Abstract
Large conductance calcium- and voltage-activated potassium channels (BK channels) activate in response to calcium influx during action potentials and contribute to the spike repolarization and fast afterhyperpolarization. BK channels targeted to active zones in presynaptic nerve terminals have been shown to limit calcium entry and transmitter release by reducing the duration of the presynaptic spike at neurosecretory nerve terminals and at the frog neuromuscular junction. However, their functional role in central synapses is still uncertain. In the hippocampus, BK channels have been proposed to act as an ‘emergency brake’ that would control transmitter release only under conditions of excessive depolarization and accumulation of intracellular calcium. Here we demonstrate that in the CA3 region of hippocampal slice cultures, under basal experimental conditions, the selective BK channel blockers paxilline (10 μm) and iberiotoxin (100 nm) increase the frequency, but not the amplitude, of spontaneously occurring action potential-dependent EPSCs. These drugs did not affect miniature currents recorded in the presence of tetrodotoxin, suggesting that their action was dependent on action potential firing. Moreover, in double patch-clamp recordings from monosynaptically interconnected CA3 pyramidal neurones, blockade of BK channels enhanced the probability of transmitter release, as revealed by the increase in success rate, EPSC amplitude and the concomitant decrease in paired-pulse ratio in response to pairs of presynaptic action potentials delivered at a frequency of 0.05 Hz. BK channel blockers also enhanced the appearance of delayed responses, particularly following the second action potential in the paired-pulse protocol. These results are consistent with the hypothesis that BK channels are powerful modulators of transmitter release and synaptic efficacy in central neurones.
BK channels are very large conductance potassium channels (∼250 pS) activated by both calcium and voltage that regulate cell excitability (Vergara et al. 1998). They are widely expressed throughout the vertebrate nervous system (Hille, 2001; Knaus et al. 1996) where they are often colocalized with voltage-dependent calcium channels (VDCCs, Robitaille et al. 1993; Lancaster & Nicoll, 1987; Storm, 1987a; Marrion & Tavallin, 1998; Stanley, 1997). The interplay between these two channels is very tight and has very important functional consequences (see for instance in the cochlea: Roberts et al. 1990). Activated by membrane depolarization during action potentials, BK channels tend to hyperpolarize the cell driving the membrane potential towards the equilibrium potential for potassium. By doing so they regulate cell excitability and contribute to action potential repolarization and spike-frequency adaptation (Adams et al. 1982; Lancaster & Nicoll, 1987; Storm, 1987b; Schwindt et al. 1988; Shao et al. 1999). Immunohistochemical and radioligand binding studies have revealed the presence of BK channels on neuronal soma, processes (but see Poolos & Johnston, 1999) and axon terminals of several brain structures including the hippocampus, where they are particularly abundant (Knaus et al. 1996; Wanner et al. 1999). Here, they have been localized in presynaptic nerve endings, on the membranes facing the synaptic cleft at Schaffer collateral—CA1 synapses (Hu et al. 2001). This presynaptic localization suggests a role for BK channels in controlling transmitter release. Thus, by shaping presynaptic action potentials they would regulate calcium signals necessary to trigger fusion of synaptic vesicles, exocytosis and transmitter release (Sabatini & Regehr, 1997; Sun et al. 1999). Indeed, BK channels have been shown to regulate secretion in exocrine tissues (Petersen & Maruyama, 1984; Obaid et al. 1989; Dopico et al. 1999) and transmitter release at the frog neuromuscular junction (Robitaille & Charlton, 1992). In this preparation, block of BK channels with charybdotoxin produced a two-fold increase of transmitter release. This suggests that under physiological conditions BK channels diminish transmitter release by narrowing presynaptic action potentials and by reducing calcium entry into the cytosol (Robitaille & Charlton, 1992; Robitaille et al. 1993). In central neurones, however, the role of BK channels in regulating transmitter release is still uncertain and has been indirectly inferred from their action at the somatic level (Shao et al. 1999). It has even been hypothesized that, under basal conditions, BK channels targeted to active zones of presynaptic glutamatergic terminals do not exert any effect on transmitter release (Hu et al. 2001). They would provide an ‘emergency brake’ only under conditions of excessive depolarization and accumulation of intracellular calcium, such as brain ischaemia and epilepsy (Hu et al. 2001; Runden-Pran et al. 2002).
In the present study we took advantage of hippocampal slice cultures to study the role of BK channels on transmitter release at monosynaptically coupled CA3–CA3 principal cells (Debanne et al. 1995; Pavlidis & Madison, 1999). While this preparation maintains morphological and functional features similar to those of native brain tissue, it has the advantage of expressing a higher level of connectivity which increases the probability of finding monosynaptically coupled neurones (Gähwiler et al. 1997; De Simoni et al. 2003). We found that blocking BK channels with iberiotoxin or paxilline increased transmitter release and synaptic efficacy in target neurones.
Methods
Organotypic hippocampal slice cultures
The hippocampus was removed from 4- to 7-day-old rats killed by decapitation and organotypic cultures were prepared following the method already described (Gähwiler, 1981; Saviane et al. 2002). The procedure is in accordance with the regulations of the Italian Animal Welfare Act and was approved by the local authority veterinary service. Transverse 400 μm thick slices were cut with a tissue chopper and attached to coverslips in a film of reconstituted chicken plasma (Cocalico, Reamstown, PA, USA) clotted with thrombin (Sigma, Milan, Italy). The coverslips were transferred to plastic tubes containing 0.75 ml of medium. The tubes were placed in a roller drum (6 revolutions h−1) inside an incubator at 36°C. The medium contained: basal medium (BME, Eagle, with Hanks's salts, without l-glutamine; Gibco, 100 ml), Hanks' balanced salt solution (HBSS; Gibco, 50 ml), horse serum (Gibco, 50 ml), l-glutamine (Gibco, 200 mm, 1 ml), 50%d-glucose in sterile water for tissue culture (Gibco, 2 ml).
Electrophysiological recordings
After 10–14 days in vitro the cultures, which had flattened near-monolayer thickness, were transferred to a recording chamber fixed to the stage of an upright microscope. Cultured slices in the recording chamber were superfused at room temperature (22–24°C) with a bath solution containing (mm): NaCl 150, KCl 3, CaCl2 2, MgCl2 1, Hepes 10, glucose 10 (pH 7.3, adjusted with NaOH). A low concentration of tetrodotoxin (TTX, 10 nm; Affinity Research Products, Nottingham, UK) was added in order to reduce polysynaptic activity. Electrophysiological experiments were performed on CA3 pyramidal cells using the whole-cell configuration of the patch-clamp technique in current- or voltage-clamp mode. CA3 neurones were identified both visually (using infrared differential interference contrast video microscopy) and on the basis of their firing properties, i.e. their ability to accommodate in response to long (800 ms) depolarizing current pulses. The identity of cells as pyramidal neurones was confirmed in some experiments in which cells were labelled with biocytin (0.2–0.3%; purchased from Sigma, Milan, Italy; see Fig. 3). Patch electrodes were pulled from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany). They had a resistance of 3–6 MΩ when filled with an intracellular solution containing (mm): KMeSO4 135 (125 when adding 10 mm BAPTA), KCl 10, Hepes 10, MgCl2 1, Na2ATP 2, Na2GTP 0.4; pH was adjusted to 7.3 with KOH. Single or pairs of action potentials (50 ms interval) were evoked in current-clamp mode by short (5 ms) depolarizing current pulses at 0.05 Hz. Spontaneous or evoked EPSCs were recorded from the postsynaptic neurones, loaded with the calcium chelator BAPTA (10 mm) in order to block activation of BK channels. EPSCs were detected in voltage-clamp mode at a holding potential of −60 mV. Under our experimental conditions the reversal potential for Cl− was −66 mV. Membrane potential values were corrected for the liquid junction potential of 9 mV.
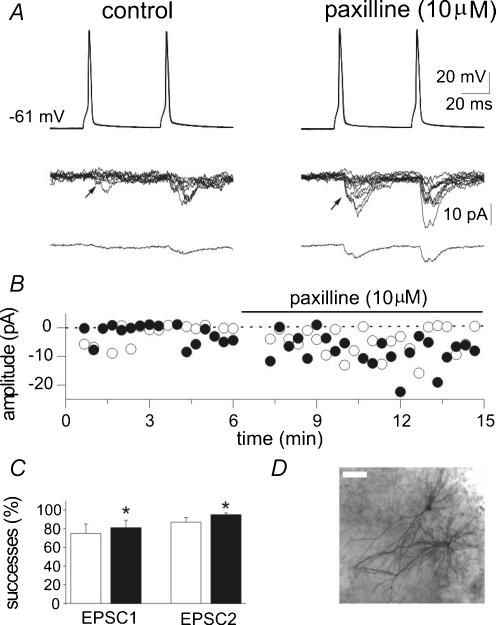
Figure 3. Blocking BK channels with paxilline increases synaptic efficacy at low probability CA3−CA3 connections.
A, pairs of action potentials are generated (50 ms intervals, 0.05 Hz) in the presynaptic cell (upper traces) while EPSCs are recorded from the postsynaptic cell in control (left) and in the presence of paxilline (10 μm, right). Eight traces are superimposed and shown in the middle, while the average of all responses (successes plus failures) is shown at the bottom. Note reduced failure rate and increased amplitude of successes after paxilline. B, time course of the peak amplitude of the first (○) and second (•) EPSCs recorded from the cell shown in A. C, mean success rate of EPSC1 and EPSC2 obtained in 7 cells under control conditions (open columns) and during paxilline application (filled columns). P <0.05. D, a pair of interconnected cells labelled with biocytin (the bar is 50 μm).
Stock solution of the BK channel blockers iberiotoxin (from Latoxan, Valence, France) and paxilline (from Sigma-Aldrich, Milan, Italy) were obtained by dissolving the drugs in water or dimethylsulphoxide (DMSO) and applied at the final concentration of 50–100 nm and 10 μm, respectively. The final concentration of DMSO in the working solution was 0.1% (v/v). At this concentration, DMSO alone did not modify the shape of action potentials or the kinetic properties of EPSCs.
Data acquisition and analysis
Data were stored on a magnetic tape and transferred to a computer after digitization with an A/D converter (Digidata 1322, Axon Instruments, Foster City, CA, USA). Data acquisition was done using pCLAMP 8.2 (Axon Instruments). Data were sampled at 20–100 kHz and filtered with a cut-off frequency of 1 kHz. Series resistance compensation was used for current-clamp recordings. Membrane input resistance was calculated by measuring the amplitude of voltage responses to steady hyperpolarizing current steps of 100–200 pA.
Spontaneous EPSCs were analysed with the AxoGraph 4.6 program (Axon Instruments), which uses a detection algorithm based on the minimization of the sum of squared errors between data and a template function approximating the width and the time course of a typical synaptic event as described by Clements & Bekkers (1997). Miniature EPSCs were recorded in the presence of TTX (1 μm) at the holding potential of −60 mV, which is close to the reversal for Cl−.
Evoked EPSCs were analysed with Clampfit software and transmission failures were identified visually. The onset of the EPSC was given by the intersection of a line through the 10 and 90% of EPSC rise time with the baseline. Onset, rise and decay times were calculated after averaging only the successes. EPSC latency was calculated as the time gap between the onset of the mean EPSC and the peak of the presynaptic spike. Mean EPSC amplitude was obtained by averaging successes and failures. The paired-pulse ratio (PPR) was calculated as the ratio between the mean amplitude of EPSC2 over EPSC1.
Action potentials were analysed with Clampfit software. They were characterized by their firing threshold, their amplitude (from threshold to peak) and their width at the threshold level.
Values are given as mean ±s.e.m. Significance of differences was assessed by Student's t test or Wilcoxon test. The differences were considered significant when P was < 0.05.
Results
BK channels contribute to action potential repolarization in CA3 neurones
Patch-clamp recordings, in whole-cell configuration and current-clamp mode, were performed from CA3 pyramidal neurones in organotypic hippocampal slice cultures. These neurones were identified as principal cells both visually and on the basis of their firing properties, i.e. their ability to accommodate in response to long depolarizing current pulses. In some experiments (n = 16), cells were morphologically identified as pyramidal neurones by biocytin injection (Fig. 3D).
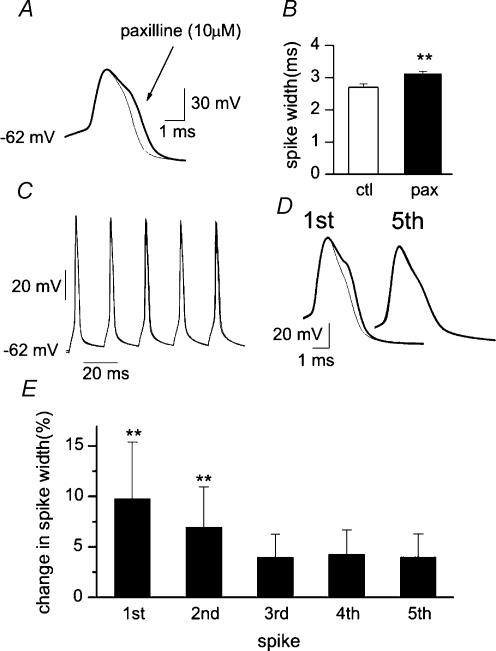
Action potentials were induced by the injection of short depolarizing current steps from the resting membrane potential. The impact of BK channels on action potentials was firstly examined by applying paxilline, a tremorgenic indole alkaloid that selectively blocks these channels (Knaus et al. 1994). As shown in Fig. 1A and B, application of paxilline (10 μm) significantly broadened the action potential with the development of a shoulder (on average spike duration increased from 2.7 ± 0.1 to 3.1 ± 0.1 ms, n = 12, P < 0.001). These data indicate that BK channels exert a strong control on spike repolarization. A similar effect was produced by iberiotoxin, a toxin from the scorpion Buthulus tamulus (Galvez et al. 1990; Candia et al. 1992) known to block BK channels and increase transmitter release at the neuromuscular junction (Robitaille et al. 1993). In four cells, iberiotoxin significantly broadened the action potential (from 2.6 ± 0.1 to 2.9 ± 0.2 ms, P < 0.05) with the development of a shoulder during the repolarizing phase (data not shown). In CA1 pyramidal neurones, fast inactivation of a transient BK channel-mediated current substantially contributes to frequency-dependent spike broadening (Shao et al. 1999). In order to see whether this also occurred in our preparation, bursts of five consecutive action potentials were generated at a frequency of 50 Hz. Spike duration increased by 4 ± 2% from the first to the second spike, but less for the last three consecutive spikes (n = 3). The involvement of BK channels in spike broadening was tested by blocking their activity with paxilline. Interestingly, paxilline (10 μm) broadened the first two spikes (by 10 ± 5 and 7 ± 4%, respectively; Figs 1C and 1D), but had only a small effect on the last three (data from 3 experiments are shown in Fig. 1E). Paxilline-induced modifications of the first spikes were similar to those obtained under control conditions with development of a shoulder during the repolarizing phase of the action potential. In agreement with a previous study on CA1 pyramidal cells (Shao et al. 1999), the present results clearly show that BK channels participate in action potential repolarization, but in CA3 neurones their role during repetitive firing is less pronounced.
Figure 1. BK channels are involved in action potential repolarization.
A, action potentials generated under control conditions (thin line) and in the presence of paxilline (thick line) are aligned at threshold and superimposed. B, mean changes in spike width before (open column) and during paxilline application (filled column; n = 10). C, two bursts of five consecutive action potentials generated by brief depolarizing current pulses (5 ms duration, each delivered at 50 Hz) under control conditions and in the presence of paxilline (10 μm) are superimposed. D, the first and the fifth spikes in the train before (thin line) and during paxilline (thick line) are superimposed. E, mean changes in spike duration (as percentage of controls) obtained in the presence of paxilline (10 μm) during repetitive firing (n = 3). Note that paxilline clearly broadened only the first two action potentials. P < 0.001.
BK channels control spontaneous action potential-dependent release of glutamate
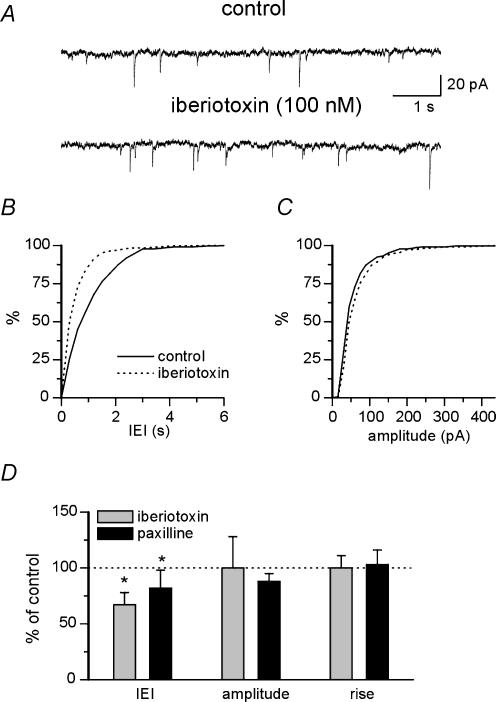
To evaluate the possibility that BK channels localized on presynaptic nerve endings may control transmitter release, spontaneous EPSCs (action potential-dependent and -independent events) were recorded from CA3 pyramidal neurones before and after application of selective BK channel blockers. Application of the non-NMDA receptor antagonist CNQX (10 μm) completely blocked spontaneously occurring synaptic events, indicating that they were mediated by non-NMDA receptors (not shown). In a first set of experiments BK channels were selectively blocked with iberiotoxin. As shown in the representative traces of Fig. 2A, iberiotoxin (100 nm) increased the occurrence of spontaneous events but did not modify their amplitude. This is shown by the cumulative distribution plots of Fig. 2B and C, where a clear shift to the left of the interevent intervals but not of the amplitude curves is seen. Overall, in seven neurones iberiotoxin significantly reduced the interevent interval from 1.2 ± 0.2 to 0.8 ± 0.1 s (P <0.05) without modifying current amplitude (23 ± 5 and 23 ± 7 pA in control and iberiotoxin, respectively) or EPSC kinetics (the rise time was 2.1 ± 0.3 ms under both conditions; Fig. 2D). In another set of experiments, BK channels were selectively blocked with paxilline (10 μm). As shown in the summary data of Fig. 2D, paxilline significantly reduced the interevent interval from 1.1 ± 0.2 to 0.9 ± 0.2 s (n = 7; P < 0.05). Again, in the presence of paxilline no significant changes in EPSC amplitude (24 ± 3 and 21 ± 2 pA, in control and in the presence of paxilline, respectively) or rise time (2.2 ± 0.3 and 2.3 ± 0.3 ms, in control and paxilline, respectively) were detected (Fig. 2D). The significant reduction in interevent interval duration after treatment with iberiotoxin and paxilline suggests that BK channels are involved in the modulation of transmitter release. In order to elucidate whether the observed effects depended on action potential broadening following BK channel block with iberiotoxin and paxilline or to changes in the release machinery downstream of calcium entry, additional experiments (n = 5) were performed on miniature (action potential-independent) EPSCs recorded in the presence of TTX (1 μm). In line with the occurrence of BK activation during action potentials, no significant change in the mean interevent interval of mini EPSCs was noticed (1.4 ± 0.1 and 1.3 ± 0.1 s, in control and paxilline, respectively; data not shown). Taken together, these results show that BK channel blockade selectively affects action potential-dependent, spontaneous synaptic activity, and suggest an involvement of BK channels in controlling synaptic release that is dependent on spike broadening.
Figure 2. BK channels increase the frequency but not the amplitude of spontaneous action potential-dependent EPSCs.
A, traces showing spontaneous EPSCs recorded from a CA3 pyramidal neurone at the holding potential of −60 mV under control conditions and in the presence of iberiotoxin (100 nm). B and C, cumulative interevent-interval (B) and amplitude distribution (C) of spontaneous EPSCs (same neurone shown in A) under control conditions (continuous line) and during iberiotoxin application (dotted line). Bin size was 0.3 s in B and 15 pA in C. D, mean changes of interevent interval (IEI), amplitude and rise time, compared to control (dotted line) during application of iberiotoxin (n = 7) or paxilline (n = 7). P <0.05.
BK channels modulate the evoked release of glutamate
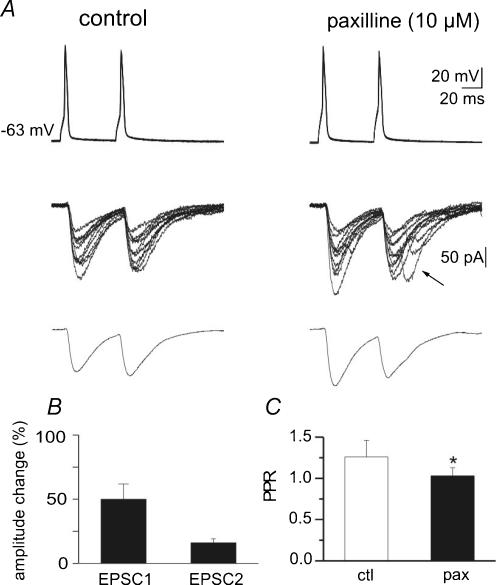
To further investigate whether BK channels modulate neurotransmitter release, double patch-clamp recordings were performed from monosynaptically interconnected CA3 pyramidal neurones. Evidence for monosynaptic connections between neurones was given by the short latency of evoked EPSCs (2.8 ± 0.7 ms, n = 12; see also Debanne et al. 1995). Thirteen different pairs of neurones were studied under control conditions and in the presence of paxilline (n = 7) or iberiotoxin (n = 6). Usually, pairs of presynaptic action potentials (50 ms apart), delivered at a frequency of 0.05 Hz, evoked two sequential EPSCs that fluctuated in amplitude from trial to trial, with occasional transmission failures. These were probably true transmitter failures and not branch point failures, since on average the amplitude of a second response was similar whether or not it was preceded by a failure or a success (mean amplitudes of second response were 21 ± 4 and 23 ± 5 pA, respectively; n = 10). This indicates that in the majority of cases the action potential did not fail to invade the axon terminals. The example of Fig. 3A and B shows a low probability synapse in which, under control conditions, the first spike evoked few small amplitude EPSCs (characterized by a first peak marked by an arrow, followed by a second one occurring with a longer delay), associated with response failures. This kind of response is not rare in recordings from CA3–CA3 connections (Debanne et al. 1995). While the first component is surely monosynaptic because of the constant latency and no changes in delay of paired-pulse facilitation, the second response could be mono- or disynaptic. Due to paired-pulse facilitation (PPF), which largely depends on presynaptic increase in release probability (Zucker, 1989), responses to the second spike were larger and associated with less transmitter failures. As shown in the examples of Fig. 3A and B, addition of paxilline (10 μm) produced a clear potentiation of the synaptic responses. It increased the success rate to both first and second spike (from 17 to 58% and from 44 to 85%, respectively) and the amplitude of individual EPSCs (from 1.9 to 6.57 pA and from 4.4 to 9.1 pA for the first and the second EPSC, respectively). As expected from an enhanced release probability due to an increased calcium entry in presynaptic terminals, in this cell paxilline induced a reduction of PPR from 2.3 to 1.4 (see averaged responses in Fig. 3A). Figure 4A illustrates a high probability CA3—CA3 synapse that under control conditions showed only successes both to the first and second spike. This type of synapse was found in two out of seven cases. In line with a high probability of release, these synapses did not exhibit paired-pulse facilitation (see averaged traces in Fig. 4A). As shown in this example, application of paxilline (10 μm) increased the peak amplitude of the responses to the first action potential (from 118 to 142 pA) and produced paired-pulse depression (PPR changed from 0.96 to 0.77; see averaged traces in Fig. 4A), suggesting that also in the case of highly reliable synapses, block of BK channels is able to increase transmitter release. Overall, as shown in the summary data of Figs 3C and 4B and C, paxilline significantly increased the percentage of successes to the first and second responses (from 75 ± 10 to 87 ± 6%, and from 81 ± 8 to 95 ± 7% for the first and second EPSC, respectively; P < 0.05; Fig. 3C), significantly reduced the paired-pulse ratio (from 1.26 ± 0.2 to 1.03 ± 0.1; P < 0.05; Fig. 4C) and increased the mean peak amplitude of the first and second EPSCs (by 50 and 16%, respectively, P < 0.001; Fig. 4B). Although the first and second EPSC amplitudes in the absence or presence of paxilline were not significantly different, a clear trend towards potentiation was observed. It should be stressed that the reported paxilline-induced increase in success rate is underestimated. In fact, summary data also include those connections with no failures (n = 2), in which the number of successes could not have been increased further with paxilline. Indeed, if these neurones are excluded, the percentage of successes increased from 66 ± 11 to 82 ± 6% and from 70 ± 9 to 92 ± 3% for the first and second spikes, respectively.
Figure 4. In high probability CA3−CA3 connections paxilline increases the amplitude of evoked EPSCs and decreases the PPR.
A, the upper traces represent pairs of 10 superimposed action potentials generated at 0.05 Hz in the presynaptic cell. Pairs of 10 EPSCs recorded from the postsynaptic cell are superimposed in the middle. Average EPSCs (in this particular neurone no failures were detected) are recorded at the bottom. Note that paxilline (right, 10 μm) increased EPSC amplitude and induced the appearance of delayed responses. B, summary data from 5 cells showing mean amplitude of EPSC1 and EPSC2 in paxilline, normalized to control values. C, mean PPR obtained in 5 neurones under control conditions (open column) and during application of paxilline (filled column). P < 0.05
As shown in Fig. 4A (arrow), in some cases in the presence of paxilline a second EPSC peak appeared with a delay. This may be due to a delayed release caused by a rise in calcium entry into the nerve terminal after blockade of BK channels with paxilline or alternatively to the activation of a previously silent connection. Paxilline did not modify the mean EPSCs latency.
In six additional experiments the effects of iberiotoxin on monosynaptically connected neurones were also tested. Two out of these neurones exhibited only successes, the remaining four successes intermingled with failures. In synapses exhibiting successes and failures, iberiotoxin (50 nm) increased the success rate to both first and second spikes (Fig. 5A). In four neurones the success rate increased from 67 ± 5 to 96 ± 3% (EPSC1; P < 0.01), and from 90 ± 4 to 95 ± 4% (EPSC2) (Fig. 5B). In Fig. 6A, a neurone exhibiting only successes is represented. Addition of iberiotoxin (50 nm) induced a strong potentiation of EPSC1 and EPSC2 (Fig. 6A and B). While in iberiotoxin the peak amplitude of EPSC1 increased more than 50% with respect to control, the enhancement of EPSC2 was less pronounced. As expected for the iberiotoxin-induced increase in release probability, the increase in the peak amplitude was associated with a decrease in PPR (Fig. 6D). Overall, in six neurones the peak amplitude of EPSCs varied significantly from 20 ± 5 to 30 ± 5 pA for EPSC1 (P <0.005) and from 27 ± 7 to 31 ± 8 pA for EPSC2 (Fig. 6C). Similarly to paxilline, the effect of iberiotoxin on EPSC amplitude was associated with a significant (P <0.05) reduction in PPR from 1.4 ± 0.2 to 0.9 ± 0.5 (n = 6; Fig. 6D).
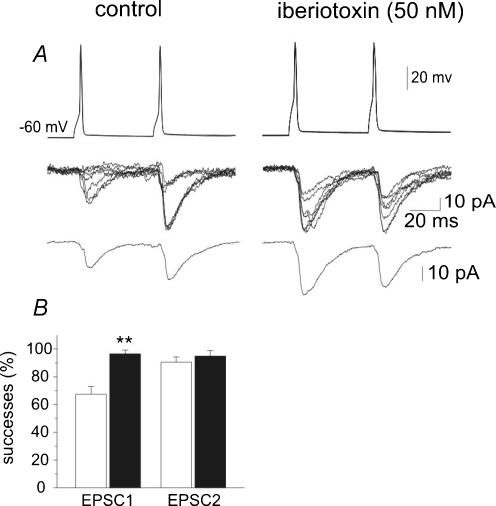
Figure 5. Blocking BK channels with iberiotoxin increases synaptic efficacy at CA3−CA3 connections.
Pairs of action potentials are generated in the presynaptic cell (upper traces) while EPSCs are recorded from the postsynaptic cell in control (left) and in the presence of iberiotoxin (50 nm, right). Seven superimposed traces are shown in the middle while average responses are shown at the bottom (average of 15 responses including failures). Note changes in PPR after iberiotoxin. B, mean success rate of EPSC1 and EPSC2 obtained in 4 cells under control conditions (open columns) and during paxilline application (filled columns). P <0.01.
Figure 6. At high probability synapses, iberiotoxin increases the amplitude of EPSC1 and EPSC2.
A, pairs of seven EPSCs recorded from the postsynaptic cell in control (left) and in the presence of iberiotoxin (right) are superimposed in the upper traces. Average EPSCs are represented in the lower traces. B, time course of the peak amplitude of the first (○) and second (•) EPSCs recorded from the cell shown in A. C, summary data from 6 cells showing mean amplitude of EPSC1 and EPSC2 in control (open column) and in the presence of iberiotoxin (filled column). D, mean PPR obtained in 6 neurones under control conditions (open column) and during application of iberiotoxin (filled column). P < 0.05; P < 0.005.
Discussion
The results presented in this study provide evidence that BK channels control transmitter release under basal conditions at CA3–CA3 connections in rat hippocampal slice cultures. We found that, in CA3 pyramidal neurones, both iberiotoxin and paxilline were able to increase the frequency but not the amplitude of spontaneous, action potential-dependent EPSCs. Moreover, broadening the action potential in presynaptic neurones with paxilline or iberiotoxin enhanced the probability of transmitter release and synaptic strength in target cells. Although evidence for distinct BK channel subtypes at soma and terminals has been provided for neurosecretory neurones (Dopico et al. 1999), an indirect estimate of how spike repolarization and presynaptic firing affect transmitter release and synaptic efficacy can be inferred with simultaneous recordings from two synaptically connected neurones, particularly in those cases in which the small size of presynaptic nerve endings precludes direct measurements with patch pipettes.
BK channels are widely expressed in the CNS and in the hippocampus. In this region receptor autoradiography and immunocytochemistry have revealed the highest level of protein expression in the middle and outer molecular layers of the dentate gyrus and in the mossy fibre pathway (Knaus et al. 1996; Wanner et al. 1999). Lower but still significant levels have been found in stratum oriens and stratum radiatum (Wanner et al. 1999) within the terminal areas, suggesting a functional role of these channels in regulating transmitter release. Further evidence in favour of a presynaptic localization of BK channels is given by the experiments of Hu et al. (2001). Using double labelling immunogold analysis with BK channel and glutamate receptor antibodies, these authors have demonstrated that the pore-forming BK channel subunits are primarily targeted to presynaptic membranes of CA1 glutamatergic synapses where they face the synaptic cleft. Interestingly, CA3 pyramidal cells give rise to the Schaffer collaterals, which form the majority of glutamatergic axons projecting to CA1 stratum radiatum, and to collaterals which synapse on neighbouring CA3 pyramidal cells (CA3−CA3 connections). Therefore, although the present experiments were performed on hippocampal slice cultures, which express higher levels of connectivity compared to acute slices (De Simoni et al. 2003), it is likely that the synapses under examination were formed by collateral of the same axons where presynaptic BK channels have been identified (Hu et al. 2001).
BK channels contribute to action potential repolarization
In the CA1 region of the hippocampus, blocking calcium entry or rapidly chelating intracellular calcium significantly slows down the repolarization of the action potential, suggesting a prominent role for calcium-activated potassium currents in action potential repolarization (Storm, 1987a,b; Poolos & Johnston, 1999; Shao et al. 1999). Further experiments using selective BK channel blockers provided evidence that BK channels are indeed involved in spike repolarization (Adams et al. 1982; Lancaster & Nicoll, 1987; Storm, 1987b; Schwindt et al. 1988; Shao et al. 1999). Our experiments with paxilline and iberiotoxin confirm and extend to CA3 pyramidal cells previous data on spike broadening obtained on the CA1 hippocampal region. Additionally, the effect of iberiotoxin suggests that the BK channels involved in spike repolarization in CA3 neurones are unlikely to contain the β4 subunit, which is expressed in the hippocamapal formation and confers resistance to the block by iberiotoxin and charybdotoxin (Meera et al. 2000; Weiger et al. 2000). Moreover, the results obtained with bursts of spikes elicited at 50 Hz suggest, in agreement with a previous report on CA1 pyramidal cells (Shao et al. 1999), that fast inactivation of a transient BK-channel current account for frequency-dependent spike broadening of the first few spikes. Such inactivation might be linked to the presence of BK channel β subunits conferring an inactivating behaviour to the channels, such as for example β2 (Wallner et al. 1999), although its expression in rat CA3 neurones has not been specifically assessed. As a consequence, during high frequency bursts BK channels would affect transmitter release predominantly during the first two or three spikes even if calcium accumulation can be enhanced by high frequency stimulation.
BK channels modulate the spontaneous release of glutamate
As shown in the cerebellum at granule cell—Purkinje cell synapses, a slight broadening of the presynaptic action potential caused by low concentrations of tetraethylammonium modestly increased presynaptic calcium fluxes that in turn led to a greatly enhanced transmitter release (Sabatini & Regehr, 1997). Therefore presynaptic spike broadening may be crucial for the enhancement in frequency of synaptic currents. Indeed, the present experiments show that both iberiotoxin and paxilline are able to enhance transmitter release, as suggested by the increase in frequency of spontaneous, action potential-dependent synaptic activity. These data are similar to those obtained at the neuromuscular junction using iberiotoxin and charybdotoxin, another BK channel blocker (Robitaille & Charlton, 1992; Robitaille et al. 1993). The increase in frequency but not in amplitude of spontaneous EPSCs suggests a presynaptic site of action of the drugs. We cannot rule out the possibility that the observed appearance of delayed EPSCs caused by iberiotoxin or paxilline may be influenced by changes in network properties. Moreover, the observed paxilline- and iberiotoxin-induced potentiation of spontaneously action potential-dependent EPSCs, but not miniature EPSCs, is consistent with the hypothesis of a presynaptic mechanism of action, and also indicates that BK channels regulate action potential waveform but do not interfere directly with the release machinery.
BK channels modulate the evoked release of glutamate
Our data on paired recordings from interconnected cells clearly show that blocking BK channels with paxilline or iberiotoxin increases synaptic efficacy both at low and high probability synapses. The effect of these drugs on EPSCs was presynaptic in origin, as shown by the decrease in transmitter failures and paired-pulse ratio, which are considered traditional indexes of presynaptic modifications (Katz, 1969; Zucker, 1989). In particular, at CA3–CA3 synapses, the PPR is inversely related to the initial release probability (Dobrunz & Stevens, 1997). Thus, it is likely that the observed reduction in PPR reflects an increased number of quanta delivered simultaneously by a single nerve pulse. In line with an increased probability of release following blockade of BK channels with paxilline or iberiotoxin is the appearance in some patches of delayed responses with multiple peaks that could be due to the activation of previously presynaptically ‘silent’ connections (see Gasparini et al. 2000; Saviane et al. 2003). In this respect, our data confirm previous work on the neuromuscular junction, where a clear increase in transmitter release was observed after BK channel block under normal experimental conditions (Robitaille & Charlton, 1992; Robitaille et al. 1993; Blundon et al. 1995).
Although we cannot exclude the possibility that at axon terminals the shape of action potentials differs from the soma (Geiger & Jonas, 2000), the present data clearly show that a small modification in spike width (induced by BK channel blockers) is associated with a large increase in transmitter release. This is in line with the observation of Sabatini & Regehr (1997) that found a supralinear relationship between calcium influx and EPSC amplitude. This relationship can be influenced by several factors including the properties of presynaptic calcium channels, calcium sensitivity of the release machinery and localization of calcium channels with respect to BK channels.
We also considered the possibility that BK channels regulate axonal conduction as reported for A-type potassium channels (Debanne et al. 1997). Thus, blocking BK channels with paxilline or iberiotoxin would facilitate release by removing propagation failures at axonal branches. Although this type of mechanism cannot be completely ruled out, it seems unlikely. In the present experiments, the observation that on average the amplitude of the second EPSCs was similar, whether or not this was preceded by a failure or a success, is consistent with real transmitter failures and not conduction failures. In the case of a conduction failure in fact, no residual calcium would have been accumulated into the cell, thus precluding facilitation of a second response, following the arrival of a second spike (Zucker, 1989). Therefore, on average the second responses would have been smaller in comparison to those occurring after successes. This suggests that in the majority of the cases the action potential did not fail to invade the axon terminal. In line with this observation it has recently been found that, at low frequency of stimulation (1 Hz), conduction of individual action potentials travelling along single CA3-to-CA1 axon branches is highly reliable, with almost no failures (Raastad & Shepherd, 2003). It should be stressed that while CA3 axonal branches are among the thinnest in the nervous system, with high axial resistance, which favours propagation failures (Shepherd & Harris, 1998), in paired-pulse experiments, failures started appearing when the spike interval was shorter than 20 ms, much shorter than that used in the present experiments (50 ms; Raastad & Shepherd, 2003).
In previous work it was shown that BK channels localized on presynaptic active zones are recruited only in extreme or rare conditions of enhanced calcium accumulation in presynaptic terminals, such as those occurring during application of 4-aminopyridine (Hu et al. 2001). Under these conditions, they would act as an ‘emergency brake’, which protects against hyperactivity, particularly in pathological conditions, such as brain ischaemia and epilepsy (Runden-Pran et al. 2002). In contrast with Hu et al. (2001), we found that BK channels are also functional in basal conditions. This apparent discrepancy may depend on the different synapse studied and/or on the different preparation used (acute hippocampal slices versus organotypic cultures). While in Hu et al. (2001) the effects of BK channel blockers were studied on CA3–CA1 synapses, the present work was performed on CA3–CA3 synapses. Thus, we cannot exclude the possibility that collaterals of the same axon may have different functional properties according to the targets they innervate (see Scanziani et al. 1998). Therefore, BK channels localized on collaterals projecting to CA3 neurones may differ from those localized on Schaffer collateral.
Moreover, according to recently published papers (Gähwiler et al. 1997; De Simoni et al. 2003) acute slices are similar to organotypic slices cultured for 1, 2 or 3 weeks. In organotypic slices, however, an increase in frequency of excitatory miniature postsynaptic currents occurs during the first week and is probably related to an increased level of complexity of high order dendritic branching. In slices cultured for more than 1 week, as those used in the present experiments, the development continues in both preparations at similar rates.
In conclusion, the present data clearly show that in hippocampal slice cultures at CA3−CA3 synapses, BK channels regulate action potential repolarization and calcium entry at the somatic level. Moreover, they provide indirect evidence that BK channels contribute to modulate transmitter release also at nerve terminals.
Acknowledgments
We wish to thank Beatrice Pastore and Micaela Grandolfo for technical support. This work was supported by a grant from Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) to E.C.
References
- Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982;296:746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Blundon JA, Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995;73:178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys J. 1992;63:583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gähwiler BH, Thompson SM. Physiology and pharmacology of unitary synaptic connections between pairs of cells in CA3 and CA1 of rat hippocampal slice cultures. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman S. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. J Physiol. 1999;519:101–114. doi: 10.1111/j.1469-7793.1999.0101o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Meth. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release. Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 2001. [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substance. Springfield, IL, USA: Thomas; 1969. [Google Scholar]
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochem. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavallin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Flores R, Salzberg BM. Calcium channels that are required for secretion from intact nerve terminals of vertebrates are sensitive to omega-conotoxin and relatively insensitive to dihydropyridines. Optical studies with and without voltage-sensitive dyes. J General Physiol. 1989;93:715–729. doi: 10.1085/jgp.93.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Madison DV. Synaptic transmission in pair recordings from CA3 pyramidal cells in organotypic culture. J Neurophysiol. 1999;81:2787–2797. doi: 10.1152/jn.1999.81.6.2787. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984;307:693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad M, Shepherd GM. Single-axon action potentials in the rat hippocampal cortex. J Physiol. 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Runden-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: a study in organotypical hippocampal slice cultures. Neuroscience. 2002;112:277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Mohajerani MH, Cherubini E. An I D-like current that is down regulated by Ca2+ modulates information coding at CA3−CA3 synapses in the rat hippocampus. J Physiol. 2003;552:513–524. doi: 10.1113/jphysiol.2003.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Savtchenko LP, Raffaelli G, Voronin LL, Cherubini E. Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3−CA3 synapses in the rat hippocampus. J Physiol. 2002;544:469–476. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci U S A. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988;59:424–449. doi: 10.1152/jn.1988.59.2.424. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- Storm JF. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987a;435:387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987b;385:733–739. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Schlichter LC, Stanley EF. Single-channel properties of BK-type calcium-activated potassium channels at a cholinergic presynaptic nerve terminal. J Physiol. 1999;518:639–651. doi: 10.1111/j.1469-7793.1999.0639p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci U S A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SG, Koch RO, Koschak A, Trieb M, Garcia ML, Kaczorowski GJ, Knaus HG. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochem. 1999;38:5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci. 2000;20:3563–3570. doi: 10.1523/JNEUROSCI.20-10-03563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]