Abstract
The mechanisms underlying metabolic inhibition of sympathetic responses within exercising skeletal muscle remain incompletely understood. The aim of the present study was to test whether α2-adrenoreceptor-mediated vasoconstriction was more sensitive to metabolic inhibition than α1-vasoconstriction during dynamic knee-extensor exercise. We studied healthy volunteers using two protocols: (1) wide dose ranges of the α-adrenoreceptor agonists phenylephrine (PE, α1 selective) and BHT-933 (BHT, α2 selective) were administered intra-arterially at rest and during 27 W knee-extensor exercise (n = 13); (2) flow-adjusted doses of PE (0.3 μg kg−1 l−1) and BHT (15 μg kg−1 l−1) were administered at rest and during ramped exercise (7 W to 37 W; n= 10). Ultrasound Doppler and thermodilution techniques provided direct measurements of femoral blood flow (FBF). PE (0.8 μg kg−1) and BHT (40 μg kg−1) produced comparable maximal reductions in FBF at rest (−58 ± 6 versus−64 ± 4%). Despite increasing the doses, PE (1.6 μg kg−1 min−1) and BHT (80 μg kg−1 min−1) caused significantly smaller changes in FBF during 27 W exercise (−13 ± 4 versus−3 ± 5%). During ramped exercise, significant vasoconstriction at lower intensities (7 and 17 W) was seen following PE (−16 ± 5 and −16 ± 4%), but not BHT (−2 ± 4 and −4 ± 5%). At the highest intensity (37 W), FBF was not significantly changed by either drug. Collectively, these data demonstrate metabolic inhibition of α-adrenergic vasoconstriction in large postural muscles of healthy humans. Both α1- and α2-adrenoreceptor agonists produce comparable vasoconstriction in the resting leg, and dynamic thigh exercise attenuates α1- and α2-mediated vasoconstriction similarly. However, α2-mediated vasoconstriction appears more sensitive to metabolic inhibition, because α2 is completely inhibited even at low workloads, whereas α1 becomes progressively inhibited with increasing workloads.
Vascular tone is controlled by interactions of sympathetic nervous and local vascular control mechanisms (Laughlin et al. 1996). In resting muscle, the importance of sympathetic activity for vascular tone is exemplified by the 50–100% increase in blood flow following sympathetic denervation or α-adrenergic blockade (Delp & Armstrong, 1988; Laughlin et al. 1996; Dinenno et al. 2002b). In exercising muscle, metabolites accumulate and become involved in the control of local blood flow. During exercise the need for dramatic increases in perfusion and oxygen delivery to the contracting units is met by increased cardiac output and redistribution of blood flow. The latter is accomplished primarily by increasing sympathetic vasoconstriction in internal organs as well as resting muscle vascular beds. The increased sympathetic vasoconstrictor stimulus is delivered in parallel to the exercising muscle (Hansen et al. 1994), but the effectiveness of this stimulus in the face of local metabolic vasodilatation has been a matter of some controversy. In some models of exercise, evidence of sympathetic vasoconstriction has been reported (Secher et al. 1977; O'Leary et al. 1991b; Joyner et al. 1992; Buckwalter & Clifford, 1999), whereas other studies find evidence for metabolic blunting of sympathetic vasoconstriction in the exercising muscle (Thomas et al. 1994; Richardson et al. 1995; Hansen et al. 1996; Thomas et al. 1998; Sander et al. 2000; Ruble et al. 2000, 2002; Chavoshan et al. 2002; Tschakovsky et al. 2002; Rosenmeier et al. 2003; Dinenno & Joyner, 2003). These seemingly conflicting results are not mutually exclusive, since metabolic blunting of sympathetic vasoconstriction does not exclude residual sympathetic effects, but rather implies less vasoconstriction than would be expected for a given sympathetic input.
In recent years there seems to be a growing consensus that metabolic inhibition of sympathetic vasoconstriction is a factor in several models of exercise in both animals and humans. Although the underlying mechanisms are incompletely understood, several lines of evidence point to inhibition of postjunctional adrenergic signalling via metabolites from active skeletal muscle. The α-adrenergic receptors can be divided into the α1 and α2 subtypes, each with vasoconstrictor properties in vivo. Microvascular preparations have identified α1-adrenoreceptors primarily in proximal and α2-adrenoreceptors primarily in the distal vasculature of skeletal muscle (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991). In both isolated microvascular preparations and intact animal models, α2-vasoconstriction seems more sensitive to metabolic inhibition than α1-vasoconstriction (Anderson & Faber, 1991; Thomas et al. 1994). However, the role of α-adrenoreceptor subtypes in humans remains unclear.
Previous studies considering the effect of exercise on α-adrenoreceptor-mediated vasoconstriction have been limited to the animal hindlimb muscles (Thomas et al. 1994; Thomas et al. 1998; Buckwalter et al. 2001), and the human forearm during rhythmic handgrip (Hansen et al. 1996; Sander et al. 2000; Chavoshan et al. 2002; Tschakovsky et al. 2002; Rosenmeier et al. 2003; Dinenno & Joyner, 2003). However, to our knowledge no previous human studies have examined α-adrenergic function in a large postural muscle group such as the quadriceps during graded, dynamic exercise. This is an important consideration, since the leg represents a vascular bed with the potential to vasodilate to such a degree that it may challenge maintenance of systemic arterial blood pressure during exercise (Saltin et al. 1998; Volianitis et al. 2003), and so may be considered more haemodynamically significant than smaller muscle vascular beds.
The aims of this study were to test the following hypotheses: (1) metabolic inhibition of α-adrenergic vasoconstriction is present in exercising human thigh muscle, and (2) α2-mediated vasoconstriction is more sensitive than α1-mediated vasoconstriction to metabolic inhibition in exercising human thigh muscle. To accomplish this, femoral blood flow was measured directly at rest and during steady-state knee-extensor exercise with superimposed administration of the selective α-receptor subtype agonists phenylephrine (PE, an α1 agonist) and BHT-933 (BHT, an α2 agonist).
Methods
Subjects and general procedures
Seventeen healthy young men (25 ± 1 years) participated in the present study. Written informed consent was obtained from all participants, and experiments were approved by the local ethics committee of Copenhagen and Frederiksberg and conformed to the Declaration of Helsinki. All studies were performed in a thermoneutral environment, with subjects in a semirecumbent position (approximately 30 deg reclined). Heart rate (HR) was recorded from an ECG (ADInstruments), and respiratory excursions from a strain gauge belt (Respitrace, ADInstruments). Blood pressure was determined both non-invasively by automated sphygmomanometry (Dinamap), and invasively (see below). Knee-extensor exercise with one leg was performed at 60 r.p.m. on a modified cycle ergometer as previously described (Andersen et al. 1985). The knee extensor force and rhythm were recorded via a strain gauge attached to the ergometer lever arm (customized signal processor, FBJ Industries). All measurements at rest were performed during vascular occlusion of the lower leg (pneumatic cuff below the knee inflated to 280 mmHg). With this procedure, a very large fraction of blood flow measured at the level of the groin is supplying the thigh muscle vascular bed.
Catheterization
The femoral artery and vein of the exercising leg were cannulated under local anaesthesia (lidocaine, 5 ml, 20 mg ml−1). The arterial (Arrow, 20 gauge) and venous (Cook, 18 gauge) catheters were inserted in the proximal direction ∼5 cm below the inguinal ligament. The arterial catheter was used for intra-arterial infusions of drugs and phasic invasive blood pressure measurements (calibrated to the level of the aortic valve and corrected for pump pressure artifacts during infusions). The venous catheter was used to perform thermodilution measurements of leg blood flow, as described below.
Blood flow measurements
Thermodilution. A sterile tip-thermistor (model 94-030-2.5-Fr, Baxter) was inserted through the femoral venous catheter and positioned 8 cm proximal to the catheter tip (at the level of the inguinal ligament). Another thermistor was placed at the venous catheter inlet and both thermistors were connected to a customized dual temperature signal processor (custombuild, FBJ Industries). This allows continuous measurements of blood temperature during constant infusion of a sterile iced saline solution (around 2–3°C at the venous inlet) administered at 100 ml min−1 by a modified Harvard pump for periods of 20 s. Dilution of this indicator in the vein of the exercising leg causes blood temperature to decrease by 0.5–3°C, depending on blood flow. This thermodilution procedure has been described and validated in detail previously and provides very accurate and reproducible measurements of leg blood flow during knee extensor exercise (Andersen & Saltin, 1985). During rest, thermodilution was not used, because the method is unsuited for determination of very low levels of blood flow, as expected during vasoconstrictor infusions. Femoral blood flow (FBFTD) was calculated by the formula:
where Fsal is saline flow; Cbl and Csal are specific heats for blood and saline, respectively; ρbl and ρsal are specific densities for blood and saline, respetively; tbb and tbd are temperatures of blood before and during the saline infusion, respectively; and tic is the saline infusate temperature, corrected to the level of the catheter tip.
Ultrasound imaging and Doppler
The ultrasound machine (model CFM 800, GE Medical) was equipped with a mechanical sector transducer operating at an imaging frequency of 7.5 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution is achieved (best theoretical resolution around 0.1 mm). The femoral artery was insonated distal to the inguinal ligament for dynamic recordings of diameter throughout a cardiac cycle. The maximum diameter (systole) was used for calculation of blood flow, because systole is the phase of blood propulsion in large arteries. The blood velocity profile was obtained using the same transducer with a Doppler frequency of 4.0–6.0 MHz, operated in the high-pulsed repetition frequency mode (4–36 kHz) with a sample volume of 5 mm in depth. Care was taken to avoid aliasing especially during exercise. All blood velocity measurements were obtained with the probe at a constant 46 deg (or in some subjects 50 deg) angle to the femoral artery during simultaneous real time 2D vessel visualization to maintain insonation angle constant and sample volume centred. At all sample points we obtained both diameter of the femoral artery (FAD) and, approximately 20–30 s later, an angle-corrected, time- and space-averaged, and intensity-weighted mean blood velocity (Vmean) (Echopac Software, GE Medical and PowerLab, ADInstruments). Using FAD and Vmean, femoral artery blood flow (FBFD) was calculated from:
The Doppler method has been reported to yield reproducible femoral artery blood flow measurements at rest and during knee-extensor exercise, previously used at up to 70% of maximal work (Radegran, 1997). We obtained FBFD both during rest and exercise. However, a priori the thermodilution method was chosen as the method of choice during exercise, since variability for the thermodilution measurements was expected to be less compared to the Doppler measurements. Thus, for the present study, blood flow values during rest are from ultrasound Doppler measurements, while values during exercise are from the thermodilution technique. A direct comparison of the results obtained from the two methods during exercise was performed (see Results).
Drugs
Phenylephrine (PE; Danish county pharmaceutical corporation, SAD) was used as a specific α1-adrenergic agonist. BHT-933 (BHT; Sigma-Aldrich, Denmark) was used as a specific α2-adrenergic agonist. Propranolol (Prop; AstraZeneca, Sweden) was used as a non-specific β-adrenergic antagonist. In validation experiments, isoproterenol (Iso; Danish county pharmaceutical corporation, SAD) was used as a non-specific β-adrenergic agonist, while yohimbine (Sigma-Aldrich, Denmark) was used as an α2-adrenergic antagonist. Drugs were dissolved and diluted as appropriate with normal saline, except yohimbine, which was diluted with sterile water. Intra-arterial infusion rates ranged from 0.2 to 6 ml min−1.
Experimental protocols
To address the aims of the present study, four separate protocols were performed. Graphic presentations of the protocols are presented in Fig. 1. Protocols 1 and 2 were designed to validate the drugs administered for β-adrenergic blockade and α2-adrenoreceptor activation, respectively. In protocol 3, wide dose ranges of the α1-agonist PE and α2-agonist BHT were administered at rest and during one level of moderate intensity (27 W) exercise. This design allows evaluation of the doses required to obtain a maximal drug response. In protocol 4, one flow-adjusted dose of PE and BHT was administered at rest and during graded (7 W to 37 W) exercise. This design allows evaluation of the exercise intensity required to attenuate each drug at a concentration which was shown to yield submaximal effects at rest. Details regarding the specific objectives and measurements for each protocol are described below.
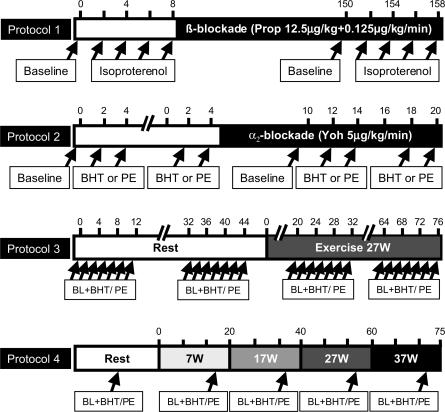
Figure 1. Experimental protocols 1–4 represented graphically.
Time courses are given in minutes on the top axis of each panel. BL, baseline; BHT, BHT-933; PE, phenylephrine; Prop, propranolol; Yoh, yohimbine. Protocol 1: the β-receptor agonist isoproterenol (1, 2, 4 and 8 ng kg−1 min−1, 2-min infusions) was administered before and after 150 min of β-blockade with propranalol (12.5 μg kg−1 bolus and 0.125 μg kg−1 min−1 maintenance). Protocol 2: the selective α2-adrenoreceptor agonist BHT-933 (BHT, 20 and 40 μg kg−1 min−1, 2-min infusions) and the selective α1-agonist phenylephrine (PE, 0.4 and 0.8 μg kg−1 min−1, 2-min infusions) were applied before and during the last 10 min of a 20 min infusion of the α2-antagonist yohimbine (5 μg kg−1 min−1). Protocol 3: dose–response for BHT (2.5–80 μg kg−1 min−1, 2-min infusions) and PE (0.025–1.6 μg kg−1 min−1, 2-min infusions) at rest and during 27 W knee extensor exercise. Protocol 4: flow-adjusted administration of BHT (15 μg kg−1 l−1, 3-min infusions) and PE (0.3 μg kg−1 l−1, 3-min infusions) at rest and during ramped exercise.
Protocol 1: Validation of β-adrenergic blockade
β-Blockade in the leg was used in one of the exercise protocols, because PE previously has been shown to have β-adrenergic agonist properties (Torp et al. 2001). Intra-arterial administration of propranolol has previously been shown to produce effective β-blockade in the human forearm after 10 min (Johnsson, 1967), and in validation experiments we extended this finding to the leg. The purpose of the present protocol was to validate the assumption that the maintenance dose of propranolol used would sustain the β-blockade in the leg for several hours. We tested this in nine resting subjects by measuring blood pressure, HR and FBF during challenges with intra-arterial isoproterenol (Iso; 1, 2, 4, and 8 ng kg−1 min−1, 2 min on each dose) before and after 150 min of intra-arterial administration of Prop (2.5 μg kg−1 min−1 for 5 min as bolus, followed by 0.125 μg kg−1 min−1 as maintenance). This dosing regimen for Prop was identical to that used in protocol 3 of the present study.
Protocol 2: Validation of α2-adrenergic specificity of BHT-933
The α-adrenergic receptor subtype specificity of PE has been studied quite extensively. In contrast, the specificity of BHT has only been documented sparingly in humans (Jie et al. 1984). The purpose of this protocol was to validate the assumption that BHT in the dose range used is specific for α2-adrenergic receptors in the human leg. We tested this in seven resting subjects by measuring blood pressure, HR and FBF during intra-arterial BHT (20 and 40 μg kg−1 min−1 each for 2 min) and PE (0.4 and 0.8 μg kg−1 min−1 each for 2 min) before and during the last 10 min of concomitant intra-arterial infusion of the α2-adrenergic antagonist yohimbine (5 μg kg−1 min−1 for 20 min). If BHT specifically acts as an α2-adrenergic receptor ligand, we would predict that yohimbine would inhibit the BHT response without affecting the PE response. All subjects participating in protocol 2 also participated in protocol 1 on the same day, and thus α-adrenergic activation and inhibition were performed during concomitant β-blockade.
Protocol 3: Subtype specific α-adrenergic agonists during rest and exercise
The purpose of this protocol was twofold: first, to determine to what degree metabolic inhibition of α-adrenergic vasoconstriction is evident in a large muscle mass during dynamic exercise in humans; second, to determine whether α2-vasoconstriction is more sensitive to metabolic inhibition than α1-vasoconstriction at a given work-load. Intra-arterial Prop was used to obtain local β-adrenergic blockade. In 13 subjects, we measured blood pressure, HR, FBF at rest and during 27 W one-legged knee-extensor exercise before and during intra-arterial administration of PE (0.025–1.6 μg kg−1 min−1, 7 doses, 2 min each dose) and BHT (2.5–80 μg kg−1 min−1, 6 doses, 2 min each dose). At rest, the highest dose of PE (1.6 μg kg−1 min−1) and BHT (80 μg kg−1 min−1) were only administered to a subset of the subjects (n= 4) due to systemic effects. The wide range of drug doses allowed evaluation of saturation kinetics at rest and during exercise, and post hoc comparison of doses normalized to flow, i.e. similar drug-concentration in femoral arterial blood despite large changes in predrug steady-state blood flow. The two agonists were separated by at least 15 min to allow washout and restoration of haemodynamic values, and the sequence of drug application was alternated. In this protocol, only the FBF measurements at rest could be performed during vascular occlusion of the lower leg, because in pilot experiments it was determined that prolonged lower leg ischaemia (around 15 min was needed for completion of the multiple doses of each drug) was too painful during knee-extensor exercise. During exercise, the first 10 min was without measurements to allow ample time for blood flow, HR and MAP to reach steady-state levels even at low to moderate exercise intensities (Radegran & Saltin, 1998). The exercise lasted a total of 80 min.
In pilot experiments, two prolonged infusions of BHT and PE were performed during supine rest with 60–90 min between infusions. There was no indication of tachyphylaxis to the vasoconstrictor effects of either drug. This is in accordance with previously published results which do not indicate development of tolerance towards intra-arterial administration of these α-agonists within the same study day (Jie et al. 1984; Lembo et al. 1994; Lembo et al. 1997).
Protocol 4: α-Adrenergic vasoconstriction during different levels of exercise
The purpose of this protocol was to determine whether metabolic inhibition of α-adrenergic vasoconstriction in the exercising thigh is dependent on exercise intensity. This protocol was performed without β-adrenergic blockade. In 10 subjects, we measured blood pressure, HR, FBF at rest and during 7, 17, 27 and 37 W one-leg knee-extensor exercise before and during intra-arterial administration of PE (flow-adjusted dosing to 0.3 μg kg−1 l−1, 3 min infusions), and BHT (flow-adjusted dosing to 15 μg kg−1 l−1, 3 min infusions). In a subset of the subjects (n= 5), the responses to increased doses of PE (0.6 μg kg−1 l−1) and BHT (30 μg kg−1 l−1) were determined during 7 W. This was done to determine if the responses seen during exercise were maximal. The brevity of drug infusions during this protocol allowed FBF measurements both at rest and during exercise to be performed during vascular occlusion of the lower leg. The subjects performed two separate bouts of exercise lasting 75–80 min, with 90 min rest between bouts. Subjects exercised for 12–15 min at each level (7 W to 37 W) using an incremental protocol. Blood flow was allowed to reach steady-state during the first 10 min of exercise at each level before measurements and drug administration began. The sequence of drug administration was alternated.
Data analysis and statistics
All data underwent analog-to-digital conversion and were sampled at 400 Hz, recorded on a PC, and analysed offline with signal processing software (PowerLab, ADInstruments). Mean arterial pressure was derived from the arterial pressure waveform and the sphygmomanometer readings (MAP = diastolic BP + 1/3 pulse pressure). Femoral vascular conductance (FVC) was calculated by the formula: FVC = FBF/MAP. During the last 30 s of each baseline or drug dose FBFTD was averaged over 5–10 s; FBFD over 8 s; and MAP, HR over at least 30 s. Within group differences were assessed by Student's paired t test and one- or two-way anova for repeated measures (i.e. repeated measures for both drug and dose). Post-hoc analysis was performed by Dunnett's procedure for one-way anova, and by Tukey's HSD procedure for two-way anova. The Doppler and thermodilution data obtained during exercise in protocols 3 and 4 were compared head to head by paired t tests. Data are expressed as the mean ±s.e.m., except when comparing the Doppler and thermodilution methods, where the mean ± 95% confidence intervals are used. Statistical significance was set at P < 0.05, adjusted by the Bonferroni method as appropriate.
Results
Lasting β-blockade in the human thigh (protocol 1)
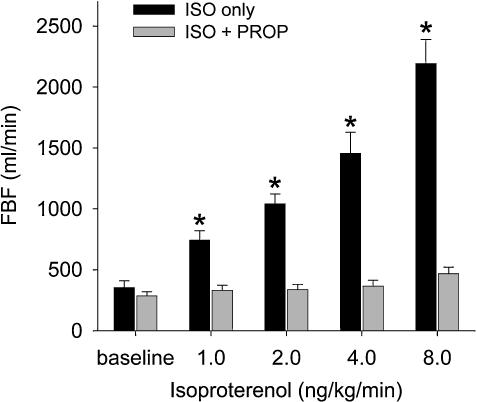
The propranolol-bolus and maintenance dose provided virtually complete blockade of isoproterenol-induced vasodilatation, even 150 min after initiation (Fig. 2).
Figure 2. Femoral blood flow (FBF) during infusion of the non-selective β-agonist isoproterenol (1–8 ng kg−1 min−1) before (black bars), and 150 min after initiation of non-selective β-blockade with propranolol (grey bars).
Propranolol provided virtually complete blockade of isoproterenol-mediated vasodilatation (n= 9). *P < 0.05 compared to baseline and compared to responses after propranolol.
α2-Adrenergic specificity of BHT-933 in the human thigh (protocol 2)
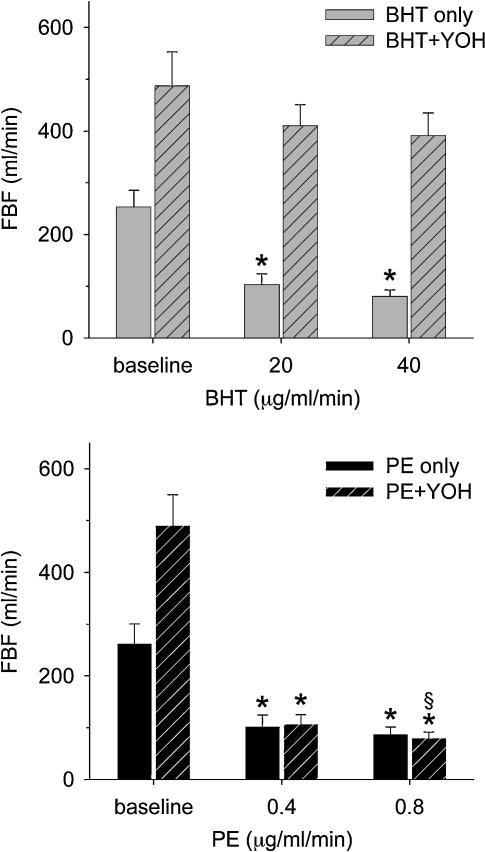
The local vasoconstrictor responses to high doses of BHT and PE both caused resting femoral blood flow to decrease from about 250 ml min−1 to 80 ml min−1, a 60% decrease in flow (Fig. 3). During concomitant yohimbine administration to provide α2-blockade, resting blood flow doubled. However, despite this increase in resting flow PE still caused flow to decrease to about 80 ml min−1, while the effect of BHT was significantly depressed to accomplish only a 20% decrease in flow (Fig. 3).
Figure 3. Specificity of BHT for α2-adrenoreceptors was validated by measurements of femoral blood flow before and after selective α2-blockade with yohimbine.
Top panel, administration of the selective α2-agonist BHT (20 and 40 μg kg−1 min−1) produced significant vasoconstriction (grey bars), and this effect was inhibited following yohimbine (5 μg kg−1 min−1) (hatched bars). Bottom panel, the selective α1-agonist phenylephrine (PE, 0.4 and 0.8 μg kg−1 min−1) produced significant vasoconstriction both before (black bars) and after administration of yohimbine (n= 7) (hatched bars). *P < 0.05 compared to baseline; §P < 0.05 compared to BHT responses after yohimbine.
Dose–response relationship for phenylephrine and BHT at rest and during 27 W exercise (protocol 3)
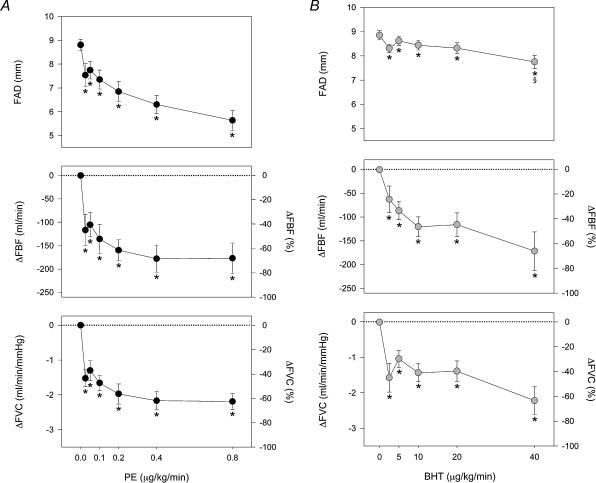
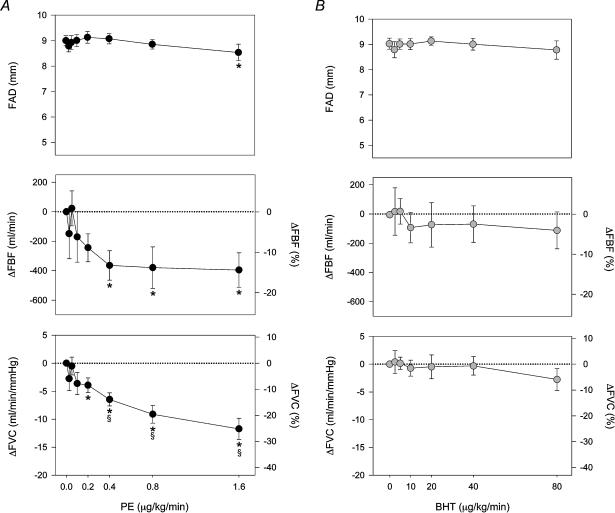
The α-adrenoreceptor agonist dose–response relationships at rest and during 27 W dynamic knee extensor exercise are presented in Figs 4 and 5, respectively. There were no significant changes in blood pressure or heart rate (HR) during infusion of the agonist drugs at rest. FAD diameter decreased following PE administration (from 8.8 ± 0.2 to 5.6 ± 0.4 mm, rest versusPE 0.8 μg kg−1 min−1, P < 0.01), while BHT caused a much smaller FAD change (from 8.9 ± 0.2 to 7.8 ± 0.3 mm, rest versus BHT 40 μg kg−1 min−1, P < 0.05). The change in FAD was significantly greater during PE compared to BHT (P < 0.01). The highest doses of PE and BHT produced comparable reductions in FBF (−58.5 ± 6.3 versus−64.4 ± 4.3% m l min−1, PE 0.8 μg m l−1 min−1versus BHT 40 μg m l−1min−1) and FVC (−57.4 ± 6.6 versus −62.4 ± 4.1%, PE 0.8versus BHT 40), with a clear plateau at the highest doses (Fig. 4A and B).
Figure 4. Dose–response relationships for intra-arterial PE and BHT at rest.
A, during incremental doses of PE, femoral artery diameter (FAD, top) decreased 35%, with concomitant reductions in femoral blood flow (FBF, middle) and femoral vascular conductance (FVC, bottom). B, during incremental doses of BHT, FAD decreased only slightly and only at the highest doses. However, FBF and FVC both decreased significantly, and of similar magnitudes to the responses seen during PE. *P < 0.05 compared to resting baseline, §P < 0.05 between comparable doses of PE and BHT. On the lower four panels the mean ±s.e. symbols are accurate for the absolute changes, whereas the alternative y axis for relative changes has been included for easier comparison to Fig. 5.
Figure 5. Dose–response relationships for intra-arterial PE and BHT during 27 W exercise.
A, during exercise, PE reduced FAD only at the highest dose (top). The changes in FBF (middle) and FVC (bottom) during PE were blunted compared to rest. A significant change in FVC occurred at the highest doses of PE compared to baseline and compared to the highest dose of BHT, and FBF also fell slightly during high doses of PE. B, during exercise, the response to BHT was completely abolished, with no significant change in FAD, FBF, or FVC, even at the highest dose. *P < 0.05 compared to exercising baseline, §P < 0.05 between comparable doses of PE and BHT. On the lower four panels the mean ±s.e. symbols are accurate for the absolute changes, whereas the alternative y axis for relative changes has been included for easier comparison to Fig. 4.
For higher doses of PE, the decrease in FAD dominated blood flow changes. For example, mean blood velocities during baseline and PE 0.8 μg kg−1 min−1 were not significantly different (7.7 ± 0.8 versus 7.6 ± 1.0 cm s−1, n.s.), and despite similar decreases in flow, the mean blood velocity was significantly higher during PE 0.8 μg kg−1 min−1 compared to BHT 40 μg kg−1 min−1 (7.6 ± 1.0 versus 2.9 ± 0.4 cm s−1, P < 0.05). We also observed a transient decrease (about 10 mmHg) in blood pressure and sleepiness following the highest dose of BHT, both lasting around 30 min with some individual variation.
Moderate intensity knee-extensor exercise (27 W) was accompanied by an increase in HR (58 ± 2 versus 78 ± 2 beats min−1, rest versus exercise, P < 0.01) with no significant change in MAP (86 ± 2 versus 83 ± 2 mmHg). Exercising FBF reached about 3000 ml min−1 with no change in FAD (8.9 ± 0.2versus9.0 ± 0.2 mm, restversus exercise). MAP, HR and FBF remained at steady-state levels throughout each exercise bout.
During 27 W exercise, PE infusion produced a small but statistically significant change in FAD (9.1 ± 0.2versus8.7 ± 0.3 mm, rest versus exercise, PE 0.8 μg kg−1 min−1, P < 0.05), and significantly decreased FBF and FVC (Fig. 5A). However, the relative change in flow to the highest dose of PE (0.8 μg kg−1 min−1) was significantly smaller than the response at rest (58 ± 6 versus 12 ± 5%, rest versus exercise, P < 0.01), while the absolute change tended to be higher (188 ± 33 versus 380 ± 141 ml min−1, rest versus exercise, n.s.). During the highest PE doses (PE 0.8 and 1.6 μg kg−1 min−1) we observed significant increases in MAP (9 ± 2 and 15 ± 4 mmHg), with concomitant decreases in HR (−9 ± 1 and −14 ± 2 beats min−1). In contrast, BHT infusion during exercise produced no significant changes in MAP, HR, FAD, FBF, or FVC (Fig. 5B). The difference in FBF response between PE and BHT did not reach significance at this level of exercise. However, the changes in FVC were significantly larger for the last three doses of PE versus BHT (Fig. 5A).
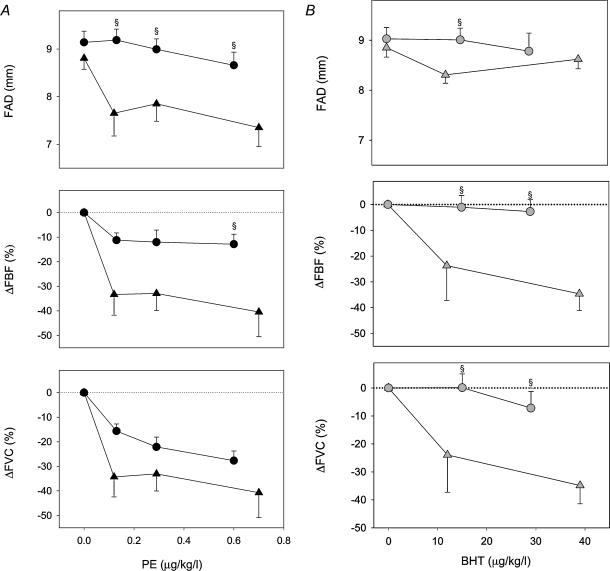
Post-hoc‘flow-adjustment’ was performed to calculate the drug doses yielding similar femoral arterial drug concentration at rest versus exercise. For PE, drug concentrations of 0.025, 0.05 and 0.1 (rest) versus 0.4, 0.8 and 1.6 μg kg−1 (exercise) were used, which resulted in drug deliveries of 0.12, 0.29 and 0.70 versus 0.13, 0.29 and 0.60 μg kg−1 l−1, respectively, n.s. Similarly for BHT, 2.5 and 5 (rest) versus 40 and 80 μg kg−1 min−1 (exercise) gave deliveries of 12 and 32 versus 15 and 29 μg kg−1 l−1, respectively, n.s. When comparing the responses to these flow-adjusted doses, the PE-induced changes in FAD were significantly smaller during exercise compared to rest for all doses (Fig. 6A). However, only the FBF response to the highest flow-adjusted dose of PE was statistically reduced during exercise, and FVC responses at rest and during exercise were not statistically different for PE (Fig. 6A). In contrast, the FBF and FVC responses to both flow-adjusted doses of BHT were significantly reduced during exercise (Fig. 6B).
Figure 6. Post-hoc flow-adjusted doses for PE and BHT at rest (triangles) and during 27 W exercise (circles).
A, PE-induced reduction in FAD (top) was significantly smaller during exercise compared to rest for all flow-adjusted doses. However, only the FBF response (middle) to the highest flow-adjusted dose of PE was statistically reduced during exercise, and FVC responses (bottom) at rest and during exercise were not statistically different during flow-adjusted doses of PE. B, BHT-induced reduction in FAD (top) was attenuated at the lower flow-adjusted dose, and the FBF (middle) and FVC (bottom) responses to both flow-adjusted doses of BHT were significantly reduced. §P < 0.05 compared to flow-adjusted dose–response at rest.
Responses of phenylephrine and BHT-933 at rest and during increasing levels of exercise (protocol 4)
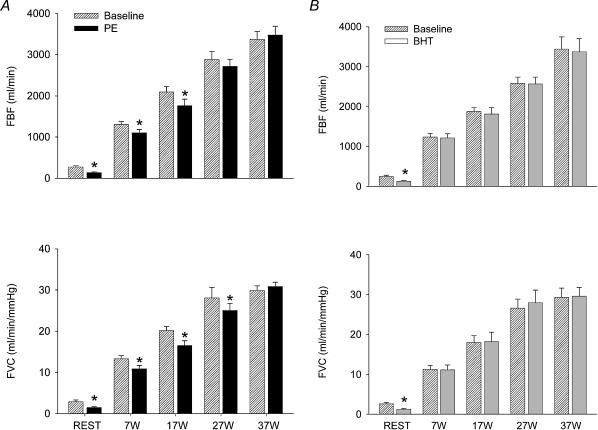
The responses of a single, flow-adjusted dose of the α-agonists PE (0.3 μg kg−1 l−1) and BHT (15 μg kg−1 l−1) during rest and ramped exercise from 7 W to 37 W are presented in Fig. 7A and B, respectively. At rest, FAD changed significantly during both PE (9.4 ± 0.3 versus 8.2 ± 0.5 mm, P < 0.01) and BHT (9.5 ± 0.3 versus 8.9 ± 0.3 mm, P < 0.01). The change in FAD was significantly greater with PE compared to BHT (P < 0.05). PE and BHT produced comparable reductions in FBF (44 ± 7 versus 50 ± 5%, PE versus BHT) and FVC (44 ± 7 versus 50 ± 5%, PE versus BHT).
Figure 7. Absolute FBF and FVC during ramped exercise of 7–37 W with superimposed administration of PE (A) and BHT (B).
Both PE and BHT were flow-adjusted to the exercise-induced increase in FBF. A, at lower workloads (7 and 17 W), PE-induced decreases in FBF and FVC were seen (black bars), and this response was abolished at higher intensities. B, BHT administration caused no changes in FBF or FVC (grey bars) at any exercise intensity. *P < 0.05 compared to predrug value for each level of exercise.
Ramped exercise at 7–37 W significantly increased HR (57 ± 4 at rest to 65 ± 2, 72 ± 3, 77 ± 2 and 82 ± 2 beats min−1 during 7, 17, 27 and 37 W) with no significant change in MAP. FBF increased during exercise to approximately 1250, 2000, 2750 and 3400 ml min−1 (7, 17, 27 and 37 W, respectively) with no significant differences between the two bouts of exercise.
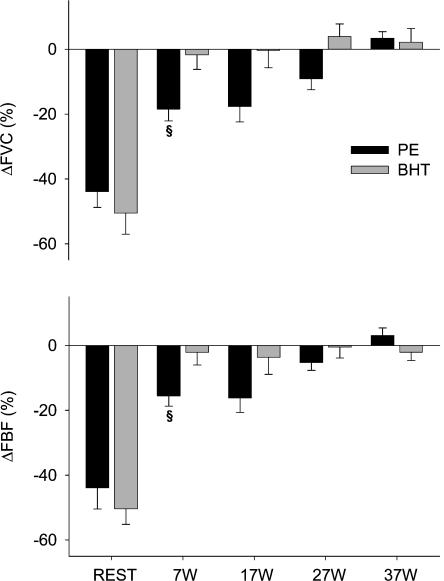
PE significantly reduced FAD at 7 W (from 9.4 ± 0.3 to 8.3 ± 0.5 mm, P < 0.01) and 17 W (from 9.5 ± 0.3 to 9.1 ± 0.4 mm, P < 0.05), decreased in FBF at 7 and 17 W, and reduced FVC at 7, 17 and 27 W (Fig. 7A). At the highest workload (37 W), the effects of PE were abolished. The doses of PE used in this protocol caused no significant changes in MAP or HR. In contrast, BHT caused no significant differences in FAD, FBF or FVC at any intensity (Fig. 7B). BHT caused small (3–4 mmHg) but statistically significant decreases in MAP at 17 and 27 W without changing HR. While the relative changes in FBF and FVC were significantly attenuated at all levels of exercise compared to rest for both agonist drugs, the differences between PE and BHT reached statistical significance only at the 7 W intensity (Fig. 8).
Figure 8. Changes in FBF (top) and FVC (bottom) during ramped exercise of 7–37 W with superimposed administration of PE (black bars) and BHT (grey bars).
All responses to BHT and PE during exercise were significantly smaller than at rest. At lower exercise intensities, PE produced significant changes in FBF and FVC, and these responses were significantly larger than the response to BHT at 7 W §P < 0.05 between PE and BHT at same level of exercise.
In a subset of subjects in protocol 4 (n= 5), the effects of double dosing of PE and BHT were studied during 7 W exercise. For all parameters, this doubling of doses caused no significant changes in the responses to the drugs, indicating that the effects seen with the doses chosen were already near-maximal.
Comparison of Doppler and thermodilution for measuring femoral blood flow during exercise
Femoral blood flows measured during exercise with ultrasound Doppler and thermodilution were always performed within the same minute of steady-state exercise. The average FBFTD and FBFD during 27 W exercise before drug infusions were 2960 ± 240 ml min−1 and 2890 ± 400 ml min−1, respectively (mean ± 95% confidence intervals) (n.s.). The PE and BHT-induced changes were small during exercise with both methods, and at no measurement point did the two methods yield significantly different results (P≥ 0.29 for all paired t tests). The difference in FVC between PE and BHT did not reach statistical significance when using FBFD as the basis for calculating FVC, but the PE effect remained significant. The two methods also yielded very similar results during the ramped exercise protocol, and at no measurement point were the averages from the two methods statistically different (P≥ 0.11 for all paired t tests). The individual and summary data for simultaneous measurements of FBF using Doppler and thermodilution during the first bout of ramped exercise at 7, 17, 27 and 37 W are compared in Fig. 9. As in protocol 3, FBFTD showed less variability within the group than FBFD with the exception of 37 W, where the variabilities were similar. The PE- and BHT-induced changes in blood flow were not different when comparing the two methods, and the PE-induced decreases in FBFD and FVC during 7 and 17 W remained significant. The difference between PE and BHT responses for FBFD during 7 W did not reach statistical significance.
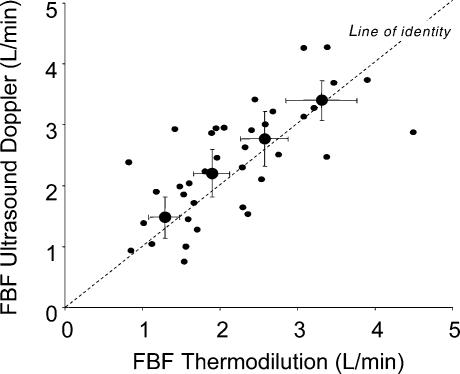
Figure 9. Comparison of ultrasound Doppler and thermodilution techniques for determining femoral blood flow during knee-extensor exercise.
The individual data shown by small filled circles are obtained within the same minute for the two methods in 10 subjects during increasing levels of exercise intensity from 7 to 37 W before administration of α-agonists. The mean values obtained by the two methods, shown by the larger filled circles, did not differ significantly at any intensity of exercise. The variation of the data obtained by the Doppler method was generally larger than the variation of the thermodilution data. Summary data are shown as means ± 95% confidence intervals.
Discussion
There are several major new findings from the present study. First, in dose–response studies α1- and α2-adrenoreceptor agonists produce comparable maximal vasoconstriction in the resting leg. Second, the small α2-mediated compared to the large α1-mediated decrease in femoral artery diameter provides functional evidence that α2-receptors are located predominantly distal in the vascular tree. Third, we demonstrate for the first time metabolic inhibition of both α1- and α2-mediated vasoconstriction in the human leg. Finally, we show that α2- is more sensitive than α1-mediated vasoconstriction to metabolic inhibition in the exercising human thigh. This latter important finding is based on complete inhibition of α2-mediated vasoconstriction even at low workloads, whereas α1-mediated vasoconstriction becomes progressively inhibited with increasing workloads. Collectively, these findings provide novel insight regarding the contribution of α-adrenoreceptor subtypes to the control of muscle blood flow at rest and during exercise.
Evidence for heterogeneous distribution of α-adrenoreceptors
Our finding that femoral artery diameter (FAD) was reduced to a much larger extent following α1-compared to α2-agonist administration provides functional evidence of a heterogeneous distribution of α-adrenoreceptors in the skeletal muscle vasculature of the human leg. This extends observations in the rat (Anderson & Faber, 1991) demonstrating a predominance of α1-adrenoreceptors in the upstream, conduit arteries and α2-receptors in the resistance arterioles of the microcirculation. Thus, the present study has advanced the concept first identified in animals (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991) that large upstream arteries contain both α1- and α2-receptors for the control of arterial blood pressure, while the nutrient arterioles contain predominantly α2-receptors for the fine control of tissue perfusion.
α1- versusα2-mediated vasoconstriction at rest
The maximal reduction in resting FBF following α2-agonist (BHT) application was similar to that seen following α1-agonist (PE) administration. This extends similar observations in the dog hindlimb following intra-arterial agonist infusion of PE (selective for α1) and clonidine (selective for α2) at rest (Buckwalter et al. 2001). Conversely, local administration of selective α-antagonist agents in the resting human forearm indicated an apparent dominance of α2-adrenoreceptor-mediated vascular tone (63% of basal tone) over α1 (Dinenno et al. 2002b). However, calculated differences in control of resting flow do not exclude similar maximal responses to α1- versusα2-agonists. Furthermore, dissimilar α1-responsiveness has recently been reported in the human arm and leg (Pawelczyk & Levine, 2002), suggesting that these two limbs exhibit differences in α1-adrenoreceptor sensitivity and distribution.
Metabolic inhibition of local vasoconstriction in exercising muscle
To our knowledge, we have provided the first pharmacological evidence of metabolic attenuation of α-adrenergic vasoconstriction during dynamic exercise in the human thigh. These findings extend results from studies in the human forearm (Hansen et al. 1996, 2000; Sander et al. 2000; Chavoshan et al. 2002; Tschakovsky et al. 2002; Rosenmeier et al. 2003; Dinenno & Joyner, 2003). We believe there are several major advantages of using knee-extensor exercise over handgrip in this context. First, direct and accurate measurements of blood flow during exercise are feasible by the thermodilution and ultrasound Doppler methods (Andersen & Saltin, 1985; Walloe & Wesche, 1988; Radegran & Saltin, 1998). Second, the fraction of blood flow in the femoral artery reaching the thigh muscles during knee-extensor exercise is more than 95% while using an occlusive cuff below the knee (Savard et al. 1988). In contrast, rhythmic handgrip cannot be sustained with an occlusive wrist cuff (Tschakovsky et al. 2002), and the fraction of brachial artery blood flow reaching contracting muscle at low intensity exercise is below 90% (Wahren, 1966). Third, knee-extensor exercise up to 30 W (around 40% of maximum) can be sustained for hours with steady-state blood flow and pressure, without fatiguing and with no significant activation of the sympathetic nervous system as measured by plasma noradrenaline (Turcotte et al. 1992; Steensberg et al. 2002). In contrast, to sustain rhythmic handgrip for more than 10 min requires very low intensities (10% of maximum) (Wahren, 1966; Rosenmeier et al. 2003). At higher intensities fatigue and evidence of increasing activation of muscle sympathetic nerve activity quickly become apparent (Tschakovsky et al. 2002). In the present study, these unique attributes of the human knee-extensor model of exercise allowed definition of the level of intensity required for metabolic inhibition of sympathetic vasoconstriction and definition of the pharmacological dose responses at a given intensity of thigh exercise.
Previous studies in humans and animals have pointed to a post-junctional site of interaction between skeletal muscle metabolic events and α-adrenergic vasoconstriction. In this regard, responses to reflex-mediated increases in muscle sympathetic nerve activity, direct sympatho-neural stimulation, indirect acting sympathomimetics (tyramine) and intra-arterial directly acting α-agonists are all attenuated during exercise (Thomas et al. 1994, 1998; Hansen et al. 1996, 2000; Sander et al. 2000; Buckwalter et al. 2001; Chavoshan et al. 2002; Tschakovsky et al. 2002; Ruble et al. 2002; Keller et al. 2003; Rosenmeier et al. 2003). A wide array of factors contributing to the observed alteration in vascular responsiveness during exercise have been proposed, including vasoactive substances such as adenosine (Laughlin et al. 1989; Saltin et al. 1998), prostaglandins and thromboxanes (Karamouzis et al. 2001), the indirect effect of increased muscle temperature (Cooke et al. 1984), hypoxia (Hansen et al. 2000), and acidosis (McGillivray-Anderson & Faber, 1990). These factors are not mutually exclusive.
It is noteworthy that several different protocols performed in both rodents and humans have identified skeletal muscle production of nitric oxide as an important metabolic event. Lack of skeletal muscle nitric oxide synthase or pharmacological blockade of nitric oxide production has been accompanied by loss of metabolic inhibition of sympathetic vasoconstriction (Thomas et al. 1998, 2003; Sander et al. 2000; Chavoshan et al. 2002; Fadel et al. 2003). One recent handgrip study was not able to demonstrate a significant effect of pharmacological blockade of nitric oxide synthase on the vasoconstriction induced by α-agonists in the human forearm (Dinenno & Joyner, 2003). This study clearly demonstrates exercise-induced inhibition of sympathetic vasoconstriction during handgrip and therefore suggests that nitric oxide may not be the only important signalling molecule mediating metabolic inhibition of sympathetic vasoconstriction.
Stimulation of the α-receptors in the exercising limb has often produced some degree of vasoconstriction. This was also the case in the present study for phenylephrine during lower exercise intensities. Conflicting evidence exists regarding the importance of functional sympatholysis versus sympathetic vasoconstriction in exercising skeletal muscle, and has been the subject of several comprehensive reviews (Laughlin et al. 1996; Rowell, 1997). The principal point of contention centres on the paradox of vasoconstriction of the exercising muscle vasculature, which seems counterproductive to the effort of increasing blood flow to meet the metabolic demands of the muscle. Several studies have addressed whether blood flow to exercising muscle or blood pressure is prioritized during heavy exercise of large muscle groups, with conflicting results (Secher et al. 1977; Savard et al. 1989; Richter et al. 1992; Strange, 1999). However, it is now well accepted that perfusion of the contracting skeletal muscle is ultimately a balance between metabolic vasodilatation and sympathetic vasoconstriction (VanTeeffelen & Segal, 2003; Joyner & Thomas, 2003). The underlying mechanisms guarding this balance are still incompletely understood.
α1- versusα2-mediated vasoconstriction during exercise
Experimental evidence in microvascular and animal preparations has suggested that α2-mediated vasoconstriction is more sensitive to metabolic inhibition than α1-mediated vasoconstriction (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991; Thomas et al. 1994; Buckwalter et al. 2001; Ruble et al. 2002). Thus, the vasoconstrictor responses to specific α2-agonists such as UK 14304 and clonidine were more effectively inhibited during exercise than α1-agonists such as phenylephrine and the non-specific agonist noradrenaline. Microvascular studies in rats have identified a heterogeneous distribution of α-receptors, with α2-receptors located predominantly in the peripheral vascular bed (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991). Taken together, these findings have prompted the hypothesis that preserved α1-responsiveness would help to maintain blood pressure by keeping vascular tone in arteries and large arterioles, while attenuation of α2-responsiveness in smaller arterioles would help increase nutrient blood flow to the exercising muscle fibres.
In humans, mild handgrip exercise was recently shown to attenuate both phenylephrine- and clonidine-mediated vasoconstriction compared to rest (Rosenmeier et al. 2003). However, this study used only one level of exercise and one dose (flow-adjusted) of each drug, and therefore was not powered to detect differences between the attenuation of α1- versusα2-mediated vasoconstriction. The present study extends these findings to the human leg, because we also found evidence for attenuation of both α1- and α2-mediated vasoconstriction, and our dose–response curves provide evidence that even the maximal responses are attenuated. Using the ramped exercise protocol, we also demonstrated a residual vasoconstriction to phenylephrine (α1-agonist) at low and moderate exercise intensity, whereas BHT-933 (α2-agonist) did not produce vasoconstriction at any exercise intensity. Thus, a major contribution of the present study is the demonstration of a greater sensitivity of α2- than α1-adrenoreceptor subtypes to the metabolic byproducts produced during exercise in the human thigh.
The mechanisms underlying differential exercise-induced inhibition of α1- than α2-mediated vasoconstriction are not understood, but could be related to either ligand–receptor affinity or the signal transduction following receptor activation. Regarding the relative affinity, we used doses of BHT-933 that were about 50 times higher than the doses of phenylephrine. This is in accordance with the differences in dosing used in human forearm (Jie et al. 1984; Lembo et al. 1994) and dog carotid artery studies (Willems et al. 2001). It is unknown whether agonist binding to the receptors is decreased during exercise. Our dose–response curves for phenylephrine during exercise argue against this mechanism for the attenuation of phenylephrine responses, because with a ‘simple’ affinity issue phenylephrine would be expected to have a similarly high maximum effect at rest and during exercise. The lack of significant responses to any dose of BHT-933 during exercise precludes any speculation on this putative mechanism for this agonist.
Degree of exercise-induced inhibition of α-adrenergic responses
Previous studies have demonstrated seemingly different degrees of exercise-induced attenuation of α-adrenergic vasoconstriction. For non-specific α-adrenergic or α1-adrenergic agonists some previous animal studies have shown very little exercise-induced attenuation of the response (Thomas et al. 1994; Buckwalter et al. 1998). In contrast, more recent animal studies conclude that phenylephrine responses are attenuated during exercise (Buckwalter et al. 2001). Furthermore, recent human forearm studies (Rosenmeier et al. 2003; Dinenno & Joyner, 2003), and the present study, agree that even mild exercise causes significant attenuation of the phenylephrine response such that maximal responses seen during exercise are around 10–20% of blood flow, compared to 50–60% at rest. The reason for this apparent development of results from earlier to more recent studies is not clear. For α2-adrenergic agonists, animal studies report different degrees of exercise-induced attenuation of the response. In rat hindlimb, heavy evoked exercise caused a complete inhibition of the response to the α2-agonist UK14304 (Thomas et al. 1994), whereas in the conscious dog, the hindlimb response to the α2-agonist clonidine remained significant, around 15% at heavy running (Buckwalter et al. 2001). The differences in these studies were several, including species and choice of α2-agonist. Regarding the latter though, in vitro studies have identified UK14304 as a full agonist but clonidine as a partial agonist (Wise et al. 1997), which would not explain why clonidine seems to cause larger in vivo effects than UK14304. In the recent human handgrip studies, the response to clonidine also remains at around 15% of brachial blood flow during mild exercise (Rosenmeier et al. 2003; Dinenno & Joyner, 2003), whereas in the present study there was no detectable response in the thigh circulation to BHT-933 even during mild exercise. The obvious differences between these studies include the choice of limb and α2-agonist. The choice of limb may explain part of the difference, because non-exercising muscle and tissue like the skin receive a larger proportion of brachial blood flow during handgrip than femoral blood flow during knee-extensor exercise. The choice of α2-agonist is also likely to be important, because clonidine has been reported to be less specific compared to BHT-933 (Jie et al. 1984). Thus, the higher flow-adjusted doses of clonidine used during exercise may cause α1-adrenergic receptor stimulation.
Significance of functional sympatholysis in the leg
The leg represents a large muscle mass with an enormous capacity to vasodilate during exercise, reaching up to 2.5 l kg−1 min−1 (Andersen & Saltin, 1985) and often receiving the majority of cardiac output. Others have observed that the increase in skeletal muscle blood flow accompanied by the increase in fraction of cardiac output with exercise means that small changes in vascular conductance can greatly influence arterial pressure (O'Leary, 1991a; Tschakovsky et al. 2002). Thus, exercise produces a condition that demands a precise balance between neural and metabolic control of flow to maintain homeostasis. The vascular bed of the leg represents a ‘sleeping giant’ that may necessitate a haemodynamic response unique to this large muscle group (Andersen & Saltin, 1985). Accordingly, the observed attenuation of α-adrenergic vasoconstriction may be a protective mechanism, insuring adequate blood flow to the exercising tissue in the face of increased sympathetic outflow.
As noted above, muscle sympathetic nervous activity, measured by plasma noradrenaline, does not increase significantly at knee-extensor exercise intensities below 30 W (Turcotte et al. 1992; Steensberg et al. 2002). Since metabolic attenuation of α-adrenergic vasoconstriction is evident in our study even at intensities well below 30 W, it would seem sympathetic neural activation does not occur until well after the thigh muscle is ‘protected’ by functional sympatholysis. This extends similar findings in studies of handgrip exercise (Hansen et al. 1996; Sander et al. 2000).
Experimental limitations
The potential experimental limitations of the present study are related to the use of exogenous vasoconstrictors administered luminally. Physiological control of peripheral adrenergic receptors takes place at the abluminal side, where noradrenaline released from sympathetic boutons primarily acts on proximate receptors located on vascular smooth muscle cells. Sympathetic boutons are more abundant at the level of the resistance arterioles compared to large arteries. When agonists are administered intra-arterially both luminal endothelial receptors and abluminal smooth muscle receptors are activated. However, in previous studies in the human forearm the responses to direct acting adrenergic agonists and the indirect sympathomimetic tyramine are attenuated to similar degrees during handgrip (Tschakovsky et al. 2002; Rosenmeier et al. 2003; Dinenno & Joyner, 2003).
In these human studies, as well as in some animal studies, the sympathomimetic dosing has been ‘flow-adjusted’ to achieve similar intra-arterial drug concentration (Buckwalter et al. 1998; Ruble et al. 2002; Tschakovsky et al. 2002; Rosenmeier et al. 2003). Furthermore, in several studies α-adrenergic responses during exercise have been compared with the responses seen during local pharmacological vasodilatation administered to match exercise hyperaemia (Tschakowsky et al. 2002; Rosenmeier et al. 2003). When phenylephrine, clonidine and tyramine are superimposed on adenosine- or nitroprusside-induced vasodilatation, the result has invariably been large decreases in blood flow. This approach has served to validate that the diminished vasoconstrictor responses during exercise are not related to changes in drug delivery during increases in blood flow.
It should be noted that flow-adjusting the dose of sympathomimetic does not secure adjustment of drug concentration in the vascular wall interstitium or receptor occupancy. The higher absolute drug dose would tend to increase and the lower transit time to decrease receptor occupancy when using flow-adjusted dosing during exercise. This may be a concern, especially when the drugs are administered as bolus injections (Buckwalter et al. 1998; Ruble et al. 2002). Another caveat to flow-adjusted doses is that the resultant intra-arterial α-agonist concentration will be different during the latter part of drug administration in a condition where flow does not change much in response to the agonist drug (i.e. exercise) compared to a condition where flow decreases by about 50% in response to the agonist drug (i.e. resting pharmacological vasodilatation) (Rosenmeier et al. 2003). In the present study, the wide dose–response ranges in combination with flow-adjusted dosing (both by design and post hoc) have minimized these potential concerns of using intra-arterial infusion of agonists.
When using pharmacological tools, the specificity of the drugs should always be considered. Phenylephrine has the ability to activate β-adrenergic receptors (Torp et al. 2001). This effect is unlikely to be of major importance in the present study, since exercise attenuated phenylephrine responses to similar degrees both with and without complete β-blockade by the non-specific β-blocker propranolol (protocols 3 and 4). We chose phenylephrine, because it is well accepted to be a relatively specific α1-agonist. We chose BHT-933 as an α2-agonist, rather than the classical drug clonidine, because in a previous study clonidine actions in the human forearm were inhibited by both the α1- (prazosin) and α2-antagonist (yohimbine), whereas BHT-933 actions were only inhibited by yohimbine (Jie et al. 1984). Our data (protocol 2) supported the specificity of PE for α1-receptors since the α2-antagonist yohimbine caused no decrease in the response to PE, and provided the first evidence in humans that BHT actions in the human thigh are largely inhibited by an α2-antagonist.
When using subtype-selective agonists, the differential location of the receptors should also be considered. Specifically, α1-receptors are almost exclusive located postsynaptically on vascular smooth muscle, while α2-receptors have been identified both on smooth muscle and presynaptically on the sympathetic boutons. Activation of the presynaptic α2-receptors is known to cause inhibition of noradrenaline release from the boutons, which may decrease effects on postsynaptic receptors (Guimaraes & Moura, 2001). In the present study, this presynaptic effect of BHT-933 is not dominant at rest, because the maximal vasoconstriction of BHT-933 is simlar to that of PE. During mild and moderate intensities of knee-extensor exercise, previous studies have demonstrated that noradrenaline levels remain similar to resting values (Turcotte et al. 1992; Steensberg et al. 2002). Furthermore, during increasing levels of sympathetic activation and noradrenaline release the presynaptic effects of α2-agonists decrease, probably because noradrenaline has already activated the receptors (Guimaraes & Moura, 2001). Thus, the presynaptic effects of BHT-933 may contribute to but are unlikely to completely explain the lack of vasoconstriction during exercise in the present study.
One disadvantage of using the knee-extensor exercise model is that the maximal drug doses may have minor systemic effects. The highest intrafemoral doses of PE caused significant changes in blood pressure and heart rate during exercise, but not during rest. The explanation for this differential effect is likely to be related to the different transit time of PE during the two conditions. At rest the mean transit time during PE administration in the thigh is likely to be more than 15 s in the leg alone, while during exercise the transit time is decreased to 5 s (Bangsbo et al. 2000). Thus, the uptake and degradation of PE may be incomplete during exercise. It is worth emphasizing, that such a ‘flow-through phenomenon’ is unlikely to explain the exercise-induced inhibition of α-agonist effects, because simply increasing flow and lowering transit time by vasodilators in other studies have not been sufficient to inhibit α-agonist vasoconstriction (Rosenmeier et al. 2003; Dinenno & Joyner, 2003).
In the present study, the dose–response and ramped exercise protocols both included the responses to phenylephrine at 0.3 μg kg−1 l−1 min−2 and to BHT-933 at 15 μg kg−1 l−1 min−2 at rest and during 27 W knee-extensor exercise. In both protocols, the overall findings were similar, because phenylephrine and BHT-933 caused large decreases in femoral blood flow and conductance at rest, and visibly smaller responses during exercise. It should be noted, however, that the blood flow decrease in response to phenylephrine during exercise reached significance only in the dose–response protocol, whereas the response to phenylephrine during exercise versus rest was only significant in the ramped exercise protocol. The reason for this difference in the responses to phenylephrine in the two protocols is unclear, but could be related to the difference in the duration and total dose of the phenylephrine infusion. The responses to BHT-933 during 27 W exercise were very similar in both protocols.
By design we have not attempted in the present study to address whether residual sympathetic vasoconstriction from endogenously released noradrenaline exists in the exercising human thigh. This could be addressed pharmacologically by using α-antagonists rather than agonists. In animals, previous studies using this approach have yielded conflicting results. The literature has been reviewed in the discussion of a recent study in exercising dogs which provided evidence for both residual α1- and α2-vasoconstriction in exercising legs (Buckwalter & Clifford, 1999). In humans, systemic α-blockade is not accompanied by increases in blood flow to exercising forearm (Hartling & Trap-Jensen, 1983). In contrast, 20–30% increases in plethysmographic blood flows were seen during heavy handgrip exercise after stellate block (Joyner et al. 1992). In pilot studies, we have found intra-arterial administration of the α1-blocker prazosin into the femoral artery yields incomplete α1-blockade in the human leg, even at doses that cause severe orthostatic hypotension. Thus, adjustments to the exercise model or to the pharmacological intervention are needed before α-antagonist studies can be performed in the human thigh.
Conclusions and potential clinical significance
We have identified a functionally differential distribution of α-adrenoreceptor subtypes in the vasculature of the human leg. Furthermore, we have demonstrated a higher sensitivity of α2-mediated vasoconstriction compared to α1-mediated vasoconstriction to the metabolic events taking place during mild thigh muscle exercise. Strikingly, we have observed that moderate intensity knee-extensor exercise completely inhibits the vasoconstrictor actions of intra-arterially administered exogenous sympathomimetics. Knowledge of these mechanisms may provide the clinician with a better understanding of the functional consequences of pathological vascular disorders associated with age, diabetes, heart failure and hypertension, and present possible diagnostic techniques and methods of treatment. For example, an age-related reduction in α-adrenergic responsiveness specific to α1-receptors has recently been described (Dinenno et al. 2002a). This emphasizes the need for a better understanding of subtype contributions to peripheral vascular control. Furthermore, it has been demonstrated that patients suffering from congestive heart failure experience diminished skeletal muscle perfusion at rest and during submaximal and maximal exercise, with accompanying increased vascular resistance (Sullivan et al. 1989). Recent experimental and clinical trials indicate that this relative hypoperfusion is related to an inability of the skeletal muscle to adequately vasodilate in opposition to the increased sympathetic nerve activity that accompanies exercise (Johnson et al. 1999; Notarius et al. 1999; Thomas et al. 2001). Since these patients might benefit from exercise as prophylaxis and as part of a cardiac rehabilitation program (Monchamp & Frishman, 2002), knowledge of α-adrenoceptor contribution to the control of blood flow might provide a therapeutic target in this population.
Acknowledgments
This study was performed at the Copenhagen Muscle Research Center with the financial support of the Danish National Research Foundation (504-14). M. Sander was supported by grants and fellowships from the Danish Heart Foundation, the Danish Medical Research Council, the Novo Foundation, and the Kaj Hansen Foundation. This study was also supported in part by funding from NIH (HL045547) and NASA (NAG 9-1262). P.J.F. is currently an individual National Institute of Health-National Research Service Award postdoctoral fellow in the Division of Hypertension at the University of Texas Southwestern Medical Center in Dallas. This work was submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy for D.W.W., as submitted to the Graduate School of Biomedical Science, University of North Texas Health Science Center at Fort Worth.
References
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KM, Faber JE. Differential sensitivity of arteriolar α1- and α2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. α-Adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol. 1999;277:H33–H39. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. α1-Adrenergic-receptor responsiveness in skeletal muscle during dynamic exercise. J Appl Physiol. 1998;85:2277–2283. doi: 10.1152/jappl.1998.85.6.2277. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JP, Shepherd JT, Vanhoutte PM. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ Res. 1984;54:547–553. doi: 10.1161/01.res.54.5.547. [DOI] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB. Blood flow in normal and denervated muscle during exercise in conscious rats. Am J Physiol. 1988;255:H1509–H1515. doi: 10.1152/ajpheart.1988.255.6.H1509. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002a;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional α-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002b;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory. J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol. 2003;549:243–253. doi: 10.1113/jphysiol.2003.038828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: An update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest. 1996;98:584–596. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol. 1994;266:H2508–H2514. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- Hartling OJ, Trap-Jensen J. Haemodynamic and metabolic effects of α-adrenoceptor blockade with phentolamine at rest and during forearm exercise. Clin Sci. 1983;65:247–253. doi: 10.1042/cs0650247. [DOI] [PubMed] [Google Scholar]
- Jie K, Van Brummelen P, Vermey P, Timmermans PB, Van Zwieten PA. Identification of vascular postsynaptic α1- and α2-adrenoceptors in man. Circ Res. 1984;54:447–452. doi: 10.1161/01.res.54.4.447. [DOI] [PubMed] [Google Scholar]
- Johnson W, Lucas C, Stevenson LW, Creager MA. Effect of intensive therapy for heart failure on the vasodilator response to exercise. J Am Coll Cardiol. 1999;33:743–749. doi: 10.1016/s0735-1097(98)00631-7. [DOI] [PubMed] [Google Scholar]
- Johnsson G. The effects of intra-arterially administered propranolol and H 56–28 on blood flow in the forearm – a comparative study of two beta-adrenergic antagonists. Acta Pharmacol Toxicol. 1967;263:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. doi: 10.1113/jphysiol.2003.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamouzis M, Langberg H, Skovgaard D, Bulow J, Kjaer M, Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiol Scand. 2001;171:71–76. doi: 10.1046/j.1365-201X.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- Keller DM, Wasmund WL, Wray DW, Ogoh S, Fadel PJ, Smith ML, Raven PB. Carotid baroreflex control of leg vascular conductance at rest and during exercise. J Appl Physiol. 2003;94:542–548. doi: 10.1152/japplphysiol.00817.2002. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol. 1989;257:H1507–H1515. doi: 10.1152/ajpheart.1989.257.5.H1507. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 705–769. [Google Scholar]
- Lembo G, Iaccarino G, Vecchione C, Barbato E, Izzo R, Fontana D, Trimarco B. Insulin modulation of an endothelial nitric oxide component present in the α2- and β-adrenergic responses in human forearm. J Clin Invest. 1997;100:2007–2014. doi: 10.1172/JCI119732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo G, Iaccarino G, Rendina V, Volpe M, Trimarco B. Insulin blunts sympathetic vasoconstriction through the α2-adrenergic pathway in humans. Hypertension. 1994;24:429–438. doi: 10.1161/01.hyp.24.4.429. [DOI] [PubMed] [Google Scholar]
- McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth muscle by α1- and α2-adrenoceptors. Implications for neural and metabolic regulation. Circ Res. 1990;66:1643–1657. doi: 10.1161/01.res.66.6.1643. [DOI] [PubMed] [Google Scholar]
- Monchamp T, Frishman WH. Exercise as a treatment modality for congestive heart failure. Heart Dis. 2002;4:110–116. doi: 10.1097/00132580-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Ando S, Rongen GA, Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J. 1999;20:880–887. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses. Am J Physiol. 1991a;260:H632–H637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Rowell LB, Scher AM. Baroreflexinduced vasoconstriction in active skeletal muscle of conscious dogs. Am J Physiol. 1991b;260:H37–H41. doi: 10.1152/ajpheart.1991.260.1.H37. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect. J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. Am J Physiol. 1995;269:H1545–H1552. doi: 10.1152/ajpheart.1995.269.5.H1545. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kies B, Hargreaves M, Kjaer M. Effect of arm-cranking on leg blood flow and noradrenaline spillover during leg exercise in man. Acta Physiol Scand. 1992;144:9–14. doi: 10.1111/j.1748-1716.1992.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Clifford PS. Dynamic exercise attenuates sympathetic responsiveness of canine vascular smooth muscle. J Appl Physiol. 2000;89:2294–2299. doi: 10.1152/jappl.2000.89.6.2294. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszcynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol. 1988;64:649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: Role of muscle mass. Am J Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand. 1977;100:288–297. doi: 10.1111/j.1748-1716.1977.tb05952.x. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Van Hall G, Keller C, Osada T, Schjerling P, Pedersen BK, Saltin B, Febbraio MA. Muscle glycogen content and glucose uptake during exercise in humans: influence of prior exercise and dietary manipulation. J Physiol. 2002;541:273–281. doi: 10.1113/jphysiol.2001.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol. 1999;514:283–291. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires α-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res. 2001;88:816–823. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- Torp KD, Tschakovsky ME, Halliwill JR, Minson CT, Joyner MJ. β-Receptor agonist activity of phenylephrine in the human forearm. J Appl Physiol. 2001;90:1855–1859. doi: 10.1152/jappl.2001.90.5.1855. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle. J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianitis S, Krustrup P, Dawson E, Secher NH. Arm blood flow and oxygenation on the transition from arm to combined arm and leg exercise in humans. J Physiol. 2003;547:641–648. doi: 10.1113/jphysiol.2002.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J. Quantitative aspects of blood flow and oxygen uptake in the human forearm during rhythmic exercise. Acta Physiol Scand. 1966;67(Suppl. 269):1–93. [PubMed] [Google Scholar]
- Walloe L, Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol. 1988;405:257–273. doi: 10.1113/jphysiol.1988.sp017332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems EW, Valdivia LF, Ramirez-San Juan E, Saxena PR, Villalon CM. Pharmacological identification of the major subtypes of adrenoceptors involved in the canine external carotid vasoconstrictor effects of adrenaline and noradrenaline. Life Sci. 2001;69:143–153. doi: 10.1016/s0024-3205(01)01105-5. [DOI] [PubMed] [Google Scholar]
- Wise A, Carr IC, Groarke DA, Milligan G. Measurement of agonist efficacy using an α2a-adrenoceptor-Gilα fusion protein. FEBS Lett. 1997;419:141–146. doi: 10.1016/s0014-5793(97)01431-2. [DOI] [PubMed] [Google Scholar]