Abstract
Neurotrophins are a family of closely related proteins that were identified initially as survival factors for sensory and sympathetic neurons, and have since been shown to control many aspects of survival, development and function of neurons in both the peripheral and the central nervous systems. Each of the four mammalian neurotrophins has been shown to activate one or more of the three members of the tropomyosin-related kinase (Trk) family of receptor tyrosine kinases (TrkA, TrkB and TrkC). In addition, each neurotrophin activates p75 neurotrophin receptor (p75NTR), a member of the tumour necrosis factor receptor superfamily. Through Trk receptors, neurotrophins activate Ras, phosphatidyl inositol-3 (PI3)-kinase, phospholipase C-γ1 and signalling pathways controlled through these proteins, such as the MAP kinases. Activation of p75NTR results in activation of the nuclear factor-κB (NF-κB) and Jun kinase as well as other signalling pathways. Limiting quantities of neurotrophins during development control the number of surviving neurons to ensure a match between neurons and the requirement for a suitable density of target innervation. The neurotrophins also regulate cell fate decisions, axon growth, dendrite growth and pruning and the expression of proteins, such as ion channels, transmitter biosynthetic enzymes and neuropeptide transmitters that are essential for normal neuronal function. Continued presence of the neurotrophins is required in the adult nervous system, where they control synaptic function and plasticity, and sustain neuronal survival, morphology and differentiation. They also have additional, subtler roles outside the nervous system. In recent years, three rare human genetic disorders, which result in deleterious effects on sensory perception, cognition and a variety of behaviours, have been shown to be attributable to mutations in brain-derived neurotrophic factor and two of the Trk receptors.
Keywords: neurotrophin, Trk receptor, p75 neurotrophin receptor, apoptosis, nerve growth factor
1. Introduction
Neurotrophins were identified as promoters of neuronal survival, but it is appreciated that they regulate many aspects of neuronal development and function, including synapse formation and synaptic plasticity (Lewin & Barde 1996; Bibel & Barde 2000; Kaplan & Miller 2000; Huang et al. 2001; Poo 2001; Shooter 2001; Sofroniew et al. 2001; Dechant & Barde 2002; Chao 2003; Huang & Reichardt 2003; Lu et al. 2005). The first neurotrophin, nerve growth factor (NGF), was discovered during a search for survival factors that could explain the deleterious effects of deletion of target tissues on the subsequent survival of motor and sensory neurons (Levi-Montalcini 1987; Shooter 2001). Its discovery validated the central model of neurotrophic factor action—that targets of neuronal innervation secrete limiting amounts of survival factors, which function to balance the size of a target tissue with the number of innervating neurons. The second neurotrophin to be characterized—brain-derived neurotrophic factor (BDNF)—was purified in a heroic effort from pig brain as a survival factor for several neuronal populations not responsive to NGF (Barde et al. 1982). Conserved features of the sequences of these two proteins made isolation of clones encoding additional members of this family possible. Four neurotrophins are expressed in mammals: NGF, BDNF, neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4). Members of numerous other families of proteins, most notably the glial cell-derived neurotrophic factor family, also regulate neuronal survival and other aspects of neuronal development and function through activation of receptor tyrosine kinases, but the neurotrophins have continued to be a focus for research and source of surprise (figures 1 and 2).
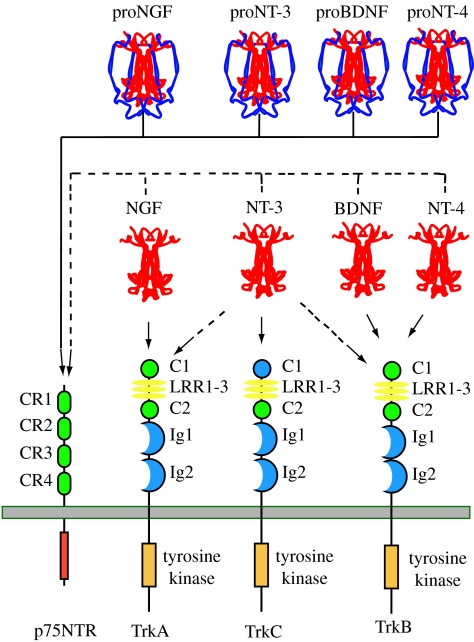
Figure 1.
Neurotrophin–receptor interactions. This illustrates the major interactions of each of the four mammalian neurotrophins. Each proneurotrophin binds p75NTR, but not the Trk receptors. Following maturation through proteolysis of the proneurotrophins, each mature neurotrophin is able to bind and activate p75NTR, but exhibits more specific interactions with the three Trk receptors. NGF binds specifically TrkA; BDNF and NT4 recognize TrkB; NT3 activates TrkC. In some cellular contexts, NT3 is also able to activate TrkA and TrkB with less efficiency. For simplicity, only the major tyrosine kinase-containing isoforms of the Trk receptors are depicted. Differential splicing generates isoforms of TrkB and TrkC that have truncated cytoplasmic domains lacking a tyrosine kinase motif. Splicing also generates an isoform of TrkC with a small insert in the kinase domain that affects substrate specificity. Splicing of exons that generate the extracellular domain of each Trk receptor results in the expression of receptors with small peptide inserts between the second immunoglobin and transmembrane domains that affect ligand-binding specificity. Ligand-binding specificity is also affected by the presence of p75NTR. Additional details are provided in the text.
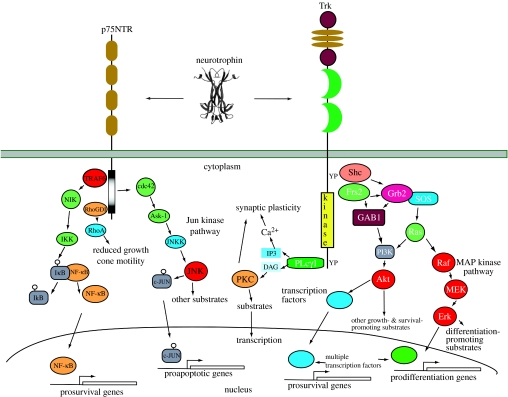
Figure 2.
Neurotrophin signalling. This depicts the interactions of each neurotrophin with Trk and p75NTR receptors and major intracellular signalling pathways activated through each receptor. The p75NTR receptor regulates three major signalling pathways. NF-κB activation results in transcription of multiple genes, including several that promote neuronal survival. Activation of the Jun kinase pathway similarly controls activation of several genes, some of which promote neuronal apoptosis. Ligand engagement of p75NTR also regulates the activity of Rho, which controls growth cone motility. Pro-apoptosis actions of p75NTR appear to require the presence of sortilin, which functions as a co-receptor for the neurotrophins. Sortilin is not depicted in this figure, but is described in the text. Each Trk receptor also controls three major signalling pathways. Activation of Ras results in activation of the MAP kinase-signalling cascade, which promotes neuronal differentiation including neurite outgrowth. Activation of PI3 kinase through Ras or Gab1 promotes survival and growth of neurons and other cells. Activation of PLC-γ1 results in activation of Ca2+- and protein kinase C-regulated pathways that promote synaptic plasticity. Each of these signalling pathways also regulates gene transcription. Many additional adaptors for p75NTR and Trk receptors have been identified which are not depicted in this figure for simplicity, but are described in more detail in the text. Interactions between p75NTR and Trk receptors are not depicted in this figure, but are described in the text. p75NTR also appears to function as constituent of other receptor complexes, most notably the Nogo receptor complex. These interactions and signalling pathways are not depicted here.
The neurotrophins have had an unusually important role in developmental neurobiology. The experiments leading to their discovery revealed the essential role of cellular interactions in controlling cell survival and differentiation (Jacobson et al. 1997). Preceding serious studies on membrane transport and endocytosis in other cells, neuroscientists have shown that NGF was internalized by receptor-dependent processes and transported along axons in membranous vesicles to the cell soma by cytoskeletal and energy-dependent processes (Thoenen & Barde 1980). Signalling locally was shown to regulate growth cone motility, while signalling in the cell soma controlled cell survival and gene expression. Ligand turnover was shown to occur in lysosomes. It is clear that all cells use similar mechanisms to control ligand and receptor trafficking, signalling and degradation. Finally, neurotrophins activate receptor tyrosine kinases (Chao 2003). During the past 15 years receptor-associated and cytoplasmic tyrosine kinases have been shown to regulate almost all aspects of neuronal development and function, including precursor proliferation and commitment, cell survival, axon and dendrite growth, membrane trafficking, synapse formation and function, as well as glial differentiation and interactions with neurons. Many of the mechanisms through which neurotrophins control neural development and function have proven to be shared with these ligand and tyrosine kinase families. As a result, studies on neurotrophins have increased our understanding of molecular mechanisms of interest to almost all modern neuroscientists. In addition, recent studies have revealed a diversity of roles for these factors outside the nervous system, most notably in cardiac development, neovascularization and immune system function (Donovan et al. 2000; Lin et al. 2000; Coppola et al. 2004; Kermani et al. 2005).
Finally, several human genetic diseases have been associated with mutations in neurotrophins or their receptors. Several different mutation types in the trkA gene, including nonsense, missense, frame shift and splice site mutations, have been identified in congenital insensitivity to pain with anhydrosis (CIPA) patients (Indo 2001). The phenotypes associated with CIPA are believed to result in large part from loss of NGF-dependent neurons, including nociceptive sensory and sympathetic neurons, during embryogenesis. A dominant mutation in trkB (Y722C) that impairs TrkB kinase signalling has recently been described in a patient with severe hyperphagic obesity and severe impairments in nociception, learning and memory (Yeo et al. 2004). Several additional missense mutations in trkB have been identified in this patient cohort that has not been characterized functionally. A polymorphism in the bdnf gene that impairs the transport of BDNF protein has been associated with impaired hippocampal function and episodic memory (Egan et al. 2003).
This review focuses on the signalling pathways stimulated by the neurotrophins that affect the survival, differentiation and function of cells within the nervous system. Several excellent reviews describe recent progress in understanding the developmental and functional roles of these proteins (Bibel & Barde 2000; Huang & Reichardt 2001; Patapoutian & Reichardt 2001; Sofroniew et al. 2001; Dechant & Barde 2002; Chao 2003; Chao & Lee 2004; Lu et al. 2005).
2. Neurotrophins and their receptors
The neurotrophins and their genes share homologies in sequence and structure. The organization of the genomic segments adjacent to these genes is also similar. Together, these observations provide compelling evidence that the neurotrophin genes have arisen through successive duplications of a portion of the genome derived from an ancestral chordate (Hallbook 1999). Their genes share many similarities, including the existence of multiple promoters. The protein product of each gene includes a signal sequence and a prodomain, followed by the mature neurotrophin sequence. Thus, each gene product must be processed by proteolysis to form a mature protein. A surprising recent work has demonstrated that regulation of their maturation is an important post-transcriptional control point that limits and adds specificity to their actions (Lee et al. 2001). The mature neurotrophin proteins are non-covalently associated homodimers. Although some neurotrophin monomers are able to form heterodimers with other neurotrophin monomers in vitro, there is no evidence that these heterodimers exist at significant concentrations in vivo.
The structures of NGF, NT-3 and NT-4 homodimers and of the BDNF monomer, associated with NT-3 and NT-4 monomers, have been solved (McDonald et al. 1991; Robinson et al. 1995; Butte et al. 1998). Each of these four proteins shares a highly homologous structure with features—tertiary fold and cystine knot—that are present in several other growth factors, including transforming growth factor-β (TGF-β) and platelet-derived growth factor. Additional neurotrophins have been isolated from fish, where NT-6 and NT-7 have been characterized. These do not have orthologues in mammals or birds and appear to interact with the same receptors as the mammalian proteins.
The neurotrophins interact with two entirely distinct classes of receptors. The first receptor to be discovered, named p75 neurotrophin receptor (p75NTR), was identified as a low-affinity receptor for NGF, but was subsequently shown to bind each of the neurotrophins with a similar affinity (Rodriguez-Tebar et al. 1990; Frade & Barde 1998). p75NTR is a member of the tumour necrosis receptor superfamily with an extracellular domain that includes four cysteine-rich motifs, a single transmembrane domain and a cytoplasmic domain that includes a ‘death’ domain similar to those present in other members of this family (Liepinsh et al. 1997; He & Garcia 2004). Although this receptor does not contain a catalytic motif, it interacts with several proteins, to be described in more detail later, that transmit signals important for regulating neuronal survival and differentiation as well as synaptic plasticity. The three-dimensional structure of the extracellular domain of p75NTR in association with an NGF dimer has demonstrated that each of the four cysteine-rich repeats participates in binding to NGF (He & Garcia 2004). Interestingly, p75NTR binds NGF along the interface between the two NGF monomers and binding results in a conformational change in NGF that alters the monomeric interface on the opposite side of the NGF dimer, eliminating the potential for binding of one NGF dimer to two p75NTR monomers. The results suggest that binding of NGF to p75NTR may result in dissociation of p75NTR multimers and are compatible with the possibility that tropomyosin-related kinase (Trk) and p75NTR monomers simultaneously bind the same neurotrophin dimer. Of recent interest, a gene related to p75NTR, named NRH-2, has recently been identified. The product of this gene lacks the extracellular cysteine-rich repeats present in p75NTR and fails to bind NGF, but it is able to interact and influence the ligand-binding properties of TrkA (Murray et al. 2004).
In mammals, the three members of the Trk subfamily of receptor tyrosine kinases constitute the second major class of neurotrophin receptors (Chao 2003; Huang & Reichardt 2003). Gene duplication has resulted in the presence of additional Trk receptors in fish. A single Trk orthologue has been characterized in Amphioxus, which is expressed in sensory neural precursors and is able to interact with each of the mammalian neurotrophins (Benito-Gutierrez et al. 2005). In contrast, neither trk nor neurotrophin genes have been identified in the genome of the ascidian Ciona (Dehal et al. 2002). Thus, Trk receptors appear to have arisen during the chordate radiation before the cephalochordate–vertebrate split. The extracellular domain of each of the Trk receptors consists of a cysteine-rich cluster followed by three leucine-rich repeats, another cysteine-rich cluster and two immunoglobulin-like domains. Each receptor spans the membrane once and is terminated with a cytoplasmic domain consisting of a tyrosine kinase domain surrounded by several tyrosines that serve as phosphorylation-dependent docking sites for cytoplasmic adaptors and enzymes. In contrast to interactions with p75NTR, the neurotrophins dimerize the Trk receptors, resulting in activation through transphosphorylation of the kinases present in their cytoplasmic domains. The four neurotrophins exhibit specificity in their interactions with the three members of this receptor family with NGF activating TrkA, BDNF and NT-4 activating TrkB, and NT-3 activating TrkC. In addition, NT-3 can activate the other Trk receptors with less efficiency. The major site at which neurotrophins interact with these receptors is in the membrane-proximal immunoglobulin-like domain. The three-dimensional structures of this domain in each of the Trk receptors have been solved (Ultsch et al. 1999). In addition, the structure of NGF bound to the TrkA Ig domain has also been determined (Wiesmann et al. 1999). The results have made it possible to identify residues in the neurotrophins and the Trk receptors that account for the specificity observed in their interactions (Urfer et al. 1998; Wiesmann & de Vos 2001).
As a first approximation, expression of a specific Trk receptor confers responsiveness to the neurotrophins to which it binds. Splicing, however, introduces some limitations to this generalization. For example, differential splicing affects ligand interactions of each of the Trk receptors through insertions of short amino acid sequences into the juxtamembrane regions of the extracellular domains of TrkA, TrkB and TrkC (Meakin et al. 1992; Clary & Reichardt 1994; Shelton et al. 1995; Garner et al. 1996; Strohmaier et al. 1996). For both TrkA and TrkB, the insertion of the sequence encoded by a small exon enhances the binding of the receptor to non-preferred ligands. TrkA and TrkB isoforms that lack these inserts are activated strongly only by NGF and BDNF, respectively. The isoform of TrkA including an insert is also activated by NT-3 in addition to NGF (Clary & Reichardt 1994), while the similar isoform of TrkB is activated by NT-3 and NT-4 in addition to BDNF (Strohmaier et al. 1996). Although the TrkA insert was not localized in the crystal structure of the membrane-proximal Ig domain complexed to NGF, the organization of this complex is compatible with the possibility that these residues participate directly in neurotrophin binding (Wiesmann et al. 1999).
Differential splicing of exons encoding portions of the intracellular domains of Trk receptors also regulates the signalling initiated by neurotrophin binding. Splicing generates isoforms of TrkB and TrkC that include comparatively short cytoplasmic motifs without a tyrosine kinase domain. Expression of a non-kinase-containing isoform has been shown to inhibit productive dimerization of kinase-containing Trk receptors, thereby inhibiting responses to neurotrophins, such as activation of phospholipase C-γ1 (PLC-γ1; Eide et al. 1996). For many years, it appeared that these truncated receptors did not directly signal, but instead functioned to restrict the diffusion of neurotrophins and possibly participate in their presentation to signal-transducing receptors. Recent work, however, has demonstrated that the BDNF-mediated activation of the truncated T1 isoform of TrkB controls release of Ca2+ from intracellular stores through a G protein and inositol tris-phosphate (IP3)-dependent pathway (Rose et al. 2003). A truncated isoform of TrkC associates with the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 (PDZ) domain of tamalin in the presence of NT3 (Esteban et al. 2006). Tamalin forms a complex with the Arf–guanine-nucleotide exchange factor (GEF) ARF nucleotide-binding site opener (ARNO), so NT3-mediated signalling through truncated TrkC results in activation of Arf6, Arf6-dependent activation of Rac and membrane ruffling. Differential splicing of trkC mRNA also results in expression of a TrkC isoform with an amino acid insert within the tyrosine kinase domain. This insert appears to modify the substrate specificity of this tyrosine kinase, inhibiting its activation of several substrates and interfering with its ability to promote neuronal differentiation (Guiton et al. 1995; Meakin et al. 1997).
Differential splicing of the Trk receptor mRNAs is not the only influence that modulates neurotrophin binding and action. In some central nervous system (CNS) neurons, many Trk receptors are localized in intracellular vesicles. Second signals, such as cAMP or Ca2+, promote insertion of the receptors into the surface membrane, where they are accessible to neurotrophins (Meyer-Franke et al. 1998; Du et al. 2000). In these neurons, responsiveness to neurotrophins may require incorporation of neurons into signalling networks that result in the production of these second messengers. TrkB has also been shown to be recruited to cholesterol-rich rafts following ligand engagement in a Trk-kinase-dependent manner (Du et al. 2003). Some of the signalling consequences of TrkB activation are not observed when these rafts are disrupted. Finally and not surprisingly, Trk receptor endocytosis is also stimulated by ligand engagement as well as cytoplasmic Ca2+ (Du et al. 2003).
Trk receptor function is regulated in several ways by the presence of the pan-neurotrophin receptor, p75NTR. Firstly, p75NTR inhibits activation of Trk receptors by non-preferred neurotrophins both in vitro and in vivo (Benedetti et al. 1993; Bibel et al. 1999; Brennan et al. 1999). Secondly, in vitro studies indicate that the presence of p75TR potentiates activation of TrkA by suboptimal concentrations of NGF, although it does not appear to potentiate the activation of other Trk receptors similarly by their ligands (Davies et al. 1993; Mahadeo et al. 1994), and that the p75NTR collaborates with TrkA to form high-affinity binding sites for NGF (Esposito et al. 2001). In addition to the promotion of binding of NGF to TrkA, p75NTR has been shown to promote retrograde transport of several neurotrophins (Curtis et al. 1995). Two recent papers provide evidence for two distinct mechanisms through which the presence of p75NTR promotes Trk signalling. In one paper, it was reported that p75NTR suppresses ubiquitination of TrkA and TrkB, thereby delaying Trk receptor internalization and degradation (Makkerh et al. 2005). The other paper presented data indicating that the presence of p75NTR promotes endocytosis of each of the Trk receptors through recruitment of E2 and E3 ubiquitin ligases that mediate ubiquitination and subsequent trafficking of the Trk receptors to endosomal compartments, resulting in enhanced cell differentiation (Geetha et al. 2005a). Although there are important discrepancies between these reports, each suggests a mechanism through which p75NTR may promote axon growth and target innervation in vivo as well as in vitro (Bentley & Lee 2000; Harrison et al. 2000). Thus, p75NTR modulates Trk receptor function on several levels—by promoting ligand binding, by promoting accessibility to neurotrophins through promotion of axon growth and target innervation, and by promoting endocytosis and retrograde transport to membrane compartments where internal engagement of neurotrophins with Trk receptors may promote signalling. Significant, but incomplete, sensory and sympathetic deficits are observed in mice lacking p75NTR (Lee et al. 1994; Stucky & Koltzenburg 1997). It is not clear which p75NTR function is the most important in preventing these deficits.
3. Control of neurotrophin responsiveness through gene expression and post-transcriptional mechanisms
Nerve growth factor was purified as a survival factor for cultured sympathetic and sensory neurons from the submaxillary glands of male mice, where it is expressed at extraordinarily high levels, greatly simplifying its purification (Levi-Montalcini 1987; Shooter 2001). The evolutionary processes that have resulted in its high, sexually dimorphic and species-specific expression in these glands remain a mystery, but there is no evidence that this is an important source for the NGF, which functions in vivo to promote neuronal survival. Discovery of the sites of NGF synthesis relevant to its roles in the nervous system required development of sensitive two-site ELISA and mRNA blot assays (Korsching & Thoenen 1983; Heumann et al. 1984; Davies et al. 1987). With these assays, it was shown that NGF is synthesized and secreted by sympathetic and sensory target organs. After secretion, it is captured through receptor interactions in nerve terminals, where it acts locally to regulate target innervation and nerve terminal function (Campenot 1977; Zhang et al. 2005). After internalization, NGF is transported to neuronal cell bodies, where it acts to promote neuronal survival and differentiation (Kuruvilla et al. 2004). Extending the observations on NGF, each of the other neurotrophins has also been shown to be a target-derived neurotrophic factor (Farinas et al. 1996; Yee et al. 2003). Within targets, expression of these proteins is localized to specialized end organs, such as hair follicles, that are innervated by the axons of responsive neurons. Through receptor interactions, internalization and retrograde transport, each of these proteins has local actions affecting growth cone behaviour and synaptic function, as well as actions at a distance in the cell body and nucleus.
There are also other sources of neurotrophins. Of special importance, during development, neurotrophins are expressed in regions innervated by sensory neuron axons en route to their final targets and their expression there is essential for survival of these neurons (Farinas et al. 1996, 1998). In addition, some sensory neurons depend transiently on neurotrophin expression by immature cells in the vicinity of their final targets, which sustain these neurons until specialized cells in the targets, such as hair cells in the inner ear, have completed differentiation (Coppola et al. 2001; Farinas et al. 2001). Neurotrophin expression is also important after injury. After peripheral nerve injury, macrophages infiltrate the nerve as part of an inflammatory response. These cells secrete cytokines that induce synthesis of NGF in both Schwann cells and fibroblasts within the nerve (Heumann et al. 1987). Nerve growth factor is also expressed in mast cells from which it is released following mast cell activation at sites of inflammation. The elevated expression of neurotrophic factors in injured nerves and targets is believed to be essential for successful survival and regeneration of injured neurons.
Many neurons also express neurotrophins. For example, many sensory and sympathetic neurons express BDNF or NT4. Following secretion, NT4 regulates the innervation of sympathetic neurons by preganglionic neuron axons emanating from the spinal cord (Schober et al. 1998; Roosen et al. 2001). Neurotrophins derived from sensory neurons affect both peripheral and central targets (Mannion et al. 1999). Within sensory ganglia, BDNF may also have paracrine or autocrine action.
Neurotrophin expression is controlled initially through cellular interactions in tissues that determine sites and levels of neurotrophin gene expression (Patapoutian et al. 1999). Each of the neurotrophin genes is regulated through multiple promoters and enhancers (Selby et al. 1987; Bishop et al. 1994). Multiple extrinsic stimuli, including Wnt and TGF-β family members (Buchman et al. 1994), thyroid hormone (Koibuchi et al. 1999), steroids (Lindholm et al. 1987; Toran-Allerand 1996) and inflammatory cytokines (Lindholm et al. 1987) have been shown to control expression from neurotrophin genes. Although many of the cis elements and transcription factors involved in neurotrophin gene expression are being identified, there is no tissue or cell type in which these interactions are completely characterized (Lei et al. 2005; Chang et al. 2006).
In studies on neurotrophin expression within neurons, particular attention has been focused on control of BDNF expression because BDNF is an important modulator of synaptic plasticity. Expression of the BDNF gene is controlled through four distinct promoters. Of particular interest, expression from one of these is regulated by neuronal activity through Ca2+ flux via L-type Ca2+-channels. Cytoplasmic Ca2+ activates BDNF transcription through protein kinase-cascades that result in the activation of several transcription factors, including cyclic AMP response element binding protein (CREB), calcium-responsive transcription factor and upstream stimulatory factors (Tao et al. 1998, 2002; Chen et al. 2003b). Activation of BDNF transcription is also enhanced by Ca2+-dependent phosphorylation of the transcriptional repressor methyl CpG-binding protein 2 (MeCP2) and its release from the BDNF promoter (Chen et al. 2003a). The MeCP2 gene is mutated in Rett syndrome, and studies in a mouse model have recently demonstrated that the reduced expression of BDNF observed in the absence of MeCP2 is important in regulating the progression of this developmental disorder (Chang et al. 2006).
Packaging, transport, secretion and processing of neurotrophins are also important post-transcriptional regulators of their actions, and again most attention has been focused upon BDNF owing to its important roles in controlling neuronal functions in the mature brain. Present evidence indicates that each of the neurotrophins can be sorted into granules whose exocytosis is regulated by signals, including ligand engagement of Trk receptors, that mobilize intracellular Ca2+ stores (Heymach et al. 1996; Canossa et al. 2001). The efficiency with which the different neurotrophins are sorted into the regulated secretion pathway varies depending upon the cell type. In hippocampal neurons, NGF and NT3 appear to be largely secreted into a constitutive secretory pathway, while BDNF is sorted into a regulated pathway (Mowla et al. 1999; Farhadi et al. 2000). Sorting of NGF and NT3 into a regulated secretion pathway appears to require the presence of the prohormone domain and, as a result, is promoted by inhibition of the protease furin.
Human genetic studies indicate that regulated trafficking and secretion of BDNF are exceedingly important for normal brain function. The presence of a single nucleotide polymorphism in the BDNF gene resulting in a V66M mutation in the BDNF prohormone domain leads to selective impairments in hippocampal episodic memory and abnormal hippocampal function (Egan et al. 2003; Hariri et al. 2003). The V66M mutation inhibits sorting of BDNF into a regulated secretion pathway and affects its transport and localization within neurons. Two proteins have been identified that direct proBDNF into a regulated secretory pathway. The large Golgi and endosomal protein sortilin interacts with the prodomain of proBDNF through a site that includes the V66M polymorphism (Chen et al. 2005). RNAi-mediated suppression of sortilin expression interferes with BDNF targeting to the regulated secretion pathway. Interestingly, sortilin interacts much less effectively with the M66 than V66 isoform of proBDNF. Sorting of proBDNF also requires interactions with carboxypeptidase E, and interestingly, the amino acid residues in BDNF responsible for this interaction reside in mature BDNF, not in the prohormone domain (Lou et al. 2005). Both carboxypeptidase E and sortilin mediate sorting of many proteins in addition to BDNF. It is not certain whether they act synergistically or sequentially to direct proBDNF to a regulated secretory compartment.
Finally, neurotrophin activity is regulated by the proteases responsible for conversion of the proneurotrophins to mature neurotrophins. Interest in these proteases has been increased tremendously by recent discoveries documenting that proneurotrophins are secreted from cells and are biologically active (Lee et al. 2001). Firstly, intracellular proneurotrophin processing is not always efficient, so substantial amounts of proneurotrophins are secreted in some tissues. Secondly, proneurotrophins bind with high affinity to the p75NTR receptor and activate signalling pathways controlled by this receptor, which in many cells results in promotion of apoptosis (Lee et al. 2001). Data suggest that secretion of proneurotrophins is increased following brain injury or degeneration, and binding of these proteins to p75NTR may increase neuronal loss in these injury and disease models (Fahnestock et al. 2001; Harrington et al. 2004; Pedraza et al. 2005).
Inside the cells, mature forms of NGF, BDNF and NT3 are generated by furin and other prohormone convertases in the trans-Golgi network (Seidah et al. 1996). Some trimming of proneurotrophins also occurs earlier in the biosynthetic pathway in the endoplasmic reticulum (Mowla et al. 2001). As a result, multiple size variants of the proneurotrophins are detected in brain extracts (Lee et al. 2001), but the significance of prohormone proteolysis that does not result in release of mature neurotrophins is not clear. Extracellular proBDNF is cleaved by plasmin following its activation by tissue plasminogen activator (Pang et al. 2004). Regulation of cleavage of the extracellular proBDNF is a topic of intense current interest because there are extensive data indicating that mature BDNF promotes hippocampal long term potentiation (LTP) through TrkB (Patterson et al. 1996; Minichiello et al. 2002; Zakharenko et al. 2003), while recent data indicate that proBDNF enhances hippocampal long term depression (LTD) through p75NTR (Patterson et al. 1996; Minichiello et al. 1999, 2002; Zakharenko et al. 2003; Pang et al. 2004). Consistent with these observations, absence of either TrkB or p75NTR compromises the performance in spatial learning paradigms (Minichiello et al. 1999; Peterson et al. 1999).
To summarize, the neurotrophins were discovered as target-derived trophic factors, and cellular interactions in these targets control the expression of the mRNAs encoding these proteins. In addition, intracellular trafficking and intra- and extracellular proteolysis have proven to be crucial controllers of their actions.
4. Trk receptor-mediated signalling pathways
The Trk receptors are typical receptor tyrosine kinases whose activation is stimulated by neurotrophin-mediated dimerization and transphosphorylation of activation loop tyrosines (Huang & Reichardt 2003). The Trk receptors are activated specifically by the mature and not the pro-forms of the neurotrophin gene products (Lee et al. 2001). Thus, the proteases that control processing of proneurotrophins control Trk receptor responsiveness. The cytoplasmic domains of the Trk receptors contain several additional tyrosines that are also substrates for phosphorylation by each receptor's tyrosine kinase. When phosphorylated, these residues form the cores of binding sites for scaffolds and enzymes that are intermediates in intracellular signalling cascades. Specifically, the vertebrate Trk receptors contain 10 evolutionarily conserved tyrosines in their cytoplasmic domains, three of which (Y670, Y674 and Y675 in human TrkA sequence) are in the autoregulatory loop of the tyrosine kinase domain, which controls tyrosine kinase activity. Similar to other receptor tyrosine kinases, phosphorylation of these tyrosines results in elevated Trk tyrosine kinase activity. Phosphorylation of other tyrosine residues creates binding sites for proteins containing phosphotyrosine-binding (PTB) or Src-homology-2 (SH2) domains. The major pathways activated by the Trk receptors are Ras, Rac, PI3-kinase, PLC-γ1 and their downstream effectors (Huang & Reichardt 2003). These include Ras stimulation of mitogen-activated protein (MAP) kinase cascades, phosphatidyl inositol-3 (PI3)-kinase stimulation of Akt and PLC-γ1-dependent generation of IP3 and diacylglycerol (DAG) that results in mobilization of Ca2+ stores and activation of Ca2+ and DAG-regulated protein kinases. Among the tyrosines not in the kinase activation loop, two (Y490 and Y785) are the major sites of endogenous phosphorylation, although five of the remaining tyrosines also contribute to promoting NGF-mediated neurite outgrowth by PC12 cells (Inagaki et al. 1995). Phospho-Y490 provides a recruitment site for both Shc and Frs2, which provide links to Ras, PI3-kinase and other pathways. Phospho-Y785 recruits the enzyme PLC-γ1, which is then activated through phosphorylation by the Trk receptors. Through generation of IP3 and DAG from phosphatidyl inositides, activation of this enzyme results in Ca2+ and protein kinase C mobilization. In addition, endocytosis and transfer of Trk receptors to different membrane compartments control the efficiency and duration of Trk-mediated signalling, in part because many adaptor proteins are localized to specific membrane compartments (York et al. 2000). Interestingly, there is a glutamine, not a tyrosine, at the site equivalent to Y785 in the single Trk homologue present in the Amphioxus genome (Benito-Gutierrez et al. 2005, 2006). Although each mammalian neurotrophin is able to activate survival and differentiation pathways through the Amphioxus Trk receptor, they do not activate the PLC-γ1 pathway. Thus, signalling through this pathway appears to be an adaptation incorporated into Trk receptors early in vertebrate evolution.
Although much of the work on Trk receptors has focused on signalling pathways initiated through phosphorylation of Y490 and Y785, analyses of mutant mice, homozygous for TrkB mutants in which F replaces Y at these sites, indicate that most of the additional docking sites have redundant roles in signalling (Minichiello et al. 1998, 2002; Medina et al. 2004). Another work has identified interactions between Grb2 and other adaptors with phosphorylated tyrosines in the kinase activation loop that provide alternative mechanisms for activation of downstream signalling pathways (Qian et al. 1998; MacDonald et al. 2000). Additional work has identified adapters and enzymes, such as c-Abl, that interact with Trk receptors at different sites, not all of which include a phosphotyrosine (Yano et al. 2000; Robinson et al. 2005). Many of the adaptors studied in cell lines are differentially expressed in various cell types, so the details of Trk receptor-stimulated signalling are likely to differ substantially between different subpopulations of neurons.
Activation of Ras is required for normal neuronal differentiation and also promotes survival of many neuronal subpopulations. Recruitment by activated Trk receptors of several different adapters promotes Ras activation. Transient activation of Ras is mediated by the adaptor protein Shc, which is recruited to phospho-Y490 on Trk receptors via interactions with the Shc PTB domain (Nimnual et al. 1998). Trk-mediated phosphorylation of Shc creates a phosphotyrosine site on Shc that recruits the adaptor Grb2, which is bound to the Ras exchange factor son of sevenless (SOS). Additional scaffold proteins, including SH2-B, adaptor protein with pleckstrin homology and Src homology-2 domains (APS) and insulin receptor substrate-1 (IRS1), also provide links to Ras in some cell types (Qian & Ginty 2001). Activation of Ras also appears to be facilitated by neurotrophin-dependent phosphorylation and activation of the Ras G protein-releasing factor, RasGRF1 (Robinson et al. 2005). Active Ras stimulates signalling through c-Raf–Erk, p38MAP kinase and class I PI3 kinase pathways (Xing et al. 1998; Vanhaesebroeck et al. 2001). Activation of Erk1 and Erk2 is mediated in a cascade initiated by Ras activation of the protein kinase Raf. Raf phosphorylates Mek1 and/or Mek2, which in turn phosphorylate and activate Erk1 and Erk2 (English et al. 1999). Adaptor proteins that link proteins together in this signalling pathway greatly increase its efficiency. In neurons, p38MAP kinase is probably activated by a pathway initiated by Ras-mediated binding and activation of the exchange factor RalGDS, which results in activation of Ral and recruitment of Src (Ouwens et al., 2002). In addition, p38MAP kinase can be activated through neurotrophin-stimulated activation of the Ras-family G proteins, Rin and Rit (Shi & Andres 2005; Shi et al. 2005). Ras also appears to promote activation of these G proteins through uncharacterized exchange factors (Spencer et al. 2002). p38MAP kinase in turn activates MAP kinase-activated protein kinase-2. Ras also triggers a signalling cascade through sequential activation of Wnk1, MEKK2 and MEK5 that results in activation of Erk5 (Watson et al. 2001; Xu et al. 2004; Wang et al. 2005). Erk5 may also be activated through a RalGDS–Ral–Src–dependent pathway (Scapoli et al. 2004). The MAP kinase cascades feedback to attenuate and terminate responses through phosphorylation of intermediates and activation of phosphatases. As an example, Erk and Rsk mediate phosphorylation of SOS, which results in dissociation of the SOS–Grb2 complex (Douville & Downward 1997).
Prolonged activation of MAP kinases appears to require recruitment of a distinct adaptor, Frs2, to phospho-Y490 on Trk receptors (Kao et al. 2001; Wu et al. 2001). Similar to Shc, Frs2 is phosphorylated by active Trk receptors. Phosphorylation provides recruitment sites for the adaptor proteins Grb2 and Crk, the tyrosine kinase Src, the cyclin-dependent kinase substrate p13 suc1 and the protein phosphatase SH-PTP2 (Meakin et al. 1999). Following recruitment to Frs2, Crk binds and activates the Rap1 exchange factor C3G. Alternatively, Crk can be recruited to activated Trk receptors through binding to the transmembrane protein ankyrin repeat-rich membrane spanning adaptor (ARMS), which is closely associated with Trk receptors in some cells (Arevalo et al. 2004). Ligand engagement of Trk receptors results in tyrosine phosphorylation of ARMS, creating a docking site for Crk, resulting in activation of Rap1 through C3G. Active Rap1 stimulates the protein kinase B-Raf, which activates the Erk kinase cascade. In addition to providing a mechanism for sustained MAP kinase activation, Frs2 also activates the Grb2–SOS–Ras pathway. Frs2 also couples Trk signalling to Src family kinases, which promote differentiation synergistically with MAP kinases. Finally, recruitment of the protein phosphatase, SH-PTP2, is believed to facilitate activation of the Erk cascade, probably by inactivation of an inhibitor, such as Ras-GAP or MAP kinase phosphatase.
Each of the MAP kinase cascades summarized earlier has overlapping, but also distinct targets within the cell (Pearson et al. 2001). For example, Erk1/2 and Erk5 phosphorylate and activate the Rsk kinases. Rsks and MAP kinase-activated protein kinase 2 each phosphorylate CREB, which activates transcription of genes essential for normal differentiation and prolonged survival of neurons. In addition to the shared targets, the different MAP kinase cascades also have distinct transcription factors as targets (Pearson et al. 2001). For example, Erk5, but not Erk1/2, activates MEF2, while Erk1/2, but not Erk5, activates Elk1. Identification of all the intermediates in signalling between proximal Trk receptor activation and various phenotypes in neurons induced by their signalling remains an interesting and important challenge.
The neurotrophins also activate several of the Rho family of GTPases that control organization of the cytoskeleton, cell motility and growth cone behaviour (Yuan et al. 2003b). Neurotrophins activate several of these GTPases, most notably, Cdc42 and Rac, through pathways that are incompletely characterized. It is clear though, that neurotrophin effects on axonal growth and growth cone guidance depend in large measure upon regulation of these monomeric G proteins, so this seems an important area for intensive studies (Yuan et al. 2003b). Trk receptor activation provides several pathways for regulation of Cdc42 and Rac. First, in Schwann cells, ligation of TrkC results in phosphorylation and activation of the Cdc42GEF Double's Big Sister (Yamauchi et al. 2005a). It seems likely that TrkC and other Trk receptors activate this and other GEFs directly in neurons. Second, the Ras GEF SOS is also a RacGEF whose RacGEF activity is regulated by phosphorylation by c-Abl and almost certainly other tyrosine kinases (Sini et al. 2004). Since c-Abl is constitutively associated with TrkA (Yano et al. 2000), it seems plausible that the RacGEF activity of SOS is activated following Trk receptor activation. Third, several observations provide evidence for a Ras-dependent pathway that results in activation of Rac involving Ras-mediated binding to and activation of Tiam1, which directly activates Rac (Yamauchi et al. 2005b). Fourth, the Ras-family G proteins Rin and Rit, each of which is stimulated by Trk receptor signalling, activate Cdc42 and Rac, most probably through a ternary complex including Par6 (Hoshino & Nakamura 2003; Hoshino et al. 2005). Finally, the monomeric G protein RhoG is activated following neurotrophin application. GTP-bound RhoG interacts with Elmo, which in turn associates with the RacGEF Dock180, resulting in activation of Rac. The RhoG–Elmo–Dock180–Rac pathway is important in promoting NGF-dependent neurite outgrowth by PC12 cells (Katoh & Negishi 2003). RhoG may also act directly on many of the same effectors as Rac (Prieto-Sanchez & Bustelo 2003). The pathway through which neurotrophins activate RhoG is not entirely clear, but appears to involve regulation of Trio, a GEF for RhoG (Estrach et al. 2002). Since there are several hundred GEF genes, many of which are differentially expressed, both redundancy and neuronal cell-type specificity will probably prove to be important in understanding the mechanisms through which neurotrophins activate Rho-family G proteins (Ma et al. 2005).
Generation of P3-phosphorylated phosphoinositides by PI3-kinase promotes survival of many neurons. Class I PI3-kinases are activated through Ras-dependent and -independent pathways, and the comparative importance of these two mechanisms differs in various subsets of neurons. In many neurons, Ras-mediated activation of PI3-kinase initiates the major pathways through which survival signals are conveyed (Vaillant et al. 1999). In addition, PI3-kinase can also be activated through Shc–Grb2 or Irs1 in Ras-independent pathways. Recruitment of Gab1 by phosphorylated Grb2 permits subsequent binding and activation of PI3-kinase (Holgado-Madruga et al. 1997). In addition, in some neurons, Trk receptor activation results in phosphorylation of IRS1, which also permits recruitment and activation of PI3-kinase (Yamada et al. 1997).
The P3-phosphorylated phosphoinosides generated by PI3-kinase have multiple effects on neuronal survival and development. First, these lipids are essential for survival of many neurons. In collaboration with phosphoinositide-dependent kinases, they activate the protein kinase Akt, which in turn controls through phosphorylation the activities of several proteins important in promoting cell survival (Yuan & Yankner 2000; Brunet et al. 2001; Yuan et al. 2003a). These include substrates that directly regulate the caspase cascade, such as BAD, a Bcl2-family member that promotes apoptosis by binding to Bcl-xL, thereby preventing Bcl-xL from inhibiting the pro-apoptotic activity of Bax. Phosphorylated BAD is sequestered by 14-3-3 proteins, preventing its pro-apoptotic actions. Akt also regulates the activity of several transcription factors. Through phosphorylation of the forkhead transcription factor, FKHRL1, it promotes association of this transcription factor with 14-3-3 proteins, thereby sequestering it in the cytoplasm and preventing it from activating transcription of several genes whose products promote apoptosis (Brunet et al. 2001). Akt-mediated phosphorylation of IκB results in its degradation, liberating NF-κB. NF-κB-promoted gene transcription has been shown to promote sensory neuron survival (Hamanoue et al. 1999).
In addition, PI3-kinase activation has many targets that promote axon growth and pathfinding, and neuronal differentiation. The 3-phosphoinositides generated by PI3-kinase recruit many signalling proteins to the membrane, including GEFs for Cdc42, Rac and Rho. These control organization of the F-actin cytoskeleton and almost certainly account for the ability of neurotrophin gradients to steer growth cones (Yuan et al. 2003b). Akt effectors include the S6 kinases, which are important for promoting translation of subsets of mRNAs (Kimball et al. 2002). Through PI3-kinase-mediated signalling, Trk receptor ligation also results in activation of integrin-linked kinase (ILK) in sympathetic neurons (Zhou et al. 2004). ILK has many targets, including glycogen synthase kinase 3-β (GSK3-β), which is inactivated as a result of ILK-mediated phosphorylation (Zhou et al. 2004). While ILK has usually been considered a target of Akt in neuronal cells (Cantley 2002), there is evidence indicating that it functions upstream of Akt and influences Akt activity (Mills et al. 2003). GSK3-β has been implicated in control of apoptosis through regulation of caspases and in control of neurite outgrowth through regulation of Rho family GTPases and the microtubule plus end-binding protein APC (Zhou et al. 2004; Chin et al. 2005; Filipenko et al. 2005).
PLC-γ1 is recruited to a docking site surrounding phosphorylated Y785 on TrkA and to similar sites on TrkB and TrkC, following Trk kinase activation. Docked PLC-γ1 is activated through Trk-mediated phosphorylation and then hydrolyses PtdIns (4,5)P2 to generate IP3 and DAG. The presence of IP3 results in Ca2+ release from cytoplasmic stores. DAG stimulates DAG-regulated isoforms of protein kinase C. Together these signalling molecules activate many intracellular enzymes, including almost all PKC isoforms, Ca2+-calmodulin-dependent protein kinases and other Ca2+-calmodulin-regulated targets. The proteins activated as a result of PLC-γ1 activation include PKC-δ, which is required for NGF-promoted activation of MEK1 and Erk1/2 (Corbit et al. 1999). Signalling through this pathway controls expression and/or activity of many proteins, including ion channels and transcription factors (Toledo-Aral et al. 1995; Minichiello et al. 2002; Klein et al. 2005).
In addition to its effects on cytoplasmic signalling pathways, PLC-γ1-mediated depletion of PtdIns (4,5)P2 directly affects the activity of membrane proteins that interact with phosphatidylinositides. Of particular interest, Trk receptor signalling through PLC-γ1 activates several members of the TRP family of cation channels. In the CNS, TRPC3 is broadly expressed and activated through TrkB (Li et al. 1999). In nociceptive sensory neurons, the heat-activated TRP channel VR1 (the capsaicin receptor) appears to be repressed by PtdIns (4,5)P2, so PLC-γ1-stimulated depletion of PtdIns (4,5)P2, as a result of TrkA activation, results in hypersensitization of this channel to thermal and mechanical stimuli (Chuang et al. 2001; Prescott & Julius 2003). TrkA and VR1 can be co-immunoprecipitated, indicating that they exist in a macromolecular complex that almost certainly increases the efficiency of Trk receptor regulation of VR1 activity. Recent work, however, has challenged the importance of this pathway in regulation of VR1, with results indicating that the major pathway through which TrkA promotes VR1 activity is through stimulation of VR1 insertion into the surface membrane from intracellular vesicles (Zhang et al. 2005). TrkA-dependent activation of PI3-kinase results in Src-dependent phosphorylation of VR1 at a specific tyrosine residue (Y200), after which VR1 is inserted into the surface membrane. The deletion of the putative binding site through which PtdIns (4,5)P2 was believed to inhibit VR1 (amino acids 777–820) was shown to increase the level of phosphorylation of VR1 at Y200, suggesting that amino acids 777–820 modulate access of kinases or phosphatases to the Y200 residue. Additional poorly defined pathways also contribute to NGF-stimulated thermal sensitization and additional work will be required to characterize these completely.
5. Regulation of signalling through compartmentalization and transport of Trk receptors
The extended geometry of neurons has attracted interest in neurotrophin transport for decades and important in vivo and in vitro experiments demonstrated in the 1970s that neurotrophins affected gene transcription through retrograde transport as well as the behaviours of axon terminals through local signalling pathways (Thoenen & Barde 1980). It is clear that neurotrophins and activated Trk receptors are transported together in endocytotic vesicles. Internalization and transport of Trk receptors serve two functions—to bring activated Trk receptors into proximity of cell compartments, such as the nucleus, where signalling is required to enable specific cellular responses, such as gene transcription and to transport activated Trk receptors to membrane compartments, where signalling effectors are concentrated (York et al. 2000; Delcroix et al. 2003; Ye et al. 2003).
On the cell surface, Trk receptors become concentrated in caveoli-like domains (Huang et al. 1999). Recruitment by activated Trk receptors of signal-transducing proteins on the cell surface appears crucial. Unusually rapid internalization of TrkA induced by exposure to a NGF-monoclonal antibody complex prevents NGF-dependent phosphorylation of Frs2, even though it does not affect phosphorylation of Shc or transient Erk1/2 activation (Saragovi et al. 1998). Frs2 is N-myristoylated and preferentially concentrated in lipid rafts, suggesting that activated Trk receptors must spend sufficient time on the surface to interact with Frs2 and other lipid raft-associated proteins.
Neurotrophin binding stimulates Trk receptor internalization through clathrin-coated pits and macropinocytosis. Exciting recent data have demonstrated that internalization of Trk receptors is regulated by ubiquitination, suggesting that the differential expression of ubiquitin E2 and E3 ligases may result in differences in Trk receptor trafficking in different populations of neurons (Makkerh et al. 2005; Geetha et al. 2005a; Arevalo et al. 2006b). It will be interesting to determine whether differential ubiquitination accounts for the surprising observation that in sympathetic neurons, retrograde transport of TrkA is promoted by NGF, but not NT3, even though both neurotrophins bind and activate TrkA on these cells (Kuruvilla et al. 2004). After internalization, neurotrophins are localized together with Trk receptors in endosomal compartments that recruit and activate signalling intermediates, such as Shc (Delcroix et al. 2003; Ye et al. 2003). Transport to endosomal compartments is required for efficient activation through Frs2 or the Crk–C3G–Rap1 signalling pathway that results in persistent activation of the MAP kinase cascade (York et al. 2000; Wu et al. 2001). Rap1 is preferentially localized to endosomes, providing an attractive explanation for this observation. The differential localization of signalling intermediates, such as Frs2 and Rap1, probably explains why treatments that either inhibit or accelerate internalization of ligated Trk receptors prevent normal neuronal responses to these complexes (Saragovi et al. 1998; Zhang et al. 2000).
Through incompletely understood mechanisms, the signalling potential of intracellular Trk receptors appears to be further regulated during endocytosis and transport, possibly by sorting into distinct populations of endosomes. Using Campenot chambers, local application of NGF to distal axons or cell bodies of sensory neurons was shown to activate both the Erk1/2 and the Erk5 kinases locally, but application of NGF to the distal axons activated Erk5, but not Erk1/2, in the cell body compartment (Watson et al. 2001). Trk receptors are internalized through clathrin-mediated endocytosis and also through pinocytosis mediated by a novel protein, Pincher (Valdez et al. 2005). Pincher-associated endosomes are associated with Erk5 and are not readily degraded by lysosomes. RNAi-mediated inhibition of Pincher severely attenuates the retrograde transport-mediated survival response of neurons to neurotrophins. Thus, clathrin and Pincher appear to promote the formation of endosomal vesicles that differ in their stability and signalling potential with Pincher promoting the formation of endosomes, which are preferentially recruited for retrograde signalling.
6. p75 Neurotrophin receptor-mediated signalling pathways
Much of the recent excitements in neurotrophin research have focused on the surprisingly diverse functions of p75NTR. As mentioned earlier, this receptor is known to bind the unprocessed proneurotrophins with ligand engagement resulting in dramatically different consequences, such as apoptosis, than those that follow ligand engagement of Trk receptors (Lee et al. 2001). The proneurotrophins actually bind with high affinity to a complex of p75NTR with sortilin, a Vps10-domain containing protein (Nykjaer et al. 2004; Chen et al. 2005). Both sortilin and p75NTR participate directly in binding to the proneurotrophins. The presence of sortilin is required to observe apoptosis following engagement of p75NTR by the proneurotrophins. Each neurotrophin also binds p75NTR with an approximately 1000-fold lower affinity. The p75NTR receptor also forms a signal-transmitting subunit of the Nogo receptor complex (NogoR, Lingo, p75NTR) that mediates inhibitory effects on axon growth of three myelin-associated glycoproteins—Nogo, myelin-associated glycoprotein and oligodendrocyte-myelin glycoprotein (Wang et al. 2002; Wong et al. 2002; Mi et al. 2004). Alternatively, a tumour necrosis factor receptor homologue named TROY can replace p75NTR to form a functional Nogo receptor complex (Shao et al. 2005).
Several signalling pathways are activated following neurotrophin binding to p75NTR. These are mediated through binding p75NTR of several adaptor proteins, including Traf6, neurotrophin receptor-interacting factor (NRIF), melanoma-associated antigen (MAGE), neurotrophin receptor p75 interacting MAGE homologue (NRAGE), Schwann cell factor 1 (SC1), RhoGDI and other proteins (Yamashita et al. 2005). One major pathway activated by p75NTR engagement by neurotrophins is the Jun kinase-signalling cascade. Signalling through this cascade results in activation of p53 and apoptosis. In addition, the Jun kinase cascade induces expression of Fas ligand in neuronal cells, promoting apoptosis through activation of the Fas receptor. p53 has multiple targets, including the pro-apoptotic gene, bax. Several essential intermediates in the pathway through which ligand engagement of p75NTR activates the Jun kinase cascade have been identified. Genetic deletion of NRIF or the E3 ubiquitin ligase Traf6 prevents activation of Jun kinase signalling in sympathetic neurons (Yeiser et al. 2004; Linggi et al. 2005). In an interesting recent work, it has been shown that these two proteins form a complex and Traf6-mediated K63 ubiquitination of NRIF is required for entry of NRIF into the nucleus (Geetha et al. 2005b). Nuclear transport of NRIF complexed to the intracellular domain of p75NTR requires ligand-dependent γ-secretase-mediated cleavage of p75NTR (Kenchappa et al. 2006). Overexpression studies have also suggested roles for MAGE, NRAGE and SC1 in promoting apoptosis. In PC12 cells and sympathetic neurons, activation of the Jun kinase cascade involves Cdc42 because apoptosis is strongly inhibited by a dominant negative Cdc42 (Bazenet et al. 1998). The MAP kinase kinase kinase named apoptosis signal-regulated kinase 1 (ASK1) is clearly downstream of Cdc42 because it strongly inhibits cell death promoted by constitutively active Cdc42 (Kanamoto et al. 2000). In oligodendrocytes, Rac activation is required for p75NTR-stimulated apoptosis, indicating that different cell types may use different Rho family GTPases as essential intermediates in the pathway that results in activation of the Jun kinase signalling pathway (Harrington et al. 2002). The Jun kinase kinase named MKK7 seems likely to provide a link between ASK1 and Jun kinase in sympathetic neurons (Kanamoto et al. 2000). Another MAP kinase kinase capable of phosphorylating JNK, MKK4, however, appears to play an anti-apoptotic role during neural development, so the role of the MKKs is slightly confusing (Chi et al. 2005). Animals lacking multiple JNK isoforms show perturbations of neuronal apoptosis during early brain development and in response to stress (Kuan et al. 1999, 2003).
Neurotrophin binding to p75NTR also promotes activation of NF-κB, thereby promoting NF-κB-dependent neuronal survival (Hamanoue et al. 1999; Middleton et al. 2000). As described earlier, neurotrophins promote the association of Traf6 with the cytoplasmic domain of p75NTR (Khursigara et al. 1999). Interleukin-1 receptor-associated kinase (IRAK) is recruited to this complex, resulting in formation of a complex of Traf6 and IRAK with atypical protein kinase C-ι (aPKC-ι) and the aPKC-interacting protein p62 (Wooten et al. 2001; Vandenplas et al. 2002). IκB kinase-β (IKK-β), a known substrate of aPKC, is recruited to and activated in this complex. IKK-mediated phosphorylation of IκB results in release of the transcription factor NF-κB. Both the p62 and the kinase activity of IRAK are necessary for activation of NF-κB.
Ligand engagement of p75NTR has been shown to activate acidic sphingomyelinase, which results in generation of ceramide (Dobrowsky et al. 1994). Ceramide has been shown to promote both apoptotic and prosurvival pathways initiated by p75NTR ligation (DeFreitas et al. 2001; Song & Posse de Chaves 2003). Ceramide is known to control many signalling pathways, including the Erk, Jun kinase, NF-κB signalling pathways as well as the activity of TrkA through phosphorylation of this receptor on serine residues (MacPhee & Barker 1997; Muller et al. 1998). Ceramide is known to bind Raf, thereby inhibiting extracellular regulated kinase (ERK) signalling (Muller et al. 1998). Evidence indicates that ceramide also inhibits or promotes signalling through PI3-kinase by a variety of mechanisms, depending upon cell type (MacPhee & Barker 1997; Zundel et al. 2000). The factors accounting for its different effects in different neural populations seem likely to involve its differential regulation of PI3-kinase signalling.
Neurotrophin engagement of p75NTR also controls Rho family GTPase activity. p75NTR has been shown to activate RhoA through a direct interaction, thereby inhibiting neurite outgrowth (Yamashita et al. 1999). Neurotrophin binding to p75NTR eliminates p75NTR-dependent activation of RhoA, stimulating neurite outgrowth. Recent work indicates that p75NTR functions as displacement factor that facilitates release of RhoA from RhoGDI (Yamashita & Tohyama 2003). p75NTR interacts directly with RhoGDI to facilitate RhoA release. This group also reported that activators of the Nogo receptor-signalling complex increased this association, thereby increasing the activation of RhoA. It will be interesting to determine whether the effects of neurotrophin binding to p75NTR are also mediated through RhoGDI. If so, the observations predict that neurotrophins would weaken the association of RhoGDI with the p75NTR–sortilin complex. While these observations provide a satisfying model to explain the direct enhancement of neurite outgrowth by ciliary neurons observed in vitro following the neurotrophin engagement of p75NTR (Yamashita et al. 1999), they do not explain the slower growth of sensory and motor neurones observed in vivo in mice lacking p75NTR (Bentley & Lee 2000). One possibility is that in vivo p75NTR is tonically associated with neurotrophins, thereby reducing levels of active RhoA. Alternatively, the presence of p75NTR has been shown to promote retrograde transport of neurotrophins in vivo and may enhance axon growth through this mechanism (Curtis et al. 1995). Finally, the presence of p75NTR has recently been shown to facilitate internalization of Trk receptors through recruitment of an E2–E3 ubiquitin ligase complex that ubiquitinates Trk receptors (Geetha et al. 2005a). As discussed earlier, internalization of Trk receptors appears necessary for neurotrophin stimulation of persistent MAP kinase activation and differentiation, including neurite outgrowth. In vivo, the most important function of p75NTR may be to facilitate Trk internalization and subsequent signalling events that promote axon growth at the axon terminal and retrograde transport and nuclear signalling at the cell soma. Disruption of this mechanism may explain the otherwise puzzling deficit in all classes of sensory neurons observed in the p75NTR mutant (Stucky & Koltzenburg 1997).
Two different mutants in the gene encoding p75NTR have been isolated with neomycin cassettes inserted into exon III or IV (Lee et al. 1992; von Schack et al. 2001). The exon III p75NTR mutant used in most experiments expresses low quantities of a short receptor isoform lacking the p75NTR extracellular domain and, as expected, does not bind NGF, but this protein retains transmembrane and cytoplasmic domains and remains able to interact with TrkA (von Schack et al. 2001). A cryptic Kozak sequence in the exon IV mutant also results in expression of a similar short receptor isoform containing the transmembrane and cytoplasmic domains of p75NTR (Paul et al. 2004). Since overexpression of the transmembrane and cytoplasmic domains together or the cytoplasmic domain alone stimulates apoptosis (Majdan et al. 1997; Paul et al. 2004), it is possible, but not likely, that some of the phenotypes observed in each mutant are caused by expression of low levels of a truncated receptor.
7. Crosstalk between neurotrophin signalling pathways activated through p75 neurotrophin receptor and Trk receptors
Neurotrophin-mediated interactions through p75NTR and the Trk receptors have frequently opposing consequences. For example, ligation of Trk receptors almost always promotes neuronal survival and differentiation (Patel et al. 2000), while engagement of p75NTR frequently, but not invariably, promotes apoptosis (DeFreitas et al. 2001; Teng et al. 2005). Similarly, BDNF-mediated activation of TrkB facilitates hippocampal LTP (Zakharenko et al. 2003), while neurotrophin interactions with p75NTR instead facilitate hippocampal LTD (Woo et al. 2005). How can one understand the opposition and synergy observed when neurotrophins activate these two receptor systems? The observation that the two different receptor classes actually have distinct preferred ligands—proneurotrophins for p75NTR and mature neurotrophins for Trk receptors—provides a partial explanation for this riddle (Lee et al. 2001). In addition, there are important mechanisms through which these receptors communicate with each other to ensure a consistent biological response. Importantly, signalling pathways initiated through Trk receptors act at several steps to suppress the major pro-apoptotic-signalling pathway stimulated by p75NTR, the Jun kinase pathway (Yoon et al. 1998). Activation of Ras in sympathetic neurons suppresses the Jun kinase cascade. In these neurons, Ras-mediated activation of PI3-kinase is required to suppress this signalling pathway (Mazzoni et al. 1999). A Jun kinase scaffold Jnk-interacting protein 1 (JIP1) has also been shown to interact with active Akt1 (Kim et al. 2002). Interactions with Akt1 displace constituents of the Jun kinase pathway, thereby reducing its efficiency. In addition, JIP1 has been shown to promote activation of Akt1 through facilitation of phosphorylation of the activation loop of Akt1 by phosphoinositide-dependent kinase-1 (Kim et al. 2003). In addition, the PI3 kinase pathway acts to suppress Jun kinase signalling by inhibition of other intermediates. For example, in some cells, c-Raf has been shown to bind and inactivate ASK1 (Chen et al. 2001). There are consequently several mechanisms through which Trk receptor activation results in suppression of pro-apoptotic p75NTR-mediated signalling. Notably, Trk activation does not inhibit induction of NF-κB-mediated signalling by p75NTR (Yoon et al. 1998). Consequently, in the presence of Trk signalling, activation of the NF-κB pathway makes a synergistic contribution to neuronal survival.
Interactions between p75NTR and Trk receptor signalling are facilitated by assembly of multiprotein complexes that include both receptors as constituents. A protein, named ARMS has been shown to interact with both receptors and to be phosphorylated following Trk activation, thereby providing a docking site for Crk, which results in prolonged activation of Rap and Erk signalling (Kong et al. 2001; Arevalo et al. 2004, 2006a). Caveolin provides another platform for interaction between Trk receptors and p75NTR because it has been shown to interact with both receptors (Bilderback et al. 1999). Finally, the p62–Traf6–IRAK complex described earlier also appears to function as a scaffold for association of these two receptors with Traf6 binding to p75NTR and p62 to TrkA (Wooten et al. 2001). As described earlier, evidence has recently been presented indicating that this complex plays an important role in promoting TrkA-mediated internalization and signalling (Geetha et al. 2005a). The E2 ubiquitin ligase UbcH7 is recruited to this complex together with Traf6, which has E3 ligase activity, and promotes ubiquitination of K485 on TrkA and corresponding residues on TrkB and TrkC that facilitate their internalization. Results from other laboratories, however, suggest that ubiquitination may actually inhibit Trk receptor internalization (Makkerh et al. 2005; Arevalo et al. 2006b). At present, it is difficult to reconcile these observations. In any event, the overall picture that emerges from these studies on p75NTR–Trk receptor interactions is that the pro-apoptotic signals of p75NTR are largely suppressed as a result of Trk receptor-mediated signalling and that elaborate mechanisms have evolved to ensure that ligand-engagement of p75NTR functions to promote the efficacy of Trk signalling in conditions where Trk receptors are ligated by neurotrophins. Specificity in signalling through the two families of receptors appears to be regulated through proteolysis of the proneurotrophins.
8. Conclusion
Neurotrophins are known to have a wide range of roles in development and function of the nervous system. Discovered as target-derived survival factors, they are known to control cell fate, axon growth and guidance, dendrite structure and pruning, synapse formation and synaptic plasticity. Characterization of their receptors—Trk receptor and p75NTR—has tremendously advanced their studies and made it possible to characterize signalling pathways and take initial steps towards associating individual signalling pathways with specific developmental or functional roles of the neurotrophins. While progress in mechanistic studies on individual molecules and pathways has been rapid, a system-wide analysis to integrate and test the completeness of our understanding of these pathways is badly needed. It also seems likely that different populations of neurons will make differential use of the various pathways owing to differences in expression of the proteins that are signalling intermediates. A major challenge is to devise approaches appropriate for studying these cell populations and how neurotrophins affect their integration into the vastness of the complete nervous system. In summary, studies of a small group of proteins—the neurotrophins—have provided insight into almost all areas of contemporary neuroscience. They seem likely to continue to do so in the future.
Acknowledgments
Work from the author's laboratory has been supported by the Howard Hughes Medical Institute and the United States National Institutes of Health. I have benefited greatly from conversations with many members of my laboratory. I especially wish to acknowledge Dr Eric Huang who has shaped my thoughts over several years during composition of previous reviews on this subject.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Arevalo J.C, Yano H, Teng K.K, Chao M.V. A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J. 2004;23:2358–2368. doi: 10.1038/sj.emboj.7600253. 10.1038/sj.emboj.7600253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo J.C, Pereira D.B, Yano H, Teng K.K, Chao M.V. Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation. J. Biol. Chem. 2006a;281:1001–1007. doi: 10.1074/jbc.M504163200. 10.1074/jbc.M504163200 [DOI] [PubMed] [Google Scholar]
- Arevalo J.C, Waite J, Rajagopal R, Beyna M, Chen Z.Y, Lee F.S, Chao M.V. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006b;50:549–559. doi: 10.1016/j.neuron.2006.03.044. 10.1016/j.neuron.2006.03.044 [DOI] [PubMed] [Google Scholar]
- Barde Y.A, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazenet C.E, Mota M.A, Rubin L.L. The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death. Proc. Natl Acad. Sci. USA. 1998;95:3984–3989. doi: 10.1073/pnas.95.7.3984. 10.1073/pnas.95.7.3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao M.V. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc. Natl Acad. Sci. USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. 10.1073/pnas.90.16.7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Gutierrez E, Nake C, Llovera M, Comella J.X, Garcia-Fernandez J. The single AmphiTrk receptor highlights increased complexity of neurotrophin signalling in vertebrates and suggests an early role in developing sensory neuroepidermal cells. Development. 2005;132:2191–2202. doi: 10.1242/dev.01803. 10.1242/dev.01803 [DOI] [PubMed] [Google Scholar]
- Benito-Gutierrez E, Garcia-Fernandez J, Comella J.X. Origin and evolution of the Trk family of neurotrophic receptors. Mol. Cell. Neurosci. 2006;31:179–192. doi: 10.1016/j.mcn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bentley C.A, Lee K.F. p75 is important for axon growth and Schwann cell migration during development. J. Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Barde Y.A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. 10.1101/gad.841400 [DOI] [PubMed] [Google Scholar]
- Bibel M, Hoppe E, Barde Y.A. Biochemical and functional interactions between the neurotrophin receptors Trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. 10.1093/emboj/18.3.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilderback T.R, Gazula V.R, Lisanti M.P, Dobrowsky R.T. Caveolin interacts with Trk A and p75 (NTR) and regulates neurotrophin signaling pathways. J. Biol. Chem. 1999;274:257–263. doi: 10.1074/jbc.274.1.257. 10.1074/jbc.274.1.257 [DOI] [PubMed] [Google Scholar]
- Bishop J.F, Mueller G.P, Mouradian M.M. Alternate 5′ exons in the rat brain-derived neurotrophic factor gene: differential patterns of expression across brain regions. Brain Res. Mol. Brain Res. 1994;26:225–232. doi: 10.1016/0169-328x(94)90094-9. 10.1016/0169-328X(94)90094-9 [DOI] [PubMed] [Google Scholar]
- Brennan C, Rivas-Plata K, Landis S.C. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat. Neurosci. 1999;2:699–705. doi: 10.1038/11158. 10.1038/11158 [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta S.R, Greenberg M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. 10.1016/S0959-4388(00)00211-7 [DOI] [PubMed] [Google Scholar]
- Buchman V.L, Sporn M, Davies A.M. Role of transforming growth factor-beta isoforms in regulating the expression of nerve growth factor and neurotrophin-3 mRNA levels in embryonic cutaneous cells at different stages of development. Development. 1994;120:1621–1629. doi: 10.1242/dev.120.6.1621. [DOI] [PubMed] [Google Scholar]
- Butte M.J, Hwang P.K, Mobley W.C, Fletterick R.J. Crystal structure of neurotrophin-3 homodimer shows distinct regions are used to bind its receptors. Biochemistry. 1998;37:16 846–16 852. doi: 10.1021/bi981254o. 10.1021/bi981254o [DOI] [PubMed] [Google Scholar]
- Campenot R.B. Local control of neurite development by nerve growth factor. Proc. Natl Acad. Sci. USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. 10.1073/pnas.74.10.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H. Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways. EMBO J. 2001;20:1640–1650. doi: 10.1093/emboj/20.7.1640. 10.1093/emboj/20.7.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. 10.1126/science.296.5573.1655 [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of MeCp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. 10.1016/j.neuron.2005.12.027 [DOI] [PubMed] [Google Scholar]
- Chao M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. 10.1038/nrn1078 [DOI] [PubMed] [Google Scholar]
- Chao M.V, Lee F.S. Neurotrophin survival signaling mechanisms. J. Alzheimer's Dis. 2004;6:S7–11. doi: 10.3233/jad-2004-6s611. [DOI] [PubMed] [Google Scholar]
- Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK–ERK independent mechanism. Proc. Natl Acad. Sci. USA. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. 10.1073/pnas.141224398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.G, Chang Q, Lin Y, Meissner A, West A.E, Griffith E.C, Jaenisch R, Greenberg M.E. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003a;302:885–889. doi: 10.1126/science.1086446. 10.1126/science.1086446 [DOI] [PubMed] [Google Scholar]
- Chen W.G, West A.E, Tao X, Corfas G, Szentirmay M.N, Sawadogo M, Vinson C, Greenberg M.E. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J. Neurosci. 2003b;23:2572–2581. doi: 10.1523/JNEUROSCI.23-07-02572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.Y, Ieraci A, Teng H, Dall H, Meng C.X, Herrera D.G, Nykjaer A, Hempstead B.L, Lee F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. 10.1523/JNEUROSCI.1017-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Sarkisian M.R, Rakic P, Flavell R.A. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc. Natl Acad. Sci. USA. 2005;102:3846–3851. doi: 10.1073/pnas.0500026102. 10.1073/pnas.0500026102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin P.C, Majdzadeh N, D'Mello S.R. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res. Mol. Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. 10.1016/j.molbrainres.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Chuang H.H, Prescott E.D, Kong H, Shields S, Jordt S.E, Basbaum A.I, Chao M.V, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. 10.1038/35082088 [DOI] [PubMed] [Google Scholar]
- Clary D.O, Reichardt L.F. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc. Natl Acad. Sci. USA. 1994;91:11 133–11 137. doi: 10.1073/pnas.91.23.11133. 10.1073/pnas.91.23.11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko M.E, Martinez-De Velasco J, Lyons W.E, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- Coppola V, et al. Ablation of TrkA function in the immune system causes B cell abnormalities. Development. 2004;131:5185–5195. doi: 10.1242/dev.01383. 10.1242/dev.01383 [DOI] [PubMed] [Google Scholar]
- Corbit K.C, Foster D.A, Rosner M.R. Protein kinase Cdelta mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol. Cell. Biol. 1999;19:4209–4218. doi: 10.1128/mcb.19.6.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, et al. Differential role of the low affinity neurotrophin receptor (p75) in retrograde axonal transport of the neurotrophins. Neuron. 1995;14:1201–1211. doi: 10.1016/0896-6273(95)90267-8. 10.1016/0896-6273(95)90267-8 [DOI] [PubMed] [Google Scholar]
- Davies A.M, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. 10.1038/326353a0 [DOI] [PubMed] [Google Scholar]
- Davies A.M, Lee K.F, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. 10.1016/0896-6273(93)90069-4 [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde Y.A. The neurotrophin receptor p75 (NTR): novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. 10.1038/nn1102-1131 [DOI] [PubMed] [Google Scholar]
- DeFreitas M.F, McQuillen P.S, Shatz C.J. A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J. Neurosci. 2001;21:5121–5129. doi: 10.1523/JNEUROSCI.21-14-05121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal P, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. 10.1126/science.1080049 [DOI] [PubMed] [Google Scholar]
- Delcroix J.D, Valletta J.S, Wu C, Hunt S.J, Kowal A.S, Mobley W.C. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. 10.1016/S0896-6273(03)00397-0 [DOI] [PubMed] [Google Scholar]
- Dobrowsky R.T, Werner M.H, Castellino A.M, Chao M.V, Hannun Y.A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Donovan M.J, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene. 1997;15:373–383. doi: 10.1038/sj.onc.1201214. 10.1038/sj.onc.1201214 [DOI] [PubMed] [Google Scholar]
- Du J, Feng L, Yang F, Lu B. Activity- and Ca (2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J. Cell Biol. 2000;150:1423–1434. doi: 10.1083/jcb.150.6.1423. 10.1083/jcb.150.6.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je H.S, Liu X.W, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J. Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. 10.1083/jcb.200305134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M.F, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. 10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- Eide F.F, Vining E.R, Eide B.L, Zang K, Wang X.Y, Reichardt L.F. Naturally occurring truncated TrkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J. Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb M.H. New insights into the control of MAP kinase pathways. Exp. Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. 10.1006/excr.1999.4687 [DOI] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens R.M, Perez P, Chao M.V, Kaplan D.R, Hempstead B.L. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 2001;276:32 687–32 695. doi: 10.1074/jbc.M011674200. 10.1074/jbc.M011674200 [DOI] [PubMed] [Google Scholar]
- Esteban P.F, et al. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J. Cell Biol. 2006;173:291–299. doi: 10.1083/jcb.200512013. 10.1083/jcb.200512013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. 10.1016/S0960-9822(02)00658-9 [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol. Cell. Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. 10.1006/mcne.2001.1016 [DOI] [PubMed] [Google Scholar]
- Farhadi H.F, Mowla S.J, Petrecca K, Morris S.J, Seidah N.G, Murphy R.A. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J. Neurosci. 2000;20:4059–4068. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Yoshida C.K, Backus C, Reichardt L.F. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. 10.1016/S0896-6273(00)80240-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Wilkinson G.A, Backus C, Reichardt L.F, Patapoutian A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron. 1998;21:325–334. doi: 10.1016/s0896-6273(00)80542-5. 10.1016/S0896-6273(00)80542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko N.R, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837–5849. doi: 10.1038/sj.onc.1208737. 10.1038/sj.onc.1208737 [DOI] [PubMed] [Google Scholar]
- Frade J.M, Barde Y.A. Nerve growth factor: two receptors, multiple functions. Bioessays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. 10.1002/(SICI)1521-1878(199802)20:2%3C137::AID-BIES6%3E3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Garner A.S, Menegay H.J, Boeshore K.L, Xie X.Y, Voci J.M, Johnson J.E, Large T.H. Expression of TrkB receptor isoforms in the developing avian visual system. J. Neurosci. 1996;16:1740–1752. doi: 10.1523/JNEUROSCI.16-05-01740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten M.W. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol. Cell. 2005a;20:301–312. doi: 10.1016/j.molcel.2005.09.014. 10.1016/j.molcel.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Geetha T, Kenchappa R.S, Wooten M.W, Carter B.D. TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor. EMBO J. 2005b;24:3859–3868. doi: 10.1038/sj.emboj.7600845. 10.1038/sj.emboj.7600845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiton M, Gunn-Moore F.J, Glass D.J, Geis D.R, Yancopoulos G.D, Tavare J.M. Naturally occurring tyrosine kinase inserts block high affinity binding of phospholipase C gamma and Shc to TrkC and neurotrophin-3 signaling. J. Biol. Chem. 1995;270:20 384–20 390. doi: 10.1074/jbc.270.35.20384. 10.1074/jbc.270.35.20384 [DOI] [PubMed] [Google Scholar]
- Hallbook F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opin. Neurobiol. 1999;9:616–621. doi: 10.1016/S0959-4388(99)00011-2. 10.1016/S0959-4388(99)00011-2 [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Middleton G, Wyatt S, Jaffray E, Hay R.T, Davies A.M. p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol. Cell. Neurosci. 1999;14:28–40. doi: 10.1006/mcne.1999.0770. 10.1006/mcne.1999.0770 [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Goldberg T.E, Mattay V.S, Kolachana B.S, Callicott J.H, Egan M.F, Weinberger D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A.W, Kim J.Y, Yoon S.O. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J. Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A.W, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc. Natl Acad. Sci. USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. 10.1073/pnas.0305755101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.M, Jones M.E, Uecker S, Albers K.M, Kudrycki K.E, Davis B.M. Levels of nerve growth factor and neurotrophin-3 are affected differentially by the presence of p75 in sympathetic neurons in vivo. J. Comp. Neurol. 2000;424:99–110. doi: 10.1002/1096-9861(20000814)424:1<99::aid-cne8>3.0.co;2-j. 10.1002/1096-9861(20000814)424:1%3C99::AID-CNE8%3E3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- He X.L, Garcia K.C. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. 10.1126/science.1095190 [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Scott J, Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984;3:3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. 10.1083/jcb.104.6.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymach J.V, Jr, Krüttgen A, Suter U, Shooter E.M. The regulated secretion and vectorial targeting of neurotrophins in neuroendocrine and epithelial cells. J. Biol. Chem. 1996;271:25 430–25 437. doi: 10.1074/jbc.271.41.25430. 10.1074/jbc.271.41.25430 [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Moscatello D.K, Emlet D.R, Dieterich R, Wong A.J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl Acad. Sci. USA. 1997;94:12 419–12 424. doi: 10.1073/pnas.94.23.12419. 10.1073/pnas.94.23.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S. Small GTPase Rin induces neurite outgrowth through Rac/Cdc42 and calmodulin in PC12 cells. J. Cell Biol. 2003;163:1067–1076. doi: 10.1083/jcb.200308070. 10.1083/jcb.200308070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Yoshimori T, Nakamura S. Small GTPase proteins Rin and Rit Bind to PAR6 GTP-dependently and regulate cell transformation. J. Biol. Chem. 2005;280:22 868–22 874. doi: 10.1074/jbc.M411592200. 10.1074/jbc.M411592200 [DOI] [PubMed] [Google Scholar]
- Huang E.J, Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.J, Reichardt L.F. Trk receptors: roles in signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang C.S, Zhou J, Feng A.K, Lynch C.C, Klumperman J, DeArmond S.J, Mobley W.C. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J. Biol. Chem. 1999;274:36 707–36 714. doi: 10.1074/jbc.274.51.36707. 10.1074/jbc.274.51.36707 [DOI] [PubMed] [Google Scholar]
- Huang E.J, Liu W, Fritzsch B, Bianchi L.M, Reichardt L.F, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Thoenen H, Lindholm D. TrkA tyrosine residues involved in NGF-induced neurite outgrowth of PC12 cells. Eur. J. Neurosci. 1995;7:1125–1133. doi: 10.1111/j.1460-9568.1995.tb01102.x. 10.1111/j.1460-9568.1995.tb01102.x [DOI] [PubMed] [Google Scholar]
- Indo Y. Molecular basis of congenital insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum. Mutat. 2001;18:462–471. doi: 10.1002/humu.1224. 10.1002/humu.1224 [DOI] [PubMed] [Google Scholar]
- Jacobson M.D, Weil M, Raff M.C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. 10.1016/S0092-8674(00)81873-5 [DOI] [PubMed] [Google Scholar]
- Kanamoto T, Mota M, Takeda K, Rubin L.L, Miyazono K, Ichijo H, Bazenet C.E. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol. Cell. Biol. 2000;20:196–204. doi: 10.1128/mcb.20.1.196-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Jaiswal R.K, Kolch W, Landreth G.E. Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 2001;276:18 169–18 177. doi: 10.1074/jbc.M008870200. 10.1074/jbc.M008870200 [DOI] [PubMed] [Google Scholar]
- Kaplan D.R, Miller F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. 10.1016/S0959-4388(00)00092-1 [DOI] [PubMed] [Google Scholar]
- Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. 10.1038/nature01817 [DOI] [PubMed] [Google Scholar]
- Kenchappa R.S, Zampieri N, Chao M.V, Barker P.A, Teng H.K, Hempstead B.L, Carter B.D. Ligand-dependent cleavage of the p75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. 10.1016/j.neuron.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Kermani P, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. 10.1172/JCI200522655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursigara G, Orlinick J.R, Chao M.V. Association of the p75 neurotrophin receptor with TRAF6. J. Biol. Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. 10.1074/jbc.274.5.2597 [DOI] [PubMed] [Google Scholar]
- Kim A, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum M, Chao M. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697. doi: 10.1016/s0896-6273(02)00821-8. 10.1016/S0896-6273(02)00821-8 [DOI] [PubMed] [Google Scholar]
- Kim A.H, Sasaki T, Chao M.V. JNK-interacting protein 1 promotes Akt1 activation. J. Biol. Chem. 2003;278:29 830–29 836. doi: 10.1074/jbc.M305349200. 10.1074/jbc.M305349200 [DOI] [PubMed] [Google Scholar]
- Kimball S.R, Farrell P.A, Jefferson L.S. Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J. Appl. Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Klein M, Hempstead B.L, Teng K.K. Activation of STAT5-dependent transcription by the neurotrophin receptor Trk. J. Neurobiol. 2005;63:159–171. doi: 10.1002/neu.20124. 10.1002/neu.20124 [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Fukuda H, Chin W.W. Promoter-specific regulation of the brain-derived neurotropic factor gene by thyroid hormone in the developing rat cerebellum. Endocrinology. 1999;140:3955–3961. doi: 10.1210/endo.140.9.6997. 10.1210/en.140.9.3955 [DOI] [PubMed] [Google Scholar]
- Kong H, Boulter J, Weber J.L, Lai C, Chao M.V. An evolutionarily conserved transmembrane protein that is a novel downstream target of neurotrophin and ephrin receptors. J. Neurosci. 2001;21:176–185. doi: 10.1523/JNEUROSCI.21-01-00176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc. Natl Acad. Sci. USA. 1983;80:3513–3516. doi: 10.1073/pnas.80.11.3513. 10.1073/pnas.80.11.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C.Y, Yang D.D, Samanta Roy D.R, Davis R.J, Rakic P, Flavell R.A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. 10.1016/S0896-6273(00)80727-8 [DOI] [PubMed] [Google Scholar]
- Kuan C.Y, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl Acad. Sci. USA. 2003;100:15 184–15 189. doi: 10.1073/pnas.2336254100. 10.1073/pnas.2336254100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel L.S, Glebova N.O, Lonze B.E, Valdez G, Ye H, Ginty D.D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. 10.1016/j.cell.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Lee K.F, Li E, Huber L.J, Landis S.C, Sharpe A.H, Chao M.V, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. 10.1016/0092-8674(92)90286-L [DOI] [PubMed] [Google Scholar]
- Lee K.F, Bachman K, Landis S, Jaenisch R. Dependence on p75 for innervation of some sympathetic targets. Science. 1994;263:1447–1449. doi: 10.1126/science.8128229. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng K.K, Hempstead B.L. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. 10.1126/science.1065057 [DOI] [PubMed] [Google Scholar]
- Lei L, Laub F, Lush M, Romero M, Zhou J, Luikart B, Klesse L, Ramirez F, Parada L.F. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev. 2005;19:1354–1364. doi: 10.1101/gad.1227705. 10.1101/gad.1227705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lewin G.R, Barde Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. 10.1146/annurev.ne.19.030196.001445 [DOI] [PubMed] [Google Scholar]
- Li H.S, Xu X.Z, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. 10.1016/S0896-6273(00)80838-7 [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Ilag L.L, Otting G, Ibanez C.F. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. 10.1093/emboj/16.16.4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.I, Das I, Schwartz G.M, Tsoulfas P, Mikawa T, Hempstead B.L. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev. Biol. 2000;226:180–191. doi: 10.1006/dbio.2000.9850. 10.1006/dbio.2000.9850 [DOI] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. 10.1038/330658a0 [DOI] [PubMed] [Google Scholar]
- Linggi M.S, Burke T.L, Williams B.B, Harrington A, Kraemer R, Hempstead B.L, Yoon S.O, Carter B.D. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J. Biol. Chem. 2005;280:13 801–13 808. doi: 10.1074/jbc.M410435200. 10.1074/jbc.M410435200 [DOI] [PubMed] [Google Scholar]
- Lou H, Kim S.K, Zaitsev E, Snell C.R, Lu B, Loh Y.P. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. 10.1016/j.neuron.2004.12.037 [DOI] [PubMed] [Google Scholar]
- Lu B, Pang P.T, Woo N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- Ma X.M, Huang J.P, Eipper B.A, Mains R.E. Expression of Trio, a member of the Dbl family of Rho GEFs in the developing rat brain. J. Comp. Neurol. 2005;482:333–348. doi: 10.1002/cne.20404. 10.1002/cne.20404 [DOI] [PubMed] [Google Scholar]
- MacDonald J.I, Gryz E.A, Kubu C.J, Verdi J.M, Meakin S.O. Direct binding of the signaling adapter protein Grb2 to the activation loop tyrosines on the nerve growth factor receptor tyrosine kinase, TrkA. J. Biol. Chem. 2000;275:18 225–18 233. doi: 10.1074/jbc.M001862200. 10.1074/jbc.M001862200 [DOI] [PubMed] [Google Scholar]
- MacPhee I.J, Barker P.A. Brain-derived neurotrophic factor binding to the p75 neurotrophin receptor reduces TrkA signaling while increasing serine phosphorylation in the TrkA intracellular domain. J. Biol. Chem. 1997;272:23 547–23 551. doi: 10.1074/jbc.272.38.23547. 10.1074/jbc.272.38.23547 [DOI] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao M.V, Hempstead B.L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- Majdan M, et al. Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J. Neurosci. 1997;17:6988–6998. doi: 10.1523/JNEUROSCI.17-18-06988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkerh J.P, Ceni C, Auld D.S, Vaillancourt F, Dorval G, Barker P.A. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6:936–941. doi: 10.1038/sj.embor.7400503. 10.1038/sj.embor.7400503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion R.J, et al. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl Acad. Sci. USA. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. 10.1073/pnas.96.16.9385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni I.E, Said F.A, Aloyz R, Miller F.D, Kaplan D. Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway. J. Neurosci. 1999;19:9716–9727. doi: 10.1523/JNEUROSCI.19-22-09716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald N.Q, Lapatto R, Murray-Rust J, Gunning J, Wlodawer A, Blundell T.L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991;354:411–414. doi: 10.1038/354411a0. 10.1038/354411a0 [DOI] [PubMed] [Google Scholar]
- Meakin S.O, Suter U, Drinkwater C.C, Welcher A.A, Shooter E.M. The rat trk protooncogene product exhibits properties characteristic of the slow nerve growth factor receptor. Proc. Natl Acad. Sci. USA. 1992;89:2374–2378. doi: 10.1073/pnas.89.6.2374. 10.1073/pnas.89.6.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin S.O, Gryz E.A, MacDonald J.I. A kinase insert isoform of rat TrkA supports nerve growth factor-dependent cell survival but not neurite outgrowth. J. Neurochem. 1997;69:954–967. doi: 10.1046/j.1471-4159.1997.69030954.x. [DOI] [PubMed] [Google Scholar]
- Meakin S.O, MacDonald J.I, Gryz E.A, Kubu C.J, Verdi J.M. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J. Biol. Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. 10.1074/jbc.274.14.9861 [DOI] [PubMed] [Google Scholar]
- Medina D.L, Sciarretta C, Calella A.M, Von Bohlen Und Halbach O, Unsicker K, Minichiello L. TrkB regulates neocortex formation through the Shc/PLCgamma-mediated control of neuronal migration. EMBO J. 2004;23:3803–3814. doi: 10.1038/sj.emboj.7600399. 10.1038/sj.emboj.7600399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson G.A, Kruttgen A, Hu M, Munro E, Hanson M.G, Jr, Reichardt L.F, Barres B.A. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. 10.1016/S0896-6273(00)80586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. 10.1038/nn1188 [DOI] [PubMed] [Google Scholar]
- Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffray E, Hay R.T, Davies A.M. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J. Cell Biol. 2000;148:325–332. doi: 10.1083/jcb.148.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J, Digicaylioglu M, Legg A.T, Young C.E, Young S.S, Barr A.M, Fletcher L, O'Connor T.P, Dedhar S. Role of integrin-linked kinase in nerve growth factor-stimulated neurite outgrowth. J. Neurosci. 2003;23:1638–1648. doi: 10.1523/JNEUROSCI.23-05-01638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Casagranda F, Tatche R.S, Stucky C.L, Postigo A, Lewin G.R, Davies A.M, Klein R. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron. 1998;21:335–345. doi: 10.1016/s0896-6273(00)80543-7. 10.1016/S0896-6273(00)80543-7 [DOI] [PubMed] [Google Scholar]
- Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. 10.1016/S0896-6273(00)80853-3 [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella A.M, Medina D.L, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. 10.1016/S0896-6273(02)00942-X [DOI] [PubMed] [Google Scholar]
- Mowla S.J, Pareek S, Farhadi H.F, Petrecca K, Fawcett J.P, Seidah N.G, Morris S.J, Sossin W.S, Murphy R.A. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla S.J, Farhadi H.F, Pareek S, Atwal J.K, Morris S.J, Seidah N.G, Murphy R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001;276:12 660–12 666. doi: 10.1074/jbc.M008104200. 10.1074/jbc.M008104200 [DOI] [PubMed] [Google Scholar]
- Muller G, Storz P, Bourteele S, Doppler H, Pfizenmaier K, Mischak H, Philipp A, Kaiser C, Kolch W. Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J. 1998;17:732–742. doi: 10.1093/emboj/17.3.732. 10.1093/emboj/17.3.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.S, Perez P, Lee R, Hempstead B.L, Chao M.V. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. J. Neurosci. 2004;24:2742–2749. doi: 10.1523/JNEUROSCI.3960-03.2004. 10.1523/JNEUROSCI.3960-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual A.S, Yatsula B.A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. 10.1126/science.279.5350.560 [DOI] [PubMed] [Google Scholar]
- Nykjaer A, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. 10.1038/nature02319 [DOI] [PubMed] [Google Scholar]
- Ouwens D.M, et al. Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras–MEK–ERK pathway and of Thr69 through RalGDS–Src–p38. EMBO J. 2002;21:3782–3793. doi: 10.1093/emboj/cdf361. 10.1093/emboj/cdf361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P.T, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. 10.1126/science.1100135 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt L.F. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. 10.1016/S0959-4388(00)00208-7 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Backus C, Kispert A, Reichardt L.F. Regulation of neurotrophin-3 expression by epithelial-mesenchymal interactions: the role of Wnt factors. Science. 1999;283:1180–1183. doi: 10.1126/science.283.5405.1180. 10.1126/science.283.5405.1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T.D, Jackman A, Rice F.L, Kucera J, Snider W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. 10.1016/S0896-6273(00)80899-5 [DOI] [PubMed] [Google Scholar]
- Patterson S.L, Abel T, Deuel T.A, Martin K.C, Rose J.C, Kandel E.R. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. 10.1016/S0896-6273(00)80140-3 [DOI] [PubMed] [Google Scholar]
- Paul C.E, Vereker E, Dickson K.M, Barker P.A. A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J. Neurosci. 2004;24:1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. 10.1523/JNEUROSCI.5397-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B.E, Karandikar M, Berman K, Cobb M.H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. 10.1210/er.22.2.153 [DOI] [PubMed] [Google Scholar]
- Pedraza C.E, Podlesniy P, Vidal N, Arevalo J.C, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR. Am. J. Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.A, Dickinson-Anson H.A, Leppert J.T, Lee K.F, Gage F.H. Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. J. Comp. Neurol. 1999;404:1–20. doi: 10.1002/(sici)1096-9861(19990201)404:1<1::aid-cne1>3.0.co;2-#. 10.1002/(SICI)1096-9861(19990201)404:1%3C1::AID-CNE1%3E3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Poo M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. 10.1038/35049004 [DOI] [PubMed] [Google Scholar]
- Prescott E.D, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. 10.1126/science.1083646 [DOI] [PubMed] [Google Scholar]
- Prieto-Sanchez R.M, Bustelo X.R. Structural basis for the signaling specificity of RhoG and Rac1 GTPases. J. Biol. Chem. 2003;278:37 916–37 925. doi: 10.1074/jbc.M301437200. 10.1074/jbc.M301437200 [DOI] [PubMed] [Google Scholar]
- Qian X, Ginty D.D. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 2001;21:1613–1620. doi: 10.1128/MCB.21.5.1613-1620.2001. 10.1128/MCB.21.5.1613-1620.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Riccio A, Zhang Y, Ginty D.D. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017–1029. doi: 10.1016/s0896-6273(00)80620-0. 10.1016/S0896-6273(00)80620-0 [DOI] [PubMed] [Google Scholar]
- Robinson R.C, Radziejewski C, Stuart D.I, Jones E.Y. Structure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimer. Biochemistry. 1995;34:4139–4146. doi: 10.1021/bi00013a001. 10.1021/bi00013a001 [DOI] [PubMed] [Google Scholar]
- Robinson K.N, Manto K, Buchsbaum R.J, MacDonald J.I, Meakin S.O. Neurotrophin-dependent tyrosine phosphorylation of Ras guanine-releasing factor 1 and associated neurite outgrowth is dependent on the HIKE domain of TrkA. J. Biol. Chem. 2005;280:225–235. doi: 10.1074/jbc.M410454200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Barde Y.A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. 10.1016/0896-6273(90)90107-Q [DOI] [PubMed] [Google Scholar]
- Roosen A, et al. Lack of neurotrophin-4 causes selective structural and chemical deficits in sympathetic ganglia and their preganglionic innervation. J. Neurosci. 2001;21:3073–3084. doi: 10.1523/JNEUROSCI.21-09-03073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C.R, Blum R, Pichler B, Lepier A, Kafitz K.W, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. 10.1038/nature01983 [DOI] [PubMed] [Google Scholar]
- Saragovi H.U, et al. A TrkA-selective, fast internalizing nerve growth factor-antibody complex induces trophic but not neuritogenic signals. J. Biol. Chem. 1998;273:34 933–34 940. doi: 10.1074/jbc.273.52.34933. 10.1074/jbc.273.52.34933 [DOI] [PubMed] [Google Scholar]
- Scapoli L, Ramos-Nino M.E, Martinelli M, Mossman B.T. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–813. doi: 10.1038/sj.onc.1207163. 10.1038/sj.onc.1207163 [DOI] [PubMed] [Google Scholar]
- Schober A, et al. TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla. J. Neurosci. 1998;18:7272–7284. doi: 10.1523/JNEUROSCI.18-18-07272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N.G, et al. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem. J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M.J, Edwards R, Sharp F, Rutter W.J. Mouse nerve growth factor gene: structure and expression. Mol. Cell. Biol. 1987;7:3057–3064. doi: 10.1128/mcb.7.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. 10.1016/j.neuron.2004.12.050 [DOI] [PubMed] [Google Scholar]
- Shelton D.L, Sutherland J, Gripp J, Camerato T, Armanini M.P, Phillips H.S, Carroll K, Spencer S.D, Levinson A.D. Human trks: molecular cloning, tissue distribution, expression of extracellular domain immunoadhesins. J. Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G.X, Andres D.A. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol. Cell. Biol. 2005;25:830–846. doi: 10.1128/MCB.25.2.830-846.2005. 10.1128/MCB.25.2.830-846.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G.X, Han J, Andres D.A. Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J. Biol. Chem. 2005;280:37 599–37 609. doi: 10.1074/jbc.M507364200. 10.1074/jbc.M507364200 [DOI] [PubMed] [Google Scholar]
- Shooter E.M. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 2001;24:601–629. doi: 10.1146/annurev.neuro.24.1.601. 10.1146/annurev.neuro.24.1.601 [DOI] [PubMed] [Google Scholar]
- Sini P, Cannas A, Koleske A.J, Di Fiore P.P, Scita G. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat. Cell. Biol. 2004;6:268–274. doi: 10.1038/ncb1096. 10.1038/ncb1096 [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V, Howe C.L, Mobley W.C. Nerve growth factor signaling, neuroprotection, neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. 10.1146/annurev.neuro.24.1.1217 [DOI] [PubMed] [Google Scholar]
- Song M.S, Posse de Chaves E.I. Inhibition of rat sympathetic neuron apoptosis by ceramide. Role of p75NTR in ceramide generation. Neuropharmacology. 2003;45:1130–1150. doi: 10.1016/s0028-3908(03)00284-3. 10.1016/S0028-3908(03)00284-3 [DOI] [PubMed] [Google Scholar]
- Spencer M.L, Shao H, Tucker H.M, Andres D.A. Nerve growth factor-dependent activation of the small GTPase Rin. J. Biol. Chem. 2002;277:17 605–17 615. doi: 10.1074/jbc.M111400200. 10.1074/jbc.M111400200 [DOI] [PubMed] [Google Scholar]
- Strohmaier C, Carter B.D, Urfer R, Barde Y.A, Dechant G. A splice variant of the neurotrophin receptor TrkB with increased specificity for brain-derived neurotrophic factor. EMBO J. 1996;15:3332–3337. [PMC free article] [PubMed] [Google Scholar]
- Stucky C.L, Koltzenburg M. The low-affinity neurotrophin receptor p75 regulates the function but not the selective survival of specific subpopulations of sensory neurons. J. Neurosci. 1997;17:4398–4405. doi: 10.1523/JNEUROSCI.17-11-04398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold D.B, Shaywitz A.J, Greenberg M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. 10.1016/S0896-6273(00)81010-7 [DOI] [PubMed] [Google Scholar]
- Tao X, West A.E, Chen W.G, Corfas G, Greenberg M.E. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. 10.1016/S0896-6273(01)00561-X [DOI] [PubMed] [Google Scholar]
- Teng H.K, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. 10.1523/JNEUROSCI.5123-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H, Barde Y.A. Physiology of nerve growth factor. Physiol. Rev. 1980;60:1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral J.J, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. 10.1016/0896-6273(95)90317-8 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C.D. Mechanisms of estrogen action during neural development: mediation by interactions with the neurotrophins and their receptors? J. Steroid Biochem. Mol. Biol. 1996;56:169–178. doi: 10.1016/0960-0760(95)00234-0. 10.1016/0960-0760(95)00234-0 [DOI] [PubMed] [Google Scholar]
- Ultsch M.H, Wiesmann C, Simmons L.C, Henrich J, Yang M, Reilly D, Bass S.H, de Vos A.M. Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J. Mol. Biol. 1999;290:149–159. doi: 10.1006/jmbi.1999.2816. 10.1006/jmbi.1999.2816 [DOI] [PubMed] [Google Scholar]
- Urfer R, Tsoulfas P, O'Connell L, Hongo J.A, Zhao W, Presta L.G. High resolution mapping of the binding site of TrkA for nerve growth factor and TrkC for neurotrophin-3 on the second immunoglobulin-like domain of the Trk receptors. J. Biol. Chem. 1998;273:5829–5840. doi: 10.1074/jbc.273.10.5829. 10.1074/jbc.273.10.5829 [DOI] [PubMed] [Google Scholar]
- Vaillant A.R, Mazzoni I, Tudan C, Boudreau M, Kaplan D.R, Miller F.D. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J. Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. 10.1083/jcb.146.5.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Akmentin W, Philippidou P, Kuruvilla R, Ginty D.D, Halegoua S. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J. Neurosci. 2005;25:5236–5247. doi: 10.1523/JNEUROSCI.5104-04.2005. 10.1523/JNEUROSCI.5104-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas M.L, Mamidipudi V, Lamar Seibenhener M, Wooten M.W. Nerve growth factor activates kinases that phosphorylate atypical protein kinase C. Cell. Signal. 2002;14:359–363. doi: 10.1016/s0898-6568(01)00261-3. 10.1016/S0898-6568(01)00261-3 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers S.J, Ahmadi K, Timms J, Katso R, Driscoll P.C, Woscholski R, Parker P.J, Waterfield M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. 10.1146/annurev.biochem.70.1.535 [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat. Neurosci. 2001;4:977–978. doi: 10.1038/nn730. 10.1038/nn730 [DOI] [PubMed] [Google Scholar]
- Wang K.C, Kim J.A, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. 10.1038/nature01176 [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol. Cell. Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. 10.1128/MCB.25.1.336-345.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F.L, Heerssen H.M, Bhattacharyya A, Klesse L, Lin M.Z, Segal R.A. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 2001;4:981–988. doi: 10.1038/nn720. 10.1038/nn720 [DOI] [PubMed] [Google Scholar]
- Wiesmann C, de Vos A.M. Nerve growth factor: structure and function. Cell. Mol. Life Sci. 2001;58:748–759. doi: 10.1007/PL00000898. 10.1007/PL00000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann C, Ultsch M.H, Bass S.H, de Vos A.M. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature. 1999;401:184–188. doi: 10.1038/43705. 10.1038/43705 [DOI] [PubMed] [Google Scholar]
- Wong S.T, Henley J.R, Kanning K.C, Huang K.H, Bothwell M, Poo M.M. A p75 (NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. 10.1038/nn975 [DOI] [PubMed] [Google Scholar]
- Woo N.H, Teng H.K, Siao C.J, Chiaruttini C, Pang P.T, Milner T.A, Hempstead B.L, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. 10.1038/nn1510 [DOI] [PubMed] [Google Scholar]
- Wooten M.W, Seibenhener M.L, Mamidipudi V, Diaz-Meco M.T, Barker P.A, Moscat J. The atypical protein kinase C-interacting protein p62 is a scaffold for NF-kappaB activation by nerve growth factor. J. Biol. Chem. 2001;276:7709–7712. doi: 10.1074/jbc.C000869200. 10.1074/jbc.C000869200 [DOI] [PubMed] [Google Scholar]
- Wu C, Lai C.F, Mobley W.C. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 2001;21:5406–5416. doi: 10.1523/JNEUROSCI.21-15-05406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kornhauser J.M, Xia Z, Thiele E.A, Greenberg M.E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B.E, Stippec S, Lenertz L, Lee B.H, Zhang W, Lee Y.K, Cobb M.H. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J. Biol. Chem. 2004;279:7826–7831. doi: 10.1074/jbc.M313465200. 10.1074/jbc.M313465200 [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohnishi H, Sano S, Nakatani A, Ikeuchi T, Hatanaka H. Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine-phosphorylated and associated with phosphatidylinositol 3-kinase in response to brain-derived neurotrophic factor in cultured cerebral cortical neurons. J. Biol. Chem. 1997;272:30 334–30 339. doi: 10.1074/jbc.272.48.30334. 10.1074/jbc.272.48.30334 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tucker K.L, Barde Y.A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. 10.1016/S0896-6273(00)81114-9 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Fujitani M, Hata K, Mimura F, Yamagishi S. Diverse functions of the p75 neurotrophin receptor. Anat. Sci. Int. 2005;80:37–41. doi: 10.1111/j.1447-073x.2005.00095.x. 10.1111/j.1447-073x.2005.00095.x [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Chan J.R, Miyamoto Y, Tsujimoto G, Shooter E.M. The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration. Proc. Natl Acad. Sci. USA. 2005a;102:5198–5203. doi: 10.1073/pnas.0501160102. 10.1073/pnas.0501160102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Tanoue A, Shooter E.M, Chan J.R. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc. Natl Acad. Sci. USA. 2005b;102:14 889–14 894. doi: 10.1073/pnas.0507125102. 10.1073/pnas.0507125102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Cong F, Birge R.B, Goff S.P, Chao M.V. Association of the Abl tyrosine kinase with the Trk nerve growth factor receptor. J. Neurosci. Res. 2000;59:356–364. doi: 10.1002/(sici)1097-4547(20000201)59:3<356::aid-jnr9>3.0.co;2-g. 10.1002/(SICI)1097-4547(20000201)59:3%3C356::AID-JNR9%3E3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- Ye H, Kuruvilla R, Zweifel L.S, Ginty D.D. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. 10.1016/S0896-6273(03)00266-6 [DOI] [PubMed] [Google Scholar]
- Yee C.L, Jones K.R, Finger T.E. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J. Comp. Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. 10.1002/cne.10589 [DOI] [PubMed] [Google Scholar]
- Yeiser E.C, Rutkoski N.J, Naito A, Inoue J, Carter B.D. Neurotrophin signaling through the p75 receptor is deficient in traf6−/− mice. J. Neurosci. 2004;24:10 521–10 529. doi: 10.1523/JNEUROSCI.1390-04.2004. 10.1523/JNEUROSCI.1390-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G.S, Connie Hung C.C, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O'Rahilly S, Farooqi I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. 10.1038/nn1336 [DOI] [PubMed] [Google Scholar]
- Yoon S.O, Casaccia-Bonnefil P, Carter B, Chao M.V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York R.D, Molliver D.C, Grewal S.S, Stenberg P.E, McCleskey E.W, Stork P.J. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol. Cell. Biol. 2000;20:8069–8083. doi: 10.1128/mcb.20.21.8069-8083.2000. 10.1128/MCB.20.21.8069-8083.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner B.A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. 10.1038/35037739 [DOI] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003a;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. 10.1016/S0896-6273(03)00601-9 [DOI] [PubMed] [Google Scholar]
- Yuan X.B, Jin M, Xu X, Song Y.Q, Wu C.P, Poo M.M, Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003b;5:38–45. doi: 10.1038/ncb895. 10.1038/ncb895 [DOI] [PubMed] [Google Scholar]
- Zakharenko S.S, Patterson S.L, Dragatsis I, Zeitlin S.O, Siegelbaum S.A, Kandel E.R, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. 10.1016/S0896-6273(03)00543-9 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moheban D.B, Conway B.R, Bhattacharyya A, Segal R.A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. 10.1038/sj.emboj.7600893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.Q, Zhou J, Dedhar S, Wu Y.H, Snider W.D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. 10.1016/j.neuron.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Zundel W, Swiersz L.M, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol. Cell. Biol. 2000;20:1507–1514. doi: 10.1128/mcb.20.5.1507-1514.2000. 10.1128/MCB.20.5.1507-1514.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]