Abstract
Although many biochemical, morphological and physiological processes in the vertebrate retina are controlled by a circadian (24 h) clock, the location of the clock and how the clock alters retinal function are unclear. For instance, several observations have suggested that dopamine, a retinal neuromodulator, may play an important role in retinal rhythmicity but the link between dopamine and a clock located within or outside the retina remains to be established. We found that endogenous dopamine release from isolated goldfish retinae cultured in continuous darkness for 56 h clearly exhibited a circadian rhythm with high values during the subjective day. The continuous presence of melatonin (1 nm) in the culture medium abolished the circadian rhythm of dopamine release and kept values constantly low and equal to the night-time values. The selective melatonin antagonist luzindole (1 μm) also abolished the dopamine rhythm but the values were high and equal to the daytime values. Melatonin application during the late subjective day introduced rod input and reduced cone input to fish cone horizontal cells, a state usually observed during the subjective night. In contrast, luzindole application during the subjective night decreased rod input and increased cone input. Prior application of dopamine or spiperone, a selective dopamine D2-like antagonist, blocked the above effects of melatonin and luzindole, respectively. These findings indicate that a circadian clock in the vertebrate retina regulates dopamine release by the activation of melatonin receptors and that endogenous melatonin modulates rod and cone pathways through dopamine-mediated D2-like receptor activation.
Virtually all organisms exhibit daily rhythms in physiology and behaviour that are shaped not only by external environmental stimuli, such as the daily light/dark cycle, but also by endogenous circadian (24 h) clocks. In vertebrates, circadian clocks have been localized to central and peripheral structures (Whitmore et al. 1998; Yamazaki et al. 2000; Abe et al. 2002), including the retina (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996). Although the molecular mechanisms that constitute clocks have recently been elucidated in detail (Panda et al. 2002), the means by which these clocks achieve their effects in the central nervous system (CNS) remain unclear.
In the vertebrate retina, a variety of biochemical, morphological and physiological processes, such as melatonin production, dopamine release and/or content, photoreceptor disc shedding, gene expression, extracellular pH and neuronal activity exhibit circadian rhythms (Cahill & Besharse, 1995; Wang & Mangel, 1996; Dmitriev & Mangel, 2000; Mangel, 2001; Ribelayga et al. 2002). Although some of these rhythmic retinal phenomena are controlled by a clock located within the retina, others may reflect the influence of extra-retinal clocks. In fact, recent work has suggested that the clock located in the suprachiasmatic nucleus regulates the expression of the clock gene period2 in the retina, but not the expression of the gene that encodes serotonin N-acetyltransferase, a key regulatory enzyme in melatonin synthesis (Sakamoto et al. 2000). Formal demonstration that the retinal clock directly regulates a rhythmic retinal process requires showing that the process in question exhibits a circadian rhythm over the course of two full circadian cycles when the retina is maintained in vitro under constant environmental conditions and in isolation from the rest of the CNS (Takahashi et al. 2001). Such a demonstration has so far been achieved only for a few rhythmic processes in the retina, such as the rhythmic production of the neurohormone melatonin (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996), the rhythmic expression of the cone photopigment (iodopsin) gene (Pierce et al. 1993) in the chick retina, and the regulation of cGMP-gated cationic channels of chick cones (Ko et al. 2001).
Extensive work has shown that the retinal clock regulates melatonin synthesis and release in most, if not all, vertebrate retinae. Due to the action of the retinal clock, the production and release of melatonin is increased at night (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996; Iigo et al. 1997b). Although these findings have clearly identified melatonin as a primary neurohormonal output of the retinal clock, the exact functional role of the clock-mediated nocturnal increase in melatonin release in the retina has remained elusive. Because melatonin can acutely inhibit dopamine release (Dubocovich, 1983; Boatright et al. 1994; Behrens et al. 2000), it has been suggested that the primary means by which melatonin alters retinal function is through dopaminergic pathways (Cahill & Besharse, 1995; Iuvone, 1995). However, attempts to confirm in vivo whether the rhythmic release of melatonin regulates the rhythm in dopamine have reported conflicting results (e.g. Doyle et al. 2002a,b). In addition, dopamine itself can acutely suppress the rhythmic production of melatonin (Cahill & Besharse, 1991; Tosini & Dirden, 2000), suggesting that the circadian-mediated interactions between melatonin and dopamine and the functional role of melatonin are not clear.
In contrast to melatonin, the functional role of dopamine in the retina is better understood. Dopamine mediates both light adaptation and the effects of a circadian clock. Dopamine release (and/or content) is higher during the subjective day due to the action of a circadian clock (Wirz-Justice et al. 1984; Ribelayga et al. 2002; Zawilska et al. 2003) and endogenous dopamine mediates the effects of the clock on neuronal activity in the retina (Manglapus et al. 1999; Ribelayga et al. 2002). However, the location of the clock that regulates the activity of dopaminergic neurones is still unknown since evidence of long-term circadian rhythms in dopamine release from in vitro retinae have not been reported to date. That is, to show that the circadian clock controlling dopamine release is located in the retina, one must demonstrate that a circadian rhythm of dopamine release occurs in isolated retinae cultured in vitro for several days. In addition, to show that the retinal rhythm in dopamine is related to the retinal rhythm in melatonin, one must demonstrate that both chronic stimulation and chronic blockade of endogenous melatonin receptors eliminate the circadian rhythm of dopamine.
To determine whether and how a circadian clock located in the retina regulates dopamine release and to determine the functional role of melatonin in the retina, we used two complementary approaches: direct measurement of endogenous dopamine release from fish neural retina maintained in culture for several days and electrophysiological recordings from fish cone horizontal cells. We report here that a circadian clock in the fish retina modulates endogenous dopamine release, that the clock uses melatonin to generate the dopamine rhythm, and that melatonin affects the light responses of cone horizontal cells through dopamine and D2-like receptor activation.
Methods
Preparation
Experiments were conducted on common goldfish (Carassius auratus) approximately 15 cm in length supplied by Ozark Fisheries, Inc. (Stoutland, MO, USA). The care and use of the fish followed all guidelines of the National Institutes of Health and the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Fish were maintained at 22 ± 1°C on a 12 h light/12 h dark cycle (with ‘lights-on’ at 03:00 h) for at least 2 weeks before each experiment. Throughout this paper, ‘subjective day’ and ‘subjective night’ refer to the day and night of the imposed light/dark cycle, respectively, when animals or isolated retinae were maintained in constant darkness.
All surgical procedures were exclusively performed in total darkness (background illumination = −10 log Io) using infrared goggles (AN/PVS-5; Night Vision Equipment, Emmaus, PA, USA), as previously described (Wang & Mangel, 1996; Ribelayga et al. 2002). Animals were deeply anaesthetized with 3-aminobenzoic acid ethyl ester (methanesulphonate salt, 100 mg l−1; Sigma), then decapitated and pithed. The eyes were quickly enucleated and hemisected along the equatorial plane so that the anterior part of the eye was removed. The eyecup was then inverted onto filter paper (model 1004 090, Whatman), the optic nerve cut, and the sclera and pigment epithelium removed.
Culture conditions
Intact neural retinae were briefly immersed in culture medium to remove cell membrane debris and quickly placed into culture wells containing 2 ml of medium (24 well cell culture cluster, Costar, Corning Inc., Corning, NY, USA). The retinae were then maintained for 56 h in total darkness under a water-saturated, 5% CO2–95% O2 atmosphere (Fisher Isotemp Plus water-jacketed CO2 incubator, Fischer Scientific, Pittsburgh, PA, USA). The temperature was maintained at 20 ± 0.1°C by using an external circulating water bath (RTE-211, Nestlab Instruments, Inc., Portsmouth, NH, USA). The CO2 sensor was thermo-sensitive, so that it did not produce any light. The Ringer-based culture medium contained (mm): 115 NaCl, 2.5 KCl, 35 NaHCO3, 0.7 CaCl2, 1.0 MgSO4, 15 glucose, 0.5 NaH2PO4, 0.1 l-glutamate, and 0.1 ascorbic acid. This medium also contained 20 ml l−1 of 50X minimum essential medium (MEM) amino acids solution (without glutamine), 10 ml l−1 of a 100X MEM vitamin solution, and antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin). All supplements were from Atlanta Biologicals (Atlanta, GA, USA). The medium was equilibrated with 5% CO2–95% O2, the pH adjusted to 7.5, and filter-sterilized. The culture medium was completely changed every 4 h and the collected samples rapidly frozen. These samples were kept frozen at –80°C until the entire experiment was completed. All of the samples were then processed at the same time.
Dopamine determinations
Reverse-phase high performance liquid chromatography (HPLC) with electrochemical detection was used to determine the total content of dopamine from retinal homogenates and the amount of endogenous dopamine released into the culture medium from explanted retinae. The liquid chromatograph and detection system consisted of: a 515 HPLC pump (Waters, Milford, MA, USA), an automatic refrigerated injector (Agilent 1100 series, Agilent, Waldbronn, Germany), an adsorbsphere HS (C18) column (250 mm × 4.6 mm, 5 μm porous silica, Alltech, Deerfield, IL, USA), and an amperometric detector (Model LC-4C, BioAnalytical System (BAS), West Lafayette, IN, USA). The potential of the carbon working electrode was set to +750 mV and its range was 2 nA V−1. We used an isocratic mobile phase composed of 0.1 m KH2PO4, 0.0235% octyl sodium sulphate (ACROS Organics, NJ, USA), 0.1 mm Na2-EDTA, pH adjusted to 3.10 with a 85% solution of o-phosphoric acid (Fisher Scientific) before addition of 15% methanol (Fisher Scientific). The solution was then filtered on a membrane filter (pore size 0.2 μm) and degassed with helium. The mobile phase flow rate was set to 0.8 ml min−1 and the column temperature to 40°C so that the pressure in the column was around 1800 p.s.i. Standards were prepared in a solution of 50 mm Na2-EDTA–1 mm sodium metabisulfite (Na2S2O5). Prior to each experiment a standard curve was established using various concentrations ranging from 5 to 1000 pg per injection (30 μl). The output signal from the electrochemical detector was digitized (Model DA-5 A/D converter, BAS). Control of the DA-5 and analysis of the chromatograms were performed using computer software (ChromGraph DA-5 and ChromGraph Report, BAS). Analysed peaks were identified by relative retention times compared to those of external standards and were quantified on the basis of their peak areas.
To determine dopamine content in retinal homogenates, retinae were briefly sonicated in 400 μl of standard solution (50 mm Na2-EDTA–1 mm Na2S2O5) containing 3333 pg of 3,4-dihydroxybenzylamine (DHBA), used as an internal standard. Fifty microlitres of the homogenate was kept for protein assay and the remainder was centrifuged (30 min, 14 000 g, 4°C), filtered on a 0.2 μm pore size filter, and 30 μl (thus containing 250 pg DHBA) of each sample was directly injected into the HPLC system.
To determine the amount of endogenous dopamine released, dopamine was extracted and concentrated from the culture medium using aluminium oxide. Briefly, 10 mg of aluminium oxide (aluminium oxide activated 150 mesh, Aldrich, Milwaukee, WI, USA) and 130 μl of an extraction solution (containing 1 m Tris (pH 8.6), 0.1 m EDTA, 10 mm Na2S2O5 and 900 pg DHBA) was added to each 1.3 ml sample of culture medium. After 30 min of rotation at 4°C and centrifugation (5 min, 3000 g at 4°C), the supernatant was removed and the pellet washed 3 times with 1 ml of cold water. Thereafter, dopamine was resuspended in 150 μl 0.05 m perchloric acid (HClO4) (Fisher Scientific) and 100 μl of the aluminium-free solution was injected into the HPLC system. The average extraction recovery was 71.6 ± 0.5 % (mean ± s.e.m; n = 4) and was calculated for each run using DHBA (600 pg run−1) as an internal standard. The detection threshold of the HPLC system was between 5 and 10 pg run−1 as determined with standard solutions. The lowest values of dopamine content measured in the retinal homogenates were above 400 pg run−1 and thus 40 times the detection threshold. The lowest values measured in an extracted sample were above 50 pg run−1 and thus at least 5 times the detection threshold. Unless indicated, all of the HPLC compounds were purchased from Sigma (St Louis, MO, USA). In all of the HPLC experiments, drugs that affect dopamine metabolism, such as dopamine reuptake inhibitors, were not used. Thus, the in vitro data illustrated in Figs 2 and 3 are measurements of endogenous dopamine release (see Discussion for additional comments).
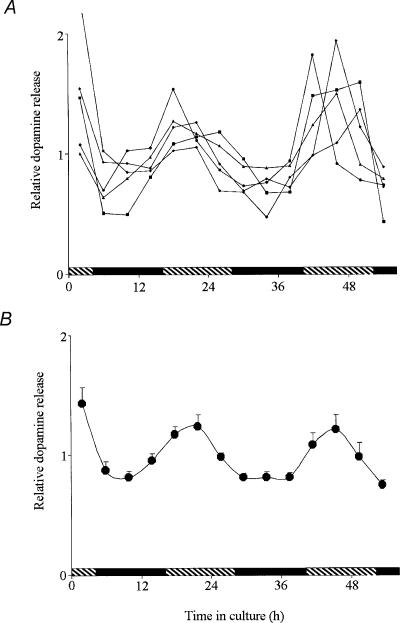
Figure 2. The clock that controls the circadian rhythm of dopamine release is located within the retina.
Isolated goldfish retinae were cultured in constant darkness and temperature for 56 h. The culture medium was changed every 4 h and dopamine present in the medium was extracted. Relative dopamine levels (dopamine content of each fraction divided by the mean) are shown for better comparison between different retinae. A, rhythms of dopamine release from 5 representative retinae from 3 separate experiments. B, mean dopamine release (± s.e.m.) from 14 retinae from 3 separate experiments as a function in time in the dark indicates that dopamine release was not constant over the course of 56 h (rm ANOVA, F13,195= 7.11; P < 0.01) and was greater during the subjective day, compared to the subjective night. Cosinor analysis (see Methods) of the data presented in B demonstrates that endogenous dopamine release under control conditions exhibited a circadian rhythm with a period of 23.3 ± 0.6 h (F3,195= 21.96; P < 0.01). Shaded and filled bars indicate the subjective day and night, respectively.
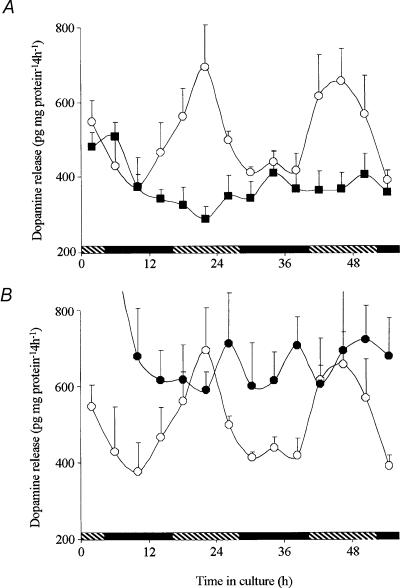
Figure 3. The retinal clock uses melatonin to control the circadian release of dopamine.
Isolated retinae were maintained for 56 h in total darkness and constant temperature in a culture medium containing no drugs, melatonin (1 nm) or luzindole (1 μm). A, continuous application of melatonin (1 nm; ▪) abolished the rhythm of dopamine release by decreasing daytime levels to the night-time values. B, continuous application of the selective melatonin antagonist luzindole (1 μm; •) abolished the rhythm of dopamine release by increasing the night-time values to the daytime levels. Each data point represents mean values ± s.e.m.. for 5 independent retinae. ○, positive control performed at the same time, but with no test drugs added. Shaded and filled bars indicate the subjective day and night, respectively. See Table 1 for cosinor analysis.
Electrical recording
Standard intracellular recording procedures were employed to record the light responses of fish l-type cone horizontal cells, as previously described (Wang & Mangel, 1996; Wang et al. 1997; Ribelayga et al. 2002). Intact, isolated retinae were placed in a chamber that had a volume of 0.5 ml and were superfused at 0.5 ml min−1 with a Ringer solution that contained (mm) 130 NaCl, 2.5 KCl, 20 NaHCO3, 0.7 CaCl2, 1.0 MgCl2, and 20 glucose, as previously described (Wang & Mangel, 1996; Wang et al. 1997; Ribelayga et al. 2002). Oxygenation of the superfusate with a mixture of 95% O2–5% CO2 maintained the superfusate at a pH of 7.6 in the retinal chamber. Retinae were dark-adapted for at least 1 h after excision, following which, horizontal cells were impaled without the aid of any light flashes. Responses of the cells to dim full-field white light flashes (ranging from –8 log Io to –5 log Io) were then recorded. Cone horizontal cells were identified by intracellular tracer injection, with spectral and intensity response curves, and by response waveform (Mangel & Dowling, 1987; Wang et al. 1997; Ribelayga et al. 2002). The maximum, unattenuated intensity (Io) of full field white light stimuli from a 100 W tungsten–halogen lamp was 2.0 × 103 μ W cm−2. Intensity values indicated in the text are relative to Io. Calibrated neutral density filters and narrow-band interference filters were used to control light intensity and stimulus wavelength, respectively.
Data analyses
All data are expressed as the mean ± s.e.m. of n values. The measured values of dopamine release and content were normalized to the protein content of each retina, following the method described by Lowry et al. (1951). However, due to the rhythmic nature of dopamine release, relative dopamine levels were calculated as the dopamine content of each fraction divided by the mean, a method used previously to calculate the rhythmic production of melatonin in the vertebrate retina (Cahill & Besharse, 1991; Hayasaka et al. 2002). In contrast, to estimate the effects of chronic application of melatonin and luzindole, absolute values of dopamine were used. To test whether dopamine release varies with the time of collection, statistical analysis was performed with a one-way repeated measurements analysis of variance (RM ANOVA) using SigmaStat 3.0 (SPSS Science, Chicago, IL, USA). To reveal the presence of a circadian rhythm and the characteristics of that rhythm, which cannot be determined by ANOVA, the data points were subjected to a non-linear regression analysis using SigmaPlot 8.0 (SPSS Science). To characterize the circadian rhythms, as has previously been done (Nelson et al. 1979; Minors & Waterhouse, 1988), individual values were fitted to the cosinor equation:
where y is the nth data point (pg dopamine released (mg protein)−1 4 h−1), x the time of the nth data point (h), M the mean (mesor; pg dopamine released (mg protein)−1 4 h−1), A the amplitude (equal to one-half of the sinusoid; pg dopamine released (mg protein)−1 4 h−1), B the acrophase (radians) and τ the endogenous period (h). The regression coefficients (M, A, B and τ) are given with their respective standard error estimates. Post hoc comparison of the regression coefficients was performed using the Student-Newman-Keuls multiple comparison test. For all analyses performed in the present study, P < 0.01 was considered significant.
Relative quantum sensitivity was determined as previously described (Naka & Rushton, 1966; Nussdorf & Powers, 1988; Wang & Mangel, 1996; Ribelayga et al. 2002). Data were normalized at the wavelength of peak sensitivity (550 or 600 nm). A 1 mV criterion response was used to minimize light sensitization of the dark-adapted state. ‘Light sensitization’ refers to the phenomenon in which bright light (photopic range) stimulation of dark-adapted retinae increases the size of cone horizontal cell light responses in the day and night (Baldridge et al. 1995) and eliminates rod input to the cells during the night (Wang & Mangel, 1996). Red (625 nm) cone spectral sensitivity data were obtained from Harosi & MacNichol (1974) and rod spectral sensitivity data were obtained from Schwanzara (1967). The maximum, unattenuated light intensity of the stimulus at 550 nm was 7.2 × 1013 photons cm−2 s−1.
Results
A circadian clock modulates rod and cone input to cone horizontal cells
A circadian clock regulates the light responses of goldfish retinal l-type cone horizontal (H1) cells (Fig. 1A), as shown previously (Wang & Mangel, 1996; Ribelayga et al. 2002). In the fish retina, cone horizontal cells are a type of second order neurone that receives synaptic contact from cones, but not from rods (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989). During the subjective day, the light responses of H1 cells are cone mediated and similar to classic responses previously reported for these cells (Naka & Rushton, 1966; Mangel & Dowling, 1987; Harsanyi et al. 1996). In contrast, during the subjective night, the responses are rod dominated. Compared to the day, the responses at night are slower, smaller in size, longer in duration, and response threshold is lower by approximately two log units. Moreover, the spectral sensitivity of the cells resembles that of red (625 nm) cones (Harosi & MacNichol, 1974) during the subjective day (see Fig. 5A below) and rods (Schwanzara, 1967) during the subjective night (Wang & Mangel, 1996; see Fig. 5B below). The identity of these cells during the subjective night when they are primarily rod driven has been conclusively established as that of H1 cells by intracellular tracer injection and by the size, spectral sensitivity and adaptability of their light responses (Wang & Mangel, 1996; Ribelayga et al. 2002).
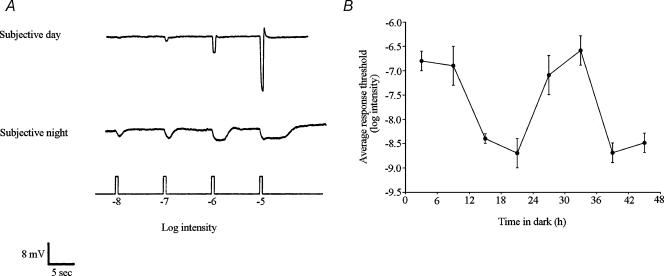
Figure 1. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells.
A, cone input to L-type cone horizontal (H1) cells predominates during the subjective day and rod input predominates during the subjective night. Compared to the day, the responses at night are slower, smaller in size, longer in duration, and response threshold is approximately two log units lower. Retinae were dark adapted for at least 1 h after excision, following which H1 cells were impaled without the aid of any light flashes. Responses of the cells to dim full-field white light flashes (ranging from –8 log Io to –5 log Io) were then recorded. The responses of two different cells are shown in the subjective day and night. Similar results were obtained from 52 other cells. B, average stimulus intensity that generated a threshold (1 mV) response from H1 cells as a function of time in the dark was lower in the subjective night than in the subjective day, indicating that a circadian clock regulates rod input to the cells. The presence of a recording from an H1 cell was confirmed following cell impalement by flashing a series of dim (<–6 log Io) lights. Following this, the threshold light response was determined by flashing full-field white lights (ranging from −9 log Io to −5 log Io; 500 ms duration at 0.1 Hz) in half log unit steps. Stimuli were presented in order of increasing intensity with stimuli presented three times at each intensity. The average response at each stimulus intensity was determined. Each data point represents the mean of the averages obtained from 6–9 cells and from at least four different retinae. If one of the light stimuli did not exactly generate a 1 mV threshold response, then the stimulus intensity that would have done so was later estimated from the stimulus–response data for each cell. Surgical isolation of each retina occurred approximately 2 h before threshold determination. Intensity values indicated in Figs 1, 4, 5 and 6 are relative to the maximum unattenuated intensity (Io) of full-field white light stimuli generated by the photostimulator (see Methods).
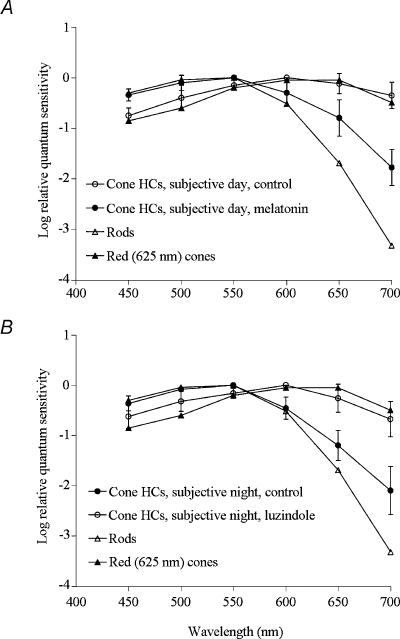
Figure 5. Endogenous activation of melatonin receptors mediates circadian clock regulation of rod and cone input to cone horizontal cells.
A, application of melatonin during the late subjective day (ZT 09, second cycle) altered the average spectral sensitivity of l-type cone horizontal cells to resemble that of goldfish rods (Schwanzara, 1967), rather than red (625 nm) cones (Harosi & MacNichol, 1974), for wavelengths ≤ 600 nm. B, in contrast, application of luzindole during the subjective night (ZT 15, second cycle) altered the average spectral sensitivity of l-type cone horizontal cells to resemble that of goldfish red (625 nm) cones and cone horizontal cells in the day (Harosi & MacNichol, 1974), rather than that of rods (Schwanzara, 1967). Each data point represents the mean ± s.e.m. of 5–7 cells (1 cell per retina).
A circadian rhythm in the threshold of cone horizontal cell light responses is illustrated in Fig. 1B, which depicts the average stimulus intensity that generated a threshold (1 mV) response as a function of time in the dark. Following constant darkness, average threshold intensities are approximately 100 times greater during the subjective day (Zeitgeber time (ZT) 03 and 09, first and second cycles) than during the subjective night (ZT 15 and 21, first and second cycles). These results reveal the presence of a circadian rhythm in rod input to cone horizontal cells and indicate that rod input is present at night, but not in the day.
A circadian clock in the fish retina controls dopamine release
Dopamine release in the fish retina is greater in the subjective day than in the night (Ribelayga et al. 2002). To determine whether the clock that controls dopamine release is located in the retina, we maintained intact in vitro goldfish neural retinae in culture for 56 h in constant darkness (i.e. background illumination =–10 log Io) and temperature (20 ± 0.1°C) and measured the amount of endogenous dopamine that was released into the culture medium every 4 h. Typical individual values from five retinae are shown in Fig. 2A and the average of 14 retinae from three independent experiments is shown in Fig. 2B. Dopamine release was not constant over the course of 56 h (RM ANOVA, F13,195 = 7.11; P < 0.01) and displayed subjective day/night variations with 50% higher values observed during the subjective day (Fig. 2B). Expressed as a fraction of the total amount of dopamine (sum of all the fractions collected during the experiment + retinal content determined at the end of the experiment), dopamine release during the subjective day (measurements after 20, 24, 28, 44, 48 and 52 h of incubation) represented on average 6.13 ± 0.30%(mean ± s.e.m) (n = 30) of the total dopamine and 4.30 ± 0.18%(n = 35) during the subjective night (measurements after 8, 12, 16, 32, 36, 40 and 56 h of incubation). In addition, endogenous dopamine release in constant darkness under control conditions varied as a function of time (RM ANOVA, F4,69= 2.54; P < 0.01), but not in the presence of melatonin (F4,69= 1.674; P = 0.112) or luzindole (F4,69= 0.698; P = 0.733) (Fig. 3). Cosinor analysis (see Methods) demonstrates that endogenous dopamine release under control conditions exhibited a circadian rhythm with a period of ∼24 h (Fig. 2B and 3, Table 1). Therefore, because dopamine release from in vitro retinae under conditions of constant darkness persisted for more than two full cycles, it can be concluded that the clock controlling the release of dopamine is located within the retina itself.
Table 1.
Cosinor analysis of endogenous dopamine release from cultured goldfish retinae
| Culture conditions | F value | P | Mean DA release (pg (mg protein)−1 4 h−1) | Amplitude DA rhythm (pg (mg protein)−1 4 h−1) | Period DA rhythm (h) |
|---|---|---|---|---|---|
| Control (no drug) | F3,69 = 7.91 | < 0.01 | 510 ± 20 | 137 ± 28 | 23.9 ± 1.0 |
| Melatonin (1 nm) | F3,69 = 0.00 | 1.00 | 378 ± 14* | — | — |
| Luzindole (1 μm) | F3,69 = 0.00 | 1.00 | 707 ± 33*$ | — | — |
The regression coefficients (mean, amplitude and period) were obtained after non-linear regression of the data illustrated in Fig. 3 using the cosinor equation and are presented with their respective standard error estimates. Only coefficients that were significant (P < 0.01) are included in the table. The phase coefficient was not significant in any of the conditions and therefore not included in the table.
P < 0.01 when compared to control
P < 0.01 when compared to melatonin (Student-Newman-Keuls multiple comparison test).
The retinal clock uses melatonin receptor activation to control the circadian release of dopamine
We investigated whether the circadian release of dopamine was related to the circadian rhythm of melatonin release. In a number of vertebrate species, including fish, melatonin has been firmly identified as an output of the retinal clock such that its production and release peak at night (Cahill & Besharse, 1995; Iigo et al. 1997b; Tosini & Fukuhara, 2002). Because melatonin acutely inhibits dopamine release (Dubocovich, 1983; Boatright et al. 1994; Behrens et al. 2000) and because dopamine release is lower at night, melatonin may act as an effector of the retinal clock through which the clock indirectly controls the circadian release of dopamine. Alternatively, dopamine might be released on a circadian basis independently of melatonin. If so, their mutual inhibitory effects might somehow consolidate the overall circadian output of the retinal clock. To test whether the circadian release of dopamine is dependent on melatonin, melatonin (1 nm) was applied continuously during the culture period while dopamine release was measured. In the presence of melatonin dopamine release was completely arrhythmic (RM ANOVA F4,69= 1.674; P = 0.112) although still detectable (Fig. 3A and Table 1). The main effect of melatonin was to inhibit the clock-controlled increase in dopamine release typically observed during the subjective day. Similarly, continuous application of the selective melatonin antagonist luzindole (1 μm) abolished the circadian rhythm of dopamine release as well (RM ANOVA F4,69= 0.698; P = 0.733; Fig. 3B and Table 1. However, in the presence of luzindole, dopamine release remained at its highest value, that is, the clock-driven decrease at night was not observed. Since the circadian rhythm of dopamine release is eliminated during the continuous presence of melatonin and during the continuous blockade of melatonin receptors, the results indicate that the circadian rhythm of dopamine is primarily driven by the circadian rhythm of melatonin and that the rhythms are in antiphase. Moreover, the luzindole results indicate that the decrease in dopamine release at night is due to the endogenous activation of melatonin receptors.
Effects of melatonin on retinal cone horizontal cells depend on the time of day
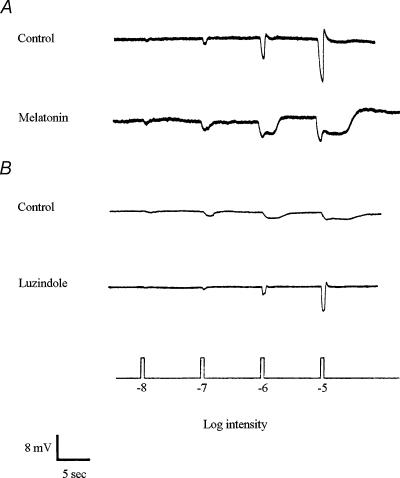
Superfusion of melatonin (0.01–10 μm) during the late subjective day (ZT 9, second cycle) increased rod input and reduced cone input to H1 cells (Fig. 4A), a state typically observed during the subjective night. In contrast, melatonin application during the early subjective day (ZT 3) or during the subjective night (ZT 15, ZT 21) had no effect (data not shown).
Figure 4. Melatonin increases rod input and decreases cone input to goldfish retinal l-type cone horizontal (H1) cells.
A, superfusion of melatonin (1 μm) during the late subjective day (ZT 09) introduced rod input and decreased cone input to H1 cells, so that light responses resembled those typically obtained during the subjective night. B, superfusion of luzindole (10 μm) during the subjective night (ZT 15, 21) decreased rod input and increased cone input to the cells. The recordings shown are representative of results obtained from 14 (A) and 8 (B) cells.
Superfusion of luzindole (1–10 μm) during the subjective night (ZT 15, second cycle) increased cone input and decreased rod input (Fig. 4B), a state typically observed during the subjective day. Luzindole application during the subjective day had no effect (data not shown). These results suggest that melatonin acts as a circadian clock signal for the night, that is, the clock increases the level of melatonin receptor activation during the night and decreases it in the day. The increase in melatonin at night then increases rod input and decreases cone input to cone horizontal cells.
Spectral sensitivity measurements demonstrate that melatonin modulates rod and cone input to cone horizontal cells. Figure 5A shows that application of melatonin during the late subjective day (ZT 09, second cycle) altered the average spectral sensitivity of l-type cone horizontal cells to resemble that of goldfish rods (Schwanzara, 1967), rather than red (625 nm) cones (Harosi & MacNichol, 1974). In contrast, application of luzindole during the subjective night (ZT 15, second cycle) altered the average spectral sensitivity of l-type cone horizontal cells (Fig. 5B) to resemble that of goldfish red cones (Harosi & MacNichol, 1974). Although the spectral sensitivity of l-type cone horizontal cells is similar to that of rods during the subjective night (Fig. 5B; see Ribelayga et al. 2002) and in the late subjective day following melatonin application (Fig. 5A), cone horizontal cells in both instances exhibit a greater relative spectral sensitivity in the far red region of the spectrum. This suggests that l-type cone horizontal cells still receive some input from red cones at night when endogenous melatonin levels are relatively high, even though they are primarily driven by rods. Similarly, electroretinogram studies of goldfish (Nussdorf & Powers, 1988) and Japanese quail (Manglapus et al. 1998) have shown that the dark-adapted night-time b-wave exhibits a greater than expected sensitivity in the far red region of the spectrum.
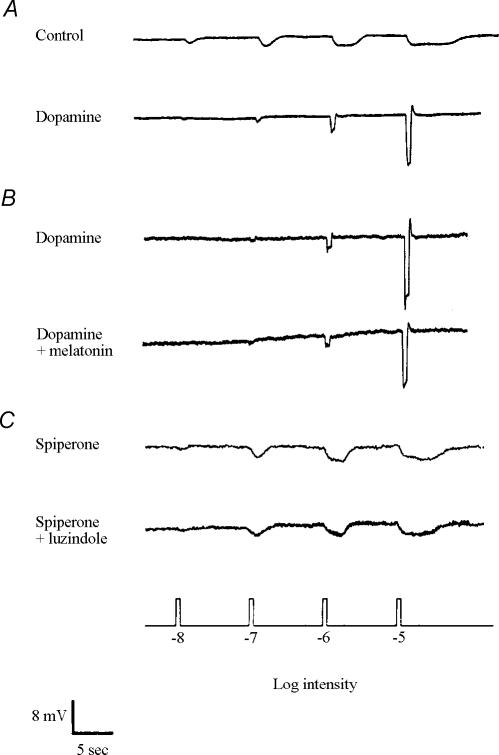
Based on the HPLC measurements described above (Figs 2 and 3), we examined whether the effects of melatonin are mediated through dopamine pathways. As previously reported (Ribelayga et al. 2002), superfusion of dopamine (0.001–1 μm) during the subjective night increased cone input and eliminated rod input to H1 cells (Fig. 6A), a state typically observed during the subjective day. In contrast, dopamine application during the subjective day had no effect or increased responses slightly (data not shown; see Ribelayga et al. 2002). Prior application of dopamine (0.1 μm) during the late subjective day (ZT 09, second cycle) blocked the effects of melatonin (10 μm) (Fig. 6B). In addition, prior application of spiperone (10 μm), a selective D2-like receptor antagonist, during the subjective night (ZT 15, second cycle) blocked the effects of luzindole (1–10 μm) (Fig. 6C), suggesting that the effects of endogenous activation of melatonin receptors by melatonin are mediated by D2-like receptor activation. Spiperone application during the subjective night did not affect the light responses of H1 cells (data not shown), as reported previously (Ribelayga et al. 2002).
Figure 6. The effects of melatonin on cone horizontal cells are mediated by activation of dopamine D2-like receptors.
A, superfusion of dopamine (0.1 μm) during the subjective night (ZT 15, second cycle) increased cone input and eliminated rod input to H1 cells. B, prior application of dopamine (0.1 μm) during the late subjective day (ZT 09, second cycle) blocked the effects of melatonin (10 μm). C, prior application of spiperone (10 μm), a selective D2-like receptor antagonist, during the subjective night (ZT 15, second cycle) blocked the effects of luzindole (10 μm). These results suggest that the effects of a clock-driven increase in melatonin at night on H1 cells are mediated by D2-like receptor activation. Similar results were obtained from 15 (A), 8 (B) and 9 (C) cells. The small depolarizing drift in B (dopamine + melatonin) was not typically observed.
Discussion
In this study, we demonstrate that a circadian clock in the vertebrate retina regulates dopamine release so that dopamine release is greater during the subjective day compared to the subjective night. In addition, the data suggest that the retinal clock uses melatonin as a primary effector that inhibits dopamine release during the night so that rod input to cone horizontal cells dominates at night and cone input dominates during the subjective day. That is, the rhythm in melatonin release precedes that of dopamine release and produces a rhythm in dopamine release that is opposite in phase.
Location of the circadian clock that controls dopamine release
Although day/night and circadian clock-mediated differences in dopamine release and/or content have been demonstrated previously in the retina (Wirz-Justice et al. 1984; Pozdeyev & Lavrikova, 2000; Doyle et al. 2002a,b; Ribelayga et al. 2002; Zawilska et al. 2003) and elsewhere in the CNS (Khaldy et al. 2002; Sellix & Freeman, 2003), the current findings clearly demonstrate the location of a clock that regulates dopamine levels. The measurement of a circadian rhythm in dopamine release from intact in vitro neural goldfish retinae maintained in constant darkness for 56 h (Fig. 2) indicates conclusively that a clock within the retina controls dopamine release. Moreover, the fact that an endogenous rhythmic process in the in vitro neural retina controls dopamine release and that a circadian rhythm of dopamine release has been observed using in oculo microdialysis (Adachi et al. 1999) suggests that a retinal circadian clock regulates dopamine release similarly in vivo.
Although the results presented here indicate that the retina itself contains a circadian clock that regulates dopamine and melatonin release, the possibility that an extra-retinal clock exerts an influence on retinal rhythmic activity cannot be excluded. For example, in goldfish, the pineal gland itself contains a circadian clock that increases melatonin production at night (Iigo et al. 1991) and the pineal clock may influence retinal melatonin synthesis (Iigo et al. 1997a) and dopamine release. In addition, retinal clock pathways can also be influenced by non-circadian sources. All vertebrate retinae are innervated by efferent projections (Reperant et al. 1989) and neural and hormonal influences of the brain on to the retina have been suggested (Faillace et al. 1995; Iigo et al. 1997a; Sakamoto et al. 2000; Ohngemach et al. 2001). The teleost retina, in particular, is innervated by a dense efferent projection from the olfactory bulb to the dopaminergic interplexiform cell that has been shown to modulate the activity of the dopaminergic system (Zucker & Dowling, 1987; Umino & Dowling, 1991).
Relationship between the clock, dopamine, and melatonin
Although a number of studies have clearly identified melatonin as an output of the retinal clock (Besharse & Iuvone, 1983; Cahill & Besharse, 1991; Tosini & Menaker, 1996; Iigo et al. 1997b), the relationship between the clock and the rhythms of melatonin and dopamine release has been unclear. The observations that melatonin release is high during the night, that dopamine release is high during the subjective day, and that melatonin can inhibit dopamine release has led to the widespread assumption that retinal melatonin generates the circadian rhythm of dopamine in the retina (reviews: Cahill & Besharse, 1995; Iuvone, 1995). However, dopamine itself can acutely suppress the rhythmic production of melatonin in the retina (Cahill & Besharse, 1991; Tosini & Dirden, 2000) and attempts to demonstrate in vivo the circadian clock pathway that utilizes melatonin and dopamine to alter physiological function remain controversial to date. For instance, a circadian rhythm of dopamine content and release is present in the retina of Royal College of Surgeons (RCS) rats (Doyle et al. 2002b). Because RCS rat retinae contain few photoreceptor cells (Dowling & Sidman, 1962) and because melatonin is synthesized primarily, and possibly exclusively, in the photoreceptor cell layer (Cahill & Besharse, 1993; Tosini & Fukuhara, 2002), these observations suggest that a second circadian clock, in addition to the retinal clock that controls melatonin synthesis (Cahill & Besharse, 1993; Tosini & Menaker, 1996), is located either in or outside the retina and regulates the activity of the retinal dopaminergic system. In contrast, a circadian rhythm of dopamine content is absent in mouse strains that are genetically incapable of rhythmically producing melatonin (C57Bl/6J, Doyle et al. 2002a; BALB/c, Nir et al. 2000), but is present in C3H/f mice that produce melatonin rhythmically (Doyle et al. 2002a), suggesting either that the retinal rhythm of melatonin might generate the dopamine rhythm in mice or that a circadian clock located elsewhere in the CNS produces the circadian rhythm in retinal dopamine.
The conflicting results obtained from in vivo experiments are likely to be due to the limitations of this approach. Indeed, in vivo experiments necessitate the sampling of different tissues at different times of the day and night, and not from the same tissue studied over the course of two full circadian cycles. In in vivo experiments, it is not known whether the observed day/night differences are due to a clock located within or outside of the retina. The fact that we were able to monitor dopamine release for up to 56 h in vitro from isolated fish retinae not only demonstrates conclusively that the circadian clock controlling dopamine release is located in the retina, but also led us to investigate in an isolated system two possibilities by which the retinal clock controls dopamine release: (1) dopamine release is decreased at night because of the action of melatonin, or (2) dopamine and melatonin rhythms are both controlled by the retinal clock but through independent pathways so that their mutual inhibitory actions reinforce the magnitude of the oscillations and maintain the oscillations in opposite phase.
The findings reported here indicate that the retinal clock decreases dopamine release at night by the activation of melatonin receptors and that the effects of the endogenous rhythm of melatonin are primarily mediated through the activation of dopamine D2-like receptors. These conclusions are supported by three observations. First, the continuous presence of melatonin eliminated the circadian rhythm of dopamine release by inhibiting the daytime increase in dopamine (Fig. 3A). Second, the continuous presence of the melatonin antagonist luzindole also eliminated the rhythm of endogenous dopamine release but by increasing the night-time levels of extracellular dopamine (Fig. 3B). This finding demonstrates that activation of melatonin receptors at night produces a decrease in dopamine release. Third, prior application of spiperone, a selective D2-like antagonist, during the subjective night eliminated the effects of luzindole on horizontal cell light responses (Fig. 6C), suggesting that dopamine-mediated activation of D2-like receptors relays the effects of melatonin. In other words, the findings reported here indicate that the clock-induced rhythm in melatonin receptor activation precedes the activation of D2-like receptors and that the primary effect of melatonin in the retina may be the inhibition of dopamine release. Although previous work has shown that dopamine can inhibit melatonin synthesis (Cahill & Besharse, 1991; Nguyen-Legros et al. 1996; Tosini & Dirden, 2000), the findings reported here suggest that the melatonin rhythm is not produced by the rhythm in dopamine. This conclusion is also consistent with the observation that the rhythmic synthesis of melatonin occurs in photoreceptors even in the absence of the inner retina (Cahill & Besharse, 1993; Thomas et al. 1993), because the cell bodies of all dopaminergic neurones are located in the inner retina. The acute suppressive and entraining effects of dopamine on melatonin rhythmic production may therefore represent a negative feedback influence on melatonin production, rather than a means by which the clock generates or eliminates melatonin rhythmicity.
Because the presence of artificially elevated levels of extracellular dopamine in the culture medium might interfere with melatonin synthesis and release (Cahill & Besharse, 1991; Tosini & Dirden, 2000), we avoided the use of drugs that affect dopamine metabolism, such as dopamine reuptake inhibitors, in all of our long-term experiments. Therefore, one could argue that the day/night changes in dopamine overflow into the medium might result from the regulation of dopamine release or dopamine reuptake, or both. However, several observations strongly suggest that the retinal clock regulates dopamine release, and not dopamine reuptake. First, we and others have observed that the effect of dark during the night, as well as the subjective day/subjective night difference in dopamine overflow measured over the course of 1–2 h from freshly isolated retinas, persist in the presence of the dopamine reuptake inhibitor nomifensine even if the level of extracellular dopamine is increased at both times (Boatright et al. 1994; C. Ribelayga & S. C. Mangel, personal observations). Second, melatonin acts indirectly on dopaminergic cells by enhancing GABAergic suppression of dopamine release (Boatright et al. 1994) and direct recordings from isolated dopaminergic cells have shown that GABA hyperpolarizes the cells and decreases dopamine release by exocytosis as measured by amperometry (Puopolo et al. 2001). Finally, the fact that exogenous dopamine at very low concentrations (1–5 nm) can affect the light responses of horizontal cells during the subjective night, but not in the subjective day (Ribelayga et al. 2002), strongly suggests that the nocturnal decrease in extracellular dopamine cannot be explained by a hyperactive transporter at night (Mangel, 2001). Taken together, these independent observations clearly indicate that the retinal clock, via melatonin action, controls dopamine overflow by regulating dopamine release and not by regulating dopamine reuptake.
Although expression of the genes that constitute the core molecular mechanism of the circadian clock has been found within the retina (Anderson & Green, 2000; Tosini & Fukuhara, 2002), the link between the core molecular mechanism of the retinal clock and the rhythm in melatonin production remains to be established. It is possible that the core mechanism of the retinal clock acts directly on gene expression of the melatonin-synthesizing enzymes, as has been suggested (Green & Besharse, 1994; Green et al. 1995; Chong et al. 1998, 2000; Hayasaka et al. 2002; Tosini & Fukuhara, 2002). This process appears to occur within photoreceptor cells, since the retinal clock that rhythmically synthesizes melatonin is located in photoreceptor cells (Cahill & Besharse, 1993; Thomas et al. 1993; Anderson & Green, 2000).
Physiological impact of the clock-controlled melatonin–dopamine pathway
Although an extensive literature already exists on the localization of melatonin synthesis and melatonin receptors in the vertebrate retina (Cahill & Besharse, 1995; Iuvone, 1995; Tosini & Fukuhara, 2002), our study clearly demonstrates the physiological consequences of melatonin action in the vertebrate retina. The results presented here (Figs 4–6) and previously (Ribelayga et al. 2002) indicate that the retinal clock uses melatonin to affect the light responses of retinal neurones by modulating dopamine release.
In the vertebrate retina, dopamine is released by a subset of amacrine or interplexiform cells and acts both locally at synaptic sites and distally by volume transmission on dopamine receptors distributed throughout the retina (Besharse et al. 1988; Djamgoz & Wagner, 1992; Witkovsky et al. 1993; Yazulla & Lin, 1995; Nguyen-Legros et al. 1999; Puopolo et al. 2001). Dopamine release is controlled by both light and a circadian clock. The clock-mediated effects of dopamine described so far have been linked to volume transmission and the activation of D2-like receptors present on the photoreceptors (Besharse et al. 1988; Cahill & Besharse, 1995; Iuvone, 1995; Nguyen-Legros et al. 1999; Mangel, 2001; Ribelayga et al. 2002), and not to the D1 receptors present on the horizontal cells (Ribelayga et al. 2002; Ribelayga & Mangel, 2003). The rhythmic production of melatonin occurs in the photoreceptors under the control of a retinal clock (Cahill & Besharse, 1995; Tosini & Fukuhara, 2002). Melatonin receptors are mainly expressed on photoreceptors as well as in the inner nuclear layer where their activation inhibits dopamine release (Dubocovich, 1983; Boatright et al. 1994; Fujieda et al. 2000; Wiechmann & Smith, 2001; Natesan & Cassone, 2002; Scher et al. 2002). Depending on the species, all of the three known high-affinity melatonin receptor subtypes (Mel1a (MT1), Mel1b (MT2), Mel1c) are present in the inner nuclear layer (Fujieda et al. 2000; Wiechmann & Smith, 2001; Natesan & Cassone, 2002; Scher et al. 2002). However, pharmacological characterization of the subtype(s) involved in the circadian control of dopamine release is handicapped by the lack of selective commercially available melatonin receptor antagonists. Although selective Mel1b (MT2) receptor antagonists (e.g. DH97, 4-P-PDOT) are commercially available, selective antagonists of the Mel1a (MT1) and Mel1c receptors are not. Recent studies have suggested that the effects of melatonin on dopamine release may be mediated through the Mel1b (MT2) subtype (Dubocovich et al. 1997; Behrens et al. 2000). Yet, these studies do not exclude a role for the other subtypes. Moreover, a novel subtype of melatonin receptor has been recently identified in a teleost fish and is expressed in the retina (Gaildrat et al. 2002). Additional experiments and identification of more specific melatonin receptor agonists/antagonists are needed to clarify this issue.
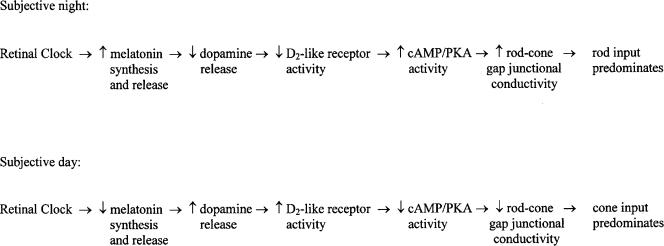
In fish, our results and other findings (Cahill & Besharse, 1995; Ribelayga et al. 2002) are consistent with the following scenario. Because (1) fish cone horizontal cells receive synaptic contacts only from cones and not from rods (Stell & Lightfoot, 1975; Downing & Djamgoz, 1989), (2) D2-like receptors are located on photoreceptor cells, and not on horizontal cells (Dearry & Burnside, 1986; Harsanyi & Mangel, 1992; Yazulla & Lin, 1995), and (3) rod–cone gap junctions are present in numerous vertebrates (Raviola & Gilula, 1973) including fish (Witkovsky et al. 1974), the retinal circadian clock likely regulates rod and cone input to cone horizontal cells by modulating rod–cone coupling (Wang & Mangel, 1996; Mangel, 2001; Ribelayga et al. 2002). As illustrated in Fig. 7, the clock located in the retina increases melatonin synthesis and release during the night (Besharse & Iuvone, 1983; Cahill & Besharse, 1993; Tosini & Menaker, 1996; Iigo et al. 1997b) so that the release of dopamine decreases (Dubocovich, 1983; Nowak, 1988; Boatright et al. 1994; Dubocovich et al. 1997; Behrens et al. 2000; this study). This event lowers extracellular levels of dopamine and activation of the D2-like receptors on photoreceptor cells (Pierce & Besharse, 1985; Dearry & Burnside, 1986; Manglapus et al. 1999; Ribelayga et al. 2002; this study). As a result, during the night intracellular cyclic AMP (cAMP) and protein kinase A (PKA) activity levels increase in photoreceptors (Schorderet & Nowak, 1990; Nir et al. 2002; Ribelayga et al. 2002), thus increasing the conductance of rod–cone gap junctions so that rod input dominates cone horizontal cells (Wang & Mangel, 1996; Mangel, 2001; Ribelayga et al. 2002; this study). During the day, the clock decreases melatonin production thereby reducing the melatonin-mediated inhibition of dopamine release. The resultant increase in extracellular dopamine activates the D2-like receptors on photoreceptors. As a consequence, intracellular cAMP and PKA activity levels in photoreceptors decrease, thus lowering the conductance of rod–cone gap junctions so that cone input dominates cone horizontal cells. We have previously suggested (Ribelayga et al. 2002) that circadian and light-adaptive phenomena are different and may regulate the conductance of rod–cone gap junctions differently (see Krizaj et al. 1998).
Figure 7. Proposed circadian clock pathway in the fish retina.
The clock in the retina increases melatonin release during the night so that the release of dopamine decreases. This lowers activation of the D2-like receptors on photoreceptor cells. As a result, intracellular cAMP and PKA activity levels in photoreceptors increase, thus increasing the conductance between rod–cone gap junctions so that rod input dominates cone horizontal cells. In the subjective day, cone input dominates cone horizontal cells because the clock has decreased melatonin levels thereby reducing inhibition of dopamine release. The increase in dopamine release increases activation of the D2-like receptors on photoreceptors. As a result, intracellular cAMP and PKA activity levels in photoreceptor cells decrease, thus lowering the conductance between rod–cone gap junctions. See Discussion for details.
In summary, our results demonstrate that a circadian clock in the retina modulates endogenous dopamine release, that the dopamine rhythm is highly linked to the rhythmic production of melatonin and to the activation of melatonin receptors, and that the effects of melatonin on cone horizontal cells are mediated through dopamine and D2-like receptor activation. Because the intact in vitro vertebrate retina can be maintained in a viable state for several days, it can therefore serve as an outstanding model system for understanding how circadian clocks alter brain function.
Acknowledgments
This investigation was supported in part by grants to S.C. Mangel from the NIH (EY005102, EY014235) and the NSF (IBN-9819981), by an NIH grant (HD38985), and by a National Eye Institute CORE Grant (EY03039) to the University of Alabama at Birmingham. C. Ribelayga and Y. Wang were supported in part by postdoctoral fellowships from Fight for Sight/Prevent Blindness America, NY (PD 01009), and the Helen Keller Eye Research Foundation, Birmingham, Alabama, respectively.
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi A, Suzuki Y, Nogi T, Ebihara S. The relationship between ocular melatonin and dopamine rhythms in the pigeon: effects of melatonin inhibition on dopamine release. Brain Res. 1999;815:435–440. doi: 10.1016/s0006-8993(98)01077-4. [DOI] [PubMed] [Google Scholar]
- Anderson FE, Green CB. Symphony of rhythms in the Xenopus laevis retina. Microsc Res Tech. 2000;50:360–372. doi: 10.1002/1097-0029(20000901)50:5<360::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Baldridge WH, Weiler R, Dowling JE. Dark-suppression and light-sensitization of horizontal cell responses in the hybrid bass retina. Vis Neurosci. 1995;12:611–620. doi: 10.1017/s0952523800008907. [DOI] [PubMed] [Google Scholar]
- Behrens UD, Douglas RH, Sugden D, Davies DJ, Wagner HJ. Effect of melatonin agonists and antagonists on horizontal cell spinule formation and dopamine release in a fish retina. Cell Tissue Res. 2000;299:299–306. doi: 10.1007/s004419900161. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone MP, Pierce ME. Regulation of rhythmic photoreceptor metabolism: a role for post-receptoral neurons. Prog Ret Eye Res. 1988;7:21–61. [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinae: regulation by a photoreceptor oscillator. Prog Ret Eye Res. 1995;14:267–291. [Google Scholar]
- Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275:32991–32998. doi: 10.1074/jbc.M005671200. [DOI] [PubMed] [Google Scholar]
- Chong NW, Cassone VM, Bernard M, Klein DC, Iuvone PM. Circadian expression of tryptophan hydroxylase mRNA in the chicken retina. Brain Res Mol Brain Res. 1998;61:243–250. doi: 10.1016/s0169-328x(98)00219-8. [DOI] [PubMed] [Google Scholar]
- Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinae. I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986;46:1006–1031. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Wagner HJ. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int. 1992;20:139–191. doi: 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. A circadian clock regulates the pH of the fish retina. J Physiol. 2000;522:77–82. doi: 10.1111/j.1469-7793.2000.0077m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing JE, Djamgoz MB. Quantitative analysis of cone photoreceptor-horizontal cell connectivity patterns in the retina of a cyprinid fish: electron microscopy of functionally identified and HRP-labelled horizontal cells. J Comp Neurol. 1989;289:537–553. doi: 10.1002/cne.902890402. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002a;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002b;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Cutrera R, Sarmiento MI, Rosenstein RE. Evidence for local synthesis of melatonin in golden hamster retina. Neuroreport. 1995;6:2093–2095. doi: 10.1097/00001756-199510010-00033. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Gaildrat P, Becq F, Falcon J. First cloning and functional characterization of a melatonin receptor in fish brain: a novel one? J Pineal Res. 2002;32:74–84. doi: 10.1034/j.1600-079x.2002.1817.x. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Tryptophan hydroxylase expression is regulated by a circadian clock in Xenopus laevis retina. J Neurochem. 1994;62:2420–2428. doi: 10.1046/j.1471-4159.1994.62062420.x. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- Harosi FI, MacNichol EF., Jr Visual pigments of goldfish cones. Spectral properties and dichroism. J General Physiol. 1974;63:279–304. doi: 10.1085/jgp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Activation of dopamine D2 receptors increases the electrical coupling between fish horizontal cells by inhibiting dopamine release. Proc Natl Acad Sci USA. 1992;89:9220–9224. doi: 10.1073/pnas.89.19.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Wang Y, Mangel SC. Activation of NMDA receptors produces dopamine-mediated changes in fish retinal horizontal cell light responses. J Neurophysiol. 1996;75:629–647. doi: 10.1152/jn.1996.75.2.629. [DOI] [PubMed] [Google Scholar]
- Hayasaka N, LaRue SI, Green CB. In vivo disruption of Xenopus CLOCK in the retinal photoreceptor cells abolishes circadian melatonin rhythmicity without affecting its production levels. J Neurosci. 2002;22:1600–1607. doi: 10.1523/JNEUROSCI.22-05-01600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Furukawa K, Hattori A, Ohtani-Kaneko R, Hara M, Suzuki T, Tabata M, Aida K. Ocular melatonin rhythms in the goldfish, Carassius auratus. J Biol Rhythms. 1997a;12:182–192. doi: 10.1177/074873049701200209. [DOI] [PubMed] [Google Scholar]
- Iigo M, Hara M, Ohtani-Kaneko R, Hirata K, Tabata M, Aida K. Photic and circadian regulations of melatonin rhythms in fishes. Biol Signals. 1997b;6:225–232. doi: 10.1159/000109132. [DOI] [PubMed] [Google Scholar]
- Iigo M, Kezuka H, Aida K, Hanyu I. Circadian rhythms of melatonin secretion from superfused goldfish (Carassius auratus) pineal glands in vitro. General Comp Endocrinol. 1991;83:152–158. doi: 10.1016/0016-6480(91)90115-m. [DOI] [PubMed] [Google Scholar]
- Iuvone PM. Cell biology and metabolic activity of photoreceptor cells: light-evoked and circadian regulation. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and Clinical Aspects of the Outer Retina. London: Chapman & Hall; 1995. pp. 25–55. [Google Scholar]
- Khaldy H, Leon J, Escames G, Bikjdaouene L, Garcia JJ, Acuna-Castroviejo D. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinology. 2002;75:201–208. doi: 10.1159/000048238. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retina cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mangel SC. Circadian clock regulation of neuronal light responses in the vertebrate retina. Prog Brain Res. 2001;131:505–518. doi: 10.1016/s0079-6123(01)31040-3. [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proc R Soc Lond B Biol Sci. 1987;231:91–121. doi: 10.1098/rspb.1987.0037. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM. Mathematical and statistical analysis of circadian rhythms. Psychoneuroendocrinol. 1988;13:443–464. doi: 10.1016/0306-4530(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. An attempt to analyse colour reception by electrophysiology. J Physiol. 1966;185:556–586. doi: 10.1113/jphysiol.1966.sp008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan AK, Cassone VM. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis Neurosci. 2002;19:265–274. doi: 10.1017/s0952523802192042. [DOI] [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996;67:2514–2520. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ. Melatonin inhibits [3H]-dopamine release from the rabbit retina evoked by light, potassium and electrical stimulation. Med Sci Res. 1988;16:1073–1075. [Google Scholar]
- Nussdorf JD, Powers MK. Spectral sensitivity of the electroretinogram b-wave in dark-adapted goldfish. Vis Neurosci. 1988;1:159–168. doi: 10.1017/s0952523800001437. [DOI] [PubMed] [Google Scholar]
- Ohngemach S, Feldkaemper M, Schaeffel F. Pineal control of the dopamine D2-receptor gene and dopamine release in the retina of the chicken and their possible relation to growth rhythms of the eye. J Pineal Res. 2001;31:145–154. doi: 10.1034/j.1600-079x.2001.310208.x. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SE. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movement. I. Interaction of melatonin and dopamine in the control of cone length. J General Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Pozdeyev NV, Lavrikova EV. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J Mol Neurosci. 2000;15:1–9. doi: 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001;30:211–225. doi: 10.1016/s0896-6273(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci USA. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant J, Miceli D, Vesselkin NP, Molotchnikoff S. The centrifugal visual system of vertebrates: a century-old search reviewed. Int Rev Cytol. 1989;118:115–171. doi: 10.1016/s0074-7696(08)60874-8. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol. 2003;467:243–253. doi: 10.1002/cne.10927. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2002;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Oishi K, Shiraishi M, Hamano S, Otsuka H, Miyake Y, Ishida N. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport. 2000;11:3995–3997. doi: 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT(1) melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- Schorderet M, Nowak JZ. Retinal dopamine D1 and D2 receptors: characterisation by binding or pharmacological studies and physiological functions. Cell Mol Neurobiol. 1990;10:303–325. doi: 10.1007/BF00711177. [DOI] [PubMed] [Google Scholar]
- Schwanzara SA. The visual pigments of fresh water fishes. Vis Res. 1967;7:121–148. doi: 10.1016/0042-6989(67)90079-x. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Freeman ME. Circadian rhythms of neuroendocrine dopaminergic neuronal activity in ovariectomized rats. Neuroendocrinology. 2003;77:59–70. doi: 10.1159/000068334. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO. Colour-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975;159:473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Turek FW, Moore RY. Handbook of Behavioural Neurobiology. Vol. 12. New York, NY, USA: Kluwer Academic/Plenum Publishers; 2001. Circadian Clocks. [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601:303–307. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci Lett. 2000;286:119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Tosini G, Fukuhara C. The mammalian retina as a clock. Cell Tissue Res. 2002;309:119–126. doi: 10.1007/s00441-002-0578-z. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Umino O, Dowling JE. Dopamine release from interplexiform cells in the retina: effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J Neurosci. 1991;11:3034–3046. doi: 10.1523/JNEUROSCI.11-10-03034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harsanyi K, Mangel SC. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. J Neurophysiol. 1997;78:439–449. doi: 10.1152/jn.1997.78.1.439. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Strahle U, Sassone-Corsi P. Zebrafish Clock rhythmic expression reveals independent peripheral circadian oscillators. Nat Neurosci. 1998;1:701–707. doi: 10.1038/3703. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Smith AR. Melatonin receptor RNA is expressed in photoreceptors and displays a diurnal rhythm in Xenopus retina. Brain Res Mol Brain Res. 2001;91:104–111. doi: 10.1016/s0169-328x(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Nicholson C, Rice ME, Bohmaker K, Meller E. Extracellular dopamine concentration in the retina of the clawed frog. Xenopus laevis. Proc Natl Acad Sci USA. 1993;90:5667–5671. doi: 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Shakib M, Ripps H. Interreceptoral junctions in the teleost retina. Invest Ophthalmol. 1974;13:996–1009. [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Lin ZS. Differential effects of dopamine depletion on the distribution of [3H]SCH 23390 and [3H]spiperone binding sites in the goldfish retina. Vision Res. 1995;35:2409–2414. [PubMed] [Google Scholar]
- Zawilska JB, Bednarek A, Berezinska M, Nowak JZ. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem. 2003;84:717–724. doi: 10.1046/j.1471-4159.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE. Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]