Abstract
Progressive changes are observed in both the composition of mammal faunas and vegetation during the Miocene epoch [24–5 mega-annum (Ma)]. These changes are usually interpreted as a response to climatic changes. In the traditional view, forests or woodlands gradually gave way to more open habitats, with grazing (grass-eating) ungulate (hoofed) mammal species replacing the browsing (leafy-vegetation-eating) species as grasslands expanded. However, data from fossil assemblages in the Great Plains region of North America show that this faunal change was not a one-for-one replacement of browsers by grazers, as usually thought. Typical late early Miocene (17 Ma) fossil communities included extraordinarily high numbers of browsing ungulate species, comprising a fauna that cannot be directly analogized with any present-day community. Both maximum species richness of all ungulates and the proportion of browsers declined steadily in ungulate communities through the middle Miocene, to levels comparable to those of the present by the late Miocene. The resulting dramatic, cumulative loss of browsing species constitutes one of the strongest faunal signals of the late Tertiary (but was not a single “event”). We suggest that the early Miocene browser-rich communities may reflect higher levels of primary productivity in Miocene vegetation, compared with equivalent present-day vegetation types. The observed decline in species richness may represent a gradual decline in primary productivity, which would be consistent with one current hypothesis of a mid-Miocene decrease in atmospheric CO2 concentrations from higher mid-Cenozoic values.
Evolution of plant and animal communities in mid to high latitudes over the past 50 myr (a unit of one million years as a simple quantity of time) is marked by a general reduction in the extent of forest and woodland ecosystems and, from around 15 mega-annum (Ma), the spread of grasslands (1–4). This biotic shift has long been attributed to increases in higher latitude aridity and temperature seasonality (5, 6).
Mammal faunas changed over this time span in response to these environmental trends (7–9). Ungulates (hoofed, herbivorous mammals such as horses, rhinos, deer, antelope, cattle, pigs, camels, elephants, etc.) are sensitive to the quality and quantity of their forage and exhibit a range of dietary specializations, from feeding purely on grass (grazers) to feeding almost entirely on leaves of dicotyledonous plants (browsers). The received view is that, as habitats became more open and grasslands expanded, grazing species simply replaced browsing species, much as one would observe today passing along a broad geographic transect from wetter to drier habitats. In this scenario, the decline in numbers of browsers and the eventual extinction of many lineages are often perceived as the inevitable fates of more primitive herbivores, with their place in the ecosystem being taken by better-adapted grazers. Significant changes in mammal faunas are also thought to have accompanied the transition at 7 Ma to grasslands dominated by plant species that used the C4 photosynthetic pathway (10–12).
Mammalian faunal composition has the potential for providing information about vegetation structure and productivity that is not readily available from paleobotanical data. Fossil floras provide taxonomic information, from which general vegetation types can often be inferred. However, within vegetation types, paleobotany provides little direct evidence for absolute abundances of plant life forms or for productivity. Moreover, plant macrofossils are seldom preserved in the same habitats as fossil vertebrates (5, 6).
We present data on community composition in Miocene faunas of the Great Plains region of North America and analyze trends in the percentage and absolute number of species of different dietary types. The results suggest that the decline of browsing species was more extreme than previously recognized and also document the unique features of the faunas of the early Miocene when the decline began. These faunas have no analog under any present-day regime of rainfall and temperatures and thus carry a signal about early Miocene environmental conditions that has hitherto been unrecognized. The detailed pattern of the loss of browsing species from North American ungulate communities may indicate changing levels of primary productivity in terrestrial ecosystems. This evolutionary pattern, in turn, may have implications both for the history of atmospheric CO2 levels and for explanations for the C3/C4 transition at 7 Ma.
Methods
Faunal Data.
Data on continent-wide generic diversity were based on new compilations from the most recently published synthesis (13). Data on species co-occurrence and community composition were assembled from the primary literature and from examination of museum collections (Table 1). Taxonomy follows ref. 13, and the time scale and dates follow ref. 14. We chose localities that contained a good sample of ungulate taxa and also represented a single, and likely attritional, faunal sample (e.g., a single quarry). Each time period is represented by three localities, with the exception of 9 Ma, 7.5 Ma, and 4.0 Ma (two localities each) and 15 Ma (four localities). The oldest faunas sampled are latest early Miocene, from the late Hemingfordian Land Mammal “age,” corresponding to the inception of the “Clarendonian chronofauna.” This chronofauna represents a period of relative ecosystem stability between 17.5 and 8.8 Ma, coinciding with the domain of the North American Miocene savanna-like ecosystem (see refs. 3, 4, and 13). The majority of ungulates in this chronofauna are members of extant clades and thus can be assumed to have comparable ecologies to living ungulates. The major exception is the oreodonts (the extinct Merycoidodontidae), but these constitute only a small percentage of the Miocene fauna.
Table 1.
Fossil faunas of the Great Plains region of North America used in this study.
| Fauna | LMA | Ma | B | SM | M | H | T |
|---|---|---|---|---|---|---|---|
| Bellville Fm., White Rock LF, KS | L. Bl. | 2.0 | 1 | 0 | 2 | 3 | 6 |
| Kiem Fm., Sand Draw LF, NB | L. Bl. | 2.0 | 1 | 0 | 2 | 4 | 7 |
| Long Pine Fm., Big Springs LF, NB | L. Bl. | 2.0 | 2 | 0 | 4 | 3 | 9 |
| Broadwater Fm., Broadwater LF, NB | E. Bl. | 3.0 | 1 | 0 | 2 | 3 | 6 |
| Deer Park Fm., Deer Park LF, KS | E. Bl. | 3.0 | 1 | 0 | 1 | 4 | 6 |
| Rexroad Fm., Rexroad LF, KS | E. Bl. | 3.0 | 4 | 0 | 1 | 4 | 9 |
| Ash Hollow Fm., Santee LF, NB | L'est Hp. | 4.0 | 2 | 2 | 2 | 4 | 10 |
| Ash Hollow Fm., Devil's Nest Airstrip LF, NB | L'est Hp. | 4.0 | 4 | 2 | 2 | 5 | 13 |
| Ash Hollow Fm., Honey Creek, NB | L. Hp. | 5.5 | 1 | 1 | 2 | 4 | 8 |
| Ogallala Fm., Edson Quarry LF, KS | L. Hp. | 5.5 | 3 | 2 | 3 | 5 | 13 |
| Ash Hollow Fm., Mailbox LF, NB | L. Hp. | 5.5 | 2 | 2 | 2 | 5 | 11 |
| Ash Hollow Fm., Minium Quarry, KS | L.E. Hp. | 6.5 | 2 | 1 | 3 | 5 | 11 |
| Ogallala Group, Wray LF, CO | L.E. Hp. | 6.5 | 3 | 1 | 2 | 7 | 13 |
| Ash Hollow Fm., Cambridge FT 40 LF, NB | L.E. Hp. | 6.5 | 4 | 2 | 3 | 7 | 16 |
| Snake Creek Fm., Aphelops Draw LF, NB | E.E. Hp. | 7.5 | 2 | 2 | 3 | 4 | 11 |
| Ash Hollow Fm., Lemoyne Quarry, NB | E.E. Hp. | 7.5 | 3 | 2 | 4 | 5 | 14 |
| Snake Creek Fm., Snake Creek LF, NB | L. Cl. | 9.0 | 4 | 4 | 2 | 6 | 16 |
| Ash Hollow Fm., Blue Jay Quarry, NB | L. Cl. | 9.0 | 3 | 3 | 3 | 5 | 14 |
| Ogallala Fm., Wakeeny Creek LF, KS | E. Cl. | 10.0 | 2 | 2 | 2 | 4 | 10 |
| Ash Hollow Fm., Big Spring Canyon LF, SD | E. Cl. | 10.0 | 3 | 3 | 4 | 7 | 17 |
| Ash Hollow Fm., Little Beaver B Quarry, NB | E. Cl. | 10.0 | 5 | 4 | 6 | 5 | 20 |
| Ash Hollow Fm., Trail Creek Quarry LF, NB | L. Ba. | 12.0 | 2 | 2 | 5 | 2 | 11 |
| Valentine Fm., Myers Farm, NB | L. Ba. | 12.0 | 9 | 4 | 3 | 2 | 18 |
| Ogallala Fm., Kennesaw LF, CO | L. Ba. | 12.0 | 4 | 2 | 3 | 2 | 11 |
| Valentine Fm., Carrot Top Quarry, NB | M. Ba. | 13.5 | 6 | 3 | 4 | 3 | 16 |
| Pawnee Creek Fm., Horse & Mastodon Quarry, CO | M. Ba. | 13.5 | 2 | 2 | 4 | 2 | 10 |
| Valentine Fm., Norden Bridge Quarry, NB | M. Ba. | 13.5 | 14 | 5 | 7 | 3 | 29 |
| Olcott Fm., Humbug Quarry, NB | E. Ba. | 15.0 | 9 | 4 | 11 | 0 | 24 |
| Olcott Fm., Echo Quarry, NB | E. Ba. | 15.0 | 10 | 5 | 10 | 0 | 25 |
| Pawnee Creek Fm., Eubanks LF, CO | E. Ba. | 15.0 | 7 | 2 | 3 | 0 | 12 |
| Sand Canyon Beds, Observation Quarry, NB | E. Ba. | 15.0 | 13 | 3 | 0 | 0 | 16 |
| Sheep Creek Fm., Thompson Quarry, NB | L. Hf. | 17.0 | 10 | 3 | 12 | 0 | 25 |
| Box Butte Fm., Foley Quarry, NB | L. Hf. | 17.0 | 8 | 1 | 4 | 0 | 13 |
| Sheep Creek Fm. equiv., Ginn Quarry, NB | L. Hf. | 17.0 | 8 | 2 | 3 | 0 | 13 |
| Running Water Fm., Aletomeryx Quarry, NB | E. Hf. | 18.0 | 2 | 3 | 1 | 0 | 6 |
| Martin Canyon Beds, Univ. of Kansas Quarry A, CO | E. Hf. | 18.0 | 7 | 2 | 1 | 0 | 10 |
| Batesland Fm., Flint Hill LF, SD | E. Hf. | 18.0 | 14 | 2 | 0 | 0 | 16 |
Fm, Formation; LF, Local fauna; CO, Colorado; KS, Kansas; NB, Nebraska; SD, South Dakota; LMA, North American Land Mammal Age; Bl., Blancan; Hp., Hemphillian; Cl., Clarendonian; Ba, Barstovian; Hf., Hemingfordian. Tooth crown height classification as defined in the text: B, brachydont species; SM, submesodont species; M, mesodont species; H, hypsodont species; T, total number of ungulate species present. Additional information is published as supplementary data on the PNAS web site, www.pnas.org.
Two major taphonomic factors might contribute to inaccuracy in the numbers of contemporaneous ungulate species recorded from any one fossil locality. First, the actual number of species may be underestimated if the fossil deposit samples relatively few individuals. We have minimized this bias by selecting localities whose fauna is represented by hundreds of specimens or more. Second, numbers of coexisting species might be overestimated if, over the time span represented in a deposit, environmental changes occurred that resulted in change of species composition (15, 16). Again, we minimized the effects of such time averaging by selecting localities that are taphonomically roughly comparable. There is no reason to believe that the severity or prevalence of taphonomic biasing factors differed systematically among time periods. Thus our primary comparisons, which are among early-mid and late Miocene localities, should be relatively unaffected. On the other hand, the problem of time averaging is likely to be more acute in comparing fossil faunas with extant ones. However, our late Miocene localities resemble modern African savanna faunas in both their ungulate species diversity and proportion of browsers. The species-level dataset is published as supplementary data on the PNAS web site, www.pnas.org.
Dietary Inferences.
Browsers are defined as species that consistently consume ≤10% grass in their year-round diet (the rest consisting of dicot leaves and stems), and grazers as species that consume ≥90% grass. Species whose diets fall between these extremes are described as “mixed” or “intermediate” feeders (17).
The crown height of ungulate cheek teeth is functionally related to diet and can be categorized as brachydont (low-crowned), mesodont (medium-crowned), or hypsodont (high-crowned). The degree of hypsodonty can be measured by the hypsodonty index, relating tooth crown height to crown surface dimensions (18). Hypsodont teeth represent adaptations to deal with increased rates of tooth wear, resulting either from chewing more abrasive plant tissues or from ingesting environmental grit adhering to the plant surface (19). All modern grazers are hypsodont or at least mesodont, and all brachydont ungulates are browsers (18, 20). However, species taking a high proportion of dietary browse may have higher tooth crowns or may even be hypsodont if feeding in open habitats where dust and grit can act to abrade the dentition (e.g., the pronghorn, Antilocapra americana).
We have classed both low-crowned and submesodont (e.g., Merycoidodontidae) fossil taxa as “brachydont, ” (hypsodonty index of <≈2.5) and consider these species to have been entirely or almost entirely browsers. Mesodont and hypsodont taxa represent species that had a wider array of diets but for the most part depended on grass to a greater extent than did browsers. Although a few (1–2 per locality) of our brachydont species represent taxa (such as peccaries) that in certain habitats today consume fruit in addition to browsing, omnivorous and frugivorous ungulates are largely absent from post-Eocene North American faunas (21). Both cranial morphological features (18) and isotopic values of dental enamel (in samples younger than 7 Ma; refs. 22 and 23) can be used to distinguish mixed feeders from grazers. Early and middle Miocene hypsodont species were not necessarily specialized grazers and most probably represent mixed feeders. Among late Miocene hypsodont ungulates, equids (horses) apparently comprised a mixture of grazers and mixed-feeding taxa; antilocaprids (= pronghorns), proboscideans (elephant-related forms), and rhinos were probably mixed feeders.
Results
Community Composition.
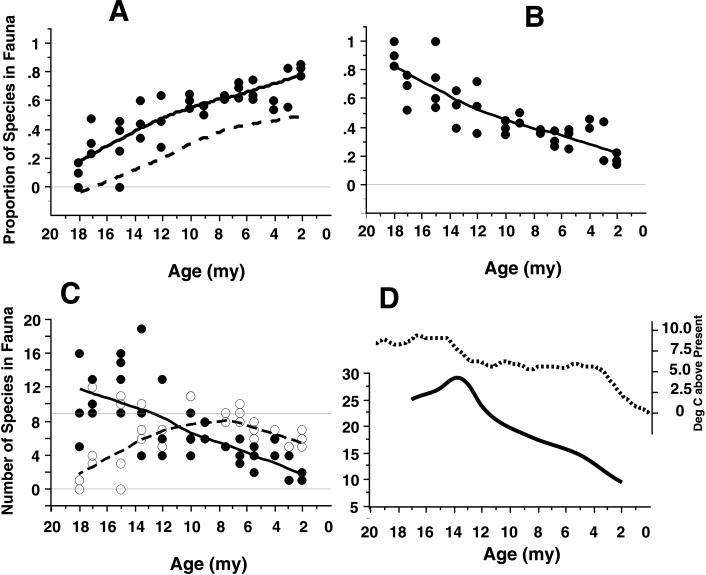
Fig. 1 shows changes in the composition of ungulate species in individual localities in the Great Plains from the late early Miocene through the Pliocene epoch. Throughout this time period, over which paleobotanical evidence shows a change from woodland-savanna to prairie ecosystems (2), nonbrowsers increase (Fig. 1A) and browsers steadily decrease (Fig. 1B) as a proportion of the total number of ungulates in localities. The proportion of browsers in present-day African grassland, bushland, or woodland habitats is 0.50 or less (24). Such low proportions of browsers do not regularly characterize Great Plains localities before around 10 Ma. Earlier localities have higher proportions of browsers, and some diverse late early Miocene localities (18–16 Ma) are composed of 100% browsing species, something not seen in modern faunas in vegetationally comparable habitats.
Figure 1.
Changes in ungulate faunas of 37 individual fossil localities in the Great Plains region of North America. Data are from ref. 13 and from research using collections at the American Museum of Natural History (Table 1). Curves drawn in A–C are LOWESS curves (tension = 66). The timespan covered is from 18 Ma (early Miocene) to 2 Ma (end of the Pliocene). (A) Nonbrowsing species as a proportion of the entire ungulate fauna at a locality. Filled circles, solid line: mesodont and hypsodont species. Lower, dashed line: hypsodont species alone. (B) Browsing (brachydont) species as a proportion of all of the ungulates at a locality. (C) Numbers of species of brachydont (filled circles, solid line) and nonbrachydont (open circles, dashed line) species in each fauna. Maximum number of browser species seen in present-day habitats is nine (upper horizontal line; ref. 25). (D) Maximum total number of ungulate species in Great Plains faunas (solid line) compared with the composite global temperature curve based on marine oxygen isotopes (55).
Absolute numbers of species in the same fossil localities show comparable patterns (Fig. 1C). The maximum number of pure browser ungulate species found in any modern faunal assemblage is nine (25). In the late early Miocene, the observed number of browsing species in many localities is as much as twice this value. Browser numbers decline steadily until reaching an absolute low at around 2 Ma. Nonbrowser species increase in numbers up to a peak at about 10 Ma (well before the expansion of C4 grasslands), then tend to decline. Furthermore, although the decline in browser representation and total species richness is broadly consistent with a decline in global temperatures, it does not precisely track the trajectory of this change (Fig. 1D).
Even the most species-rich present-day faunas in non-forest habitats (e.g., savanna-woodlands of East Africa) contain neither the absolute numbers of browsers nor the proportion of browsing species found in many late early and middle Miocene localities. The decline in the predominance of browsers was a long-term, unpunctuated trend, and browsers were not replaced one-for-one by species of other feeding types. Rather, browsers commenced their decline long before hypsodont species had reached peak diversity, and their decline continued uninterrupted during the later hypsodont decline. Thus the progressive Miocene decline of both the numbers and the proportions of the browsing species does not reflect simply the rise and later fall of hypsodont species. Early and early middle Miocene habitats supported very different ungulate faunas than exist today, and species richness and dietary composition steadily converged on modern values as the Miocene progressed.
Continent-Wide Patterns and Evidence Outside of North America.
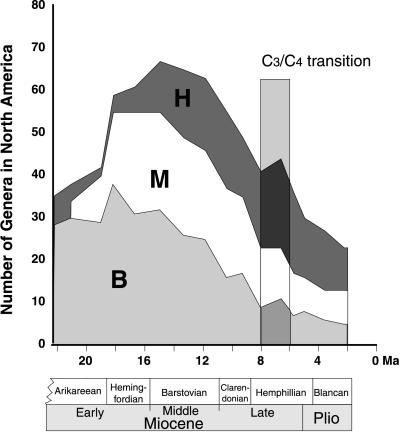
Fig. 2 illustrates the continent-wide generic diversity of North American ungulates, grouped by crown type, over the past 22 million years (excluding the Pleistocene). Although these continent-wide diversity patterns may not faithfully represent changes occurring in individual communities, they nevertheless demonstrate that the browsers (brachydonts) decreased over the whole continent in a pattern very similar to that seen in individual localities of the Great Plains.
Figure 2.
North American continent-wide ungulate generic diversity. H, hypsodont genera; M, mesodont genera; B, brachydont genera. For reference, the time range of the C3/C4 transition (11) is marked by the vertical bar. Hypsodont taxa are here interpreted as either grazers or open-habitat mixed feeders; mesodont taxa as mixed feeders of all types (and possibly including some true grazers); and brachydont taxa as browsers, based on study of diets and crown heights of modern species (18, 20). Generic data are from ref. 13.
Brachydont taxa predominated until the middle Miocene, reaching peak diversity in the late early Miocene (around 19 Ma), and declined in diversity after that time. Overall ungulate generic diversity decreased from the start of the middle Miocene, reflecting mainly the decline of brachydont taxa. Hypsodont ungulates had been present in low diversity since the late Eocene, but these earlier taxa were not ancestral to the Miocene hypsodont taxa. The latter were derived either from immigration (antilocaprids, rhinos, and proboscideans) or from evolution from endemic mesodont taxa (horses). After increasing rapidly in the late early Miocene, numbers of hypsodont taxa remained relatively constant (with a slight decline from a mid-Miocene high) through the Miocene and Pliocene. Thus, as seen in the species-level analysis of Great Plains communities, at the generic-level, continent-wide scale hypsodont taxa were not simply invading ecosystem space vacated by brachydont ones. Additionally, the late Miocene decline in browser diversity was associated with an increase in the average body size of remaining browsers. Simulation models of ungulate digestive physiology indicate that browse quality progressively decreased during this time (26).
A variety of published observations suggest that the patterns we describe for Miocene ungulate faunas are not merely a North American phenomenon. A mid-Miocene decline in the dominance of brachydont ungulates has been reported in western Eurasian faunas (27). Middle Miocene faunas of South America appear “over rich” in the number of large terrestrial browsers (28). Also, unusually high species richness characterizes small-mammal faunas of the middle Miocene of Pakistan, and these faunas decline in richness in the later Miocene (29).
Discussion
Species Richness and Productivity.
Although there are some empirical patterns that seem quite general, ecologists do not yet agree on the factors controlling species richness. Nevertheless, most recognize a relationship between species richness and productivity (30). Accordingly, we suggest that the most likely factor leading to higher species richness in the early and middle Miocene is greater habitat primary productivity. Other potential factors known to influence species richness, such as changes in regional topographic diversity or increases in habitable land area, would seem to be less applicable here (30). Additionally there are no obvious changes in predator diversity during the middle Miocene, at least on the generic, continent-wide level (14, 31). Furthermore, Miocene ungulate species, closely related to living forms, were unlikely to have tolerated levels of resource subdivision unmatched in extant assemblages, unless concomitant changes in productivity or habitat structure permitted it. Likewise, minimum population sizes sufficient for persistence in given ecological settings are unlikely to have differed substantially from present-day values.
Some theoretical models suggest that productivity changes directly alter the determinants of minimum viable niche overlap (and hence species packing) within ecosystems, which leads to predictable species-richness changes (32, 33). Other models suggest merely that higher productivity permits relatively rarer species to attain population densities sufficient for long-term survival in the ecosystem (30). Among vertebrates, and at the regional geographic scales most relevant to this research, species richness tends either to increase monotonically with increasing primary productivity or exhibits a “unimodal” pattern (increase in richness followed by decrease as productivity continues to rise; refs. 30 and 34).
For African savanna faunas, annual precipitation is usually used as a proxy for primary productivity, and positive relationships between rainfall levels and ungulate biomass and energy-use are well-known (35, 36). The unimodal richness/productivity pattern has been observed among ungulate faunas of Africa (30, 33). Paleobotanical- and paleosol-based estimates of annual rainfall levels for the Miocene of the Great Plains vary between 400 and 800 mm (6, 37). Among African ungulate communities over the same range of rainfall, the relationship between rainfall (productivity) and species richness is strongly positive and monotonic (33).
Because of this consistent correlation of ungulate species richness with primary productivity in non-forest habitats of the modern world, we conclude that the higher species richness of browsers in the mid-Miocene likely reflects the fact that these species were living in habitats of higher primary productivity than is supported in modern habitats with similar vegetation structure and climatic regime. In particular, these habitats had to be particularly productive with respect to dicot (C3) vegetation, which is the primary trophic resource base for browsing ungulates. We argue below that higher levels of atmospheric CO2 would be the simplest explanation for these inferred differences in terrestrial primary productivity, although we acknowledge current conflicting geochemical evidence and discuss other possibilities for this observation.
Atmospheric CO2 Levels and Primary Productivity.
Atmospheric CO2 levels have varied considerably over the Phanerozoic (38–40). Models of CO2 levels based on the global carbon cycle suggest that values of CO2 concentration for the early Paleozoic atmosphere may have been as much as 20 times the current (preindustrial) level of 270 ppm. After recovery from a Carboniferous low, CO2 concentrations were apparently high again (>1000 ppm) in the Mesozoic and declined throughout the course of the Cenozoic. According to some estimates, the atmospheric CO2 concentration was near 700 ppm at the start of the Miocene (39, 41). However, more recent evidence indicates that essentially modern levels had been reached by the beginning of the Miocene (42, 43).
Elevated CO2 can be shown to promote greater levels of plant productivity in laboratory studies. Studies on individual plant species (44–46) show that elevated CO2 tends to cause higher rates of photosynthesis and vegetative growth in plants that use the C3 photosynthetic pathway (but not in ones using the C4 pathway; refs. 47–49). These gains in vegetative growth are partly offset, for herbivores, by a reduction in leaf nitrogen levels. This nitrogen reduction has been shown to affect insect herbivory rates, but effects on mammalian herbivory are still largely unknown (44, 50). However, ungulate herbivores derive a substantial amount of their energy intake from microbial fermentation of plant structural tissues (51), not merely the easily digestible components of plant forage, so it is reasonable to expect a net gain in resource availability for ungulates under CO2 enrichment.
Elevated CO2 levels also affect plant water use efficiency; in both C3 and C4 pathways, a given level of photosynthesis requires less water at high CO2 concentrations (52, 53). This effect on water use means that geographic distribution of vegetation types and that correlations between climatic variables (especially rainfall) and vegetation type would be different under different CO2 concentrations. For example, drops in the atmospheric CO2 concentration in the Pleistocene apparently affected distribution of vegetation types through effects on water use efficiency (54). Under conditions of elevated CO2, a savanna-woodland might be found thriving in a region with lower annual rainfall than under current CO2 levels, where a different vegetation type (e.g., a scrub or bushland) might be found today.
If, on the other hand, early Miocene atmospheric CO2 levels were equal to or lower than those at present (42, 43), then an alternative explanation must be sought for these nonanalogous faunas. One possibility is that the vegetation of early and middle Miocene habitats in the Great Plains was radically unlike vegetation types in the forest-woodland-grassland continuum known today. High browser diversity requires one of two conditions: either that this Miocene vegetation type be one of higher productivity of browse than a modern tropical forest or forest-woodland ecotone; or that it represents a habitat in which vegetation structure was such as to make a greater percentage of leafy vegetation available to the browsing ungulate community than is available in any modern woodland or bushland. It thus must represent a vegetation type not observed supporting a significant terrestrial browser fauna at present. Combinations of relatively low seasonality, high temperatures, and moderate rainfall thought to characterize the early Miocene (6, 37) seem unlikely to support a novel vegetation type with the appropriate characteristics (instead of forest or woodland), unless other factors are involved. Furthermore, such an explanation is inconsistent with a considerable body of evidence from paleobotany, palynology, paleopedology, and the locomotor adaptations of some mammals. Workers have long believed that these data together suggest that early Miocene vegetation types and floral composition in this region structurally resembled modern woodland-savannas with various degrees of development of open vegetation, accompanied by riparian forest (2, 5, 6, 37).
Ungulate Diversity and the C3/C4 Transition.
Studies of stable carbon isotopes have revealed a striking and significant late Miocene biotic event at about 7 Ma: the “C3/C4 transition.” The isotopic composition of the teeth of grazing mammals (primarily horses) shows a change in δC13 levels in the dental enamel corresponding to a similar change in levels in soil carbonates, signifying a shift in plant photosynthetic biochemistry from the C3 cycle to the C4 cycle. This isotopic change indicates that these mammals were now feeding on C4 grasses, which presumably evolved at about this time. Both low CO2 and high temperatures favor the use of the C4 cycle in modern plant communities (47), and there are conflicting opinions as to whether the event at 7 Ma relates to declining levels of atmospheric CO2 (11–13, 48) or to an episode of widespread, severe aridity (42).
Interestingly, we find little evidence for a change in taxonomic diversity or community structure among ungulate mammals in North America occurring at or near the time of the C3/C4 shift (7 Ma). Although the C3/C4 transition ushers in a major floral change and possibly new types of grassland vegetation (6, 37), any effects on the ungulate fauna were evidently relatively subtle. The changes in community composition reported here, involving the gradual loss of browsing species, continued without interruption through the transition period. Also, we see no faunal evidence for a sudden or severe episode of climatic change near 7 Ma. Severe aridity would have turned habitats to desert or near desert conditions, resulting in significant changes in the ungulate fauna. The fact that the directional trends in community composition are continuous, suggesting some factor gradually changing over the 18–5 Ma timespan, is more consistent with early Miocene elevated CO2 than with a climatic event near 7 Ma.
Conclusion
There is no habitat today, no combination of vegetation growing under different combinations of temperature and rainfall, that supports the number of browsing ungulates observed in early and middle Miocene localities. The patterns of ungulate species richness observed in these Miocene C3 ecosystems are consistent with the hypothesis that these ecosystems had higher levels of primary productivity than in any corresponding present-day woodland or savanna. Because these levels of species richness are not matched under any present-day climatic regime, we suggest that factors other than temperature and rainfall seasonality were involved, possibly including higher levels of atmospheric CO2. The C3/C4 transition at 7 Ma, although apparently important for plants, is not captured in the detailed pattern of diversification and extinction of large herbivorous mammals of North America. The decline of the extremely species-rich browsing communities of the mid-Miocene is one of the strongest signals in the faunal history of the North American Cenozoic, suggestive of an early to mid Miocene world very different from today's world.
Supplementary Material
Acknowledgments
We thank T. Cerling, the late J. J. Sepkoski, Jr., Steve D'Hondt, and two anonymous reviewers for comments on earlier versions of the manuscript, and the National Center for Ecological Analysis and Synthesis (NCEAS) working group on mammal communities for general input. This work was supported by a Salomon Faculty Research Award (Brown University) to C.M.J. This paper is a GRIPS (Greater Rhode Island Paleontological Society) contribution.
Abbreviations
- Ma
mega-annum, a unit of one million years before the present in the radioisotopic time scale
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Janis C M. Annu Rev Ecol Syst. 1993;24:467–500. [Google Scholar]
- 2.Jacobs B F, Kingston J D, Jacobs L L. Ann Mo Bot Gard. 1999;86:590–643. [Google Scholar]
- 3.Webb S D, Opdyke N D. In: Effects of Past Global Change on Life, National Academy of Sciences Studies in Geophysics. Kennett J, Stanley S, editors. Washington, DC: Natl. Acad. Press; 1995. pp. 184–208. [Google Scholar]
- 4.Webb S D, Hulbert R C, Lambert W D. In: Paleoclimate and Evolution, with Emphasis on Human Origins. Vrba E S, Denton G H, Partridge T C, Burckle L H, editors. New Haven, CT: Yale Univ. Press; 1995. pp. 91–108. [Google Scholar]
- 5.Wing S L. In: Evolution of Tertiary Mammals of North America. Janis C M, Scott K M, Jacobs L L, editors. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. 37–60. [Google Scholar]
- 6.Leopold E B, Denton M F. Ann Mo Bot Gard. 1987;74:841–867. [Google Scholar]
- 7.Webb S D. In: Coevolution. Nitecki M H, editor. Chicago, IL: Univ. Chicago Press; 1983. pp. 267–306. [Google Scholar]
- 8.Webb S D. Annu Rev Ecol Syst. 1977;8:355–380. [Google Scholar]
- 9.Janis C M. Palaeontology. 1989;32:463–481. [Google Scholar]
- 10.Cerling T E, Harris J M, MacFadden B J, Leakey M G, Quade J, Eisenmann V, Ehleringer J R. Nature (London) 1997;389:153–158. [Google Scholar]
- 11.Cerling T E, Ehleringer J R, Harris J M. Philos Trans R Soc Lond B Biol Sci. 1998;353:159–171. doi: 10.1098/rstb.1998.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerling T E, Harris J M, MacFadden B J. In: Stable Isotopes: Integration of Biological, Ecological and Geochemical Processes. Griffiths H, editor. Oxford, U.K.: BIOS Scientific Publishers; 1998. pp. 363–379. [Google Scholar]
- 13.Janis C M, Scott K M, Jacobs L L, editors. Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, Evolution of Tertiary Mammals of North America. Cambridge, U.K.: Cambridge Univ. Press; 1998. [Google Scholar]
- 14.Woodburne M O, Swisher C C., III . In: Geochronology, Time Scales and Global Stratigraphic Correlation. Berggren W A, Kent D V, Aubry M-P, Hardenbol J, editors. Tulsa, OK: Society for Sedimentary Geology; 1995. , Special Publication No. 54, pp. 335–364. [Google Scholar]
- 15.Aslan A, Behrensmeyer A K. Palaios. 1996;11:411–421. [Google Scholar]
- 16.Cutler A H, Behrensmeyer A K, Chapman R E. Palaeogeog Palaeoclimat Palaeoecol. 1999;149:359–372. [Google Scholar]
- 17.Hofmann R R, Stewart D R M. Mammalia. 1972;36:226–240. [Google Scholar]
- 18.Janis C M. In: Functional Morphology in Vertebrate Paleontology. Thomason J J, editor. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 76–98. [Google Scholar]
- 19.Janis C M, Fortelius M. Biol Rev. 1988;63:197–230. doi: 10.1111/j.1469-185x.1988.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 20.Janis C M. In: Teeth Revisited, Proceedings of the VIIth International Symposium on Dental Morphology. Russell D E, Santoro J-P, Sigogneau-Russell D, editors. Vol. 53. Paris: Mém. Mus. Nat. Hist. Nat. Sér. C; 1988. pp. 367–387. [Google Scholar]
- 21.Janis C M. Zool Analysis Complex Syst. 1999/98;100:203–220. [Google Scholar]
- 22.MacFadden B J, Solounias N, Cerling T E. Science. 1999;283:824–827. doi: 10.1126/science.283.5403.824. [DOI] [PubMed] [Google Scholar]
- 23.MacFadden B J, Cerling T E. J Vert Paleontol. 1996;16:103–115. [Google Scholar]
- 24.Cumming D H M. In: Ecology of Tropical Savannas. Huntley B J, Walker B H, editors. Berlin: Springer; 1982. pp. 217–245. [Google Scholar]
- 25.Owen-Smith N. In: Ecology of Tropical Savannas. Huntley B J, Walker B H, editors. Berlin: Springer; 1982. pp. 359–404. [Google Scholar]
- 26.Janis C M, Gordon I J, Illius A W. Hist Biol. 1994;8:15–29. [Google Scholar]
- 27.Fortelius M, Werdelin L, Andrews P, Bernor R L, Gentry A, Humphrey L, Mittman H-W, Viranta S. In: The Evolution of Western Eurasian Neogene Mammal Faunas. Bernor R L, Falbusch V, Mittman H W, editors. New York: Columbia Univ. Press; 1996. pp. 414–448. [Google Scholar]
- 28.Kay R F, Madden R H. J Hum Evol. 1997;32:161–199. doi: 10.1006/jhev.1996.0104. [DOI] [PubMed] [Google Scholar]
- 29.Flynn L J, Downs W, Morgan M E, Barry J C, Pilbeam D. In: Advances in Vertebrate Paleontology and Geochronology, National Science Museum Monographs. Tomida Y, Flynn L J, Jacobs L L, editors. Tokyo: National Science Museum; 1998. , No. 14, pp. 167–180. [Google Scholar]
- 30.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 31.Van Valkenburgh B. Annu Rev Earth Planet Sci. 1999;27:463–493. [Google Scholar]
- 32.Prins H H T, Olff H. In: Dynamics of Tropical Communities. Newbery D M, Prins H H T, Brown N D, editors. Oxford: Blackwell Scientific; 1998. pp. 449–490. [Google Scholar]
- 33.Ritchie M E, Olff H. Nature (London) 1999;400:557–560. doi: 10.1038/23010. [DOI] [PubMed] [Google Scholar]
- 34.Waide R B, Willig M R, Steiner C F, Mittelbach G, Gough L, Dodson S I, Juday G P, Parmenter R. Annu Rev Ecol Syst. 1999;30:257–300. [Google Scholar]
- 35.Coe M J, Cumming D H, Phillipson J. Oecologia. 1976;22:341–354. doi: 10.1007/BF00345312. [DOI] [PubMed] [Google Scholar]
- 36.Fritz H, Duncan P. Proc R Soc Lond Ser B Biol Sci. 1994;256:77–82. doi: 10.1098/rspb.1994.0052. [DOI] [PubMed] [Google Scholar]
- 37.Retallack G J. Palaios. 1997;12:380–390. [Google Scholar]
- 38.Berner R A. Am J Sci. 1994;294:56–91. [Google Scholar]
- 39.Berner R A. Philos Trans R Soc Lond B Biol Sci. 1998;353:75–82. [Google Scholar]
- 40.Ekart D D, Cerling T E, Montañez I P, Tabor N J. Am J Sci. 1999;299:805–827. [Google Scholar]
- 41.Cerling T E. Am J Sci. 1991;291:377–400. [Google Scholar]
- 42.Pagani M, Freeman K H, Arthur M A. Science. 1999;285:876–878. doi: 10.1126/science.285.5429.876. [DOI] [PubMed] [Google Scholar]
- 43.Pagani M, Arthur M A, Freeman K H. Paleoceanography. 1999;14:273–292. [Google Scholar]
- 44.Bazzaz F A. Annu Rev Ecol Syst. 1990;21:167–196. [Google Scholar]
- 45.Bazzaz F A, Baslow S L, Berntson G M, Thomas S C. In: Global Change and Terrestrial Ecosystems. Walker B, Steffan W, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 43–76. [Google Scholar]
- 46.Idso K E, Idso S B. Agric Forest Meteorol. 1994;69:153–203. [Google Scholar]
- 47.Ehleringer J R, Sage R F, Flanagan L B, Pearcy R W. Trends Ecol Evol. 1991;6:95–99. doi: 10.1016/0169-5347(91)90183-X. [DOI] [PubMed] [Google Scholar]
- 48.MacFadden B J, Cerling T E. Trends Ecol Evol. 1994;9:481–486. doi: 10.1016/0169-5347(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 49.Sage R F, Monson R K, editors. C4 Plant Biology. San Diego: Academic; 1999. [Google Scholar]
- 50.Coley P D. Climatic Change. 1998;39:455–472. [Google Scholar]
- 51.Illius A W, Gordon I J. Oecologia. 1992;89:428–434. doi: 10.1007/BF00317422. [DOI] [PubMed] [Google Scholar]
- 52.Woodward F I, Thompson G B, McKee I F. Ann Bot (London) 1991;67,Suppl. 1:23–38. [Google Scholar]
- 53.Farquhar G D. Science. 1997;278:1411. [Google Scholar]
- 54.Street-Perrott F A, Huang Y, Perrott R A, Eglinton G, Barker P, Khelifa L B, Harkness D D, Olago D O. Science. 1997;278:1422–1426. doi: 10.1126/science.278.5342.1422. [DOI] [PubMed] [Google Scholar]
- 55.Miller K G, Fairbanks R G, Mountain G S. Paleoceanography. 1987;2:1–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.