Abstract
Infection by HIV-1 causes persistent, long-term high virus production in macrophages. Major evidence, both in humans and in primate models, shows the crucial role of macrophages in sustaining virus production and in mediating a cytopathic effect on bystander CD4+ T lymphocytes and neuronal cells. In the present study, we used severe combined immunodeficient (SCID) mice engrafted with human peripheral blood lymphocytes (hu-PBL-SCID mice) to investigate the in vivo effect of HIV-1-infected macrophages on virus spread and CD4+ T lymphocyte depletion, and the ability of a mAb against nerve growth factor (NGF, a neurokine essential for the survival of HIV-1-infected macrophages) to suppress the pathogenetic events mediated by infected macrophages. Injection of mice with as few as 500 HIV-exposed macrophages causes (i) complete depletion of several millions of autologous CD4+ T lymphocytes, (ii) sustained HIV viremia, and (iii) spreading of HIV-1 DNA in mouse lymphoid organs. In contrast, in vivo treatment with an anti-NGF Ab completely abrogates all effects mediated by HIV-infected macrophages. Taken together, the results demonstrate the remarkable power of macrophages in sustaining in vivo HIV-1 infection, and that such a phenomenon can be specifically abrogated by an anti-NGF Ab. This may open new perspectives of experimental approaches aimed at selectively eliminating persistently infected macrophages from the bodies of HIV-infected patients.
Clinical evidence shows that the sole persistence of latent HIV-1 infection in resting CD4+ memory T lymphocytes cannot completely explain the reemergence of the virus after cessation of antiretroviral therapy (1, 2). This supports the existence of reservoirs, other than resting CD4 lymphocytes, able to play a key role in the pathogenesis of HIV infection (3, 4).
Cells of monocyte/macrophage lineage (M/M) have long been known to be strategic targets (the Trojan horse theory) of HIV-1 in infected patients (5–9). Such cells are widely recognized as an important reservoir and source of virus during HIV-1 infection, even in patients receiving antiretroviral therapy (10). Once infected by HIV-1, M/M survive and produce large amounts of infectious viral particles (an average of 400 virions released daily by each infected cell) for a long period (11). Their ability to transfer HIV to CD4+ T lymphocytes with which they come in contact in the context of antigen presentation is well recognized (12). Therefore, M/M sustain persistent and continuously productive HIV infection (13).
The factor(s) allowing M/M to survive HIV infection are thus far poorly elucidated. In our attempt to clarify these factors, we concentrated our attention on nerve growth factor (NGF). NGF belongs to a family of neurokines involved in the differentiation processes and survival of neurons and neural crest-derived cells (14, 15). Moreover, NGF plays a relevant role in inhibiting apoptosis not only of neurons, but also of cells of bone-marrow origin, such as memory B lymphocytes (16). In addition, a recent study led by our group has demonstrated that NGF is an autocrine survival factor produced and released by HIV-1-infected M/M during the first hours/days of infection (17). NGF starvation of M/M causes apoptosis of HIV-infected, but not of uninfected M/M, via expression on their surface of p75 low-affinity NGF receptor. Such selective killing is associated with a dramatic decrease of virus production and release from M/M cultures (17). The demonstration and application of this in vitro evidence to in vivo models of HIV infection may provide grounds for therapeutic applications aimed at the selective killing of persistently HIV-infected cells.
To apply these concepts to an in vivo situation, we used severe combined immunodeficient (SCID) mice, which, being engrafted with human peripheral blood lymphocytes (hu-PBL-SCID mice), can be successfully infected with HIV-1, and the immunological and viral parameters of which can be easily monitored (18). This nonprimate animal model has contributed to the understanding of relevant aspects of the pathogenesis and therapy of HIV-1 infection. However, despite its multiple uses in AIDS research, the hu-SCID mouse model has not yet been used for studying the in vivo ability of HIV-1-infected macrophages to infect human target cells and to induce immune dysfunction. We thus performed these studies and also evaluated the ability of a neutralizing anti-NGF Ab to interfere with the pathogenetic events induced by HIV-infected M/M.
Materials and Methods
Cells. Human peripheral blood leukocytes (hu-PBLs) were obtained from the peripheral blood of healthy donors screened for HIV-1 and hepatitis before donation. After separation over Ficoll-Paque density gradient centrifugation, 30 million of these PBLs were resuspended in 0.5 ml of RPMI medium 1640 and injected i.p. into all recipient mice used in the experiments described in this paper.
Macrophages were obtained as described (11). Briefly, PBLs were seeded in six-well plates (Falcon) at 1.5 × 107 cells per well in RPMI medium 1640 (GIBCO) with the addition of 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, and 20% heat-inactivated, mycoplasma- and endotoxin-free FCS (HyClone), hereafter called complete medium. Cells were incubated at 37°C in humidified air containing 5% CO2. After 5 days, nonadherent cells were carefully removed by repeated gentle washings with warm medium. M/M obtained showed a purity >96% as tested by cytofluorimetric analysis. M/M were then infected with 1,000 tissue culture 50% infective dose per ml of an HIV-1 C-C chemokine receptor-5-dependent macrophagetropic strain (HIV-1BaL). After 2 h, virus excess was removed by repeated washings, then M/M were cultured in complete medium until injected into the mice. Where requested for in vitro studies, macrophage cultures were carefully washed every 5 days to completely remove virus particles and proteins, and were replenished with fresh complete medium. After infection, ≈40–65% of the M/M were found positive by cytofluorimetric analysis for the intracellular HIV-1-p24 antigen as described (11).
In selected experiments, CD4+ T lymphocytes were purified by negative separation with a single immunomagnetic depletion step by using a panel of antibodies in combination with magnetic cell sorting beads. Cells were cultured with the same medium as M/M, supplemented with 2 μg/ml phytohemagglutinin. Stimulation was carried out for 72 h; afterward, the medium was discarded, cells were washed three times with RPMI medium 1640, and the concentration was adjusted to 5 × 105 cells per ml of medium supplemented with 50 U/ml recombinant interleukin-2 (IL-2). Cells were infected with 1,000 tissue culture 50% infective dose per ml of the prototypic lymphocytotropic strain of HIV-1 (HIV-1IIIB). After 2 h, cells were washed, counted, and seeded in 25-cm2 plastic flasks with complete medium at concentration of 106 cells per ml until injection in mice.
Assessment of virus replication in in vitro cell cultures before injection in mice was performed by a commercially available HIV-1-p24 ELISA (DuPont).
Animals. CB17 scid/scid female mice (Harlan, Milan) were used at 4 weeks of age and were kept under specific pathogen-free conditions. SCID mice were housed in microisolator cages; all food, water, and bedding were autoclaved before use.
Animal Infection. Macrophages were gently scraped from plastic plates, suspended in PBS, and washed twice. After counting, M/M were resuspended in RPMI medium 1640 and injected in mice at different concentrations where requested.
In selected experiments, CD4+ T lymphocytes were washed twice in PBS, then counted, resuspended in RPMI medium 1640, and injected at different concentrations in PBL-reconstituted mice.
NGF Production in hu-PBL-SCID Mice. hu-PBL-SCID mice were injected i.p. with HIV-infected or mock-infected M/M. At different time points, blood was collected and NGF titration of plasma pools from each different group of mice was performed by a two-site enzyme immunoassay (EIA) by using a commercially available NGF mouse mAb 27/21 (Chemicon) as described (19).
Reagent Anti-NGF. The αD11 hybridoma was prepared according to Cattaneo et al. (20). The αD11 IgG1 Ig was purified from hybridoma supernatant by precipitation with 29% ammonium sulfate followed by affinity chromatography by using a protein G-Sepharose column (Amersham Pharmacia) and eluted by low pH buffer (10 mM HCl) (20, 21). αD11 IgG1 fractions were pooled and dialyzed across a Spectra/Por Membrane 12/14K (Spectrum, Rancho Dominguez, CA) against 10 mM Tris, pH 7.0.
The anti-NGF mAb (400 μg) was inoculated i.p. in mice immediately before injection of HIV-infected M/M and again after 3 days.
Cell Recovery from Peritoneal Cavity and Organs of the SCID Mice. hu-PBL-SCID mice were killed 14 days after HIV infection (unless differently specified), and cells were collected from the peritoneal cavity, spleen, and lymph nodes. At each time, a two-step peritoneal lavage was done. The first washing was performed with 1 ml of cold RPMI medium 1640. The recovered volume was centrifuged, and the supernatant was stored at –20°C while the cells were pooled with those obtained from a second 4-ml washing. Spleen and lymph nodes were disrupted with the blunt end of a 5-ml syringe plunger. Connective tissue and debris were allowed to settle, and the single-cell suspensions were washed twice in RPMI medium 1640 (18).
Flow Cytometric Analysis. Cells recovered from the peritoneum of the hu-PBL-SCID mice were resuspended in PBS and incubated with the appropriate f luorochrome-conjugated mAbs for 30 min. The cells were then washed with a mixture of PBS, 2% FCS, and 0.1% sodium azide and fixed with 2.5% paraformaldehyde. Two-color flow cytometry was performed with a FACSort fluorescence-activated cell-sorter cytometer (Becton Dickinson); cells were analyzed with cellquest (Becton Dickinson) software. A total of 5,000 events per sample were collected. Cells were analyzed according to forward and side scatter properties to gate the live lymphocyte population, allowing for the exclusion of erythrocytes, dead cells, and cell and tissue debris. The mAbs used were anti-human CD4 and CD8 (Becton Dickinson).
Detection of Viral Infection in SCID Mice. The chimeras were killed after 4 weeks and analyzed for HIV-1 infection by (i) cocultivation of cell suspensions from peritoneal lavage with human phytohemagglutinin-IL-2-stimulated peripheral blood mononuclear cells for 10 days, with positivity of cocultivation determined by detection of p24 antigen by ELISA (DuPont) in culture supernatants; and (ii) PCR detection of HIV-1 proviral sequences. DNA was extracted from spleens and lymph nodes of the hu-PBL-SCID mice. The presence of human sequences was determined by DNA-PCR by using specific primers (GH26 and GH27) for the HLA-DQα gene, whereas HIV-1 proviral DNA was detected by specific amplification of HIV-1 gag sequences as described (18). The sensitivity and specificity of the assay was tested by amplifying DNA prepared from 8E5 cells (which harbor one proviral HIV copy per cell) serially diluted into SCID mouse cell DNA.
Determination of HIV Viremia. Plasma samples were obtained from EDTA-preserved whole blood, separated within 4 h of collection, divided into multiple 100-μl aliquots and stored at –80°C until testing. To assess plasma HIV-1 RNA viral load we used a Versant HIV-1 RNA version 3.0 (Bayer Diagnostics, Emeryville, CA) procedure, according to the manufacturer's instructions. The assay was performed with 50 μl of plasma. The lower detection limit of quantification for the bDNA is 250 copies per ml.
Statistical Analysis. Student's t test was used for statistical analysis.
Results
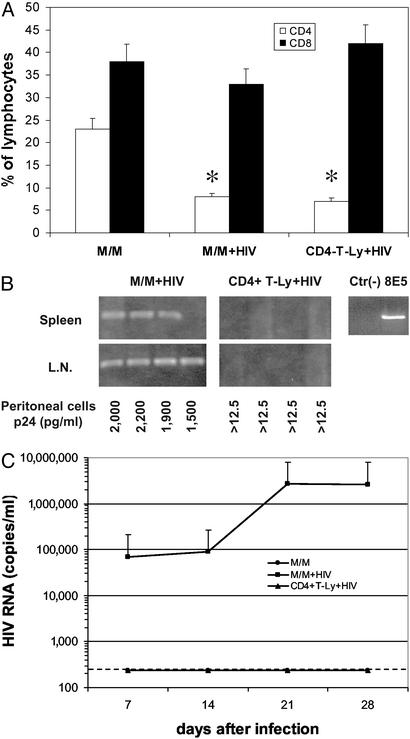
Macrophages, but Not CD4+ T Lymphocytes, Induce a Stable HIV Infection in hu-PBL-SCID Mice. As shown in Fig. 1A, inoculation in hu-PBL-SCID mice of either HIV-infected M/M or HIV-infected CD4+ T lymphocytes results in a severe depletion of CD4+ T lymphocytes, with only 8% and 7% of human CD4+ cells detectable, respectively, at day 28 after infection (when four animals for each group were killed), compared with 23% of CD4+ cells in control uninfected animals.
Fig. 1.
HIV-infected macrophages induce infection and CD4+ depletion in hu-PBL-SCID mice. (A) Percentages of CD4+ T lymphocytes (open bars) and CD8+ T lymphocytes (filled bars) were measured in hu-PBL-SCID mice at day 28 after challenge with 5,000 HIV-infected macrophages (M/M+HIV), 5,000 HIV-infected CD4+ T lymphocytes (CD4-T-Ly+HIV), or mock-infected macrophages (M/M); *, P < 0.001 compared with CD4 of mice challenged with uninfected M/M. (B) Mice were killed 4 weeks after infection, then the spleen and the lymph nodes (L.N.) were analyzed for the presence of HIV-1 proviral copies by DNA PCR. HIV-1 p24 antigen was measured by ELISA in supernatants of peritoneal cells isolated from hu-PBL-SCID mice and cocultivated (peritoneal cells) with donor autologous PBLs. Controls for the DNA PCR experiments included the DNA from uninfected CEM cells [Ctr(-)] and DNA from 8E5 HIV-infected cells, which harbor one proviral HIV copy per cell (8E5). (C) HIV-1 RNA was measured in the plasma of hu-PBL-SCID mice challenged with HIV-infected M/M (▪), HIV-infected CD4+ T lymphocytes (▴), or mock-infected macrophages (•) at different time points after cell injection. The dashed line defines the threshold of HIV RNA detection. Four mice were used in each group.
In the case of mice inoculated with HIV-infected M/M, the loss of CD4+ cells was associated with the presence of HIV-DNA in spleens and lymph nodes (Fig. 1B). Cocultivations of cells recovered from peritoneal lavage with allogeneic human lymphocytes also were positive for HIV-p24 (Fig. 1B). Consistent with these results, plasma HIV viremia increased during the first 2 weeks and was followed by a plateau until day 28 (when mice were killed), with a peak of 2.67 × 106 HIV-RNA copies per ml 21 days after inoculation (Fig. 1C).
By contrast, and despite the loss of CD4+ lymphocytes, spleens and lymph nodes of hu-PBL-SCID mice challenged with HIV-infected CD4+ T lymphocytes (instead of HIV macrophages) turned out to be negative for HIV DNA; similarly, cocultivations with allogeneic lymphocytes and plasma viremia also were negative (Fig. 1 B and C).
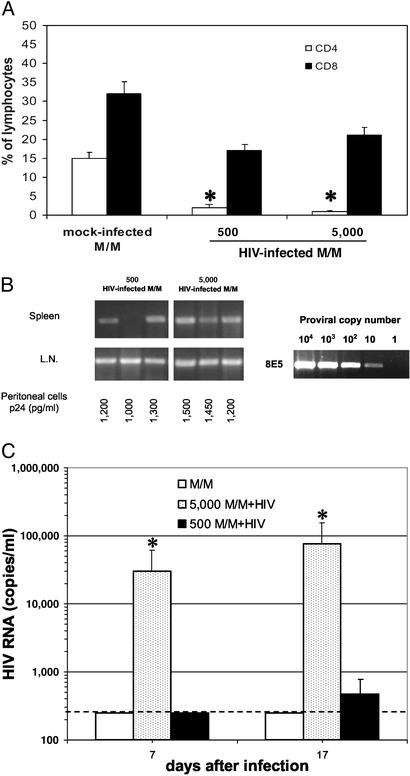
Few HIV-Infected M/M Can Sustain Plasma Viremia in hu-PBL-SCID Mice. The efficiency of depletion of CD4+ cells by HIV-infected M/M was similar when 500 or 5,000 HIV-infected M/M were inoculated in three mice for each group (Fig. 2A). Moreover, no significant differences between 500 and 5,000 infected M/M were found when spleens and lymph nodes were analyzed for HIV DNA, with the exception of one spleen found negative in a mouse receiving the lowest dose of HIV-infected M/M (Fig. 2B). HIV p24 Ag was assessed and found positive in supernatants of all cocultivations of both groups (Fig. 2B). Plasma viremia was measured, and 3.04 × 104 and 7.73 × 104 RNA copies per ml of HIV RNA were achieved in mice receiving 5,000 HIV-infected M/M, at days 7 and 17, respectively. Plasma viremia was instead undetectable in two of three animals challenged with 500 HIV-infected M/M (one mouse showed 1.4 × 103 RNA copies per ml at day 17; Fig. 2C).
Fig. 2.
Few M/M are sufficient to efficiently spread HIV infection in hu-PBL-SCID mice. (A) Percentage of CD4+ T lymphocytes (open bars) and CD8+ T lymphocytes (filled bars) were measured in hu-PBL-SCID mice at day 21 after challenge with 500 or 5,000 HIV-infected macrophages, or 5,000 mock-infected macrophages; *, P < 0.01 compared with CD4 of mice challenged with uninfected M/M. (B) HIV-1 proviral copies were evaluated in spleen and lymph nodes (L.N.) by DNA PCR; as a control, several dilutions of DNA from 8E5 HIV-infected cells are reported. Cocultivation of peritoneal cells with donor autologous PBLs was also performed. (C) HIV-1 RNA was measured in the plasma of hu-PBL-SCID mice at days 7 and 17 after challenge with 5,000 HIV-infected macrophages (5,000 M/M+HIV, stippled bars), 500 HIV-infected macrophages (500 M/M+HIV, filled bars), or 5,000 mock-infected macrophages (M/M, open bars); *, P < 0.001 compared with CD4 of mice challenged with uninfected M/M. The dashed line defines the threshold of HIV-RNA detection. Three mice were used in each group.
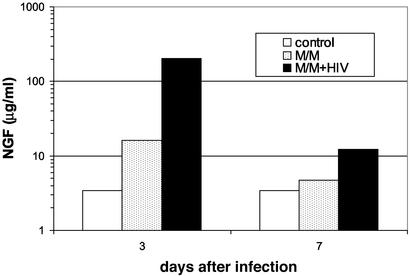
In Vivo Detection of Autocrine NGF Production by HIV-Infected M/M. When HIV-infected M/M were inoculated in hu-PBL-SCID mice, a production of 202 μg/ml of NGF was found at day 3 after infection (Fig. 3), at least 12-fold higher than that achieved in controls showing a basal NGF production. In hu-PBL-SCID mice that did not receive M/M (either HIV-infected or mock-infected), 3.4 μg/ml NGF was measured at days 3 and 7 (Fig. 3). NGF production in HIV-infected M/M-exposed mice dropped from 200 to ≈12 μg/ml at day 17, a concentration still 3-fold higher than controls (Fig. 3).
Fig. 3.
Macrophages produce and release NGF once infected by HIV. NGF production was measured in hu-PBL-SCID mice 3 and 7 days after challenge with HIV-infected macrophages (M/M+HIV, filled bars) or mock-infected microphages (M/M, stippled bars). Control mice were not injected with macrophages (control, open bars). Samples were run in pool for each different group of three mice. Results presented are from one representative experiment of two.
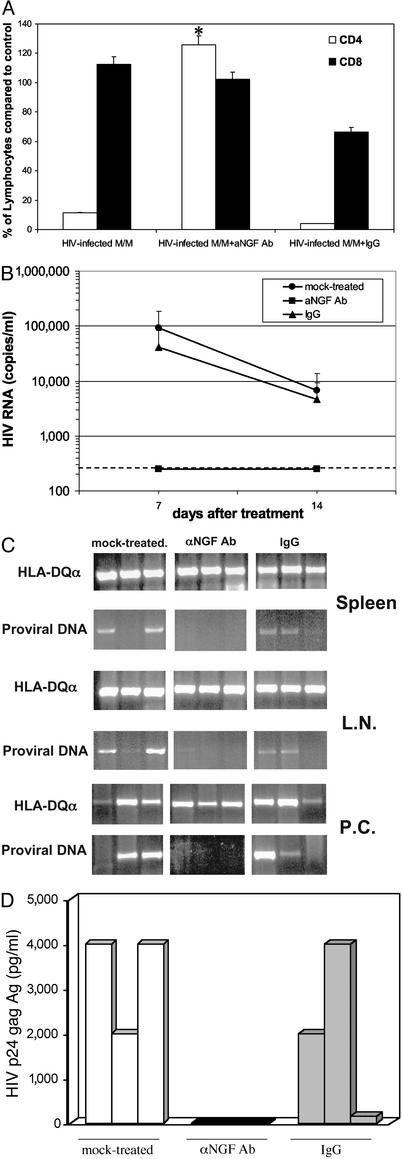
In Vivo Treatment with a Neutralizing Anti-NGF Ab Abrogates the Effects Induced by HIV-Infected M/M. Because the mice inoculated with HIV-infected M/M showed a dramatic loss of CD4+ cells, accompanied by both an overwhelming infection and a sustained plasma viremia, we next evaluated whether apoptosis of HIV-infected M/M caused by NGF starvation blocks these pathogenetic events. This event was already demonstrated in an in vitro model, in which anti-NGF Ab induced apoptosis in HIV-infected M/M by depleting the autocrine NGF produced and released by M/M during the first hours/days after virus challenge (17).
The effects of treatment of hu-PBL-SCID mice with anti-NGF Ab given at the time of challenge with infected M/M are shown in Fig. 4. CD4+ cell depletion in hu-PBL-SCID mice exposed to HIV-infected M/M was completely inhibited by treatment with the monoclonal anti-NGF Ab. Indeed, the rate of CD4+ T lymphocytes 2 weeks after infection with HIV-infected M/M and treatment with anti-NGF Ab was comparable to that found in control uninfected mice (Fig. 4A). As an additional control, the rate of CD4+ T lymphocytes in mice injected with HIV-infected macrophages and treated with an IgG isotypic Ab was similar to that achieved in mice exposed to HIV-infected M/M in the absence of anti-NGF Ab. This further confirms that, in line with the in vitro observations previously published (17), the protective effect of anti-NGF Ab is specifically related to the inhibition of NGF production by infected M/M.
Fig. 4.
NGF starvation prevents HIV infection in hu-PBL-SCID mice challenged with infected macrophages. (A) Percentage of CD4+ T lymphocytes (white bars) and CD8+ T lymphocytes (black bars) were measured in hu-PBL-SCID mice at day 14 after challenge with HIV-infected macrophages and treatment with anti-NGF mAb (HIV-infected M/M+aNGF Ab). A group of mice was treated with an isotypic Ab (HIV-infected M/M+IgG); *, P < 0.05 compared with CD4 in infected but untreated hu-PBL-SCID mice. (B) HIV-1 RNA was measured in hu-PBL-SCID plasma at days 7 and 14 after HIV macrophage challenge and treatment with either anti-NGF Ab (▪) or an isotypic IgG Ab (▴), or no treatment (•); P < 0.05. The dashed line defines the threshold of HIV-RNA detection. (C) Mice were killed 2 weeks after infection, then the spleen, lymph nodes (L.N.), and peritoneal cells (P.C.) were analyzed for the presence of HIV-1 proviral copies and HLA-DQ expression by DNA PCR. (D) HIV-1 p24 antigen was measured by ELISA in supernatants of peritoneal cells isolated from hu-PBL-SCID mice challenged with HIV-infected M/M treated with aNGF Ab (black bars) or an IgG isotypic Ab (gray bars), or mock-treated (white bars). In this experiment, three mice for each group were used. Each bar corresponds to a single mouse.
In accordance with these results, HIV RNA was not detectable in plasma of mice treated with anti-NGF Ab (Fig. 4B), at day 7 or day 14 after infection. On the contrary, plasma viremia at day 14 after infection was 6.80 × 104 (±0.52 × 104) and 4.62 × 104 (±0.29 × 104) HIV RNA copies per ml, respectively, in mock- and IgG isotype-treated mice infected with HIV macrophages (Fig. 4B). DNA PCR of lymph nodes and spleens collected from these animals revealed the presence of HIV provirus in all mice injected with HIV-infected M/M, either mock-treated or IgG isotype-treated (Fig. 4C). Moreover, cells collected from peritoneal lavage of all mice challenged with HIV-infected M/M were able to transfer HIV-1 infection to new PBL, except those collected from HIV macrophages-infected mice exposed to anti-NGF Ab (Fig. 4D). Thus, the results of virus isolation, HIV provirus, plasma viremia, and CD4 depletion all confirm that the in vivo treatment with anti-NGF Ab specifically abrogates the infection and the cytopathic effect induced by HIV-infected macrophages.
Discussion
The experiments described in this paper demonstrate the ability of M/M not only to sustain a long-term persistent virus replication, but also to play a major role in the selective death of CD4+ T lymphocytes. It remains to be elucidated whether virus transfer from HIV-infected M/M is the only factor causing the death of CD4+ T lymphocytes. M/M are indeed able to transfer HIV to CD4+ T lymphocytes (12). At the same time, HIV-infected M/M produce factors that can mediate, directly or indirectly, the activation of programmed cell death on bystander cells both in vitro and in vivo (22–26). As a relevant example, HIV-infected M/M release HIV-nef protein, which favors the recruitment and activation of CD4+ T lymphocytes (27), eventually causing their massive death. The ability of M/M to communicate with, and transfer HIV to, CD4+ T lymphocytes is based on their anatomical location in lymph nodes and tissues and on their physiological interaction with CD4+ T lymphocytes in the context of antigen presentation and immune response. It is therefore not surprising that very few (as few as 500) HIV-infected M/M injected in hu-PBL-SCID mice rapidly (within a couple of weeks) trigger a nearly complete depletion of circulating human CD4+ T lymphocytes (derived from the injection of 30 million PBLs), accompanied by a remarkable level of viremia and distribution of proviral DNA in all tissues and organs examined. The proviral DNA is always associated with HLA-DQ, a sign of efficient colonization of human CD4+ T lymphocytes in animal lymphoid tissues. Our data also show the remarkable ability of macrophages, injected i.p., to invade lymphatic vessels and distribute throughout the whole mouse body. Preliminary evidence shows that human M/M injected in the mouse peritoneum migrate and can be found in tissues and organs, including the brain (data not shown; S.A., C.L., F.B., C.-F.P., and E.G., unpublished data). This ability to migrate and distribute in all tissues increases the likelihood of contacts between HIV macrophages and circulating human CD4 lymphocytes, which in turn may activate mechanisms, whether mediated or not by virus transfer, that ultimately bring about their apoptotic death.
hu-PBL-SCID mice exhibit unique advantages for in vivo studies on the interactions between HIV and human immune cells; however, the rapid spread of virus infection and the limited extent of human immune functions, including cell turnover and replenishment, render difficult any attempt to show clear-cut antiviral activities in this model. For instance, it has been shown that an incomplete antiviral effect of either 3′-azido-3′-deoxythymidine or IFN-α can be achieved in HIV-infected hu-PBL-SCID mice only at very high doses and with treatments repeated before and after viral infection (28). Despite this potential limitation, the hu-PBL-SCID mice model is widely recognized as a reliable system to study the pathogenesis of HIV infection (29–31). In addition, there is evidence showing that most of the events described in hu-PBL-SCID mice also occur in humans, as well as in primate models of lentiviral infection. Indeed, two recent papers from the same group describe the ability of simian immunodeficiency virus/HIV type 1 chimera (SHIV)-infected macrophages to induce both virus replication and immunological damage in experimentally infected monkeys (32, 33). In addition, the role of macrophages in the pathogenesis of HIV encephalitis has been clearly described in humans and confirmed in experimental models of monkeys and hu-PBL-SCID mice (34–38). Taken together, overall data support the utilization of hu-PBL-SCID mice as a reliable model of HIV infection that may shed light on pathogenetic events (such as those described in this paper) not yet elucidated in primate models and in humans.
The mechanisms underlying the ability of macrophages to sustain long-term HIV replication have been explored, and in part clarified, by a study demonstrating the production of autocrine NGF by HIV-infected (but not uninfected) macrophages. The binding of NGF to NGF low-affinity p75 receptors on the macrophage surface rescues HIV macrophages from apoptotic death. The in vitro deprivation of NGF by an anti-NGF Ab leads to a selective apoptosis-mediated death of HIV macrophages, thus confirming the essential role of this autocrine factor in HIV macrophage survival (17). Studies conducted in another model of bone marrow-derived cells (memory B lymphocytes) have demonstrated that the expression of p75 receptor in conditions of NGF deprivation activates a series of events based on p38 mitogen-activated protein kinase activation and translocation onto the mitochondria, whereby it combines with phosphorylated Bcl-2, triggering the apoptotic cascade (39). A similar mechanism may play a role in HIV-infected macrophages, although its demonstration is still required.
The transfer of in vitro observations of the role of NGF on cell survival to in vivo models represents an essential step to scale up toward the clinical applications of experimental data. Along this line, it has already been demonstrated that in vivo treatment of immunocompetent mice with anti-NGF Ab leads to the selective death of memory B lymphocytes, and then to the disruption of B lymphocyte-associated immunological memory (16). Our hu-PBL-SCID mice model shows that in vivo administration of neutralizing anti-NGF Ab leads to the abrogation of virus spread mediated by HIV-infected macrophages. These experiments do not directly demonstrate that anti-NGF Ab has also in vivo a proapoptotic effect on injected HIV-infected macrophages, yet all evidence (both in vivo, and in vitro in other papers) strongly suggests that the abrogation of CD4 lymphocyte depletion, of spread of HIV in all tissues and organs, and of HIV viremia, is caused by the selective death of HIV-infected macrophages deprived of a main survival factor such as NGF.
Based on these observations and results, the clinical implications of these findings may be substantial. The issue of latent and persistent reservoirs represents the major obstacle to the eradication of virus infection, or to the achievement of a remarkable and sustained control of virus replication. For these reasons, several attempts have been initiated, directed at the selective elimination of infected cells, or to the cytokine-mediated activation of latently infected lymphocytes to increase their elimination (40–46). Our data demonstrate the importance of taking into account therapeutic strategies aimed at the selective elimination of reservoirs, such as HIV-infected macrophages, able to sustain and support persistent HIV replication and spreading. Confirmation of these observations in reliable and more advanced animal models (such as monkeys infected by simian immunodeficiency virus/simian/human immunodeficiency virus) may open the way to clinical experimental approaches based on treatment with factors and substances (anti-NGF Ab, or its peptidic derivatives) potentially able to eliminate this viral source, and then to achieve a greater and longer control of virus replication.
Acknowledgments
We thank Prof. A. Cattaneo (Scuola Internazionale Superiore di Studi Avanzati, Trieste, Italy) for supplying the monoclonal anti-NGF Ab used in this study, Dr. S. M. Santini (Istituto Superiore di Sanità, Rome) for his expertise and excellent assistance in fluorescence-activated cell-sorter analysis, Prof. F. Pica (University of Rome “Tor Vergata”) for the assessment of NGF levels in the plasma of hu-PBL-SCID mice, and Mrs. T. Guenci and Mrs. F. Di Santo for their technical help. This work was supported in part by grants from the Italian Ministry of Health (AIDS Project of the Istituto Superiore di Sanità, and Progetto Finalizzato no. ICS 120.5/RF00.123), the Italian Ministry of Education, University, and Research (National Research Council and 40%), and the European Community.
Abbreviations: SCID, severe combined immunodeficient; hu-PBL-SCID, SCID mice engrafted with human peripheral blood lymphocytes; M/M, monocyte/macrophage lineage; NGF, nerve growth factor.
References
- 1.Chun, T.-W., Davey, R. T., Ostrowski, M., Shawn Justement, J., Engel, D., Mullins, J. I. & Fauci, A. S. (2000) Nat. Med. 6, 757–761. [DOI] [PubMed] [Google Scholar]
- 2.Zhang, L., Chung, C., Hu, B.-S., He, T., Guo, Y., Kim, A. J., Skulsky, E., Jin, X., Hurley, A., Ramratnam, B., et al. (2000) J. Clin. Invest. 106, 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharkey, M. E., Teo, I., Greenough, T., Sharova, N., Luzuriaga, K., Sullivan, J. L., Bucy, R. P., Kostrikis, L. G., Haase, A., Veryard, C., et al. (2000) Nat. Med. 6, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambotte, O., Taoufik, Y., de Goer, M. G., Wallon, C., Goujard, C. & Delfraissy, J. F. (2000) J. Acquired Immune Defic. Syndr. 23, 114–119. [DOI] [PubMed] [Google Scholar]
- 5.Herbein, G., Coaquette, A., Perez-Bercoff, D. & Pancino, G. (2002) Curr. Mol. Med. 2, 723–738. [DOI] [PubMed] [Google Scholar]
- 6.Lane, J.-H., Sasseville, V. G., Smith, M. O., Vogel, P., Pauley, D. R., Heyes, M. P. & Lackner, A. A. (1996) J. Neurovirol. 2, 423–432. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer, M. S. & Gendelman, H. E. (1992) Curr. Top. Microbiol. Immunol. 181, 239–263. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer, M. S., Skillman, D. R., Gomatos, P. J., Kalter, D. C. & Gendelman, H. E. (1990) Annu. Rev. Immunol. 8, 169–194. [DOI] [PubMed] [Google Scholar]
- 9.Aquaro, S., Balestra, E., Cenci, A., Francescani, M., Caliò, R. & Perno, C.-F. (1997) J. Biol. Regul. Homeost. Agents 11, 69–73. [PubMed] [Google Scholar]
- 10.Sonza, S., Mutimer, H. P., Oelrichs, R., Jardine, D., Harvey, K., Dunne, A., Purcell, D.-F., Birch, C. & Crowe, S. M. (2001) AIDS 15, 17–22. [DOI] [PubMed] [Google Scholar]
- 11.Aquaro, S., Bagnarelli, P., Guenci, T., De Luca, A., Clementi, M., Balestra, E., Caliò, R. & Perno, C.-F. (2002) J. Med. Virol. 68, 479–488. [DOI] [PubMed] [Google Scholar]
- 12.Crowe, S. M., Mills, J., Kirihara, J., Boothman, J., Marshall, J. A. & McGrath, M. S. (1990) AIDS Res. Hum. Retroviruses 6, 1031–1037. [DOI] [PubMed] [Google Scholar]
- 13.Li, S., Juarez, J., Alali, M., Dwyer, D., Collman, R., Cunningham, A. & Naif, H. M. (1999) J. Virol. 73, 9741–9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi-Montalcini, R. (1987) Science 237, 1154–1162. [DOI] [PubMed] [Google Scholar]
- 15.Levi-Montalcini, R., Aloe, L. & Alleva, E. (1990) Prog. Neuroendocrinimmunol. 3, 1–10. [Google Scholar]
- 16.Torcia, M., Bracci-Laudiero, L., Lucibello, M., Nencioni, N., Labardi, D., Rubartelli, A., Cozzolino, F., Aloe, L. & Garaci, E. (1996) Cell 85, 345–356. [DOI] [PubMed] [Google Scholar]
- 17.Garaci, E., Caroleo, M. C., Aloe, L., Aquaro, S., Piacentini, M., Costa, N., Amendola, A., Micera, A., Caliò, R., Perno, C.-F. & Levi-Montalcini, R. (1999) Proc. Natl. Acad. Sci. USA 96, 14013–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizza, P., Santini, S.-M., Logozzi, M., Lapenta, C., Sestili, P., Gherardi, G., Lande, R., Spada, M., Parlato, S., Belardelli, F., et al. (1996) J. Virol. 70, 7958–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pica, F., Volpi, A., Serafino, A., Fraschetti, M., Franzese, O. & Garaci, E. (2000) Blood 95, 2905–2912. [PubMed] [Google Scholar]
- 20.Cattaneo, A., Rapposelli, B. & Calissano, P. (1988) J. Neurochem. 50, 1003–1010. [DOI] [PubMed] [Google Scholar]
- 21.Ruberti, F., Capsoni, S., Comparini, A., Di Daniel, E., Franzot, J., Gonfloni, S., Rossi, G., Berardi, N. & Cattaneo, A. (2000) J. Neurosci. 20, 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastino, A., Grelli, S., Piacentini, M., Oliverio, S., Favalli, C., Perno, C.-F. & Garaci, E. (1993) Cell. Immunol. 152, 120–130. [DOI] [PubMed] [Google Scholar]
- 23.Badley, A. D., Dockrell, D., Simpson, M., Schut, R., Lynch, D. H., Leibson, P. & Paya, C. V. (1997) J. Exp. Med. 185, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbein, G., Mahlknecht, U., Batliwalla, F., Gregersen, P., Pappas, T., Butler, J., O'Brien, W. A. & Verdin, E. (1998) Nature 395, 189–194. [DOI] [PubMed] [Google Scholar]
- 25.Shi, B., De Girolami, U., He, J., Wang, S., Lorenzo, A., Busciglio, J. & Gabuzda, D. (1996) J. Clin. Invest. 98, 1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aquaro, S., Panti, S., Caroleo, M. C., Balestra, E., Cenci, A., Forbici, F., Ippolito, G., Mastino, A., Testi, R., Mollace, V., et al. (2000) J. Leukocyte Biol. 68, 429–435. [PubMed] [Google Scholar]
- 27.Swingler, S., Mann, A., Jacque, J.-M., Brichacek, B., Sasseville, V. G., Williams, K., Lackner, A. A., Janoff, E. N., Wang, R., Fisher, D., et al. (1999) Nat. Med. 5, 997–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapenta, C., Santini, S. M., Proietti, E., Rizza, P., Logozzi, M., Spada, M., Parlato, S., Fais, S., Pitha, P. M. & Belardelli, F. (1999) Virology 263, 78–88. [DOI] [PubMed] [Google Scholar]
- 29.Tyor, W. R., Power, C., Gendelman, H. E. & Markham, R. B. (1993) Proc. Natl. Acad. Sci. USA 90, 8658–8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persidsky, Y., Ghorpade, A., Rasmussen, J., Limoges, J., Liu, X. J., Stins, M., Fiala, M., Way, D., Kim, K. S., Witte, M. H., et al. (1999) Am. J. Pathol. 155, 1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persidsky, Y. & Gendelman, H. E. (1997) J. Leukocyte Biol. 62, 100–106. [DOI] [PubMed] [Google Scholar]
- 32.Igarashi, T., Brown, C. R., Endo, Y., Buckler-White, A., Plishka, R., Bischof-berger, N., Hirsch, V. & Martin, M. A. (2001) Proc. Natl. Acad. Sci. USA 98, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamichi, H., Igarashi, T., Imamichi, T., Donau, O. K., Endo, Y., Nishimura, Y., Willey, R. L., Suffredini, A. F., Lane, H. C. & Martin, M. A. (2002) Proc. Natl. Acad. Sci. USA 99, 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, T. H., Donaldson, Y.-K., Brettle, R. P., Bell, J. E. & Simmonds, P. (2001) J. Virol. 75, 11686–11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabuzda, D. H., Ho, D. D., de la Monte, S. M., Hirsch, M. S., Rota, T. R. & Sobel, R. A. (1986) Ann. Neurol. 20, 289–295. [DOI] [PubMed] [Google Scholar]
- 36.Persidsky, Y., Buttini, M., Limoges, J., Bock, P. & Gendelman, H. E. (1997) J. Neurovirol. 3, 401–416. [DOI] [PubMed] [Google Scholar]
- 37.Persidsky, Y., Limoges, J., McComb, R., Bock, P., Baldwin, T., Tyor, W., Patil, A., Nottet, H. S., Epstein, L., Gelbard, H., et al. (1996) Am. J. Pathol. 149, 1027–1053. [PMC free article] [PubMed] [Google Scholar]
- 38.Williams, K. C., Corey, S., Westmoreland, S. V., Pauley, D., Knight, H., deBakker, C., Alvarez, X. & Lackner, A. A. (2001) J. Exp. Med. 193, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torcia, M., De Chiara, G., Nencioni, L., Ammendola, S., Labardi, D., Lucibello, M., Rosini, P., Marlier, L.-N., Bonini, P., Dello Sbarba, P., et al. (2001) J. Biol. Chem. 276, 39027–39036. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein, H., Pettoello-Mantovani, M., Bera, T. K., Pastan, I. H. & Berger, E. A. (2000) J. Infect. Dis. 181, 921–926. [DOI] [PubMed] [Google Scholar]
- 41.Ashorn, P., Moss, B., Weinstein, J. N., Chaudhary, V. K., FitzGerald, D. J., Pastan, I. & Berger, E. A. (1990) Proc. Natl. Acad. Sci. USA 87, 8889–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake, K., Iijima, O., Suzuki, N., Matsukura, M. & Shimada, T. (2001) Hum. Gene Ther. 12, 227–233. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Serrano, J., Folgueira, L., Lain de Lera, T., Pedraza, M.-A., Lemichez, E., Sanchez-Palomino, S., Noriega, A. R., Boquet, P. & Alcami, J. (1998) AIDS 12, 859–863. [DOI] [PubMed] [Google Scholar]
- 44.Batchu, R. B. & Hinds, T. (1997) Med. Hypotheses 49, 35–39. [DOI] [PubMed] [Google Scholar]
- 45.Caruso, M. & Klatzmann, D. (1992) Proc. Natl. Acad. Sci. USA 89, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feinberg, R. W., Wahl, S. M., Allen, J. B., Soman, G., Strom, T. B., Murphy, J. R. & Nichols, J. C. (1991) Science 252, 1703–1705. [DOI] [PubMed] [Google Scholar]