the graphs, and the region containing beads that hybridized to both probes is labeled blue. FL1, fluorescent channel 1; FL2, fluorescent channel 2; PE, R-phycoerythrin.
BEAMing could also be used to analyze variations in expression from the two alleles of a heterozygous individual. Heritable variations in the expression from individual alleles of the same gene have been shown to occur often in humans (18) and mice (19) and can have significant phenotypic effects (20). The results shown in Fig. 4 C and D show that PCR products made from reverse-transcribed mRNA can be used for BEAMing. In this case, calpain-10 transcripts differing by a single nucleotide polymorphism were analyzed. For single nucleotide polymorphisms such as these, probes that incorporated an extra mismatched nucleotide adjacent to the polymorphic nucleotide (see Table 1) can enhance the distinction between alleles (21, 22). The results from two independent emulsions made with aliquots of the same RT-PCR product are shown to illustrate reproducibility. Although the number of beads that functioned as templates in BEAMing varied up to 3-fold among experiments with identical templates, the proportion of beads representing the two alleles was reproducible (1,383 A allele beads to 1,380 G allele beads in Fig. 4C and 777 A allele beads to 775 G allele beads in Fig. 4D, respectively).
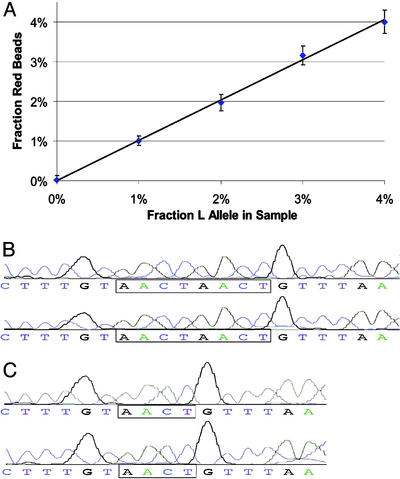
Analysis of Minor Variants in a DNA Population. The analysis of uncommon variations is ideally suited for analysis via BEAMing because of the large number of molecules that can be analyzed independently while retaining a high signal-to-noise ratio. Fig. 5A shows representative data from templates representing 1–4% of the L allele of MID42. The linearity of these measurements, with a correlation coefficient of 0.99, demonstrates the utility of this approach for such applications. We also applied this analysis to the detection of KRAS and could easily observe 0.1% mutants when spiked into a population of wild-type molecules (data not shown).
Fig. 5.
Detection and validation of variants present in a minor fraction of the DNA population. (A) Mixtures of PCR products containing 0–4% L alleles of MID42 were used for BEAMing. Flow cytometry such as that shown in Fig. 3 was used to determine the fraction of singlet beads that were red (y axis). The proportion of singlet beads that hybridized to at least one of the probes varied from 3.2% to 4.3%. (B and C) Beads were sorted with the FACSVantage SE instrument, and individual red or green beads were used as templates for conventional PCR by using the forward and reverse primers listed in Table 1. Red beads generated only the S allele sequence, whereas green beads generated only the L allele sequence.
The rare beads representing the mutant alleles could not only be quantified but also purified for subsequent analysis. As a demonstration, samples of the beads enumerated in Fig. 5A were assessed by using a flow cytometer equipped with sorting capabilities. Beads were sorted and individual beads were used as templates for conventional PCR by using the same primers used for BEAMing. Because each bead contains thousands of bound template molecules, single beads were expected to generate robust PCR products (23), and this was confirmed experimentally. These PCR products then were subjected to sequencing. As shown in Fig. 5 B and C, green and red beads generated PCR products exclusively of the L and S types, respectively.
Discussion
The results described above show that BEAMing provides a reliable and sensitive assay for measuring variations in genes and transcripts. It requires no instrumentation other than a magnetic stirrer, a temperature cycler, and a flow cytometer, all of which are widely available. There are several other advantages of this approach. First, the sensitivity can be increased to meet the specifications of the experiment simply by analyzing more beads. Such sensitivity is limited only by the error rate of the polymerases used for amplification. Second, BEAMing data can be used not only to demonstrate that a variant is present in a particular population of DNA molecules but also quantifies the fraction of variant DNA molecules in that population (Fig. 5A). Such quantification is not possible with techniques that destroy or ignore the wild-type molecules as part of the assay, such as those that use allele-specific priming or endonuclease digestion during PCR. Third, the beads containing variant alleles can be purified easily through flow sorting and used for subsequent experimentation. Such recovery is difficult with digital techniques that count molecules deposited on microscope slides. Finally, the BEAMing approach, in principle, is automatable.
Several modifications of the basic principles described here can be envisioned that will simplify the technology further or widen its applications. For example, microemulsions were made by stirring water/oil/detergent mixes. The sizes of the resultant aqueous compartments were somewhat heterogeneous, as illustrated in Fig. 2. A relatively large number of beads containing PCR products of both alleles are obtained from large compartments because they are more likely to contain more than one template molecule than smaller compartments. Although this is not a problem for the analysis of uncommon variants, it does pose a problem when the variant to be analyzed is present in a substantial fraction of the DNA molecules. For example, it is easy to distinguish a population containing 2% of allele A and 98% of allele B from one that contains 0% of allele A (Fig. 5A). But it is more difficult to distinguish a population that contains 48% of allele A and 52% of allele B from a population that contains 50% of allele A; the large number of heterozygote beads formed in the latter analysis diffuses the boundaries of the pure red and green channels. This limit to accuracy theoretically can be overcome through the preparation of more uniformly sized aqueous compartments, perhaps through sonication or pressure-driven emulsifiers.
Although flow cytometry requires only seconds to minutes per sample, multiple parallel analyses could facilitate throughput. Novel particle-counting designs may prove useful for this purpose (24, 25). Another way to increase throughput would be to physically separate the beads that contained PCR products before flow cytometry, which could be accomplished with antibodies to modified nucleotides incorporated into the PCR product during BEAMing.
Although we focused on issues related to human variation, BEAMing technology obviously could be applied to genes or transcripts of any organism or population of organisms. It could also be used to quantify epigenetic alterations such as methylation if DNA was first treated with bisulfite to convert methylated cytosine residues to thymidine. Beads generated from random fragments of whole genomes (26) rather than from individual genes as described above could be used to identify gene segments that bind to specific DNA-binding proteins (27). If the beads made by BEAMing were used in compartmentalized in vitro transcription-translation reactions, variant proteins would be bound to beads containing the corresponding variant DNA sequences (23), which could allow facile flow-cytometric evaluation of rare mutations by using antibodies that distinguish between wild-type and mutant gene products (28).
Supplementary Material
Acknowledgments
We thank Leslie Meszler, Christoph Lengauer, and Lee Blosser for expert advice and assistance with flow cytometry. G.T. is a recipient of a Junior Research Fellowship from Trinity College, University of Cambridge (Cambridge, U.K.). This work was supported by the National Colorectal Cancer Research Alliance, the Clayton Fund, and National Institutes of Health Grants CA 43460, CA 57345, and CA 62924.
Abbreviation: BEAM, beads, emulsion, amplification, and magnetics.
References
- 1.Collins, F. S., Patrinos, A., Jordan, E., Chakravarti, A., Gesteland, R. & Walters, L. (1998) Science 282, 682–689. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein, B. & Kinzler, K. W. (2002) The Genetic Basis of Human Cancer (McGraw–Hill, Toronto).
- 3.Sidransky, D., Von Eschenbach, A., Tsai, Y. C., Jones, P., Summerhayes, I., Marshall, F., Paul, M., Green, P., Hamilton, S. R., Frost, P., et al. (1991) Science 252, 706–709. [DOI] [PubMed] [Google Scholar]
- 4.Ahlquist, D. A. & Shuber, A. P. (2002) Clin. Chim. Acta 315, 157–168. [DOI] [PubMed] [Google Scholar]
- 5.Sidransky, D. (2002) Nat. Rev. Cancer 2, 210–219. [DOI] [PubMed] [Google Scholar]
- 6.Chomyn, A. & Attardi, G. (2003) Biochem. Biophys. Res. Commun. 304, 519–529. [DOI] [PubMed] [Google Scholar]
- 7.Vogelstein, B. & Kinzler, K. W. (1999) Proc. Natl. Acad. Sci. USA 96, 9236–9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra, R. D., Butty, V. L., Shendure, J., Williams, B. R., Housman, D. E. & Church, G. M. (2003) Proc. Natl. Acad. Sci. USA 100, 5926–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, H. H., Gyllensten, U. B., Cui, X. F., Saiki, R. K., Erlich, H. A. & Arnheim, N. (1988) Nature 335, 414–417. [DOI] [PubMed] [Google Scholar]
- 10.Ruano, G., Kidd, K. K. & Stephens, J. C. (1990) Proc. Natl. Acad. Sci. USA 87, 6296–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffreys, A. J., Allen, M. J., Armour, J. A., Collick, A., Dubrova, Y., Fretwell, N., Guram, T., Jobling, M., May, C. A., Neil, D. L., et al. (1995) Electrophoresis 16, 1577–1585. [DOI] [PubMed] [Google Scholar]
- 12.Lizardi, P. M., Huang, X., Zhu, Z., Bray-Ward, P., Thomas, D. C. & Ward, D. C. (1998) Nat. Genet. 19, 225–232. [DOI] [PubMed] [Google Scholar]
- 13.Jurinke, C., van den Boom, D., Cantor, C. R. & Koster, H. (2002) Adv. Biochem. Eng. Biotechnol. 77, 57–74. [DOI] [PubMed] [Google Scholar]
- 14.Tawfik, D. S. & Griffiths, A. D. (1998) Nat. Biotechnol. 16, 652–656. [DOI] [PubMed] [Google Scholar]
- 15.Ghadessy, F. J., Ong, J. L. & Holliger, P. (2001) Proc. Natl. Acad. Sci. USA 98, 4552–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi, S., Bratu, D. P. & Kramer, F. R. (1998) Nat. Biotechnol. 16, 49–53. [DOI] [PubMed] [Google Scholar]
- 17.Weber, J. L., David, D., Heil, J., Fan, Y., Zhao, C. & Marth, G. (2002) Am. J. Hum. Genet. 71, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan, H., Yuan, W., Velculescu, V. E., Vogelstein, B. & Kinzler, K. W. (2002) Science 297, 1143. [DOI] [PubMed] [Google Scholar]
- 19.Cowles, C. R., Joel, N. H., Altshuler, D. & Lander, E. S. (2002) Nat. Genet. 32, 432–437. [DOI] [PubMed] [Google Scholar]
- 20.Yan, H., Dobbie, Z., Gruber, S. B., Markowitz, S., Romans, K., Giardiello, F. M., Kinzler, K. W. & Vogelstein, B. (2002) Nat. Genet. 30, 25–26. [DOI] [PubMed] [Google Scholar]
- 21.Okimoto, R. & Dodgson, J. B. (1996) BioTechniques 21, 20–26. [DOI] [PubMed] [Google Scholar]
- 22.Luo, J., Bergstrom, D. E. & Barany, F. (1996) Nucleic Acids Res. 24, 3071–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sepp, A., Tawfik, D. S. & Griffiths, A. D. (2002) FEBS Lett. 532, 455–458. [DOI] [PubMed] [Google Scholar]
- 24.Jackson, W. C., Bennett, T. A., Edwards, B. S., Prossnitz, E., Lopez, G. P. & Sklar, L. A. (2002) Biotechniques 33, 220–226. [DOI] [PubMed] [Google Scholar]
- 25.Fu, A. Y., Chou, H. P., Spence, C., Arnold, F. H. & Quake, S. R. (2002) Anal. Chem. 74, 2451–2457. [DOI] [PubMed] [Google Scholar]
- 26.Kinzler, K. W. & Vogelstein, B. (1989) Nucleic Acids Res. 17, 3645–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, X., Li, X., Prow, T. W., Reece, L. M., Bassett, S. E., Luxon, B. A., Herzog, N. K., Aronson, J., Shope, R. E., Leary, J. F. & Gorenstein, D. G. (2003) Nucleic Acids Res. 31, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gite, S., Lim, M., Carlson, R., Olejnik, J., Zehnbauer, B. & Rothschild, K. (2003) Nat. Biotechnol. 21, 194–197. [DOI] [PubMed] [Google Scholar]
- 29.Chetverina, H. V., Samatov, T. R., Ugarov, V. I. & Chetverin, A. B. (2002) BioTechniques 33, 150–152, 154, 156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.