Abstract
Mechanisms of long-term potentiation (LTP) maintenance are discussed in the light of the phenomenon of silent synapses. Evidence that LTP is associated with the insertion of new AMPA receptors (AMPARs) in postsynaptically silent (deaf) synapses expressing only NMDA receptors (NMDARs) before LTP induction has led to the assumption that the debate on pre- versus postsynaptic locus of LTP expression has been resolved in favour of the latter. However, recent data indicate that these synapses are mainly presynaptically silent (mute or whispering), because the probability of glutamate release (Pr) or glutamate concentration in the cleft is too low to activate AMPARs. In this case LTP could be explained by an increase in Pr or enhanced glutamate concentration to activate low affinity AMPARs. Optical methods to probe calcium transients in dendritic spines have revealed an increase in Pr during LTP with concomitant postsynaptic modifications. A hypothesis is considered that accounts for the differences in both the initial failure rates between AMPAR- and NMDAR-mediated responses, and the LTP-associated decrease in failures of AMPAR-mediated responses. According to this hypothesis, glutamate release is potentiated by the strong postsynaptic depolarization used to identify NMDAR-mediated responses. We suggest that the expression of LTP may depend on coordinated pre- and postsynaptic modifications whose relative contributions vary according to the initial state of the synapse, the experimental protocol and time after induction.
Activity-dependent changes in synaptic strength such as long-term potentiation (LTP) are critical for information storage in the brain and for development of neuronal circuits. LTP is a persistent increase in synaptic efficacy triggered by a short synaptic activation (Bliss & Collingridge, 1993). While there is a general agreement about the mechanisms of LTP induction, those for its maintenance (expression) are still uncertain. In particular, it is not clear whether the site of LTP expression is primarily pre- or postsynaptic. This review discusses current views in favour of both pre- and postsynaptic mechanisms of LTP expression, with particular reference to the phenomenon of ‘silent’ synapses in the hippocampus. Conversion of a silent into a functional synapse is one way to persistently increase synaptic efficacy.
Synapses can be silent in principle through post- or presynaptic mechanisms. Postsynaptically silent (‘deaf’) synapses are unable to detect glutamate release and do not conduct at rest because of the lack of AMPA receptors (AMPARs) in the subsynaptic membrane (Fig. 1A). Presynaptically silent synapses do not conduct because the probability of glutamate release (Pr) is close to zero (‘mute’ synapses, Fig. 1B) or the concentration of released glutamate is insufficient to produce a detectable quantal response, Q (‘whispering’ synapses, Fig. 1C). Here, the assumption is that both NMDARs and AMPARs are colocalized and are simultaneously activated. The degree of receptor activation depends on the amount and temporal profile of the transmitter in the cleft. In the case of a low rate of glutamate release (from a slow adapting fusion pore; Choi et al. 2000; Fig. 1C, green) or remoteness of the release site from the receptors (spillover from neighbouring synapses, Kullmann, 2003; Fig. 1C, yellow) the amount of glutamate in the cleft would be sufficient to activate high affinity NMDARs but not AMPARs.
Figure 1. Models of silent synapses and possible mechanisms of their unsilencing with LTP induction.
A, postsynaptically silent (deaf) synapse lacking AMPARs, but expressing functional NMDARs (N in the box). Functional AMPARs were delivered to the subsynaptic membrane after LTP induction (A with arrow). B, presynaptically silent (mute) synapse. In this case, both AMPAR and NMDAR were present on the subsynaptic membrane, but they were activated only rarely because of the very low release probability (Pr) (no docked vesicles). If no vesicle release occurs during the experimental period such synapse would show 100% failure rate before LTP. LTP would lead to an increased Pr (arrows). C, two types of presynaptic silent (whispering) synapses. Here, either the amount of glutamate released by one vesicle is too small or the release is too slow to be detected by low affinity AMPARs. This could occur either because of a low conductance fusion pore (green terminal) or because of glutamate spillover from remote synapses (dashed arrow from yellow terminal). A change in the operation mode of the fusion pore from slow to fast adapting (see docked vesicle with black arrow in the green terminal) or an increased Pr (vesicles with arrows in the yellow terminal) from remote synapses would account for synapse unsilencing. (Modified, with permission, from Figure 3 from Kullmann, 2003, copyright 2003 The Royal Society.)
LTP in postsynaptically silent synapses would result from the appearance of new AMPARs in the subsynaptic membrane (Fig. 1A, arrow). This model assumes no changes in Pr (Isaac, 2003). In the case of presynaptically silent synapses, Pr, Q and/or the number of effective release sites, n, would increase during LTP (Fig. 1B and C). This may involve concomitant postsynaptic changes, e.g. when an increased n is matched by the appearance of new subsynaptic receptors.
Synapse unsilencing may involve insertion of new AMPA receptors on the subsynaptic membrane of ‘deaf’ synapses
Pairing afferent stimulation with postsynaptic depolarization has been shown to convert silent synapses into functional ones and thus to induce (Fig. 2A and B) or to contribute to LTP where some synapses are already active at rest (Fig. 2C). The number of silent synapses varies widely in different studies, and depends on the experimental conditions, e.g. the testing frequency (Fig. 2D). According to the most accepted view, LTP would be due to insertion of new AMPARs into the subsynaptic membrane. In favour of this hypothesis is the observation that in control conditions the coefficient of variation (CV) of the amplitude of AMPAR-mediated synaptic currents is higher than NMDA responses and becomes similar to NMDA after LTP induction (Kullmann, 1994). The value CV−2 measures the probabilistic behaviour of synaptic signals and is equal to the quantal content (m) for the simplest model (Poisson) of transmitter release (Redman, 1990). Therefore, before LTP, AMPARs ‘see’ less quantal release of glutamate than NMDARs. LTP is also accompanied by changes in the number of transmission failures (Fig. 2C), CV−2 and m that have been interpreted as resulting from the addition of AMPARs (Malinow & Malenka, 2002) rather than presynaptic modifications of Pr or n (Voronin, 1993; Larkman & Jack, 1995). This would account for the larger LTP of AMPAR-mediated responses in comparison with NMDA (Kullmann, 1994; Liao et al. 1995) but not for the strong increase in Pr observed during LTP.
Figure 2. Evidence for postsynaptically silent synapses and their conversion into functional ones during LTP.
A and B, at silent synapses on hippocampal CA1 pyramidal cell, LTP induces the rapid appearance of AMPAR-mediated responses at resting membrane potential (−60 mV). A, individual recordings of excitatory postsynaptic currents (EPSCs) in acute hippocampal slices at −60 mV before and after pairing. B, plot of amplitude versus time of EPSCs evoked before and after pairing protocol (100 stimuli at 1 Hz at a holding potential of 0 mV). (From Isaac, 2003; copyright 2003 Elsevier.) C, superimposed recordings of minimal EPSCs presumably generated by one or few presynaptic fibres. At +60 mV failure rate is lower than at −65 mV. On the right, averaged EPSCs recorded at +60 mV and at −65 mV. The vertical dotted line indicates initial EPSC slopes. Notice the symmetrical rise time for synaptic responses evoked at depolarizing and the hyperpolarizing potentials suggesting contribution of AMPAR-mediated responses to EPSCs recorded at +60 mV. (Modified from Liao et al. 1995; reproduced with permission from the Nature Publishing Group (http://www.nature.com)). D, the incidence of apparently silent synapses varies and depends on experimental conditions. The graph shows percentage of silent synapses reported in different publications and plotted against the frequency of repeated testing stimulation used to activate presynaptic fibres. Filled triangles represent data obtained from CA1 synapses in acute hippocampal slices from 2-week-old rats (at room temperature). Open triangles represent data obtained in other experimental conditions and at different testing frequencies: at 0.05 Hz (acute slices at 32°C; Gasparini et al. 2000) and at 0.2 Hz (pair recordings from interconnected neurones in organotypic hippocampal slices at room temperature; Montgomery et al. 2001). Overall these data suggest that testing frequency influences the incidence of apparently silent synapses, in line with low-frequency depression (LFD) that can lead to silence of actually non-silent synapses with functional AMPARs (Gasparini et al. 2000). E, tetanic stimulation induces spine delivery and clustering of glutamate receptors. Neurones in cultured hippocampal slices were infected with GluR1–green fluorescent protein (GFP) and their dendrites were visualized before and after tetanic stimulation as shown at two different amplifications (upper and lower images). In separate experiments, similar protocol was shown to induce LTP (with ∼40% EPSC amplitude increase). The arrows marked a and b on the left illustrate the same loci before and 30 min after tetanus, respectively. Notice the appearance of a clear spine at the locus a. Scale bar 2 μm. F, quantification of GluR1–GFP signal intensity of spines before and after tetanus. Spines were identified in images obtained 15 min after the tetanus. Fluorescence was integrated over 2 or 3 optical sections containing spine and also from equivalent places before tetanus. Spines were selected from 5 similar independent experiments. Units are arbitrary fluorescence units. (Adapted with permission from Shi et al. 1999, copyright 1999 American Association for the Advancement of Science (http://www.sciencemag.org).)
The observation that in pairs of interconnected CA3–CA3 neurones in organotypic hippocampal cultures the postsynaptic responsiveness to exogenous application of AMPA increases immediately after LTP induction directly supports the possibility that synapse unsilencing may occur via an increase in postsynaptic AMPAR function (Montgomery et al. 2001). This may be produced either by insertion of new AMPARs into the subsynaptic membrane or by an increase in the conductance of single AMPAR channels (Benke et al. 1998). Both these processes may require phosphorylation of AMPARs (Esteban et al. 2003). However, the time from tetanus to receptor phosphorylation, depending as it does on the activation of NMDARs, mobilization of calcium/calmodulin kinase II and increased phosphorylation of surface GluR1, is likely to be several minutes (Liao et al. 2001), whereas the underlying fast conversion of silent into functional synapses occurs in seconds (Fig. 2B). Moreover, the increase in single channel conductance was not found in all potentiated synapses (Benke et al. 1998) and the increase in AMPAR sensitivity was 3–4 times smaller than LTP magnitude (Montgomery et al. 2001). Taken together, these results throw doubt on the common assumption that the early phase of LTP proceeds by phosphorylation of existing receptors or by insertion of new receptors.
Even if synapse unsilencing does reflect the delivery of AMPARs to dendritic spines, how this happens is still unknown. In particular, it is unclear whether new receptors are present in cytoplasmic clusters or are prepackaged in selective organelles for rapid delivery. Under normal conditions, surface expression of AMPARs is under control of several proteins bearing single or multiple PDZ domains that interact with the intracellular C-terminus of AMPAR subunits (Isaac, 2003).
Recent studies on activity-dependent AMPAR trafficking (Malinow & Malenka, 2002) have demonstrated that recombinant green fluorescent protein-tagged GluR1 subunits can be mobilized from the cytoplasm to dendritic spines within 10 min of tetanic stimulation (Fig. 2E and F). Receptor insertion requires activation of calcium/calmodulin kinase II and the interaction of a PDZ domain protein with GluR1 receptors (Hayashi et al. 2000). Point mutation in the PDZ binding region of GluR1 receptors prevents synaptic delivery (Piccini & Malinow, 2002). Whether endogenous GluR1 and other receptors can also be delivered to dendritic spines in an activity-dependent way remains uncertain.
Synapse unsilencing, due to insertion of new postsynaptic AMPARs, has been suggested to be particularly relevant during postnatal development when a significant fraction of excitatory synapses are presumably ‘deaf’ because they express only NMDARs and no AMPARs (Durand et al. 1996). The molecular basis for this phenomenon has been provided by immunogold labelling studies, which have shown that the fraction of synapses containing NMDAR but not AMPAR immunoreactivity decreased from 84% at postnatal day 2 to 50% at 5 weeks with little changes in NMDAR immunoreactivity (Petralia et al. 1999). The vast majority of connections devoid of AMPARs belong to relatively small synapses thought to have low Pr (Schikorski & Stevens, 1997). This does not fit with the low failure rate of NMDAR-mediated responses (Liao et al. 1995; Isaac et al. 1995; Durand et al. 1996). Therefore, the interpretation of immunogold data may be uncertain. First, the synapses without AMPARs may represent non-functional structures. Second, the anatomical evidence critically relies on the sensitivity of the antibody used: a failure to detect AMPARs could be due to the lack of sensitivity of the technique rather than to the lack of AMPARs (Isaac, 2003). Again, we are led to the conclusion that the evidence for the proposition that LTP expression involves the addition of new AMPARs is less compelling than commonly assumed.
Synapse unsilencing may involve an enhancement of transmitter release from ‘mute’ and ‘whispering’ synapses
Several lines of evidence suggest that both AMPARs and NMDARs may be functional while synapses appear silent because of low Pr and/or Q. Powerful evidence comes from experiments with minimal paired-pulse stimulation delivered to Schaffer collateral or to mossy fibres (Gasparini et al. 2000; Maggi et al. 2003). Neurones exhibiting responses at +40 mV but only failures at −60 mV (defined as ‘postsynaptically silent’) occasionally responded to a second pulse delivered 50 ms after the first (Fig. 3A). This paired-pulse facilitation (PPF) phenomenon is known to depend largely on an increase in Pr. In line with the initial definition (Redman, 1990), these synapses are presynaptically rather than postsynaptically silent, and increasing Pr can lead to their unsilencing. Thus, ‘mute’ and ‘deaf’ synapses cannot be reliably distinguished by experiments using single pulse stimulation. An increase in Pr can also be achieved by other experimental means, such as raising the temperature, applying cyclothiazide (a drug known to block AMPAR desensitization (see also Choi et al. 2000) and to enhance transmitter release), activating presynaptic (α7 nicotine receptors with nicotine (Fig. 3B) or with endogenously released acetylcholine (Gasparini et al. 2000; Maggi et al. 2003). In all these cases, a decrease in failures has been observed, consistent with ‘dumb’ rather than ‘deaf’ synapses.
Figure 3. Evidence for presynaptically silent synapses and their conversion into functional ones during LTP.
A, recordings from a synapse (CA1 pyramidal cell in hippocampal slice from a 3-day-old rat) responding to a single pulse stimulation only at +40 mV (left panel) but not at −60 mV (central panel). This synapse would be defined as ‘postsynaptically silent’. However the right panel shows occasional responses to a second pulse delivered 50 ms after the first, indicating the presence of AMPARs. Therefore, synaptic silence is due to low Pr that increases in paired trials due to presynaptic paired-pulse facilitation. Notice also that at +40 mV, EPSCs show fast rise time characteristic of AMPAR-mediated responses (E. Sola & E. Cherubini, unpublished results). B, brief application of nicotine (1 μm), known to enhance Pr, induces the appearance of responses to the first and second pulse at a presynaptically silent synapse (hippocampal slice from a 2-day-old rat). This effect is associated with an apparent decrease in paired-pulse facilitation. (From Maggi et al. 2003; copyright 2003 National Academy of Sciences, USA.) C, repeated test stimulation suppresses unitary EPSCs recorded from interconnected CA3–CA3 neurones in cultured hippocampal slice in a frequency-dependent manner and converts PPF into paired-pulse depression. Average EPSCs and graph show complete response suppression at 1 Hz stimulation with a slow response recovery at very low (0.025 Hz) testing stimulation. Peak amplitudes of the first and second EPSCs recorded in the paired pulse protocol are shown in the graph as filled and empty circles, respectively. (From Saviane et al. 2002; copyright 2002 The Physiological Society.) D, the degree of inhibition of NMDAR-mediated EPSCs (evoked at 0.5 Hz) by the fast unbinding antagonist l-(+)-2-amino-5-phosphonopentanoic acid (LAP-5) decreases after LTP, suggesting increased cleft glutamate concentration. Groups of 10 consecutive records taken at +40 mV before and after pairing (left and right panels) and before and after exposure to 250 μml-AP5 (upper and lower traces) are represented. The two central panels illustrate EPSCs, taken at −60 mV, showing conversion from silent to non-silent transmission. (From Choi et al. 2000; reproduced with permission from the Nature Publishing Group (http://www.nature.com)). E, the involvement of NMDARs or AMPARs may depend on the temporal profile of glutamate released in the cleft. A slow flux of glutamate (left) would activate only NMDAR-mediated responses, thus imitating apparently postsynaptically silent synapses, while a fast pulse (right) activates both AMPARs and NMDARs. The same synapse shows failures of presynaptic activation (square, centre). Glutamate was iontophoretically applied within 1 μm of a visualized synapse. Filled horizontal (left) and vertical (right) bars represent ‘slow’ (10 ms, 1 nA) and ‘fast’ (1 ms, 100 nA) iontophoretic applications, respectively. The presence of AMPARs was revealed in a synapse judged as ‘postsynaptically silent’ (only NMDARs and transmitter failures) when the transmitter concentration was enhanced. (From Renger et al. 2001; copyright 2001 Elsevier.)
An increase in Pr is commonly associated with a decrease in PPF. However, estimates of PPF during LTP have led to contradictory results. Either no change or a reduction of PPF has been reported (Schulz, 1997). This apparent contradiction may depend on the way synaptic responses were recorded (field potentials, multifibre or single fibre responses). While field potentials often do not show any PPF change, minimal responses exhibit a clear reduction in PPF correlated with LTP magnitude (Kuhnt & Voronin, 1994). Modelling studies have suggested that an increase in Pr at original release sites may be masked by a concomitant increase in n (Schulz, 1997).
Another issue to be considered when dealing with silent connections is the possibility that synapses can undergo short-term low frequency-dependent depression (LFD) largely of presynaptic origin that can persist. Therefore, LFD could contribute to the variability in the proportion of apparently silent synapses (Fig. 2D). For example, functional synapses can be reversibly switched off by increasing stimulation frequency (Fig. 3C). It is worth noting that in cultured slices, LFD can be associated with the conversion of PPF into paired-pulse depression (Saviane et al. 2002). This conversion together with low PPF and LFD in cultured slices (Fig. 3C) may explain why Montgomery et al. (2001) could not reproduce the appearance of successes using paired pulses as reported by Gasparini et al. (2000) and why they observed a significant number of apparently silent synapses using repeated 0.2 Hz stimulation.
The hypothesis of AMPAR insertion has also been applied to explain general LTP mechanisms at non-silent synapses (Kullmann, 1994; Liao et al. 1995). However, in non-silent synapses, the use of optical methods has provided evidence that, at Schaffer associational synapses, LTP is associated with an increase in the probability of synaptically evoked calcium transients in single dendritic spines and a decrease in PPF (Emptage et al. 2003). According to these authors, the increased Pr does not reflect insertion of AMPARs in a synapse with only NMDARs since the synapse was not silent to start with. Compatible with these observations, an increased rate of release of FM1-43, a fluorescent marker of presynaptic function, has been observed at CA3–CA1 synapses during LTP induced by tetraethylammonium or by high frequency (200 Hz) electrical stimulation (Zakharenko et al. 2001). However, no changes in FM1-43 unloading rate have been found with lower frequency tetanus (100 Hz) suggesting that the presynaptic component of LTP may require a higher level of postsynaptic calcium for induction as compared to the postsynaptic one. Alternatively, FM1-43 measurements might fail to reveal a presynaptic component when induction protocols are weaker (Zakharenko et al. 2001). Likewise, in hippocampal culture, potentiation of unitary responses by glutamate was associated with an increase in the number of puncta immunoreactive for the presynaptic protein synaptophysin and for GluR1 subunits (Antonova et al. 2001).
If LTP involves an increase in Pr, this should affect both the AMPAR and NMDAR components of the EPSCs. However, LTP is in many cases associated with changes of only the AMPAR-mediated component (Kullmann, 1994). How can this be reconciled with an increase in glutamate release during LTP? An important consideration is that the two components are usually measured at different membrane potentials. Thus, while the AMPAR component is measured at resting membrane potential, the NMDAR-mediated component is revealed at positive membrane potentials (Fig. 3A and D). We suggest that the act of depolarization may itself lead to a short- or long-lasting increase in Pr. One way in which this could occur is that membrane depolarization enhances glutamate release via a Ca2+-dependent short- or long-term potentiation, similar to that produced by depolarizing pulses even in the absence of synaptic activation (Berretta et al. 1999). This hypothesis is supported by the slow growth of EPSCs at +40 mV (associated with a decrease in failure rate, see Fig. 1 in Voroni & Cherubini, 2003; Voronin et al. 2004), decrease in PPF, and the block of these effects when the intracellular Ca2+ is chelated with BAPTA (20 mm; Voronin et al. 2004). Note that on this model the act of measuring the NMDAR-mediated component by depolarization leads to its potentiation, explaining the apparent discrepancy between the degree of potentiation of the NMDAR- and AMPAR-mediated components.
Furthermore, the rapid appearance of EPSCs typical of AMPAR-mediated responses suggests that both AMPA and NMDA components are present at depolarizing potentials (compare initial rise time of the average traces in Fig. 2C; see also Fig. 3A). Therefore, the hypothesis of potentiated transmitter release at depolarizing membrane potentials may account for the apparent differences in CV−2 (Kullmann, 1994) and failure rates (Liao et al. 1995) between AMPAR- and NMDAR-mediated EPSCs measured at hyperpolarized and depolarized membrane potentials, respectively.
Thus, the hypothesis of potentiated transmitter release at depolarizing membrane potentials provides a presynaptic interpretation for the appearance of responses when silent synapses are depolarized. On this interpretation, such synapses contain both AMPA and NMDA receptors but are presynaptically silent. Depolarization leads to a potentiation of transmitter release, and the appearance of both NMDAR- and AMPAR-mediated responses.
However, the presynaptic view of LTP expression seems to contradict a number of observations aimed at assessing changes in glutamate release during LTP. This was indirectly estimated by monitoring electrogenic transporter currents in astrocytes that are very sensitive to synaptically released glutamate. These currents increased during post-tetanic potentiation (PTP), but did not change during LTP (Nicoll, 2003). It is worth noting that electrogenic transporter currents reflect the activity of multiple inputs so that an increase in glutamate release at potentiated synapses may be counterbalanced by a concomitant decrease at non-potentiated synapses (Zhang & Poo, 2001). Glutamate release was also estimated with MK-801, a use-dependent irreversible antagonist of NMDARs. Here the assumption was that the rate of receptor blockade during synaptic stimulation should have been directly related to Pr. Changes in the rate of block of NMDA-mediated EPSCs were found during PTP, but not in LTP (Manabe & Nicoll, 1994). However, these results were later challenged by Kullmann et al. (1996) who, in line with a presynaptic mechanism, found an increase in the rate of depression of NMDAR-mediated responses by MK-801 during LTP.
Two more sets of arguments in favour of presynaptic origin came from studies on whispering synapses (Fig. 1C, green terminal). First, using a fast unbinding NMDA antagonist, Choi et al. (2000) demonstrated LTP-induced increase in cleft glutamate concentration (from < 170 μm to the millimolar range) able to activate AMPARs (Fig. 3D). This is consistent with changes in the dynamics of the fusion pore from a non-expanding to a rapidly expanding mode of operation (Choi et al. 2003). The idea that AMPAR activation in apparently silent synapses depends on the concentration profile of glutamate in the cleft has been directly tested in cultured hippocampal neurones by slow and fast applications of glutamate (Renger et al. 2001). The former were found to evoke only NMDA currents, while the latter evoked both NMDA and AMPA currents (Fig. 3E). By alternating glutamate application with synaptic stimulation, Renger et al. (2001) found that when a synapse was silent, fast application of glutamate was still able to elicit AMPA responses, indicating that AMPARs were functional. The second set of arguments is based on glutamate spillover from remote synapses (Fig. 1C, yellow terminal), although this phenomenon is probably negligible at physiological temperature. This hypothesis has been carefully investigated (Kullmann & Asztely, 1998).
Early in postnatal life synapse unsilencing may contribute to the development of neuronal circuits
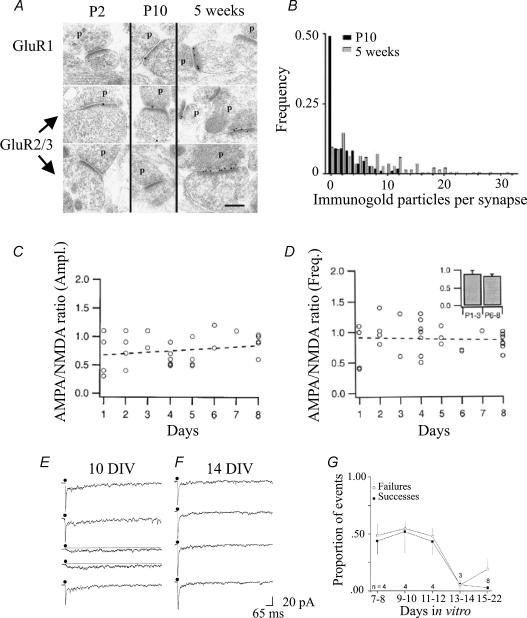
Silent synapses are common during postnatal development and have been observed in a variety of structures (Malinow & Malenka, 2002). In the hippocampus, the number of apparently silent synapses is quite high at birth and decreases during development (Durand et al. 1996). Immunogold studies indicate that maturation requires incorporation of new AMPARs at pure NMDA synapses (Fig. 4A and B). However this view has been recently challenged by the following observations which strongly support presynaptic modifications in the release machinery. Thus, Groc et al. (2002) found that spontaneous AMPAR- and NMDAR-mediated synaptic events were present from birth and their relative amplitude and frequency remained constant during the first postnatal week (Fig. 4C and D). Experiments on evoked responses in which AMPAR-mediated currents to a second of two paired stimuli can be detected in apparently silent synapses allow similar conclusions to be drawn (Fig. 3A and B; see also Gasparini et al. 2000; Maggi et al. 2003). Maturation of presynaptic machinery may also account for the increased number of AMPA responses observed during development of cultured neurones (Renger et al. 2001; Fig. 4E–G). Interestingly, interfering with presynaptic vesicle fusion by exposing mature cultures to tetanus toxin (which cleaves SNARE proteins) was able to reconvert functional into silent synapses (Renger et al. 2001).
Figure 4. During postnatal development synapse unsilencing may contribute to the development of neuronal circuits.
A and B, immunogold labelling of GluR1 and GluR2/3 AMPA receptors in stratum radiatum of the CA1 hippocampal region from 2-day-, 10-day- and 5-week-old animals. Line scale is 0.2 μm; p, presynaptic terminal. Labelling was more pronounced at 5 weeks in comparison to P2/P10. B, frequency distribution of AMPAR immunogold particles at P10 (filled bars) and at 5 weeks (open bars). (From Isaac, 2003; copyright 2003 Elsevier.) C and D, AMPAR- and NMDAR-mediated components of spontaneous quantal events (miniature excitatory postsynaptic currents or mEPSCs) are equally regulated during postnatal maturation. Amplitude (C) and frequency (D) ratio of AMPAR- and NMDAR-mediated mEPSCs recorded from CA1 pyramidal neurones during the first postnatal week. Dashed lines represent linear regressions through the data points. Although frequencies and amplitudes of both AMPAR-mediated mEPSCs and NMDA-mediated mEPSCs increased over the first postnatal week their ratio remained constant. Inset in D represents the average ratio of mEPSC frequency obtained at P1–P3 and P6–P8. (Modified from Groc et al. 2002; copyright 2002 by the Society for Neuroscience.) E and F, EPSCs evoked in cultured neurones at 10 (10 DIV) and 14 days (14 DIV) in culture. Note that at 10 DIV evoked responses varied between dual (AMPAR- and NMDAR-mediated EPSCs, black traces) and slow NMDAR-mediated EPSCs (grey traces), while at 14 DIV a higher proportion of synapses exhibited dual EPSCs. G, the proportion of NMDAR-mediated EPSCs and the rate of transmission failures decreased over the same period of development. (Modified from Renger et al. 2001.)
Synapse unsilencing may involve both pre- and postsynaptic mechanisms
Uncertainty about the contributions of pre- and postsynaptic mechanisms to LTP expression leaves many questions open. Nevertheless this discussion has boosted valuable research on the basic mechanisms controlling transmitter release, postsynaptic responsiveness and receptor trafficking (Malinow & Malenka, 2002). A definite demonstration that silent synapses do not contain functional AMPARs is still lacking (Renger et al. 2001). Interestingly, the existence of ‘AMPAR-only’ synapses has been well documented (Cottrell et al. 2000; Clark & Cull-Candy, 2002).
Several observations indicate that most of the apparently silent synapses are presynaptically (mute or whispering) rather than postsynaptically silent (deaf). The suggested model of depolarization-induced potentiation may account for the basic observations supporting the existence of the former, but pre- or postsynaptic mechanisms per se cannot entirely explain LTP maintenance. Although we favour the importance of presynaptic mechanisms (Voronin & Cherubini, 2003), coordinated postsynaptic modifications may be involved. They may also include insertion of NMDARs so far not carefully studied.
The relative contribution of pre- and postsynaptic mechanisms may vary according to the initial state of the synapse, experimental protocol and time after induction. In organotypic hippocampal slices (Emptage et al. 2003), LTP-induced increase in probability of synaptically evoked Ca2+ transients was associated with an increase in their amplitude. This may reflect an enhanced Ca2+ release from internal stores. This novel postsynaptic aspect of LTP is different from the insertion of functional AMPARs, but could in turn trigger calcium-sensitive processes in dendritic spines as well as changes in presynaptic release via retrograde messengers.
Acknowledgments
We thank P. Bregestovski, G. L. Collingridge and J. G. Nicholls for useful suggestions and numerous colleagues for discussions. This work was supported by grants from Ministero Instruzione Università & Ricerca, Italy (MIUR), International Association for the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union (INTAS), Russian Foundation for Basic Research (RFBR) and The Wellcome Trust.
References
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Berretta N, Rossokhin AV, Cherubini E, Astrelin AV, Voronin LL. Long-term synaptic changes induced by intracellular tetanization of CA3 pyramidal neurons in the hippocampal slices from juvenile rats. Neuroscience. 1999;93:469–477. doi: 10.1016/s0306-4522(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model for memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nature Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Fusion pore modulation as a presynaptic mechanism contributing to expression of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:695–705. doi: 10.1098/rstb.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22:4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell JR, Dube GR, Egles C, Liu G. Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–1587. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A, Bliss TV. Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer associational synapses. Neuron. 2003;38:797–804. doi: 10.1016/s0896-6273(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: Lack of functional AMPA receptors or low probability of glutamate release ? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. Spontaneous unitary synaptic activity in CA1 pyramidal neurons during early postnatal development: constant contribution of AMPA and NMDA receptors. J Neurosci. 2002;22:5552–5562. doi: 10.1523/JNEUROSCI.22-13-05552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Isaac JT. Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology. 2003;45:450–460. doi: 10.1016/s0028-3908(03)00229-6. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Kuhnt U, Voronin LL. Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea-pig hippocampal slices: application of quantal analysis. Neuroscience. 1994;62:391–397. doi: 10.1016/0306-4522(94)90374-3. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Silent synapses: what are they telling us about long-term potentiation? Philos Trans R Soc Lond B Biol Sci. 2003;358:727–733. doi: 10.1098/rstb.2002.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Erdeuli G, Asztely F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynoptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Larkman AU, Jack JJ. Synaptic plasticity: hippocampal LTP. Curr Opin Neurobiol. 1995;5:324–334. doi: 10.1016/0959-4388(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–403. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux J-P, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Manabe T, Nicoll RA. Long-term potentiation: evidence against an increase in transmitter release probability in the CA1 region of the hippocampus. Science. 1994;265:1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view. Philos Trans R Soc Lond B Biol Sci. 2003;358:721–726. doi: 10.1098/rstb.2002.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nature Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Piccini A, Malinow R. Critical postsynaptic density 95/disc large/zonula occludens-1 interactions by glutamate receptor 1 (GluR1) and GluR2 required at different subcellular sites. J Neurosci. 2002;22:5387–5392. doi: 10.1523/JNEUROSCI.22-13-05387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman SV. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990;70:165–122. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Egles C, Liu G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Saviane C, Savtchenko LP, Raffaelli G, Voronin LL, Cherubini E. Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3–CA3 synapses in the rat hippocampus. J Physiol. 2002;544:469–476. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE. Long-term potentiation involves increases in the probability of neurotransmitter release. Proc Natl Acad Sci U S A. 1997;94:5888–5893. doi: 10.1073/pnas.94.11.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Voronin LL. On the quantal analysis of hippocampal long-term potentiation and related phenomena of synaptic plasticity. Neuroscience. 1993;56:275–304. doi: 10.1016/0306-4522(93)90332-a. [DOI] [PubMed] [Google Scholar]
- Voronin LL, Altinbaev RS, Bayazitov IT, Gasparini S, Kasyanov AV, Saviane C, Savtchenko L, Cherubini E. Postsynaptic depolarisation enhances transmitter release and causes the appearance of responses at ‘silent’ synapses in rat hippocampus. Neuroscience. 2004. in the press. [DOI] [PubMed]
- Voronin LL, Cherubini E. ‘Presynaptic silence’ may be golden. Neuropharmacology. 2003;45:439–449. doi: 10.1016/s0028-3908(03)00173-4. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nature Neurosci. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nature Neurosci. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]