Abstract
For much of the 20th century, lactate was largely considered a dead-end waste product of glycolysis due to hypoxia, the primary cause of the O2 debt following exercise, a major cause of muscle fatigue, and a key factor in acidosis-induced tissue damage. Since the 1970s, a ‘lactate revolution’ has occurred. At present, we are in the midst of a lactate shuttle era; the lactate paradigm has shifted. It now appears that increased lactate production and concentration as a result of anoxia or dysoxia are often the exception rather than the rule. Lactic acidosis is being re-evaluated as a factor in muscle fatigue. Lactate is an important intermediate in the process of wound repair and regeneration. The origin of elevated [lactate] in injury and sepsis is being re-investigated. There is essentially unanimous experimental support for a cell-to-cell lactate shuttle, along with mounting evidence for astrocyte–neuron, lactate–alanine, peroxisomal and spermatogenic lactate shuttles. The bulk of the evidence suggests that lactate is an important intermediary in numerous metabolic processes, a particularly mobile fuel for aerobic metabolism, and perhaps a mediator of redox state among various compartments both within and between cells. Lactate can no longer be considered the usual suspect for metabolic ‘crimes’, but is instead a central player in cellular, regional and whole body metabolism. Overall, the cell-to-cell lactate shuttle has expanded far beyond its initial conception as an explanation for lactate metabolism during muscle contractions and exercise to now subsume all of the other shuttles as a grand description of the role(s) of lactate in numerous metabolic processes and pathways.
Introduction
In 1950, von Muralt distinguished four different eras in the development of muscle chemistry: pre-lactic acid, lactic acid, phosphorylation, and myosin. The pre-lactic acid era began in 1808 with Berzelius's discovery of an elevated concentration of lactate in ‘ the muscles of hunted stags’ (see Brooks & Gladden, 2003). Although there were several studies of lactic acid (HLa) in the next 99 years (see Brooks & Gladden, 2003), confusion reigned until the landmark studies of Fletcher & Hopkins (1907). Their paper ushered in the lactic acid era during which A. V. Hill's studies suggested that HLa was the immediate energy donor for muscle contractions and Meyerhof demonstrated that glycogen was the precursor of lactate (La−) (e.g. Meyerhof, 1920). Between 1926 and 1932, ATP and PCr were discovered and investigations were begun to determine which of these phosphagens might be the direct energy donor for muscle contraction (see Brooks & Gladden, 2003). These discoveries and new ideas changed the field of muscle energetics so profoundly that A. V. Hill (1932) called the experiments over the 1926–32 time period ‘the revolution in muscle physiology’. Accordingly, the 1930s marked the beginning of the phosphorylation period of muscle chemistry. In 1939, the myosin period began with the finding that the enzyme responsible for ATP hydrolysis was associated with the muscle protein, myosin (see von Muralt, 1950 for details and references). By the early 1940s, the full Emben-Meyerhof (glycolytic) pathway had also been elaborated.
If we restrict our considerations to HLa and its metabolism, we might term the period from the 1930s to approximately the early 1970s the dead-end waste product era. During this period, La− was largely considered to be a dead-end metabolite of glycolysis resulting from muscle hypoxia (Wasserman, 1984). Lactic acid was also believed to be the primary cause of the slow component of the O2 debt (Margaria et al. 1933) and a major cause of muscle fatigue (Hermansen, 1981). Since the early 1970s, a ‘lactate revolution’ has occurred. At present, we are in the midst of a lactate shuttle era which began in 1984 with the introduction of the lactate shuttle hypothesis by George Brooks (1985a).
In the following sections, I will attempt to capsulize some of the new ideas that are currently at the cutting-edge of continued investigations into La− metabolism in this lactate shuttle era. In order to limit the list of references, I will often cite examples and recent reviews rather than using complete, chronological sets of citations.
Lactate and O2 during exercise: is lactate an anaerobic metabolite?
Numerous studies beginning with those of Pasteur (see Keilin, 1966) in the 18th century demonstrated that anoxia and hypoxia stimulate cellular HLa production. For example, in 1891, Araki (cited in Karlsson, 1971) reported elevated La− levels in the blood and urine of a variety of animals subjected to hypoxia. Then, Fletcher & Hopkins (1907) found an accumulation of La− in anoxia as well as after prolonged stimulation to fatigue in amphibian muscle in vitro. Subsequently, based on the work of Fletcher & Hopkins (1907) as well as his own studies, Hill et al. (1924) postulated that HLa increased during muscular exercise because of a lack of O2 for the energy requirements of the contracting muscles.
There is no disagreement that PO2 values in the range of ∼0.5 Torr or less result in O2-limited cytochrome turnover, and therefore O2-limited oxidative phosphorylation, a condition termed dysoxia (Connett et al. 1990). However, problems have arisen because of the application of the converse of this construct, i.e. that elevated HLa production and accumulation necessarily indicate the presence of dysoxia. This supposition formed the groundwork for the anaerobic threshold concept, which was introduced and detailed by Wasserman and colleagues in the 1960s and early 1970s (see Wasserman, 1984). The basic anaerobic threshold paradigm is that elevated HLa production and concentration during muscular contractions or exercise are the result of O2-limited oxidative phosphoryation. Similarly, standard medical practice has accepted an elevated blood La− concentration ([La−]) as the herald of O2 insufficiency (Mizock & Falk, 1992).
Over the past 35 years, considerable evidence has mounted against the idea of dysoxia as the primary cause of increased HLa production and accompanying increases in muscle and blood [La−] during submaximal exercise (e.g. Connett et al. 1986) and in some clinical situations as well (see below). Recently, Richardson et al. (1998) used proton magnetic resonance spectroscopy (1H-MRS) to determine myoglobin saturation (and thereby estimate intramuscular PO2) during progressive single-leg quadriceps exercise in humans. Increasing La− efflux with increasing work rate did not appear to be the result of inadequate O2 and thereby O2-limited oxidative phosphorylation. Instead, as the intramuscular PO2 (iPO2) decreases, oxidative metabolism becomes O2 dependent (see Gladden, 1996). Within some low range of iPO2 (< 20 Torr?), larger increases in [NADH]/[NAD+] and ([ADP][Pi]/[ATP]) are required to maintain adequate stimulation of cellular respiration to meet the aerobic ATP demand. The connection to increasing La− production and higher muscle and blood [La−] is that the requisite increase in ([ADP][Pi]/[ATP]), to compensate for the lower iPO2, is a potent stimulus of glycolysis. (For further details of this paradigm, see Connett et al. 1990; Gladden, 1996.) Accordingly, the best evidence indicates that O2 is only one of several interacting factors that cause an increase in muscle and blood [La−] at submaximal exercise intensities. Additional factors are listed in Table 1 and some are discussed in detail by Gladden (2003).
Table 1.
Causes of increased lactate accumulation with increasing exercise intensity
| I | Accelerating glycolysis | ||
| 1. | a. | La− production depends on a competition for pyruvate and NADH between LDH and the NADH shuttles (malate–aspartate and glycerol phosphate) and the pyruvate transporter (e.g. Gladden, 1996). and/or | |
| b. | High activity of LDH and Keq of pyruvate to lactate reaction guarantees HLa production particularly with increasing glycolytic rate (e.g. Brooks, 1998, 2000;Brooks et al. 1999a,b). | ||
| 2. | a. | More broadly, La− production can be viewed as dependent on the balance of biochemical competition between the activities of Phos/PFK versus the activity of PDH (Parolin et al. 1999). | |
| b. | Phos is activated by increased work rate probably due to ↑[Ca2+], ↑[Pi] and ↑[AMP]; this increases glycolytic rate ⇒↑ La− production (e.g. Spriet, 1992; Parolin et al. 1999; Rush & Spriet, 2001). | ||
| c. | With increased exercise intensity; [ATP]↓, [ADP]↑, [AMP]↑, [Pi]↑, and [ammonia]↑⇒ PFK activation and ↑ La− production (e.g. Spriet, 1991). | ||
| d. | Sympathoadrenal activity increases with work rate; adrenaline activates Phos and thereby ↑ glycolysis and La− production (e.g. Drummond et al. 1969; Spriet, 1992; Parolin et al. 1999). | ||
| e. | ↑[Ca2+] may act in a feed-forward manner to activate Phos and PFK independently of metabolic feedback (e.g. Parolin et al. 1999). | ||
| 3. | Coordinated changes in the activities of other glycolytic enzymes occur via mechanisms that are not fully understood (metabolic control analysis) (e.g. Thomas & Fell, 1998)? | ||
| 4. | Intracellular perfusion rates increase in proportion to escalating ATP demand, thus increasing enzyme-substrate encounter rates (Hochachka, 1999)? | ||
| 5. | Na+–K+-ATPase is progressively stimulated by increasing exercise intensity (via ↑[Na+i]?) and by increasing hormonal concentrations, particularly the catecholamines (Nielsen & Clausen, 2000)? Increased Na+–K+-ATPase activity leads to increased lactate production perhaps by way of an association of glycolytic enzymes with Na+–K+ pumps (James et al. 1999a,b)? | ||
| II. | O2-dependent metabolism | ||
| With increasing exercise intensity, intramuscular PO2 decreases either progressively or sharply at ∼60% of V̇O2max. Regardless of pattern, oxidative phosphorylation becomes O2-dependent (not O2-limited) and greater increases in ([ADP][Pi]/[ATP]) are required to stimulate the needed oxidative phosphorylation rate. This same increase in ([ADP][Pi]/[ATP]) is a potent stimulus of glycolysis leading to ↑ La− production (see Gladden, 1996). | |||
| III. | Lactate removal | ||
| 1. | Sympathoadrenal activity causes vasoconstriction and ↓ blood flow to liver, kidney, and inactive muscle ⇒↓ La− oxidation and removal (e.g. Nielsen et al. 2002). | ||
| 2. | Adrenaline decreases lactate removal by exercising muscles and perhaps by resting muscles (Hamann et al. 2001). | ||
| 3. | Increased frequency of stimulation of previously recruited muscle fibres places more fibres in La− production mode instead of removal mode. | ||
| 4. | La− production exceeds removal ⇒↑ muscle and blood [La−] (e.g. Brooks, 1985b). | ||
| IV | Fast twitch fibre recruitment | ||
| More fast twitch fibres are recruited as exercise intensity increases. These fibres are more suited to La− production (e.g. Armstrong, 1988). |
Abbrevlatlons: LDH: lactate dehydrogenase; Phos: phosphorylase; PFK: phosphofructokinase; PDH: pyruvate dehydrogenase; ?: newer ideas that are more Gypothetical and await more direct evidence.
So, is La− an anaerobic metabolite? Yes, in the presence of anoxia; but La− is also an hypoxic metabolite in the presence of dysoxia, and an aerobic metabolite in the presence of an adequate O2 supply and utilization of glucose or glycogen as a fuel.
Lactic acid, lactate and fatigue
Lactic acidosis and fatigue.
Lactic acid is more than 99% dissociated into La− anions and protons (H+) at physiological pH. During exercise and muscle contractions, muscle and blood [La−] and [H+] can rise to very high levels (Fitts, 2003; Sahlin et al. 1976). Most researchers have argued that any detrimental effects of HLa on muscle and exercise performance are due to H+ rather than La− (Fitts, 2003). There is a vast literature in which a decline in maximal muscle force generation is correlated with a decrease in muscle pH (Hermansen, 1981; Sahlin, 1992). Evidence from numerous experimental approaches (Fitts, 2003) suggests that an elevated muscle [H+] could depress muscle function by (1) reducing the transition of the cross-bridge from the low- to the high-force state, (2) inhibiting maximal shortening velocity, (3) inhibiting myofibrillar ATPase, (4) inhibiting glycolytic rate, (5) reducing crossbridge activation by competitively inhibiting Ca2+ binding to troponin C, and (6) reducing Ca2+ re-uptake by inhibiting the sarcoplasmic ATPase (leading to subsequent reduction of Ca2+ release).
Particularly over the last 10 years, the role of acidosis as an important cause of fatigue has been challenged (see Westerblad et al. 2002). These studies have reported that the effect of increased [H+] to reduce Ca2+ sensitivity, maximal tension, and shortening velocity in skinned muscle fibres in vitro is absent when the experiments are performed at temperatures that are closer to those encountered physiologically. There is also a report that muscle acidity does not reduce muscle glycogenolysis/glycolysis during intense exercise in man (Bangsbo et al. 1996). One study in isolated rat soleus muscles in vitro observed that, rather than decreasing force generation, lactic acidosis actually protected against the detrimental effects of elevated external [K+] on muscle excitability and force (Nielsen et al. 2001).
In place of acidosis, studies on skinned muscle fibres are pointing to inorganic phosphate (Pi) as a major cause of muscle fatigue (see review by Westerblad et al. 2002 for details). Pi increases during intense muscle contractions or exercise due to breakdown of PCr. However, these studies have not evaluated the effects of high [H+] on peak power or the combined effects of a reduced Ca2+ release, a low pH and an elevated Pi (Fitts, 2003). Accordingly, Fitts (2003) notes that it is premature to dismiss H+ as an important factor in muscle fatigue. Further, at least two questions arise concerning the role of Pi as a primary fatigue agent during short-term intense exercise in intact humans. First, since most of the PCr breakdown occurs within the first 10 s of such intense exercise, would the primary role of Pi be restricted to that time frame? Second, can changes in Pi explain the reduction in performance observed in humans following prior intense exercise with different muscle groups? Despite well over 150 years of active research, the exact causes of muscle fatigue remain elusive.
Lactic acid and pH.
As noted in the preceding section, HLa is more than 99% dissociated at physiological pH. This has led to the incorrect notion that the donation of a proton by each HLa causes a decreased pH during conditions such as exercise. In 1981, Peter Stewart reintroduced and clarified the concept of physicochemical analysis of body fluid acid–base status; this represented a return to the thinking of Henderson and van Slyke and other lesser-known investigators of acid–base balance in the early 20th century (Lindinger, 2003; Johnson et al. 1996). Stewart (1981) emphasized that [H+] (pH) and [HCO3−] are dependent acid–base variables; that is, they are not causative factors. Instead, acid–base status is determined by the independent effects of carbon dioxide (PCO2), the concentration of weak acid buffers ([Atot], in plasma mainly the amino acids in plasma proteins), and the strong ion difference [SID]. [SID] is the sum of the strong cations minus the sum of the strong anions; e.g. [SID]= ([Na+] + [K+] + [Ca2+]) − ([Cl−] + [La−]) (Kowalchuk et al. 1988). In this method, it is obvious that although La− can be a significant component of [SID] acting to increase the [H+], it is definitely not the only factor involved in pH changes. The utility of this approach for determining underlying mechanisms in the study of acid–base balance during exercise has been clearly demonstrated in numerous studies (e.g. Kowalchuk et al. 1988; Lindinger et al. 1992).
Lactate anion and fatigue.
Over the years, La− has been considered unimportant in the development of fatigue. However, in the 1990s, several studies raised the possibility that La−per se might play some role in the fatigue process. In isolated dog gastrocnemii in situ, perfusion with l-(+)-lactate reduced twitch contraction force by 15% even though muscle pH was not altered from control conditions (Hogan et al. 1995). These results were subsequently supported by studies on muscles in vitro (Spangenburg et al. 1998), skinned muscle fibres (Andrews et al. 1996), and sarcoplasmic reticulum vesicles (e.g. Favero et al. 1995; Spangenburg et al. 1998). In Langendorff perfused rat hearts, La− appeared to irreversibly depress developed pressure (Samaja et al. 1999). More recently, studies of skinned mammalian muscle fibres (e.g. Posterino et al. 2001) have reported minimal effects (5% or less) of La− on muscle contractility. While these recent studies on skinned fibres suggest a minimal role for La− in the fatigue process, further studies on more intact systems are needed.
Shuttles, shuttles, everywhere
Cell-to-cell lactate shuttle.
What is now known as the cell-to-cell lactate shuttle was introduced by Brooks (1985a) simply as the lactate shuttle. Since its introduction, this hypothesis has been repeatedly supported by studies using a wide variety of experimental approaches. It posits that La− formation and its subsequent distribution throughout the body is a major mechanism whereby the coordination of intermediary metabolism in different tissues, and cells within those tissues, can be accomplished. The importance of La− as a carbohydrate fuel source is underscored by the fact that during moderate intensity exercise, blood La− flux may exceed glucose flux (Brooks, 2000). Because of its large mass and metabolic capacity, skeletal muscle is probably the major component of the lactate shuttle, not only in terms of La− production but also in terms of net La− uptake and utilization as well. At rest, muscles slowly release La− into the blood on a net basis, although at times they may show a small net uptake. During exercise, particularly short-term, high-intensity exercise, muscles produce La− rapidly while La− clearance is slowed. This results in an increased intramuscular [La−] and an increased net output of La− from muscles into the blood. Later, during recovery from short-term exercise, or even during continued, prolonged exercise, there is net La− uptake from the blood by resting muscles or by other muscles that are exercising at a low to moderate intensity (Richter et al. 1988; Brooks, 2000; Gladden, 2000). During prolonged exercise of low to moderate intensity, the muscles that originally released La− on a net basis at the onset of the exercise may actually reverse to net La− uptake (Stainsby & Welch, 1966; Gladden, 1991; Gladden et al. 1994; Brooks, 2000). Particularly during moderate to high intensity exercise, glycolytic muscle fibres are likely to be producing and releasing La−. While some of the La− escapes into the circulation, some of it may diffuse to neighbouring oxidative muscle fibres which can take up the La− and oxidize it (Baldwin et al. 1977; Stanley et al. 1986; Brooks, 2000). Clearly, La− exchange is a dynamic process with simultaneous muscle uptake and release at rest and during exercise (Jorfeldt, 1970; Brooks, 1985a; Stanley et al. 1986; Van Hall et al. 2002). Most of the La− taken up by muscles is removed via oxidation with the absolute rate depending on the metabolic rate of both exercising and resting muscles (Stanley et al. 1986; Mazzeo et al. 1986; Bergman et al. 2000; Kelley et al. 2002). Oxidative skeletal muscles that are contracting in a submaximal steady state condition are ideally suited for La− consumption. Since cardiac muscle is more highly oxidative than even the most oxidative skeletal muscle, it is not surprising that the heart is an active La− consumer. Evidence from several different experimental approaches suggests that as blood [La−], myocardial blood flow and myocardial increase, La− becomes the preferred fuel for the heart, accounting for as much as 60% of the substrate utilized (Stanley, 1991; Chatham et al. 1999). Tracer studies indicate that essentially all of the La− taken up by the heart is oxidized (Stanley, 1991). Even the brain can take up La− from the blood. Recently, Ide & Secher (2000) provided compelling evidence for net La− uptake by the brain, particularly during intense exercise; this uptake continued during a 30-min recovery period. Although the contribution of brain uptake to whole body La− uptake is negligible, it is of great interest in the consideration of brain metabolism per se as noted below in the discussion of the astrocyte–neuron lactate shuttle.
Several studies by Gladden and colleagues (Gladden, 1991, 2000; Gladden et al. 1994; Hamann et al. 2001; Kelley et al. 2002) have demonstrated that isolated, blood-perfused oxidative skeletal muscle readily consumes exogenously infused La− as a fuel. Recently, these findings have been confirmed and extended in lactate clamp (LC) studies in humans. Miller et al. (2002a,b) investigated subjects exercising at a moderate exercise intensity (∼55% V˙O2peak) with La− infusion to maintain [La−] at ∼4 mm. Overall, they (Miller et al. 2002a) found a significant increase in La− oxidation accompanied by a decrease in glucose oxidation; the interpretation is that La− competes successfully with glucose as a carbohydrate fuel source, thus sparing blood glucose for use by other tissues. Additionally, Miller et al. (2002b) found that although La− served as a gluconeogenic substrate, the absolute rate of gluconeogenesis was unchanged by LC. In contrast, LC increased the absolute gluconeogenic rate during low intensity exercise, ∼34% V̇O2peak (Roef et al. 2003). At both low and moderate intensities, La− was an important gluconeogenic precursor. These LC studies along with many other investigations of different types emphasize the role of La− as arguably the most important substrate for gluconeogenesis. The obvious conclusion from numerous studies is that La− is a useful metabolic intermediate that can be exchanged rapidly among tissue compartments. The cell-to-cell lactate shuttle provides the basic framework for interpretation of La− metabolism.
Blood provides the route by which tissues throughout the body are linked together in the cell-to-cell lactate shuttle. During exercise, intense exercise in particular, La− and H+ move out of contracting muscles primarily via monocarboxylate transporters MCT1 and MCT4 (Halestrap & Price, 1999; Juel & Halestrap, 1999; Bonen, 2001; Dubouchaud et al. 2000; Juel, 2001). Diffusion of undissociated HLa constitutes a smaller component of La− and H+ transport at physiological La− concentrations (Gladden, 1996). From the interstitial fluid, La− and H+ gain access to the blood through endothelial clefts and probably across endothelial cells as well. La− uptake and utilization as a fuel have been reported in endothelial cells (Krützfeldt et al. 1990), and monocarboxylate transporters MCT1 and MCT2 have been detected in brain endothelial cells (Mac & Nalecz, 2003) and in immortalized rat retinal capillary endothelial cells (Hosoya et al. 2001). Nevertheless, the extent to which La− and H+ move from interstitial fluid to blood or vice versa by way of facilitated transport through endothelial cells in most tissues is unknown.
From interstitial fluid of active muscles, La− enters the plasma. During intense exercise, a system designed to cotransport La− and H+ from the plasma into the red blood cells (RBCs) could aid in establishing a gradient between the plasma and interstitial fluid, and enhancing the available space for efflux of La− and H+ ions from the exercising muscles (Gladden, 1996). Indeed, the transport of La− across the RBC membrane proceeds by three distinct pathways: (1) non-ionic diffusion of undissociated HLa, (2) an inorganic anion exchange system, often referred to as the Band 3 system, and (3) a monocarboxylate-specific carrier mechanism (MCT) (Deuticke et al. 1982). MCT1 is the monocarboxylate transporter in RBC membranes (Garcia et al. 1994, 1995; Halestrap & Price, 1999) and it is the primary pathway of La− transport (Skelton et al. 1995, 1998). As blood circulates through the body to liver, heart, inactive and active skeletal muscles, and all tissues, the pathway is typically reversed with La− exiting the plasma into the interstitial fluid and on into the various tissues down the [La−] gradient. As plasma [La−] declines, La− will leave the RBCs. Several investigations have nicely illustrated the role of plasma and RBCs in picking up La− from active muscles and delivering it to inactive muscles (see Lindinger et al. 1995 for review).
Under most conditions, including exercise, RBCs can take up La− at a rate that is proportional to its rate of entry into the plasma. As a result, RBC [La−] reaches an equilibrium with plasma [La−] and the RBC/plasma ratio is relatively constant and similar to its value under normal, rest conditions. This ratio is approximately 0.5 and reflects a gradient between plasma [La−] and RBC [La−] in which the [La−] in plasma is approximately twice that inside the RBCs (Smith et al. 1997, 1998), a condition that appears to be largely established by a Donnan equilibrium (Johnson et al. 1945). At elevated [La−], such as occur during exercise or other conditions, the gradient between plasma and RBCs can become substantial as the RBC: plasma [La−] ratio remains unchanged (Smith et al. 1997, 1998). The result is that under most conditions, the plasma will contain ∼70% and the RBCs ∼30% of the whole blood La− content (Gladden, 1996). An exception to [La−] equilibration between plasma and RBCs occurs immediately following intense ‘all-out’ exercise, when La− entry into the plasma occurs at a proportionally faster rate than uptake of La− into the RBCs (Juel et al. 1990).
Intracellular lactate shuttle
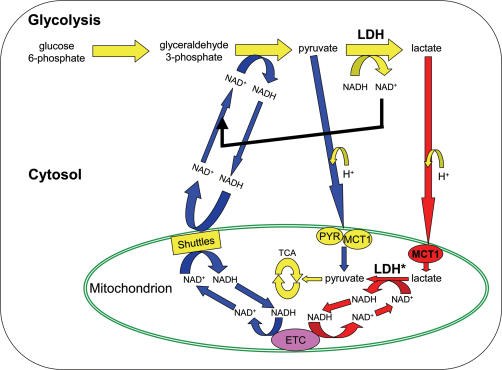
Brooks (1998) proposed an intracellular lactate shuttle and provided supportive data in a subsequent paper (Brooks et al. 1999b). This hypothetical shuttle is illustrated in Fig. 1. A central tenet of this intracellular shuttle is that HLa is an inevitable product of glycolysis, particularly during rapid glycolysis; this is so because LDH has the highest Vmax of any enzyme in the glycolytic pathway and the Keq for pyruvate to La− is far in the direction of La− (Brooks, 1998, 2000; Brooks et al. 1999a,b). Given this information, Brooks et al. (1999a) questioned how the well-known oxidation of La− by well-perfused tissues could occur through net lactate-to-pyruvate conversion in the cytosol. Brooks et al. (1999a,b) and Dubouchaud et al. (2000) have reported the following evidence for key components of an intracellular lactate shuttle in skeletal muscle: (1) direct uptake and oxidation of La− by isolated mitochondria without prior extramitochondrial conversion of La− to pyruvate, (2) presence of an intramitochondrial pool of LDH, and (3) presence of the La− transporter, MCT1, in mitochondria, presumably in the inner mitochondrial membrane.
Figure 1. Illustration of the essential elements of the hypothetical intracellular (intramuscular) lactate shuttle in comparison to the well-established malate–aspartate NAD+/NADH shuttle.
Note that for purposes of clarity the well-established glycerol phosphate shuttle is not shown. Redrawn with permission from Gladden (2001). The H+ ions for pyruvate and lactate are inserted to emphasize that the MCT1 and presumably PYR symports a proton; MCT1 can transport both pyruvate and lactate. Note that operation of such an intracellular lactate shuttle would deliver both reducing equivalents and substrate for oxidation to mitochondria. Key components of this hypothesis are in bold lettering and/or red fill for comparison to the malate–aspartate shuttle in normal font and blue fill: a high activity of cytosolic LDH is considered to guarantee La− formation in the cytosol under virtually all conditions but especially during exercise; MCT1 has been reported to be present in mitochondrial membrane allowing La− transport from cytosol into mitochondria; LDH inside mitochondria is required to complete the intracellular shuttle by converting La− to pyruvate. The asterisk beside the mitochondrial LDH denotes that the presence of LDH inside mitochondria is disputed and that some investigators consider operation of such a shuttle to be thermodynamically unfeasible. MCT1: monocarboxylate transporter 1; PYR: the mitochondrial pyruvate transporter; ETC: electron transport chain; Shuttles: the malate–aspartate NAD+ /NADH shuttle and the glycerol phosphate shuttle, which is not shown.
In the intracellular lactate shuttle scenario (Gladden, 2001), HLa would be produced constantly in the cytosol and its production rate would increase with increases in glycolytic rate. Due to its higher concentration, La− would be the primary monocarboxylate diffusing to mitochondria where it would be transported across the inner mitochondrial membrane by MCT1. Once inside the mitochondria, in the matrix, mitochondrial LDH would catalyse the conversion of La− back to pyruvate, which would be oxidized through the PDH reaction to acetyl-CoA. The acetyl-CoA would then continue through the TCA cycle. Note that the intracellular lactate shuttle would not only deliver substrate in the form of La− for conversion to pyruvate; it would also deliver reducing equivalents thus supplanting the role of the malate–aspartate and glycerol phosphate shuttles to varying degrees depending on the rate of La− formation and its rate of transport into mitochondria.
Can this fascinating and exciting hypothesis be accepted as the explanation for net La− oxidation by aerobic muscle? Two direct tests of the hypothesis by other laboratories (Rasmussen et al. 2002; Sahlin et al. 2002) have failed to confirm its central tenets. Both Sahlin et al. (2002) and Rasmussen et al. (2002) found (1) no evidence that mitochondria can use La− as a substrate without prior conversion to pyruvate in the cytosol, and (2) insignificant activities of LDH in the mitochondrial fraction. Further, both groups argue that the idea of La− conversion to pyruvate inside mitochondria is not feasible on the basis of thermodynamic principles. They point to a much higher reduction of the NAD+ /NADH redox couple inside mitochondria; so much higher that in fact it would theoretically eliminate the possibility of La− to pyruvate conversion. Sahlin et al. (2002) go on to suggest that if LDH were present in the mitochondrial matrix, it would lead to a futile cycle in which pyruvate would be reduced to La− in mitochondria and vice versa in the cytosol, oxidizing mitochondrial NADH and finally removing the driving force for the electron transport chain. An earlier report on human skeletal muscle by Popinigis et al. (1991) also failed to show either mitochondrial LDH or La− oxidation coupled to mitochondria.
Can these criticisms be countered? Brooks (Brooks et al. 1999b; Brooks, 2000, 2002a,b) points out that the intracellular lactate shuttle concept is compatible with most of the available data on La− oxidation in cardiac and skeletal muscles of humans and other mammals gathered through the use of isotopic tracers, arterial–venous mass balance techniques, and the combination of the two methods. The experimental models included perfused muscles and intact humans. A key example is the recent study of Chatham et al. (2001) in which [3-13C]lactate was used to study the perfused rat heart. Their data implied that glycolytically derived pyruvate was preferentially metabolized to La− rather than to acetyl-CoA whereas pyruvate derived from exogenous La− was preferentially directed to acetyl-CoA formation, results that are consistent with the concept of an intracellular lactate shuttle. Additional support for compartmentation of cardiac carbohydrate metabolism has come from studies of the metabolic effects of insulin and dichloroacetate (DCA) on fuel oxidation by perfused rat hearts (Lloyd et al. 2003). In these studies, insulin and DCA (a stimulator of pyruvate dehydrogenase activity) increased the oxidation of glucose and exogenous pyruvate, but not of exogenous La−. The implication is that lactate-derived pyruvate is oxidized by way of a different pathway from either exogenous pyruvate or glycolytically derived pyruvate. However, it must be recognized that while most of the results of the wide literature on La− transport and metabolism can be viewed as broadly consistent with the intracellular lactate shuttle hypothesis, this does not provide any direct proof of the hypothesis, nor does it address the thermodynamics question.
Could microcompartmentation explain the intracellular shuttle hypothesis? In other words, could there be a ‘sink’ for pyruvate in the mitochondria that would allow pyruvate concentration to be low enough (and correspondingly NADH/NAD+) to allow La− conversion to pyruvate? For this type of compartmentation to be feasible, pyruvate concentration would still have to be high enough to accomodate the PDH reaction, and [NADH] would still have to be sufficient to drive the electron transport chain.
Baba & Sharma (1971) combined histochemical and electron microscopy techniques and were apparently the first to find LDH localized in the mitochondria of rat heart and skeletal muscle. Subsequently, Kline et al. (1986) and Brandt et al. (1987) used cell fractionation techniques to demonstrate the presence of LDH in rat liver, kidney, and heart mitochondria. Szczesna-Kaczmarek (1990) reported both mitochondrial LDH and oxidation of La− by isolated mitochondria. Interpretation of these results varies as might be expected. Brooks (2002a,b) cites many of these earlier findings as support for the intracellular La− shuttle and contends (Brooks, 2002a) that Sahlin et al. (2002) and Rasmussen et al. (2002) most likely lost LDH in their isolation procedures to obtain mitochondria. To the contrary, Sahlin et al. (2002) and Rasmussen et al. (2002) speculate that the mitochondria of Brooks et al. (1999b) and others (see above) were contaminated with cytosolic LDH.
Another complicating factor is the nature of pyruvate/lactate transport into the mitochondria. Brooks et al. (1999a) present evidence of MCT1 in the mitochondria and suggest that MCT1 has a higher affinity for La− than for pyruvate (Brooks, 1998). However, a recent study of MCT1 (Bröer et al. 1998) reports a Km for pyruvate that is about one-third that found for La− although the pyruvate Vmax is 45% of the La−Vmax. Further, the ‘traditional’ mitochondrial pyruvate carrier apparently has a very high affinity for pyruvate and is not a member of the MCT family; it has a six-transmembrane-helix structure as compared to the 12-transmembrane-helix structure of the MCT family (Kuan & Saier, 1993; Palmieri et al. 1996; Halestrap & Price, 1999; Sugden & Holness, 2003). A single candidate protein for this mitochondrial pyruvate carrier has recently been identified in yeast (Hildyard & Halestrap, 2003; Sugden & Holness, 2003); further studies are required in mammalian systems.
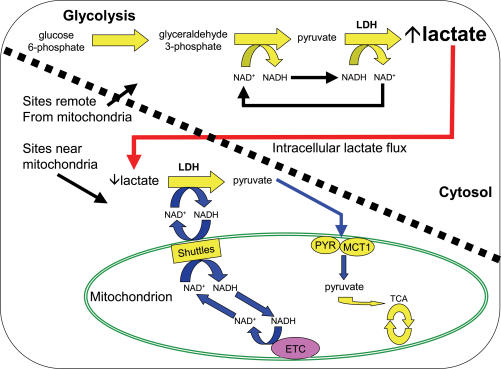
No doubt future experimentation will provide additional evidence either supporting or refuting the hypothesis. In the meantime, the original intracellular lactate hypothesis proposed by Stainsby & Brooks (1990) should receive attention as well. In this older hypothesis, illustrated in Fig. 2, pyruvate and NADH concentrations would be greatest in cytosolic locations that are farthest removed from mitochondria. Pyruvate and NADH concentrations would be lowest adjacent to mitochondria where the pyruvate carrier and the NADH shuttles would be moving pyruvate and NADH equivalents, respectively, into the mitochondria. This situation could lead to the highest La− production and concentration in remote cytosolic locations. Then, due to the relatively higher La− concentration as compared to pyruvate, La− would be the primary species diffusing to areas near mitochondria. Adjacent to mitochondria, La− and NAD+ would be converted back to pyruvate and NADH for uptake into the mitochondria. Such a scheme would accommodate ready La− production with subsequent oxidation and less transport of La− out of the cell.
Figure 2. Illustration of a simpler intracellular (intramuscular) lactate shuttle hypothesis originally proposed by Stainsby & Brooks (1990).
Note that the space above and to the right of the diagonal dashed line denotes sites that are remote from mitochondria and/or compartmentalized while the space down and to the left of the line denotes sites near mitochondria. La− is in large, bold lettering in the sites remote from mitochondria indicating that (a) [La−] should be highest here, and (b) [La−] is much greater than pyruvate concentration, especially during exercise. In this model, La− would be the predominant species diffusing from sites of glycolytic formation to low [La−] areas just outside mitochondrial membranes where La− would be converted back to pyruvate with delivery of NADH to the malate–aspartate (and glycerol phosphate) NAD+/NADH shuttles. This model does not require intramitochondrial LDH. MCT1 is shown because pyruvate might enter mitochondria via this transporter in addition to the traditional pyruvate carrier (PYR). LDH: lactate dehydrogenase; MCT1: monocarboxylate transporter 1; PYR: the mitochondrial pyruvate transporter; ETC: electron transport chain; Shuttles: the malate–aspartate NAD+/NADH shuttle and the glycerol phosphate shuttle, which is not shown for purposes of clarity. Redrawn with permission from Gladden (1996) from Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems, edited by Loring B. Rowell & John T. Shepherd, copyright 1996 by The American Physiological Society. Used by permission of Oxford University Press, Inc.
Astrocyte–neuron lactate shuttle
Increased nervous system activity requires increased energy metabolism in neurons. The conventional view is that neuronal energy metabolism is fuelled by glucose oxidation (Chih et al. 2001). The action potentials of neuron activity result in Na+ entry and K+ efflux which activates Na+–K+-ATPase in the neuronal plasma membrane; this ATPase pump activity in turn leads to ↓[ATP], ↑[ADP], ↑[Pi], and ↑[AMP], standard activators of glycolysis, the TCA cycle and mitochondrial oxidative phosphorylation. ATP synthesis will increase via these energetic pathways with a concomitant utilization of intracellular glucose that lowers [glucose], leading to an increased uptake of glucose into neurons via the neuronal glucose transporter, GLUT3 (Chih et al. 2001), which is found in both pre- and postsynaptic elements. In this scenario, rapid glycolysis is likely to result in some La− production and inhibition of oxidation of exogenously supplied La−. The primary excitatory neurotransmitter in the brain is glutamate. Glutamate is taken up by surrounding astrocytes via a carrier that cotransports Na+. In a process similar to that in the neurons, astrocyte metabolism (glycolysis, TCA cycle, oxidative phosphorylation) is activated to supply ATP for restoring Na+–K+ balance; metabolism is also stimulated to supply energy for glutamine synthesis from the glutamate that has been taken up.
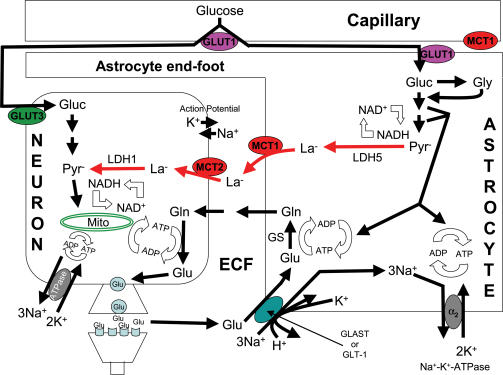
Pellerin & Magistretti (1994) challenged this conventional scheme of nervous system energetics with their introduction of the astrocyte–neuron lactate shuttle hypothesis (ANLSH); see Fig. 3. In this model (Pellerin & Magistretti, 1994, 2003; Magistretti & Pellerin, 1999; Magistretti et al. 1999; Bouzier-Sore et al. 2002; Pellerin, 2003), glutamate that is released as a neurotransmitter from the neurons is primarily taken up into astrocytes via a transporter that carries one glutamate, three Na+, and one H+ inward while one K+ is moved out of the cell (Attwell, 2000). This astrocytic transport activity then leads to Na+–K+-ATPase activation (perhaps via an increase in [Na+]i) to restore ionic balance, and to glutamine synthesis from the glutamate that has been taken up. The energy costs of the ATPase pump and glutamine synthesis result in ↓[ATP], ↑[ADP], ↑[Pi], and ↑[AMP], which largely stimulate glycolysis in the astrocytes with resultant La− production. Here, glycolytic enzymes may be compartmentalized with the ATPase pump and glutamine synthesis pathway, allowing preferential activation of the glycolytic energy system. The increased [La−] moves La− outward along its concentration gradient via MCT1 transporters in the astrocyte plasma membrane. Next, extracellular space [La−] rises, driving La− into the neighbouring neurons via MCT2 transporters in the neuronal plasma membrane. Inside the neurons, La−, along with glucose, serves as an oxidative fuel for elevated neuronal energy metabolism that has been triggered by an activated Na+–K+-ATPase to restore ionic balance and resynthesis of glutamate from glutamine, largely derived from astrocytes. While glucose can be taken up by neurons via their GLUT3 transporters, larger amounts of glucose may be used by astrocytes, and taken up into astrocytes via their GLUT1 transporters. In fact, Loaiza et al. (2003) have reported that glutamate stimulates glucose transport into cultured hippocampal astrocytes more rapidly than any stimulation of mammalian glucose transport yet known. In short, in the ANLSH, much of the fuel for increased energy demands of neurons is supplied by La− from surrounding astrocytes. As a result, metabolism of the astrocytes is largely glycolytic while that of the neurons is largely oxidative. The ANLSH has also been proposed for other support cell–neuron/receptor energetic interactions such as those between Schwann cells and peripheral neurons (Véga et al. 1998), and between Müller glial cells and photoreceptors (Poitry-Yamate et al. 1995).
Figure 3. Illustration of the putative astrocyte–neuron lactate shuttle.
The basic outline of the astrocyte–neuron lactate shuttle hypothesis is as follows. Blood glucose is a major energy substrate that can be taken up by both neurons and astrocytes via their specific glucose transporters (GLUT3 in neurons and GLUT1 in astrocytes); note that GLUT1 is also present in the plasma membrane of endothelial cells making up capillaries. Blood glucose may be more readily available to astrocytes because the surface of intraparenchymal capillaries is covered by specialized astrocytic end-feet. Release of the neurotransmitter, glutamate, at glutamatergic synapses leads to glutamate uptake into surrounding astrocytes via glutamate transporters GLT-1 and GLAST to terminate the action of glutamate on postsynaptic receptors. Glutamate entry into astrocytes is powered by the Na+ concentration gradient and current evidence suggests that one glutamate enters with three Na+ and one H+ while one K+ is simultaneously extruded. The resulting increase in intra-astrocytic [Na+] activates a glia-specific Na+–K+-ATPase α2 subunit. Glutamate is converted to glutamine by glutamine synthetase. Both the ATPase pump activation and the glutamine synthesis activate astrocytic glycolysis that is possibly compartmentalized with these processes; presence of LDH5, the muscle form of LDH, is argued to promote La− formation. The end result is La− accumulation and efflux into the extracellular fluid, facilitated by MCT1. Subsequently, La− is taken up into neurons via MCT2. Glutamine also diffuses from astrocytes into the extracellular fluid and on into neurons where it is used to resynthesize glutamate. La− taken up into neurons is preferentially converted to pyruvate, arguably because of pyruvate utilization as an aerobic fuel and the presence of LDH1, the heart form of LDH. In this hypothesis, the energy metabolism of neurons is largely aerobic with La− serving as the major fuel. See text and Table 2 for further details of the hypothesis, and Table 3 for concerns/questions about the hypothesis. GLUT1 and GLUT3: specific glucose transporters located in the membranes of brain endothelial cells and astrocytes (GLUT1), and neurons (GLUT3); MCT1 and MCT2: specific monocarboxylate transporters located in the membranes of brain endothelial cells and astrocytes (MCT1), and neurons (MCT2); Gluc: glucose; Gly: glycogen; Pyr−: pyruvate; La−: lactate; Glu: glutamate; Gln: glutamine; GS: glutamine synthetase; LDH1 and LDH5: specific forms of lactate dehydrogenase in neurons (LDH1) and astrocytes (LDH5); ECF: extracellular fluid; GLT-1 and GLAST: glutamate transporters; α2: glia-specific Na+–K+-ATPase subunit. Redrawn with permission from Pellerin (2003), Lactate as a pivotal element in neuron-glia metabolic cooperation, Neurochemistry International 43, 331–338. Used by permission of Elsevier.
The implications of the ANLSH are extremely broad. For example, apart from its inferences concerning basic mechanisms of nervous system metabolism, the ANLSH offers intriguing explanations for functional brain imaging (Magistretti & Pellerin, 1999; Magistretti et al. 1999). Local brain activity is monitored by visualization of changes in blood flow, glucose usage, and oxygen consumption via positron emission tomography (PET), changes in blood oxygenation via functional magnetic resonance imaging (fMRI), and spatio-temporal patterns of metabolic intermediates such as glucose and La− via magnetic resonance spectroscopy (MRS). Magistretti & Pellerin (1999) argue that the ANLSH ‘provides a cellular and molecular basis for … functional brain imaging techniques’. Is it possible that neuroimaging is a more direct reflection of astrocyte function rather than neuronal function (Meeks & Mennerick, 2003)?
Since the introduction of the ANLSH (Pellerin & Magistretti, 1994), there has been a virtual explosion of publications on the topic, with many supporting its basic propositions. However, criticisms and questions remain such that it has not been universally embraced. Table 2 summarizes key points of evidence offered in support of the ANLSH whereas Table 3 summarizes the most salient criticisms. In its strictest rendition, the model suggests that glial cells account for about 6.5% (two ATP from substrate phosphorylation in the glycolytic conversion of one glucose to two HLa) of nervous system activity while neuronal energy expenditure accounts for the other ∼93.5% (29 ATP from oxidation of two HLa; Salway, 1999). For the glial cells, it has been proposed that one glycolytic ATP is used to extrude three Na+ ions via the Na+–K+-ATPase pump while the second ATP is spent to convert glutamate to glutamine (Magistretti & Pellerin, 1999). It seems unlikely that metabolism is so tightly coupled and compartmentalized as to allow such strict stoichiometry. A more probable scenario may be a mixture of the ANLSH with the conventional view. In this scheme, astrocytes would utilize at least some of their own glycolytic products in oxidative metabolism and neurons would utilize some La− from astrocytes in addition to endogenous, neuronal glycolytic products (Mangia et al. 2003b). Nevertheless, as Table 2 illustrates, evidence is mounting that the ANLSH constitutes a major metabolic pathway in neural tissue.
Table 2.
Some of the key evidence supporting the astrocyte–neuron lactate shuttle hypothesis
| 1. | Rate at which metabolized glucose enters the neuronal TCA cycle equals the rate of glial glutamate cycling (Sibson et al. 1998). | |
| 2. | In cultured mouse astrocytes (Pellerin & Magistretti, 1994) and glial Müller cells in retina (Poitry-Yamate et al. 1995), uptake of exogenous glutamate is strongly associated with increased La− production. | |
| 3. | Neuronal tissue can use La− as a fuel and may prefer it. | |
| a. | Studies on brain tissue, isolated nerves, and sympathetic ganglia have reported La− utilization in replacement of glucose (e.g. McIlwain, 1956; Carpenter, 1959; Brown et al. 2001). | |
| b. | La− can substitute for, or is preferred to, glucose, in cultured cortical neurons (Pellerin et al. 1998; Bouzier-Sore et al. 2003), chick sympathetic ganglia (e.g. Larrabee, 1995), vagus nerve (Véga et al. 1998), and photoreceptors in the retina (Poitry-Yamate et al. 1995) and human brain in vivo (Smith et al. 2003). | |
| c. | La− is metabolized through the TCA cycle in GABAergic and glutamatergic neurons with labelling of TCA cycle intermediates and several amino acids derived from cycle intermediates (Schousboe et al. 1997; Waagepetersen et al. 2000). | |
| d. | LDH1 is the predominant isoform of LDH in neurons and it has been argued that this isoform is more likely to convert La− to pyruvate because of its lower Km for La−. LDH5, the predominant isoform in astrocytes, is arguably more suited for pyruvate to La− conversion (e.g. Bittar et al. 1996; Pellerin et al. 1998). | |
| e. | Nuclear magnetic resonance spectroscopy has provided evidence of La− utilization as an energy substrate in brain tissue, specifically as a neuronal fuel (e.g. Hassel & Brathe, 2000; Qu et al. 2000). | |
| 4. | Glutamate is the primary excitatory neurotransmitter of the cerebral cortex. Some observations suggest a specific mechanism for detection of glutamatergic activity by astrocytic processes surrounding glutamatergic synapses, and a resulting La− production and release. | |
| a. | An α2 Na+–K+-ATPase is expressed together with glutamate transporters (GLT-1 and GLAST) in astrocytic processes surrounding glutamatergic synapses (e.g. Robinson & Dowd, 1997; Cholet et al. 2002). | |
| b. | Astrocytic glutamate transport is largely electrogenic with one glutamate molecule transported inward with three Na+ ions (Bouvier et al. 1992). Increased intracellular [Na+] stimulates astrocytic Na+–K+-ATPase (Kimelberg et al. 1993). | |
| c. | Mobilization of a ouabain-sensitive isoform, akin to the α2 Na+–K+-ATPase, appears responsible for the glutamate-uptake-stimulated aerobic glycolysis of cortical astrocytes (Pellerin & Magistretti, 1997). | |
| d. | Ouabain completely inhibits glutamate-evoked 2-deoxyglucose uptake by astrocytes (Pellerin & Magistretti, 1994). | |
| e. | Glial glutamate transporter knockout mice show reduced glucose utilization in the somatosensory cortex and cortical astrocytes from the same mice show abolition of glutamate-stimulated glucose utilization and La− production (Voutsinos-Porche et al. 2003). | |
| 5. | There is a cell-specific expression of monocarboxylate transporters (MCTs) in the central nervous system. In cultured mouse cortex preparations, MCT1 and MCT1 RNA are found almost exclusively in astrocytes while MCT2 and its RNA are exclusive to neurons. (Bröer et al. 1997; Debernardi et al. 2003). Adult rat brain cells show a similar pattern with MCT1 also present in endothelial cells of the blood–brain barrier (Mac & Nalecz, 2003). | |
| 6. | Neurons, but not glia, respond to La− with elevation of cytosolic ATP (Ainscow et al. 2002). | |
| 7. | Anatomical considerations suggest that astrocytes are an important intermediary between capillaries, neurons, and the synapses of the neurons (Magistretti & Pellerin, 1999). | |
| a. | In the brain, the entire surface of intraparenchymal capillaries is covered by specialized astrocytic end-feet (Peters et al. 1991). | |
| b. | Specialized astrocytic processes are wrapped around synaptic contacts (Rohlmann & Wolff, 1996; Bushong et al. 2002). | |
| c. | In most brain regions, the astrocyte:neuron ratio is 10: 1 (Bignami, 1991). |
This table is derived heavily from Magistretti & Pellerin (1999), Chih et al. (2001), Bouzier-Sore et al. (2002) and Pellerin (2003).
Table 3.
Some key concerns/questions about the astrocyte–neuron lactate shuttle hypothesis
| 1. | There is no solid explanation as to why neural activity either in situ or in vivo should activate glial glycolysis but not neuronal glycolysis (Chih et al. 2001). | |
| a. | Neurons have high levels of the key glycolytic enzyme, hexokinase (Lai et al. 1999). | |
| b. | The predominant neuronal glucose transporter, GLUT3, transports glucose much faster than does the primary glial transporter, GLUT1. | |
| 2. | Is the LDH isoform (LDH1 in neurons and LDH5 in astrocytes) relevant? LDH catalyses a near-equilibrium reaction; therefore, the particular LDH isoform may have little effect on flux through the reaction in vivo (Newsholme, 2003). | |
| 3. | Neuronal activity has been reported to increase cytoplasmic pyruvate and NADH levels, making it unclear that increases in [La−] are sufficient to drive the LDH reaction towards pyruvate and NAD+ formation (see Chih et al. 2001). | |
| 4. | Does sufficient Na+ enter astrocytes during the transient presence of extracellular glutamate to stimulate significant Na+–K+-ATPase activity and thereby significant glycolysis? (Meeks & Mennerick, 2003). | |
| 5. | Some results from cultured astrocytes indicate that glutamate uptake fuels oxidative metabolism, primarily of glutamate itself while glycolysis may be slightly inhibited (Meeks & Mennerick, 2003). | |
| 6. | Dienel & Hertz (2001) argue that the evidence suggests that ‘metabolic trafficking’ of La− is at most 25% of the rate of glucose oxidation at physiologically occurring La− concentrations. They suggest that brain activation in vivo is often accompanied by overflow of glycolytically generated La− to different brain areas and sometimes to circulating blood. Further, [La−] appears to increase equally in neurons and astrocytes. | |
| 7. | Not all studies find that La− replaces glucose as a cerebral metabolic substrate or that La− is of significance for total net brain energy consumption (Leegsma-Vogt et al. 2003). | |
| 8. | Not all studies find that glutamate stimulates glycolysis in brain tissue (Gramsbergen et al. 2003). | |
| 9. | The end feet of astrocytes are not a part of the blood–brain barrier and therefore do not direct glucose utilization specifically to the astrocytes (see Gjedde & Marrett, 2001). | |
| 10. | Some find that astrocytic metabolism is not particularly less oxidative than the metabolism of neurons, and that neurons use pyruvate derived directly from neuronal glycolysis rather than from astrocytic glycolysis (Gjedde & Marrett, 2001). | |
| 11. | Whereas the astrocyte–neuron lactate shuttle predicts an initial La− overproduction, recent experiments using time-resolved proton magnetic resonance spectroscopy found a significant decrease in [La−] 5 s after visual stimulation in humans (Mangia et al. 2003a). | |
| 12. | MCT isoforms do not confer directionality to La− flux (Juel, 2001). |
Lactate–alanine shuttle
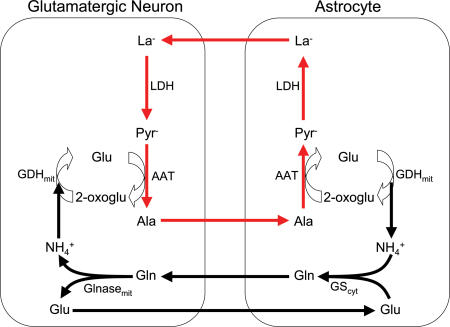
On the basis of stable isotope tracer studies in cultured astrocytes, neurons, and cocultures, Zwingmann et al. (2000) and Waagepetersen et al. (2000) independently proposed a lactate–alanine shuttle between astrocytes and neurons. Zwingmann et al. (2000) studied GABAergic neurons whereas Waagepetersen et al. (2000) studied glutamatergic neurons. Such a shuttle would supplement the well-known glutamine–glutamate cycle (Berl & Clarke, 1983). In glutamatergic neurons, glutamate is released as a neurotransmitter. As described above for the ANLSH, much of this glutamate is then taken up into surrounding astrocytes. Astrocytes synthesize glutamine from glutamate and ammonia (NH4+) via cytosolic glutamine synthetase. This glutamine is released from astrocytes and taken up by neurons where it is converted back to glutamate with ammonia formation via mitochondrial glutaminase. This series of reactions (the glutamine–glutamate cycle) describes the pathway of the carbon skeleton for this interaction between neurons and astrocytes but does not account for the nitrogen. This is where the proposed lactate–alanine cycle would play a role. In this shuttle, the ammonia from glutamine breakdown in neurons is combined with 2-oxoglutarate for conversion to glutamate via mitochondrial glutamate dehydrogenase. The resulting glutamate is then used to transaminate pyruvate and form 2-oxoglutarate and alanine. The alanine is released from neurons, taken up by astrocytes, combined with 2-oxoglutarate, and converted to pyruvate and glutamate via transamination. This glutamate formation returns to the starting point described above for the glutamine–glutamate cycle. The astrocytic pyruvate is now converted to La− which can be released and taken up into the neurons where it goes back to pyruvate thus completing the lactate–alanine shuttle. This shuttle would provide a pathway for the transfer of ammonia from neurons to astrocytes, a requirement of the glutamine–glutamate cycle. See Fig. 4 for a diagrammatic outline of the lactate–alanine shuttle as proposed for glutamatergic neurons and astrocytes. Schousboe et al. (2003) have questioned the synthesis of glutamate in GABAergic neurons via the glutamate dehydrogenase reaction because the ammonia concentration is likely to be low in these neurons. They (Schousboe et al. 2003) have also noted that alanine formation in glutamatergic neurons may account for only about 25% of the conversion of glutamine to glutamate plus ammonia. Accordingly, further experimentation is warranted to fully clarify the lactate–alanine shuttle.
Figure 4. Illustration of the proposed lactate–alanine shuttle between astrocytes and glutamatergic neurons.
This diagram focuses on the recycling of glutamate and ammonia. Glutamate released by neurons as a neurotransmitter is taken up by astrocytes and incorporated with ammonia (NH4+) to form glutamine. The glutamine is released to be taken up by neurons to re-form glutamate with ammonia release. The ammonia is used to synthesize glutamate via glutamate dehydrogenase and this glutamate then transaminates pyruvate to alanine. The alanine can leave the neurons to be taken up by astrocytes and combined with 2-oxoglutarate to be transaminated back to glutamate with accompanying pyruvate formation. Pyruvate in the astrocytes forms La− that is released and taken up into neurons. In the neurons, La− is converted back to pyruvate thus completing the lactate–alanine shuttle. LDH: lactate dehydrogenase; AAT: alanine aminotransferase; GDH: glutamate dehydrogenase; GS: glutamine synthetase; Glnase: glutaminase; mit: mitochondrial; cyt: cytosolic; Pyr−: pyruvate; Ala: alanine; Glu: glutamate; 2-oxoglu: 2-oxoglutarate; Gln: glutamine. Redrawn with permission from Waagepetersen et al. (2000), a possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons, Journal of Neurochemistry 75, 471–479. Used by permission of Blackwell Publishing Ltd.
Peroxisomal lactate shuttle
Lazarow & de Duve (1976) were the first to confirm β-oxidation of fatty acids in mammalian peroxisomes. Current thinking is that about 90% of short- and medium-chain length fatty acids are oxidized in mitochondria while the remaining 10% are oxidized in peroxisomes under basal conditions. However, the main function of peroxisomal β-oxidation is believed to be chain-shortening of very-long-chain fatty acids (i.e. C22 and longer) in preparation for subsequent oxidation by mitochondria (Salway, 1999). Additionally, the acetyl-CoA from peroxisomal β-oxidation is known to supply substrate for the synthesis of bile acids, phospholipids, cholesterol and fatty acids (Hayashi & Takahata, 1991). In order for this β-oxidation to continue, both FADH2 and NADH must be reoxidized. Unlike the case for mitochondria, FADH2 that is formed in the peroxisomal β-oxidation process is re-oxidized by a direct transfer of electrons to O2 (Mathews et al. 2000). However, the mechanism for NADH reoxidation has been puzzling.
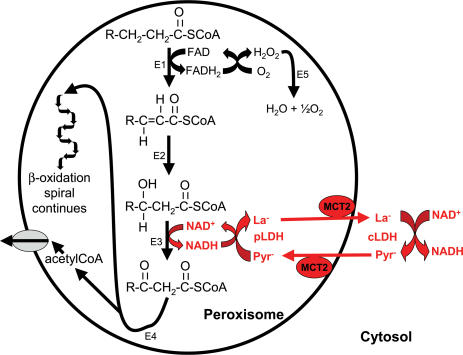
Tolbert's group (McGroarty et al. 1974) first suggested the association of LDH with rat liver peroxisomes but their methodology did not permit a firm conclusion, allowing the assumption that LDH activity in peroxisomal fractions was due to the adsorption of the cytosolic enzyme to the outer surface of the peroxisomal membrane. Accordingly, it was considered possible that NADH from peroxisomal β-oxidation passed through the peroxisomal membrane for reoxidation to NAD+ in the cytosol with subsequent entry of the NAD+ back into the peroxisome (Osmundsen et al. 1994). However, two other findings brought consideration of a peroxisomal lactate shuttle to the forefront: (1) the peroxisomal membrane in the yeast Saccharomyces cerevisiae was reported to be impermeable to NAD+/NADH in vivo (Van Roermund et al. 1995), and (2) the addition of pyruvate to a liver peroxisomal assay in vitro was found to stimulate the β-oxidation of palmitoyl-CoA while the addition of exogenous LDH had no effect (Osmundsen, 1982). Confirmation of the presence of LDH in the peroxisomal matrix and evidence of its participation in the reoxidation of NADH came in an elegant study by Baumgart et al. (1996). Three different approaches clearly demonstrated that LDH was present in the matrix of rat liver peroxisomes: (1) analytical subcellular fractionation with determination of enzyme activity, (2) immunodetection of LDH in isolated subcellular fractions using a monospecific antibody, and (3) immunoelectron microscopy applied to liver sections and to isolated peroxisomal fractions. Additional experiments demonstrated direct involvement of peroxisomal LDH in the reoxidation of NADH produced by the β-oxidation of palmitoyl-CoA: (a) NADH reoxidation increased in response to increasing pyruvate concentration before LDH inhibition occurred at concentrations above 2 mm, and (b) NADH reoxidation was reduced by the LDH inhibitor, oxamate. Subsequently, Brooks's group (McClelland et al. 2003) confirmed the presence of LDH in peroxisomes and stimulation of peroxisomal β-oxidation by pyruvate. As further evidence of the shuttle, they found peroxisomal La− generation upon the addition of pyruvate. Most importantly, McClelland and Colleagues (2003) reported that peroxisomal membranes contain the monocarboxylate transporters, MCT1 and MCT2, thus providing a mechanism for pyruvate entry into, and La− efflux from, peroxisomes. The significance of these transporters was demonstrated by the fact that the rate of pyruvate-stimulated peroxisomal β-oxidation was inhibited by the MCT blocker, α-cyano-4-hydroxycinnamate. Figure 5 illustrates the proposed peroxisomal lactate shuttle. To be complete, it should be noted that other redox shuttles are likely to be operative in peroxisomes as well (Baumgart et al. 1996).
Figure 5. Illustration of the proposed peroxisomal lactate shuttle.
Based on (McClelland et al. 2003), Salway (1999) and Baumgart et al. (1996). The outline of reactions displays the β-oxidation of very-long-chain fatty acids with acetyl-CoA release to the cytosol. Although not shown, shortened fatty acyl-CoA molecules could be released to the cytosol as well. Key elements of the peroxisomal lactate shuttle are highlighted in red indicating that NADH is reoxidized to NAD+ inside the peroxisome by the conversion of pyruvate to La−; La− then leaves the peroxisome via the monocarboxylate carrier, MCT2. In the cytosol, La− is converted back to pyruvate with concomitant conversion of NAD+ to NADH, thus delivering reducing equivalents from the peroxisome to the cytosol. The resulting pyruvate returns to the peroxisome via the MCT2 to continue the shuttle. This shuttle provides an avenue for NADH reoxidation in the peroxisome, a necessary process for the continuation of peroxisomal β-oxidation of fatty acids. E1: acyl-CoA oxidase; E2: enoyl-CoA hydratase; E3: L-3-hydroxyacyl-CoA dehydrogenase; E4: thiolase; E5: catalase; pLDH: LDH located inside the peroxisome; cLDH: LDH located in the cytosol, outside the peroxisome; Pyr−: pyruvate; La−: lactate.
Spermatogenic lactate shuttles
For 40 years, it has been known that mammalian spermatozoa can use La− as an aerobic energy source (see Storey & Kayne, 1977). In fact, La− stimulates respiration in ejaculated bovine sperm (Halangk et al. 1985) and maintains bovine sperm motility as well as glucose does (Inskeep & Hammerstedt, 1985). La− metabolism by sperm apparently involves La− transport into mitochondria followed by oxidation of the La− to pyruvate and subsequent intramitochondrial metabolism of the pyruvate via aerobic pathways. In a flurry of activity in 1977–78, evidence for such a lactate shuttle was reported for rabbit sperm (Storey & Kayne, 1977), bovine sperm (Milkowski & Lardy, 1977), and boar sperm (Calvin & Tubbs, 1978). All three of these studies utilized hypotonically treated spermatozoa (Keyhani & Storey, 1973) to generate part or all of the results. This treatment breaks plasma membranes but leaves the cell structure and mitochondria intact, including the outer and inner mitochondrial membranes (Storey & Kayne, 1977); these sperm-type mitochondria behave similarly in many ways to mitochondria isolated from other cells (Keyhani & Storey, 1973).
Hypotonically treated rabbit spermatozoa were found to oxidize external La− rapidly as evidenced by oxygen consumption measurements. Fluorometric studies demonstrated intramitochondrial reduction of NAD+ in response to the addition of extramitochondrial La− (Storey & Kayne, 1977). Other fluorometric studies of both whole bovine sperm and bovine sperm mitochondria (hypotonically treated spermatozoa) demonstrated that the redox state was altered in a manner consistent with a pyruvate/lactate redox couple (Milkowski & Lardy, 1977). In hypotonically treated boar spermatozoa, external NADH was oxidized in the presence of external La−, and this oxidation was inhibited by the monocarboxylate transport inhibitor, α-cyano-4-hydroxycinnamate, and by oxamate, an inhibitor of LDH (Calvin & Tubbs, 1978). More recently, these results were supported by experiments on sperm-type mitochondria from rats and rabbits; these experiments also showed an inhibition of the lactate–pyruvate shuttle by the monocarboxylate transport inhibitor, mersalyl (Gallina et al. 1994). Function of the shuttle was further confirmed in intact boar sperm and hypotonically treated spermatozoa (Jones, 1997). The boar sperm oxidatively metabolized fructose, glucose, glycerol, glycerol 3-phosphate and La− to CO2 while only small amounts of CO2 were produced from pyruvate. La− was the most preferred of the substrates and was oxidized preferentially in the presence of pyruvate. Mersalyl, an inhibitor of La− transport, reduced La− metabolism in a dose–response manner. To be complete, it should be noted that the lactate shuttle is not present in mouse spermatozoa (Gallina et al. 1994) and that a malate–aspartate shuttle is likely to be present in some types of spermatozoa in addition to the lactate shuttle (Calvin & Tubbs, 1978).
Function of an ‘intracellular’ lactate shuttle of course requires the presence of LDH inside the mitochondria as well as in the cytosol and typically involves carrier proteins for membrane flux. Numerous studies have substantiated the presence of a unique form of LDH (LDH C4, previously LDH X), both in the cytosol and inside the mitochondria of spermatozoa (for references see Storey & Kayne, 1977; Jones, 1997). Although monocarboxylates can traverse membranes by simple diffusion in their undissociated forms, transporters (MCTs) typically account for the majority of transmembrane traffic at physiological La− concentrations (Juel & Halestrap, 1999). Accordingly, the presence of such transporters would offer additional support for a spermatozoan lactate shuttle. Two different isoforms of the monocarboxylate transporter have been found in the testis and epididymis of hamsters (Garcia et al. 1995). Relative to the present issue, MCT1 was present on sperm heads but disappeared with maturation whereas MCT2 was present on the tails of sperm and never disappeared. Presumably, then, it is MCT2 that would be involved in the spermatozoan lactate shuttle although more specific studies have not been done and other species have not been studied. As noted earlier in the discussion of a possible intracellular lactate shuttle in skeletal muscle, tissues typically maintain a more reduced redox state inside mitochondria as compared to the cytosol; this facilitates cytosolic glycolysis and intramitochondrial oxidation (Milkowski & Lardy, 1977; Dawson, 1979). Usually, some irreversible step is necessary within a shuttle system in order to maintain these separate redox states (Milkowski & Lardy, 1977; Dawson, 1979); such a step has not been identified in the spermatozoan lactate shuttle. An obvious question is raised: Why does an ‘intracellular’ lactate shuttle appear to operate in spermatozoa but perhaps not in muscle, based on the evidence to date?
Intriguingly, the enzyme activities of the metabolic pathways (glycolysis versus TCA cycle) of germ cells appear to change during the differentiation and maturation process (Bajpai et al. 1998). Although the physiological significance of these changes is not clear, on the whole it appears that the germ cells are more dependent on a direct supply of La− as a fuel than are mature spermatozoa. For example, numerous studies have reported that La− is a preferred, and perhaps essential, substrate for spermatocytes and spermatids (see Nakamura et al. 1984; Grootegoed et al. 1984 and references therein). Recently it was reported that spermatogenesis is improved by intratesticular infusion of La− into the adult cryptorchid rat testis (Courtens & Plöen, 1999). In this context, the fluid of seminiferous tubules is reported to be rich in La− but low in glucose and pyruvate (see Bajpai et al. 1998; Courtens & Plöen, 1999 and references therein). Further, Sertoli cells that line the seminiferous tubules and extend to the lumen actively metabolize glucose with the majority being converted to La− (e.g. see Grootegoed et al. 1986; Bajpai et al. 1998). Whether or not the germ cells metabolize the La− provided by the Sertoli cells via the same lactate shuttle as spermatozoa is unclear, but it would seem to be a reasonable hypothesis. Provision of La− by Sertoli cells to the germ cells adds a cell-to-cell aspect that may not be present in the spermatozoan lactate shuttle.
Lactate transport
Shuttling of La− among neighbouring cells, among tissues, between tissues and blood, and among intracellular compartments raises important questions concerning how and at what rates La− is able to cross the membranes of cells and organelles. Definitive evidence of carrier-mediated transport of La− came from studies of sarcolemmal vesicles by Roth & Brooks (1990a,b). They demonstrated that sarcolemmal La− transport was concentration dependent, saturable, stereospecific, competitively inhibited by other monocarboxylates, blocked by known inhibitors of monocarboxylate transport, sensitive to temperature, and stimulated by [H+] gradients. Since these pioneering studies, there has literally been an explosion of research on membrane transport of La−. Several excellent reviews (e.g. Halestrap & Price, 1999; Juel & Halestrap, 1999; Brooks, 2000; Bonen, 2001; Juel, 2001) have been published in recent years. The study of La− transport was stimulated by the serendipitous cloning of the first transporter (MCT1) by Garcia et al. (1994). They were studying a mutant allele that encoded a transporter protein that transported mevalonate, an intermediate in cholesterol synthesis, in Chinese hamster ovary cells. Subsequently they found that the mevalonate wild-type gene coded for a monocarboxylate transporter. Shortly thereafter, Jackson et al. (1995) reported the independent cloning of MCT1 from rat skeletal muscle. Since then, in rapid succession, a family of nine MCTs, MCT1–MCT9, has been cloned (Halestrap & Price, 1999). At present, there is active investigation into the factors that determine MCT density in membranes as well as the possible role of MCTs in metabolic regulation.
Lactate in injury, sepsis, and haemorrhage
Lactate is an important intermediate in the process of wound repair and regeneration, a role that may not be generally familiar to researchers in the area of energy metabolism. As early as 1964, Green & Goldberg (1964) reported that collagen synthesis is increased almost twofold when [La−] rises to 15 mm in cultured fibroblasts. Notably, healing wounds produce and accumulate La− with concentrations sometimes rising to the range of 10–15 mm (Hunt et al. 1978; Gibson et al. 1997). Significantly, La− is not simply a sequel to hypoxia in wounds (Trabold et al. 2003). In fact, it is well-established that oxygen level has a relatively small effect on wound [La−] (Constant et al. 2000; Sheikh et al. 2000; Ghani et al. 2003; Trabold et al. 2003). Wound La− is raised only marginally by hypoxaemia (Hunt et al. 1978) and hyperoxia leaves wound [La−] essentially unchanged (Sheikh et al. 2000). So what is the source of La− in wound tissue and fluid? While some cells in wounds shift towards La− production in hypoxia, other cells are heavily reliant on aerobic glycolysis regardless of O2 level (Ghani et al. 2003; Trabold et al. 2003). For example, large amounts of La− are produced in rapidly multiplying cells in a process that is not fully understood, the Warburg effect. Perhaps most importantly, the ‘oxidative burst’ of leucocytes is powered largely by aerobic glycolysis because leucocytes contain few mitochondria. This ‘oxidative burst’ produces superoxide and is a key component of wound immunity. Oxidant production by leucocytes accounts for about 98% of the O2 consumed by activated cells and is dependent on PO2 up to approximately 600 mmHg. Accordingly, La− production by leucocytes increases with increased oxygenation apparently offsetting any decrease in La− production by fibroblasts due to the alleviation of hypoxia (Sheikh et al. 2000; Trabold et al. 2003).
What is the proposed role for La− in wound healing? La− enhances both collagen deposition and angiogenesis (see Constant et al. 2000; Trabold et al. 2003). Hunt's San Francisco team has proposed two separate mechanisms to explain the stimulation of collagen synthesis by La− in fibroblasts (see Ghani et al. 2003; Trabold et al. 2003 for review and references). First, La− provokes an increase in collagen promoter activity leading to an increased procollagen mRNA production and collagen synthesis. Second, La− activates prolyl hydroxylase independently of its enhancement of collagen transcription; this enzyme converts proline into hydroxyproline in collagen peptide. Apparently the underlying mechanism for both of these mechanisms is the same, down-regulation of ADP-ribosylation. ADP-ribosylation is a widespread type of post-translation modification of proteins; in this process the source of adenosine diphosphoribose (ADPR) is nicotinamide adenine dinucleotide (NAD+). The ADPR moiety can be enzymatically transferred onto certain acceptor proteins, thus modifying their structures and activities. In nuclei, numerous ADPR moieties can be added to target amino acid residues of proteins to form polyADPR (pADPR). In the case of wound healing, it is hypothesized that pADPR down-regulates collagen gene transcription in fibroblasts and that in a similar but separate process, ADPR inhibits prolyl hydroxylase in the cytoplasm. These inhibitory effects of ADP-ribosylation are reversed by elevated [La−] in the following manner. High [La−] shifts the equilibrium of the LDH reaction away from La− plus NAD+ and towards pyruvate plus NADH. The resulting decline in the NAD+ pool down-regulates NAD+-mediated pADPR and ADPR, and collagen synthesis and deposition are promoted. NADH is not a substrate for the ribosylation enzymes.
In the case of La−-stimulated angiogenesis in wounds, the major pathway appears to be enhanced vascular endothelial growth factor (VEGF) production in macrophages (Constant et al. 2000; Sheikh et al. 2000; Ghani et al. 2003; Trabold et al. 2003). Similarly to the case for collagen synthesis above, it appears that pADPR inhibits the transcription and synthesis of VEGF and that the activity of VEGF released from macrophages is inhibited by covalently bound ADPR (Constant et al. 2000; Ghani et al. 2003; Trabold et al. 2003). Again, it is proposed that elevated [La−] diminishes the NAD+ pool and decreases the inhibitory effects of pADPR on VEGF synthesis and of mono-ADPR on VEGF activity (Constant et al. 2000; Ghani et al. 2003; Trabold et al. 2003). In addition to the ADP-ribosylation pathway, La− may promote wound healing by increasing O2 supply to wounds because La− is a pH-independent vasodilator (Mori et al. 1998; Trabold et al. 2003).
Trabold et al. (2003) postulated that La− effects on the ADP-ribosylation pathway as described for wound healing may be applicable to the angiogenesis and collagen deposition that follow exercise. This novel proposal deserves consideration and data collection.
Clinically, the classical explanation for increased blood [La−] (hyperlactataemia) has been anaerobic glycolysis due to insufficient O2 delivery (e.g. Mizock & Falk, 1992) and patients have been managed accordingly. Recently, James et al. (1996, 1999a,b) have offered evidence that hyperlactataemia following injury and sepsis may in fact be the result of an adrenaline surge that stimulates sarcolemmal Na+–K+-ATPase activity and coupled aerobic glycolysis. Persistent hyperlactataemia in the face of haemodynamic stability may reflect adrenaline-stimulated aerobic glycolysis rather than tissue hypoxia (James et al. 1999a). As reviewed by James et al. (1999a), plasma adrenaline can be elevated for prolonged periods in patients who are in shock due to sepsis, trauma, or haemorrhage. Adrenaline binds to β2-adrenoceptors, activating adenylate cyclase, which catalyses the conversion of ATP to cAMP. In turn, cAMP activates protein kinase A, which triggers a conformational change in the Na+–K+-ATPase; i.e. the Na+–K+-ATPase is activated (Clausen, 2003).
Central to the relationship between Na+–K+-ATPase activation and increased blood [La−] is the notion that the ATPase pump derives its energy heavily from glycolysis that is compartmentalized in association with the pump (James et al. 1999a). Such compartmentalization of glycolysis has been reported for red blood cells (Parker & Hoffman, 1967), smooth muscle (Paul et al. 1989), and sarcoplasmic reticulum (Entman et al. 1980; Xu et al. 1995). In support of such a scenario for the skeletal muscle Na+–K+-ATPase pump, James et al. (1996) reported that aerobically incubated muscles from septic or endotoxin-treated rats displayed an increased rate of La− production that was partially inhibited by the Na+–K+-ATPase blocker, ouabain. In further studies, both adrenaline- and amylin-stimulated glycolysis and glycogenolysis by aerobically incubated rat muscles was found to be closely linked to stimulation of the muscles' Na+–K+-ATPase activity (James et al. 1999b). Finally, in a recent study (Bundgaard et al. 2003), endotoxaemia was induced in a group of healthy human subjects. In response, both plasma [adrenaline] and [La−] were increased; the increased [La−] was associated with a measured increase in La− release by the legs. Activation of Na+–K+-ATPase was suggested by a significant decrease in plasma [K+] and a relative increase in K+ uptake by the legs. There was no evidence of hypoperfusion or hypoxia.
The Cincinnati group (Luchette, James et al.) has also presented evidence that adrenaline-stimulated Na+–K+-ATPase activity may be an important contributor to increased blood [La−] during haemorrhage. In one study (McCarter et al. 2001), rats were bled to a mean arterial blood pressure of 40 mmHg; one group of these haemorrhaged rats was also administered the adrenergic blockers, propranolol and phenoxybenzamine. This adrenergic blockade significantly reduced muscle La− accumulation and plasma [La−]. In haemorrhaged rats without the blockers, the intracellular Na+: K+ ratio was decreased, implying an increase in Na+–K+ pump activity. The intracellular Na+: K+ ratio was higher in the haemorrhaged rats that received the adrenergic blockers, suggesting that the blockade reduced pump activity and thereby La− production. In a second study (Luchette et al. 2002), microdialysis catheters were placed in the muscle of both thighs of anaesthetized rats. Rats were then either haemorrhaged or treated with a local perfusion of adrenaline; both of these conditions caused an increase in dialysate [La−]. Local administration of the Na+–K+-ATPase blocker, ouabain, reduced the dialysate [La−] response to both haemorrhage and adrenaline. The important implication is that a significant portion of the increased La− production during haemorrhage may be due to an elevated adrenaline concentration that stimulates Na+–K+-ATPase pump activity and compartmentally associated glycolysis. Tissue hypoperfusion, hypoxia and resulting anaerobic glycolysis are probably not the only causes of increased La− production during shock.
In summary, there is now strong evidence that clinical hyperlactataemia can be the result of adrenaline-stimulated Na+–K+-ATPase activity that is fuelled by coupled aerobic glycolysis. The fact that Na+–K+-ATPase activity is activated during exercise, probably by both an increased intramuscular [Na+] (Nielsen & Clausen, 2000) and by increasing adrenaline concentration, invites investigation of the possible role of pump-associated aerobic glycolysis in the progressive increase in blood [La−] during incremental exercise.
The glucose paradox of cerebral ischaemia
In the 1970s and early 1980s, a lactic acidosis hypothesis of cerebral ischaemic damage was developed (Siesjo, 1981; Schurr, 2002). The paradigm is simple; ischaemia leads to a lack of O2 with resulting stimulation of glycolysis, followed by an accumulation of HLa and concomitant acidosis with an end result of cell damage. In the same time frame, Myers & Yamaguchi (1977) reported the serendipitous discovery that pre-ischaemic hyperglycaemia resulted in an increase in post-ischaemic brain damage. This finding was easily incorporated into the lactic acidosis hypothesis; more glucose provided more fuel for ischaemic glycolysis causing higher tissue HLa levels, more severe acidosis and greater nerve cell damage (Schurr, 2002). This finding has been repeated in numerous experiments in many animal models since its initial report. This oft-verified result became known as ‘the glucose paradox of cerebral ischaemia’. The primary brain energy pathway during ischaemia is, of necessity, glycolysis from glucose (and glycogen); the paradox is that an increased supply of fuel (glucose) at a time when it is most needed, would also cause greater post-ischaemic brain damage.
This paradox is currently undergoing serious re-examination, largely because of a series of findings from Shurr's laboratory at the University of Louisville. First, it was reported that both glucose and lactic acidosis protect neuronal tissue against hypoxia in vitro (Schurr et al. 1987, 1988). Later studies (see Schurr & Rigor, 1998) demonstrated that La− is used as an aerobic energy substrate by neural tissue immediately following hypoxia in vitro. Even more recently, La− was found to be a critical oxidative energy substrate immediately post-ischaemia in rat brain in vivo (Schurr et al. 2001). Clearly, as also outlined above with regard to the ANLSH, far from being simply a noxious agent to neural tissue, La− is often a valuable neuronal energy substrate.
The history of the glucose paradox of cerebral ischaemia illustrates the danger of equating correlation with causality; post-ischaemic neural damage was almost without fail associated with elevated brain La− levels, with more severe damage corresponding to higher La− concentrations. If La− in fact is not the culprit in hyperglycaemic post-ischaemic cerebral damage, what is? Schurr's laboratory has provided provocative evidence that a corticosteroid, corticosterone in rats and possibly cortisol in humans, may be the causative factor in hyperglycaemic post-ischaemic damage. First, a disregarded result of the original experiments of Myers & Yamaguchi (1977) was brought to light (Payne et al. 2003). Myers & Yamaguchi (1977) had reported that glucose infusion prior to cardiac arrest caused greater post-ischaemic brain damage when the infusion was terminated shortly before the cardiac arrest. What was ignored was the additional result that there was no aggravated brain damage when the glucose infusion was terminated 90 min prior to the cardiac arrest. Schurr et al. (2001) followed this lead with experiments in a rat model of cardiac-arrest-induced transient global cerebral ischaemia (TGI). They found that hyperglycaemia that was induced 120–240 min prior to TGI actually reduced post-TGI neuronal damage as compared to normoglycaemia. However, when hyperglycaemia was induced 15–60 min pre-TGI, post-TGI neuronal damage was significantly increased. Interestingly, brain La− levels in both hyperglycaemic conditions were similar and significantly higher than in the normoglycaemic condition; in other words, the enhanced post-TGI damage was independent of the brain [La−]. Both hyperglycaemic conditions triggered a significant increase in blood corticosterone concentration with the peak increase occurring 15–30 min after the glucose loading and a return to baseline concentration by 60–120 min. Pre-TGI administration of an inhibitor of corticosterone synthesis reduced post-TGI neuronal damage in both hyperglycaemic (glucose loading 15 min pre-TGI) and normoglycaemic animals. In a subsequent study, administration of corticosterone in the same rat model of TGI caused neuronal damage that was comparable to that caused by glucose loading 15 min pre-TGI even though plasma glucose levels were normoglycaemic and not different from control levels following the corticosterone injection (Payne et al. 2003). On the whole, current evidence suggests that hypotheses to explain the glucose paradox of cerebral ischaemia should now focus on the elevation of plasma corticosterone (cortisol) concentration elicited by pre-ischaemic hyperglycaemia, and not on glucose per se, or La− as the product of glycolysis from glucose. Once again, La− is exonerated of the charge of being a noxious waste product of glycolytic metabolism.
Conclusion and summary
The La− paradigm has shifted. Evidence from a vast array of experimental approaches regarding a wide variety of physiological processes argues that increased La− production and [La−] as the result of anoxia or dysoxia are the exception rather than the rule. La− accumulation during exercise is most often the result of a host of interacting physiological and biochemical processes rather than simply O2-limited oxidative phosphorylation. La− and its concomitant acidosis may not be the primary culprit in muscle fatigue as previously believed. La− affects pH through its contribution to the [SID] and is only one of the factors involved in acid–base status. Surprisingly, La− is likely to be a key player in wound healing through its effect on down-regulation of ADP-ribosylation. In some cases of injury and sepsis, La− accumulation may relate, not to an O2 limitation, but to an adrenaline surge and a resulting stimulation of the Na+–K+-ATPase pump fuelled largely by a functionally linked aerobic glycolysis. Extra damage caused by hyperglycaemia preceding cerebral ischaemia may be the result of a corticosteroid surge instead of lactic acidosis. Essentially unanimous experimental support for the cell-to-cell lactate shuttle, along with mounting evidence for astrocyte–neuron, lactate–alanine, peroxisomal and spermatogenic lactate shuttles strongly suggest that La− is an important intermediary in numerous metabolic processes, a particularly mobile fuel for aerobic metabolism, and perhaps a mediator of redox state among various compartments both within and between cells. La− can no longer be considered the usual suspect for metabolic ‘crimes’, but is instead a central player in cellular, regional and whole body metabolism. Overall, the cell-to-cell lactate shuttle has expanded far beyond its initial conception as an explanation for muscle and exercise metabolism to now subsume all of the other shuttles as a grand description of the role(s) of La− in numerous metabolic processes and pathways. In the words of Thomas Kuhn (1970), paradigms must be ‘sufficiently unprecedented to attract an enduring group of adherents away from competing modes of scientific activity’, and ‘sufficiently open-ended to leave all sorts of problems for the redefined group of practitioners to resolve’, an apt description of the present state of research in La− metabolism.
References
- Ainscow EK, Mirshamsi S, Tang T, Ashford JLJ, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurons: evidence for ATP-independent control of ATP-sensitive K+ channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MAW, Godt RE, Nosek TM. Influence of physiological L(+)-lactate concentrations on contractility of skinned striated muscle fibers of rabbit. J Appl Physiol. 1996;80:2060–2065. doi: 10.1152/jappl.1996.80.6.2060. [DOI] [PubMed] [Google Scholar]
- Armstrong RB. Muscle fiber recruitment patterns and their metabolic correlates. In: Horton ES, Terjung RL, editors. Exercise, Nutrition, and Energy Metabolism. New York: Macmillan Publishing Co.; 1988. pp. 9–26. [Google Scholar]
- Attwell D. Brain uptake of glutamate: food for thought. J Nutr. 2000;130:1023S–1025S. doi: 10.1093/jn/130.4.1023S. [DOI] [PubMed] [Google Scholar]
- Baba N, Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol. 1971;51:621–635. doi: 10.1083/jcb.51.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai M, Gupta G, Setty BS. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur J Endocrinol. 1998;138:322–327. doi: 10.1530/eje.0.1380322. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Campbell PJ, Cooke DA. Glycogen, lactate, and alanine changes in muscle fiber types during graded exercise. J Appl Physiol. 1977;43:288–291. doi: 10.1152/jappl.1977.43.2.288. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart E, Fahimi HD, Stich A, Völkl A. L-Lactate dehydrogenase A4- and A3B isoforms are bona fide peroxisomal enzymes in rat liver. J Biol Chem. 1996;271:3846–3855. doi: 10.1074/jbc.271.7.3846. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol. 2000;278:E244–E251. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- Berl S, Clarke DD. The metabolic compartmentation concept. In: Hertz L, Kvamme E, McGeer EG, Schousboe A, editors. Glutamine, Glutamate and GABA in the Central Nervous System. New York: Liss; 1983. pp. 205–217. [Google Scholar]
- Bignami A. Glial cells in the central nervous system. In: Magistretti PG, editor. Discussions in Neuroscience. 1. III. Amsterdam: Elsevier; 1991. pp. 1–45. [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86:6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360:471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore A-K, Merle M, Magistretti PJ, Pellerin L. Feeding active neurons: (re)emergence of a nursing role for astrocytes. J Physiol Paris. 2002;96:273–282. doi: 10.1016/s0928-4257(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore A-K, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin J-L, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT1) expressing Xenopus laevis oocytes: expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Bröer S, Schneider H-P, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Lactate: glycolytic product and oxidative substrate during sustained exercise in mammals – the ‘lactate shuttle.’. In: Gilles R, editor. Comparative Physiology and Biochemistry: Current Topics and Trends, Respiration-Metabolism-Circulation. A. Berlin: Springer; 1985a. pp. 208–218. [Google Scholar]
- Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985b;17:22–31. [PubMed] [Google Scholar]
- Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc. 2000;32:790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttle – between but not within cells. J Physiol. 2002a;541:333. doi: 10.1113/jphysiol.2002.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002b;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol. 1999a;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A. 1999b;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Gladden LB. The metabolic systems: anaerobic metabolism (glycolytic and phosphagen) In: Tipton CM, editor. Exercise Physiology. People and Ideas. New York: Oxford University Press; 2003. pp. 322–360. chap. 8. [Google Scholar]
- Brown AM, Wender R, Ransom BR. Metabolic substrates other than glucose support axon function in central white matter. J Neurosci Res. 2001;66:839–843. doi: 10.1002/jnr.10081. [DOI] [PubMed] [Google Scholar]
- Bundgaard H, Kjeldesen K, Krabbe KS, Van Hall G, Simonsen L, Qvist J, Hansen CM, Møller K, Fonsmark L, Madsen PL, Pedersen BK. Endotoxemia stimulates skeletal muscle Na+-K+-ATPase and raises blood lactate under aerobic conditions in humans. Am J Physiol. 2003;284:H1028–H1034. doi: 10.1152/ajpheart.00639.2002. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin J, Tubbs PK. Mitochondrial transport processes and oxidation of NADH by hypotonically-treated boar spermatozoa. Eur J Biochem. 1978;89:315–320. doi: 10.1111/j.1432-1033.1978.tb20929.x. [DOI] [PubMed] [Google Scholar]
- Carpenter FG. Substrates supporting activity in immature nerve fibers. Am J Physiol. 1959;197:813–816. doi: 10.1152/ajplegacy.1959.197.4.813. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol. 2001;281:E794–E802. doi: 10.1152/ajpendo.2001.281.4.E794. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Gao Z-P, Forder JR. Impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol. 1999;277:E342–E351. doi: 10.1152/ajpendo.1999.277.2.E342. [DOI] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+/K+-ATPase α2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Gayeski TEJ, Honig CR. Lactate efflux is unrelated to intracellular PO2 in a working red muscle in situ. J Appl Physiol. 1986;61:402–408. doi: 10.1152/jappl.1986.61.2.402. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Honig CR, Gayeski TEJ, Brooks GA. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar]
- Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen. 2000;8:353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- Courtens JL, Plöen L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol Reprod. 1999;61:154–161. doi: 10.1095/biolreprod61.1.154. [DOI] [PubMed] [Google Scholar]
- Dawson AG. Oxidation of cytosolic NADH formed during aerobic metabolism in mammalian cells. Trends Biochem Sci. 1979;4:171–176. [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Deuticke B, Beyer E, Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982;684:96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Drummond GI, Harwood JP, Powell CA. Studies on the activation of phosphorylase in skeletal muscle by contraction and by epinephrine. J Biol Chem. 1969;244:4235–4240. [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Entman ML, Keslensky SS, Chu A, Van Winkle WB. The sarcoplasmic reticulum-glycogenolytic complex in mammalian fast twitch skeletal muscle. J Biol Chem. 1980;255:6245–6252. [PubMed] [Google Scholar]
- Favero TG, Zable AC, Bowman MB, Thompson A, Abramson JJ. Metabolic end products inhibit sarcoplasmic reticulum Ca2+ release and [3H]ryanodine binding. J Appl Physiol. 1995;78:1665–1672. doi: 10.1152/jappl.1995.78.5.1665. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Mechanisms of muscular fatigue. In: Poortmans JR, editor. Principles of Exercise Biochemistry. 3. Basel: Karger; 2003. pp. 279–300. [Google Scholar]
- Fletcher WM, Hopkins FG. Lactic acid in amphibian muscle. J Physiol. 1907;35:247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina FG, de Burgos NMG, Burgos C, Coronel CE, Blanco A. The lactate/pyruvate shuttle in spermatozoa: operation in vitro. Arch Biochem Biophys. 1994;308:515–519. doi: 10.1006/abbi.1994.1072. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Ghani QP, Wagner S, Hussain MZ. Role of ADP-ribosylation in wound repair. The contributions of Thomas K. Hunt, MD. Wound Repair Regen. 2003;11:439–444. doi: 10.1046/j.1524-475x.2003.11608.x. [DOI] [PubMed] [Google Scholar]
- Gibson DR, Angeles AP, Hunt TK. Increased oxygen tension on wound metabolism and collagen synthesis. Surg Forum. 1997;48:696–699. [Google Scholar]
- Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J Cereb Blood Flow Metab. 2001;21:1384–1392. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Net lactate uptake during progressive steady-level contractions in canine skeletal muscle. J Appl Physiol. 1991;71:514–520. doi: 10.1152/jappl.1991.71.2.514. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate transport and exchange during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 614–648. section 12. [Google Scholar]
- Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc. 2000;32:764–771. doi: 10.1097/00005768-200004000-00008. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactic acid: new roles in a new millennium. Proc Natl Acad Sci U S A. 2001;98:395–397. doi: 10.1073/pnas.98.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden LB. Lactate metabolism during exercise. In: Poortmans JR, editor. Principles of Exercise Biochemistry. 3. Basel: Karger; 2003. pp. 152–196. [Google Scholar]
- Gladden LB, Crawford RE, Webster MJ. Effect of lactate concentration and metabolic rate on net lactate uptake by canine skeletal muscle. Am J Physiol. 1994;266:R1095–R1101. doi: 10.1152/ajpregu.1994.266.4.R1095. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Leegsma-Vogt G, Venema K, Noraberf J, Korf J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. J Neurochem. 2003;85:399–408. doi: 10.1046/j.1471-4159.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Green H, Goldberg B. Collagen and cell protein synthesis by established mammalian fibroblast line. Nature. 1964;204:347–349. doi: 10.1038/204347a0. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Jansen R, van der Molen HJ. The role of glucose, pyruvate and lactate in ATP production by rat spermatocytes and spermatids. Biochim Biophys Acta. 1984;767:248–256. doi: 10.1016/0005-2728(84)90194-4. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil. 1986;77:109–118. doi: 10.1530/jrf.0.0770109. [DOI] [PubMed] [Google Scholar]
- Halangk W, Bohnensack R, Frank K, Kunz W. Effect of various substrates on mitochondrial and cellular energy state of intact spermatozoa. Biomed Biochim Acta. 1985;44:411–420. [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Kelley KM, Gladden LB. Effect of epinephrine on net lactate uptake by contracting skeletal muscle. J Appl Physiol. 2001;91:2635–2641. doi: 10.1152/jappl.2001.91.6.2635. [DOI] [PubMed] [Google Scholar]
- Hassel B, Brathe A. Cerebral metabolism of lactate in vivo: evidence for a neuronal pyruvate carboxylation. J Cereb Blood Flow Metab. 2000;20:327–336. doi: 10.1097/00004647-200002000-00014. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Takahata S. Role of peroxisomal fatty acyl-CoA beta-oxidation in phospholipids biosynthesis. Arch Biochem Biophys. 1991;284:326–331. doi: 10.1016/0003-9861(91)90303-z. [DOI] [PubMed] [Google Scholar]
- Hermansen L. Effect of metabolic changes on force generation in skeletal muscle during maximal exercise. In: Porter R, Whelan J, editors. CIBA Foundation Symposium 82. Human Muscle Fatigue: Physiological Mechanisms. London: Pitman Medical; 1981. pp. 75–88. [DOI] [PubMed] [Google Scholar]
- Hildyard JCW, Halestrap AP. Identification of the mitochondrial pyruvate carrier in Saccharomyces cerevisiae. Biochem J. 2003;374:607–611. doi: 10.1042/BJ20030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The revolution in muscle physiology. Physiol Rev. 1932;12:56–67. [Google Scholar]
- Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Part VI. The oxygen debt at the end of exercise. Proc R Soc Lond B Biol Sci. 1924;97:127–137. [Google Scholar]
- Hochachka PW. The metabolic implications of intracellular circulation. Proc Natl Acad Sci U S A. 1999;96:12233–12239. doi: 10.1073/pnas.96.22.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Gladden LB, Kurdak SS, Poole DC. Increased [lactate] in working dog muscle reduces tension development independent of pH. Med Sci Sports Exerc. 1995;27:371–377. [PubMed] [Google Scholar]
- Hosoya K, Kondo T, Tomi M, Takanaga H, Ohtsuki S, Terasaki T. MCT1-mediated transport of L-lactic acid at the inner blood–retinal barrier: a possible route for delivery of monocarboxylic acid drugs to the retina. Pharm Res. 2001;18:1669–1676. doi: 10.1023/a:1013310210710. [DOI] [PubMed] [Google Scholar]
- Hunt TK, Conolly WB, Aronson SB, Goldstein P. Anaerobic metabolism and wound healing: an hypothesis for the initiation and cessation of collagen synthesis in wounds. Am J Surg. 1978;135:328–332. doi: 10.1016/0002-9610(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol. 2000;61:397–414. doi: 10.1016/s0301-0082(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Inskeep PB, Hammerstedt RH. Endogenous metabolism by sperm in response to altered cellular ATP requirements. J Cell Physiol. 1985;123:180–190. doi: 10.1002/jcp.1041230205. [DOI] [PubMed] [Google Scholar]
- Jackson VN, Price NT, Halestrap AP. cDNA cloning of MCT1, a monocarboxylate transporter from rat skeletal muscle. Biochim Biophys Acta. 1995;1238:193–196. doi: 10.1016/0005-2736(95)00160-5. [DOI] [PubMed] [Google Scholar]
- James JH, Fang C-H, Schrantz SJ, Hasselgren P-O, Paul RJ, Fischer JE. Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. J Clin Invest. 1996;98:2388–2397. doi: 10.1172/JCI119052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999a;354:505–508. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- James JH, Wagner KR, King J-K, Leffler RE, Upputuri RK, Ambikaipakan B, Friend LA, Shelly DA, Paul RJ, Fischer JE. Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol. 1999b;277:E176–E186. doi: 10.1152/ajpendo.1999.277.1.E176. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Edwards HT, Dill DB, Wilson JW. Blood as a physicochemical system: the distribution of lactate. J Biol Chem. 1945;157:461–473. [Google Scholar]
- Johnson RL, Jr, Heigenhauser GJF, Hsia CCW, Jones NL, Wagner PD. Determinants of gas exchange and acid-base balance during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 515–584. section 12. [Google Scholar]
- Jones AR. Metabolism of lactate by mature boar spermatozoa. Reprod Fertil Dev. 1997;9:227–232. doi: 10.1071/r96102. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L. Metabolism of L(+)-lactate in human skeletal muscle during exercise. Acta Physiol Scand. 1970;338(suppl.):1–67. [PubMed] [Google Scholar]
- Juel C. Current aspects of lactate exchange: Lactate/H+ transport in human skeletal muscle. Eur J Appl Physiol. 2001;86:12–16. doi: 10.1007/s004210100517. [DOI] [PubMed] [Google Scholar]
- Juel C, Bangsbo J, Graham T, Saltin B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand. 1990;140:147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. J Physiol. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. Lactate and phosphagen concentrations in working muscle of man with special reference to oxygen deficit at the onset of work. Acta Physiol Scand Suppl. 1971;358:1–72. [PubMed] [Google Scholar]
- Keilin D. The History of Cell Respiration and Cytochrome. Cambridge: Cambridge University Press; 1966. p. 68. [Google Scholar]
- Kelley KM, Hamann JJ, Navarre C, Gladden LB. Lactate metabolism in resting and contracting canine skeletal muscle with elevated lactate concentration. J Appl Physiol. 2002;93:865–872. doi: 10.1152/japplphysiol.01119.2001. [DOI] [PubMed] [Google Scholar]
- Keyhani E, Storey BT. Energy conservation capacity and morphological integrity of miotchondria in hypotonically treated rabbit epididymal spermatozoa. Biochim Biophys Acta. 1973;305:557–569. doi: 10.1016/0005-2728(73)90075-3. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Jalonen T, Walz W. Regulation of brain microenvironment: transmitters and ions. In: Murphy S, editor. Astrocytes: Pharmacology and Function. San Diego CA: Academic Press; 1993. pp. 193–228. [Google Scholar]
- Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB, Waters MG. Localization of L-lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1986;246:673–680. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- Kowalchuk JM, Heigenhauser GJF, Lindinger MI, Sutton JR, Jones NL. Factors influencing hydrogen ion concentration in muscle after intense exercise. J Appl Physiol. 1988;65:2080–2089. doi: 10.1152/jappl.1988.65.5.2080. [DOI] [PubMed] [Google Scholar]
- Krützfeldt A, Spahr R, Mertens S, Siegmund B, Piper HM. Metabolism of exogenous substrates by coronary endothelial cells in culture. J Mol Cell Cardiol. 1990;22:1393–1404. doi: 10.1016/0022-2828(90)90984-a. [DOI] [PubMed] [Google Scholar]
- Kuan J, Saier MH. The mitochondrial carrier family of transport proteins: structural, functional and evolutionary relationships. Crit Rev Biochem Mol Biol. 1993;28:209–233. doi: 10.3109/10409239309086795. [DOI] [PubMed] [Google Scholar]
- Kuhn TS. The Structure of Scientific Revolutions. Chicago: University of Chicago Press; 1970. p. 10. [Google Scholar]
- Lai JC, Behar KL, Liang BB, Hertz L. Hexokinase in astrocytes: kinetic and regulatory properties. Metab Brain Dis. 1999;14:125–133. doi: 10.1023/a:1020761831295. [DOI] [PubMed] [Google Scholar]
- Larrabee MG. Lactate metabolism and its effect on glucose metabolism in an excised neural tissue. J Neurochem. 1995;64:1734–1741. doi: 10.1046/j.1471-4159.1995.64041734.x. [DOI] [PubMed] [Google Scholar]
- Lazarow PB, de Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegsma-Vogt G, Venema K, Korf J. Evidence for a lactate pool in the rat brain that is not used as an energy supply under normoglycemic conditions. J Cereb Blood Flow Metab. 2003;23:933–941. doi: 10.1097/01.WCB.0000080650.64357.8F. [DOI] [PubMed] [Google Scholar]
- Lindinger MI. Exercise: a paradigm for multi-system control of acid-base state. J Physiol. 2003;550:334. doi: 10.1113/jphysiol.2003.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJF, McKelvie RS, Jones NL. Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol. 1992;262:R126–R136. doi: 10.1152/ajpregu.1992.262.1.R126. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, McKelvie RS, Heigenhauser GJF. K+ and Lac− distribution in humans during and after high-intensity exercise: role in muscle fatigue attenuation? J Appl Physiol. 1995;78:765–777. doi: 10.1152/jappl.1995.78.3.765. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Brocks C, Chatham JC. Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in rat heart. Am J Physiol. 2003;285:H163–H172. doi: 10.1152/ajpheart.01117.2002. [DOI] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchette FA, Jenkins WA, Friend LA, Su C, Fischer JE, James JH. Hypoxia is not the sole cause of lactate production during shock. J Trauma. 2002;52:415–419. doi: 10.1097/00005373-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Mac M, Nalecz KA. Expression of monocarboxylic acid transporters (MCT) in brain cells. Implications for branched chain alpha-ketoacids transport in neurons. Neurochem Int. 2003;43:305–309. doi: 10.1016/s0197-0186(03)00016-0. [DOI] [PubMed] [Google Scholar]
- McCarter FD, James JH, Luchette FA, Wang L, Friend LA, King J-K, Evans JM, George MA, Fischer JE. Adrenergic blockade reduces skeletal muscle glycolysis and Na+,K+-ATPase activity during hemorrhage. J Surg Res. 2001;99:235–244. doi: 10.1006/jsre.2001.6175. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Khanna S, González GF, Butz CE, Brooks GA. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun. 2003;304:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- McGroarty E, Hsieh B, Wied DM, Gee R, Tolbert NE. Alpha hydroxyl acid oxidation by peroxisomes. Arch Biochem Biophys. 1974;161:194–210. [Google Scholar]
- McIlwain H. Electrical influences and speed of chemical changes. Physiol Rev. 1956;36:355–375. doi: 10.1152/physrev.1956.36.3.355. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mangia S, Garreffa G, Bianciardi M, Giove F, Di Salle F, Maraviglia B. The aerobic brain: lactate decrease at the onset of neural activity. Neuroscience. 2003a;118:7–10. doi: 10.1016/s0306-4522(02)00792-3. [DOI] [PubMed] [Google Scholar]
- Mangia S, Giove F, Bianciardi M, Di Salle F, Garreffa G, Maraviglia B. Issues concerning the construction of a metabolic model for neuronal activation. J Neurosci Res. 2003b;71:463–467. doi: 10.1002/jnr.10531. [DOI] [PubMed] [Google Scholar]
- Margaria R, Edwards RHT, Dill DB. The possible mechanisms of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol. 1933;106:689–715. [Google Scholar]
- Mathews CK, van Holde KE, Ahern KG. Biochemistry. New York: Addison-Wesley Longman, Inc; 2000. p. 648. [Google Scholar]
- Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol. 1986;60:232–241. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Feeding hungry neurons: astrocytes deliver food for thought. Neuron. 2003;37:187–189. doi: 10.1016/s0896-6273(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Meyerhof O. Die Energieumwandlungen im Muskel. I. Über die Beziehungen der Milchsaure zur Warmebildung and Arbeitsleistung des Muskels in der Anaerobiose. Pflugers Arch Ges Physiol Mensch Tiere. 1920;182:232–283. [Google Scholar]
- Milkowski AL, Lardy HA. Factors affecting the redox state of bovine epididymal spermatozoa. Arch Biochem Biophys. 1977;181:270–277. doi: 10.1016/0003-9861(77)90505-7. [DOI] [PubMed] [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol. 2002a;544:963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Suh S-H, Navazio F, Brooks GA. Metabolic and cardiorespiratory responses to ‘the lactate clamp’. Am J Physiol. 2002b;283:E889–E898. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]
- Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20:80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- Mori K, Nakaya Y, Sakamoto S, Hayabuchi Y, Matsuoka S, Kuroda Y. Lactate-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. J Mol Cell Cardiol. 1998;30:349–356. doi: 10.1006/jmcc.1997.0598. [DOI] [PubMed] [Google Scholar]
- von Muralt A. The development of muscle-chemistry, a lesson in neurophysiology. Biochim Biophys Acta. 1950;4:126–129. doi: 10.1016/0006-3002(50)90015-1. [DOI] [PubMed] [Google Scholar]
- Myers RE, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Arch Neurol. 1977;34:65–74. doi: 10.1001/archneur.1977.00500140019003. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Okinaga S, Arai K. Metabolism of round spermatids: evidence that lactate is preferred substrate. Am J Physiol. 1984;247:E234–E242. doi: 10.1152/ajpendo.1984.247.2.E234. [DOI] [PubMed] [Google Scholar]
- Newsholme EA. Enzymes, energy and endurance. In: Poortmans JR, editor. Principles of Exercise Biochemistry. 3. Basel: Karger; 2003. pp. 1–35. [Google Scholar]
- Nielsen OB, Clausen T. The Na+/K+-pump protects muscle excitability and contractility during exercise. Exerc Sport Sci Rev. 2000;28:159–164. [PubMed] [Google Scholar]
- Nielsen HB, Clemmesen JO, Skak C, Ott P, Secher NH. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. J Appl Physiol. 2002;92:1677–1683. doi: 10.1152/japplphysiol.00028.2001. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H. Factors which can influence beta-oxidation by peroxisomes isolated from livers of clofibrate treated rats. Some properties of peroxisomal fractions isolated in a self-generated Percoll gradient by vertical rotor centrifugation. Int J Biochem. 1982;14:905–914. doi: 10.1016/0020-711x(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Osmundsen H, Hovik R, Bartlett K, Pourfazam M. Regulation of flux of acyl-CoA esters through peroxisomal β-oxidation. Biochem Soc Trans. 1994;22:436–441. doi: 10.1042/bst0220436. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Bisaccia F, Capobianco L, Dolce V, Fiermonte G, Iacobazzi V, Indiveri C, Palmieri L. Mitochondrial metabolite transporters. Biochim Biophys Acta. 1996;1275:127–132. doi: 10.1016/0005-2728(96)00062-x. [DOI] [PubMed] [Google Scholar]
- Parker JC, Hoffman JF. The role of membrane phosphoglycerate kinase in the control of glycolytic rate by active cation transport in human red blood cells. J General Physiol. 1967;50:893–916. doi: 10.1085/jgp.50.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJF. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Physiol. 1999;277:E890–E900. doi: 10.1152/ajpendo.1999.277.5.E890. [DOI] [PubMed] [Google Scholar]
- Paul RJ, Hardin CD, Raeymaekers L, Wuytack F, Casteels R. Preferential support of Ca2+ uptake in smooth muscle plasma membrane vesicles by an endogenous glycolytic cascade. FASEB J. 1989;3:2298–2301. doi: 10.1096/fasebj.3.11.2528493. [DOI] [PubMed] [Google Scholar]
- Payne RS, Tseng MT, Schurr A. The glucose paradox of cerebral ischemia: evidence for corticosterone involvement. Brain Res. 2003;971:9–17. doi: 10.1016/s0006-8993(03)02276-5. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+/K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem. 1997;69:2132–2137. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. How to balance the brain energy budget while spending glucose differently. J Physiol. 2003;546:325. doi: 10.1113/jphysiol.2002.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin J-L, Stella N, Magistretti PJ. Evidence supporting the existence of an astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H de F. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. Philadelphia: Saunders; 1991. [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popinigis J, Antosiewiez J, Crimi M, Lenaz G, Wakabayashi T. Human skeletal muscle: participation of different metabolic activities in oxidation of L-lactate. Acta Biochim Pol. 1991;38:169–175. [PubMed] [Google Scholar]
- Posterino GS, Dutka TL, Lamb GD. L(+)-lactate does not affect twitch and tetanic responses in mechanically skinned mammalian muscle fibres. Pflugers Arch. 2001;442:197–203. doi: 10.1007/s004240100528. [DOI] [PubMed] [Google Scholar]
- Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U. 13C NMR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci. 2000;22:429–436. doi: 10.1159/000017472. [DOI] [PubMed] [Google Scholar]
- Rasmussen HN, Van Hall G, Rasmussen UF. Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J Physiol. 2002;541:575–580. doi: 10.1113/jphysiol.2002.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol. 1998;85:627–634. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kiens B, Saltin B, Christensen NJ, Savard G. Skeletal muscle glucose uptake during dynamic exercise in humans: role of muscle mass. Am J Physiol. 1988;254:E555–E561. doi: 10.1152/ajpendo.1988.254.5.E555. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- Roef MJ, de Meer K, Kalhan SC, Straver H, Berger R, Reijngoud D-J. Gluconeogenesis in humans with induced hyperlactatemia during low-intensity exercise. Am J Physiol. 2003;284:E1162–E1171. doi: 10.1152/ajpendo.00425.2002. [DOI] [PubMed] [Google Scholar]
- Rohlmann A, Wolff JR. Subcellular topography and plasticity of gap junction distribution on astrocytes. In: Spray DC, Dermietzel R, editors. Gap Junctions in the Nervous System. Austin TX: RG Landes; 1996. pp. 175–192. [Google Scholar]
- Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990a;279:377–385. doi: 10.1016/0003-9861(90)90505-s. [DOI] [PubMed] [Google Scholar]
- Roth DA, Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990b;279:386–394. doi: 10.1016/0003-9861(90)90506-t. [DOI] [PubMed] [Google Scholar]
- Rush JWE, Spriet LL. Skeletal muscle glycogen phosphorylase a kinetics: Effects of adenine nucleotides and caffeine. J Appl Physiol. 2001;91:2071–2078. doi: 10.1152/jappl.2001.91.5.2071. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Metabolic factors in fatigue. Sports Med. 1992;13:99–107. doi: 10.2165/00007256-199213020-00005. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Fernström M, Svensson M, Tonkonogi M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J Physiol. 2002;541:569–574. doi: 10.1113/jphysiol.2002.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Salway JG. Metabolism at a Glance. London: Blackwell Science; 1999. pp. 84–85. pp. 21. [Google Scholar]
- Samaja M, Allibardi S, Milano G, Neri G, Grassi B, Gladden LB, Hogan MC. Differential depression of myocardial function and metabolism by lactate and H+ Am J Physiol. 1999;276:H3–H8. doi: 10.1152/ajpheart.1999.276.1.H3. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sonnewald U, Waagepetersen HS. Differential roles of alanine in GABAergic and glutamatergic neurons. Neurochem Int. 2003;43:311–315. doi: 10.1016/s0197-0186(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Waagepetersen HS, Larsson OM, Bakken IJ, Sonnewald U. Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia. 1997;21:99–105. [PubMed] [Google Scholar]
- Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10:131–136. [PubMed] [Google Scholar]
- Schurr A, Dong W-Q, Reid KH, West CA, Rigor BM. Lactic acidosis and recovery of neuronal function following cerebral hypoxia in vitro. Brain Res. 1988;438:311–314. doi: 10.1016/0006-8993(88)91354-6. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Tseng MT, Rigor BM. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001;895:268–272. doi: 10.1016/s0006-8993(01)02082-0. [DOI] [PubMed] [Google Scholar]
- Schurr A, Rigor BM. Brain anaerobic lactate production: a suicide note or a survival kit? Dev Neurosci. 1998;20:348–357. doi: 10.1159/000017330. [DOI] [PubMed] [Google Scholar]
- Schurr A, West CA, Reid KH, Tseng MT, Reiss SJ, Rigor BM. Increased glucose improves recovery of neuronal function after cerebral hypoxia in vitro. Brain Res. 1987;421:135–139. doi: 10.1016/0006-8993(87)91283-2. [DOI] [PubMed] [Google Scholar]
- Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg. 2000;135:1293–1297. doi: 10.1001/archsurg.135.11.1293. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1:155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells of athletic and nonathletic species. Am J Physiol. 1995;268:R1121–R1128. doi: 10.1152/ajpregu.1995.268.5.R1121. [DOI] [PubMed] [Google Scholar]
- Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells from trained and untrained human subjects. Med Sci Sports Exerc. 1998;30:536–542. doi: 10.1097/00005768-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism. In Vivo J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- Smith EW, Skelton MS, Kremer DE, Pascoe DD, Gladden LB. Lactate distribution in the blood during progressive exercise. Med Sci Sports Exerc. 1997;29:654–660. doi: 10.1097/00005768-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Smith EW, Skelton MS, Kremer DE, Pascoe DD, Gladden LB. Lactate distribution in the blood during steady-state exercise. Med Sci Sports Exerc. 1998;30:1424–1429. doi: 10.1097/00005768-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Ward CW, Williams JH. Effects of lactate on force production by mouse EDL muscle: Implications for the development of fatigue. Can J Physiol Pharmacol. 1998;76:642–648. doi: 10.1139/cjpp-76-6-642. [DOI] [PubMed] [Google Scholar]
- Spriet LL. Phosphofructokinase activity and acidosis during short-term tetanic contractions. Can J Physiol Pharmacol. 1991;69:298–304. doi: 10.1139/y91-046. [DOI] [PubMed] [Google Scholar]
- Spriet LL. Anaerobic metabolism in human skeletal muscle during short-term, intense activity. Can J Physiol Pharmacol. 1992;70:157–165. doi: 10.1139/y92-023. [DOI] [PubMed] [Google Scholar]
- Stainsby WN, Brooks GA. Control of lactic acid metabolism in contracting muscles and during exercise. Exerc Sport Sci Rev. 1990;18:29–63. [PubMed] [Google Scholar]
- Stainsby WN, Welch HG. Lactate metabolism of contracting dog skeletal muscle in situ. Am J Physiol. 1966;211:177–183. doi: 10.1152/ajplegacy.1966.211.1.177. [DOI] [PubMed] [Google Scholar]
- Stanley WC. Myocardial lactate metabolism during exercise. Med Sci Sports Exerc. 1991;23:920–924. [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- Stewart PA. How to Understand Acid-Base: A Quantitative Acid-Base Primer for Biology and Medicine. New York: Elsevier; 1981. [Google Scholar]
- Storey BT, Kayne FJ. Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod. 1977;16:549–556. [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Trials, tribulations and finally, a transporter: the identification of the mitochondrial pyruvate transporter. Biochem J. 2003;374:e1–e2. doi: 10.1042/bj20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna-Kaczmarek A. L-lactate oxidation by skeletal muscle mitochondria. Int J Biochem. 1990;22:617–620. doi: 10.1016/0020-711x(90)90038-5. [DOI] [PubMed] [Google Scholar]
- Thomas S, Fell DA. A control analysis exploration of the role of ATP utilisation in glycolytic-flux control and glycolytic-metabolite-concentration regulation. Eur J Biochem. 1998;258:956–967. doi: 10.1046/j.1432-1327.1998.2580956.x. [DOI] [PubMed] [Google Scholar]
- Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, Seremetiev A, Becker HD, Hunt TK. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11:504–509. doi: 10.1046/j.1524-475x.2003.11621.x. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Calbet JAL, Søndergaard H, Saltin B. Skeletal muscle carbohydrate and lactate metabolism after 9 wk of acclimatization to 5,260 m. Am J Physiol. 2002;283:E1203–E1213. doi: 10.1152/ajpendo.00134.2001. [DOI] [PubMed] [Google Scholar]
- Van Roermund CWT, Elgersma Y, Singh N, Wanders RJA, Tabak HF. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 1995;14:3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véga C, Poitry-Yamate CL, Jirounek P, Tsacopoulos M, Coles JA. Lactate is released and taken up by isolated rabbit vagus nerve during aerobic metabolism. J Neurochem. 1998;71:330–337. doi: 10.1046/j.1471-4159.1998.71010330.x. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton J-Y, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem. 2000;75:471–479. doi: 10.1046/j.1471-4159.2000.0750471.x. [DOI] [PubMed] [Google Scholar]
- Wasserman K. The anaerobic threshold to evaluate exercise performance. Am Rev Respir Dis. 1984;129(suppl.):S35–S40. doi: 10.1164/arrd.1984.129.2P2.S35. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lännergren J. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res. 1995;77:88–97. doi: 10.1161/01.res.77.1.88. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Richter-Landsberg C, Brand A, Leibfritz D. NMR spectroscopic study on the metabolic fate of [3-13C]alanine in astrocytes, neurons, and cocultures: implications for glia–neuron interactions in neurotransmitter metabolism. Glia. 2000;32:286–303. doi: 10.1002/1098-1136(200012)32:3<286::aid-glia80>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]