Abstract
Nerve growth factor (NGF) has been shown to regulate plasticity in the visual cortex of monocularly deprived animals. However, to date, few attempts have been made to investigate the role of NGF in synaptic plasticity at the cellular level. In the study reported here we looked at the effects of exogenously applied NGF on synaptic plasticity of layer II–III regular spiking (RS) neurones in visual cortex of 16- to 18-day-old rats. We found that local application of NGF converted high frequency stimulation (HFS)-induced long-term potentiation (LTP) into long-term depression (LTD). We showed that this shift of synaptic plasticity was also obtained with bath application of NGF during HFS. Application of NGF subsequent to HFS left LTP unaffected, conferring temporal constraints on NGF efficacy. NGF effects on LTP were mediated by TrkA receptors. Indeed, blockade of TrkA by monoclonal antibody prevented NGF from inducing LTD following HFS. Low frequency stimulation (LFS) elicited LTD in RS cells. We found that NGF or blockade of NGF signalling by anti-TrkA antibody did not change the amplitude of the LTD induced by LFS. Thus, the NGF effect is selective for synaptic modifications induced by HFS in RS cells. The present results indicate that NGF may modulate the sign of long-term changes of synaptic efficacy in response to high frequency inputs.
Neurotrophic factors of the nerve growth factor (NGF) family (neurotrophins) consist of structurally related proteins, which control survival, development and differentiation of neural cells. The prototype of these factors is NGF discovered by Levi-Montalcini (for a review, see Levi-Montalcini, 1998). Beyond its classical role as a trophic factor, in recent years it has been shown that NGF modulates plastic processes in CNS. In the visual cortex, an exogenous supply of NGF prevents monocular deprivations effects (Domenici et al. 1991; Maffei et al. 1992; Domenici et al. 1993), including ocular dominance shift and shrinkage of lateral geniculate neurones (LGN). Blockade of endogenous NGF alters the maturation of functional properties of visual cortical neurones, such as ocular dominance, induces shrinkage of LGN neurones and prolongs the critical period for monocular deprivation (Berardi et al. 1994; Domenici et al. 1994). The effects of NGF on monocular deprivation are mediated by NGF receptors expressed in visual cortex, as demonstrated by Pizzorusso et al. (1999) by using local infusion of antibodies activating TrKA. These results suggest that NGF is a factor controlling neuronal plasticity in developing visual cortex. More recently, it has been reported that neurotrophins are capable of modulating synaptic plasticity (for a review see Lessmann et al. 2003). Long-term potentiation (LTP) elicited by stimulation of the white matter (WM) represents a form of NMDA-dependent cortical synaptic plasticity (Kirkwood & Bear, 1994). In the rat, this form of LTP is expressed during postnatal development and is down-regulated once the functional maturation of visual cortex is complete (Artola & Singer, 1987; Kirkwood et al. 1995; for a review see Fox, 1995). In a previous paper, it has been reported that the LTP normally elicited by stimulation of WM is inhibited by an exogenous in vitro local supply of NGF (Pesavento et al. 2000). These results suggest that high NGF levels in the visual cortex reduce the capacity of visual cortical neurones to be strengthened in response to precise patterns of afferent inputs. However, in Pesavento et al. (2000), LTP was measured as the change of neuronal population responses (field potentials) leaving open the question as to the type of cortical neurones involved in NGF effects.
In the study reported here, we investigated the action of NGF on regular spiking (RS) neurones of visual cortex, which represents one of the principal functional cell types present in many cortical areas (Connors et al. 1982; McCormick et al. 1985; Connors & Gutnick, 1990; Schwindt & Crill, 1999). We recorded excitatory postsynaptic potentials (EPSPs) from regular spiking neurones of layer II–III using intracellular recordings. EPSPs were evoked by electrical stimulation of WM. To elicit long-term changes of postsynaptic potentials we used high frequency stimulation (HFS) and low frequency stimulation (LFS) of WM. NGF was supplied by (i) using a newly designed electrode for local release of pharmacological compounds, i.e. a coaxial glass electrode formed of an inner pipette used for intracellular recordings and an outer pipette used for local release of NGF or denatured NGF, and (ii) perfusion with NGF-containing medium. We found that NGF supplied during HFS causes a shift from LTP to LTD in RS cells. This effect was mediated by TrkA receptor as shown by using a blocking antibody. Furthermore, NGF and antibody against TrkA did not change the LTD elicited by LFS, suggesting that this neurotrophin modulates the sign of synaptic plasticity in response to high frequency inputs in selected populations of visual cortical neurones.
Methods
Slice preparation
Primary visual cortex slices were prepared from post-natal days (P) 15–20 Wistar rats. Procedures involving animals were conducted in accordance with NIH guidelines and the regulations of the Italian Animal Welfare Act DL 27/1/92 n. 116 implementing the European Community directives n. 86/609 and 93/88, and they were approved by the local authority veterinary service. Animals were deeply anaesthetized with urethane (20% in saline, intraperitoneal injection) and then decapitated. The brain was rapidly removed, and coronal sections of the occipital poles (400 μm thick) were sliced by a vibratome. All steps were performed in icy artificial cerebral spinal fluid (ACSF) solution gassed with 95% O2 and 5% CO2. The ACSF composition was (mm): NaCl 126, KCl 3.5, CaCl2 2, MgCl2 1.3, NaH2PO4 1.2, NaHCO3 25, glucose 11. Slices were stored prior to recording for at least 1 h in a recovery chamber containing oxygenated ACSF solution, at room temperature. During electrophysiological recordings, slices were perfused at 3–4 ml min−1 with oxygenated ACSF, at 33–34°C.
Intracellular recordings
Regular spiking neurones of the visual cortex (layer III–IV) were recorded in the current clamp mode with 2 m potassium acetate-filled sharp electrodes pulled from thin walled borosilicate tubes (outer diameter 1.5 mm, Hilgenberg). When filled with 2 m potassium acetate the resistance of electrodes ranged from 70 to 120 MΩ. Current-clamp studies were performed with an Axoclamp-2B amplifier (Axon Instruments, USA). Selected traces were stored on a PC for data analysis using the LTP software (Anderson & Collingridge, 2001). Several criteria had to be met before cells were accepted for further analysis: typical firing pattern of pyramidal neurones, stable resting membrane potentials of at least −60 mV, no spontaneous firing of action potentials, no sudden drops in the input resistance (indicating cell damage) and constant amplitude of the spike (> 70 mV) obtained by direct activation of the cell. Postsynaptic excitatory potentials were evoked by orthodromic stimulation (80 μs stimulus duration, 0.05 Hz frequency) of the white matter with a tungsten concentric bipolar electrode. We averaged evoked responses from three sweeps and measured the peak amplitude. The usual testing protocol was: (a) recording of excitatory postsynaptic potentials (EPSPs) for 10 min (baseline), obtained with a stimulation intensity that yielded 30–40% of the maximal subthreshold amplitude; (b) high frequency stimulation (HFS) (three trains of 100 pulses at 100 Hz, 10 s interval; during the HFS the intensity of stimulation was doubled) for the induction of LTP, or low frequency stimulation (900 pulse 1 Hz) for the induction of LTD; (c) recording of EPSPs for at least 50 min
Values are expressed as mean ± standard error (s.e.m.) percentage change relative to the mean baseline amplitude. For each experimental group we compared relative EPSP amplitudes from the last 10 min with averaged EPSP amplitudes measured before HFS or LFS (baseline values). Statistical comparison between EPSP amplitude measured during baseline and EPSP amplitude measured during the last 10 min after HFS within each group was performed by applying Student's t test. The t test was also used for statistical comparisons among different groups. Differences were considered significant with P < 0.05.
Substance delivery
NGF was supplied using two procedures. Local supply of NGF was achieved by using a coaxial electrode. The inner pipette (70–120 MΩ) was used for intracellular recordings and it was filled with 2 m potassium acetate as described above. The outer pipette was used to deliver NGF (β-NGF, Alomone Labs, Israel) and denatured NGF (100 ng ml−1), biocytin (0.5%, Sigma in ACSF). NGF (100 ng ml−1 in ACSF) was also supplied by general perfusion; in this case NGF perfusion lasted 10 min. Anti-TrkA antibodies (Cattaneo et al. 1999; Pesavento et al. 2000) were used at concentration of 10 μg ml−1. Slices were incubated with ACSF containing anti-TrkA antibodies for at least 2 h (Sermasi et al. 2000).
Biocytin (0.5%) was injected intracellularly through the recording electrode using short (10 ms) hyperpolarizing pulses (50–100 pA at 0.2 Hz) for 10 min. After 1 h of incubation slices were fixed overnight at 4°C in 4% paraformaldehyde in 0.1 m phosphate buffer. After rinsing with phosphate buffer, slices were then incubated for 1–2 h in ABC solution (ABC kit; Vector Laboratories, Burlingame, CA, USA) with Triton X-100 (0.5%) and developed using the DAB reaction.
Purification of the anti-TrkA monoclonal antibody MNAC13
The MNAC13 hybridoma was prepared according to Cattaneo et al. (1999) and purified according to Covaceuszach et al. (2001). In detail, the MNAC13 monoclonal antibody was purified from hybridoma supernatant by precipitation with 29% ammonium sulphate followed by affinity chromatography using a Protein G Sepharose column (Amersham Pharmacia Biotech) and eluted with low-pH buffer (10 mm HCl). Fractions were assessed for homogeneity by Coomassie blue staining after separation by SDS-PAGE. Then MNAC13 fractions were pooled, dialysed across a Spectra-Por Membrane 12/14K (Spectrum) against 10 mm Tris (pH 7.0) and concentrated by Centriprep 100K concentrators (Amicon). The amounts of purified protein were determined by the Lowry assay (Bio-Rad).
Results
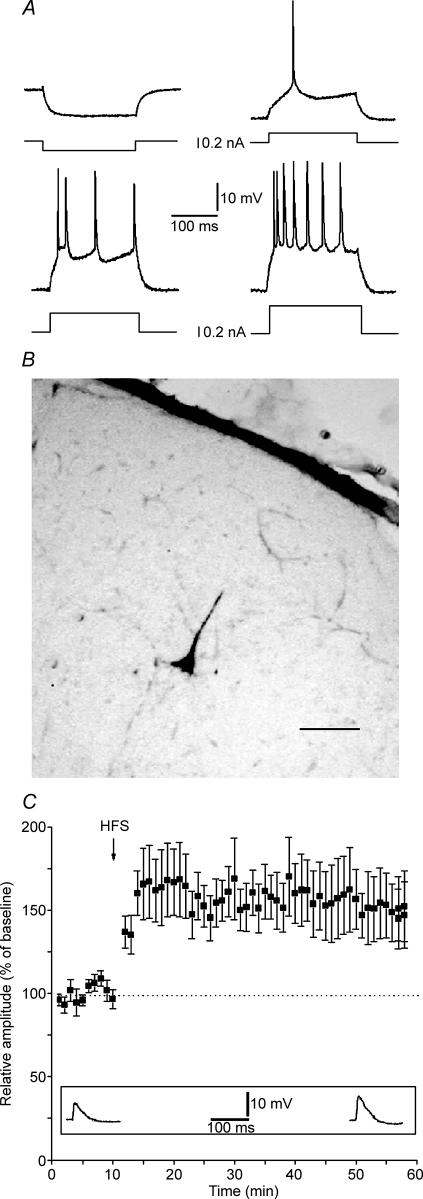
Intracellular recordings were obtained from 66 RS neurones of slices containing the primary visual cortex. Mean resting membrane potential (Vm) and input resistance (Rm) were −73 ± 4 mV and 69 ± 7 MΩ, respectively. These values were similar to previously reported data (Volgushev et al. 1995). RS neurones located in layer II–III were selected on the basis of their firing rate (Fig. 1A, McCormick et al. 1985). Some of the recorded cells were stained with biocytin and all were identified as pyramidal neurones (Fig. 1B). High frequency stimulation (HFS) of WM elicited a stable LTP in control cells (Fig. 1C; n = 10, mean change of EPSP amplitude = 150 ± 19%).
Figure 1. Regular spiking cells (RS) of the rat visual cortex express LTP.
A, characteristic RS response to intracellular current pulses of different intensities. Spike discharge pattern of typical RS neurone in layer II–III of area 17 during injection of current pulses (200 ms). Recording was done at Vm = −70. Different intensities and polarity of injected currents are reported in the four panels. B, intracellularly recorded RS neurone labelled by injection of 0.5% biocytin dissolved in 2 m potassium acetate solution. Scale bar = 80 μm. C, long-term potentiation of EPSPs in RS following HFS. Values indicate mean EPSP amplitudes ± s.e.m. Inset depicts EPSPs from a representative neurone recorded before HFS and at the 50th minute after HFS.
Local NGF application converts HFS-induced LTP into HFS-induced LTD
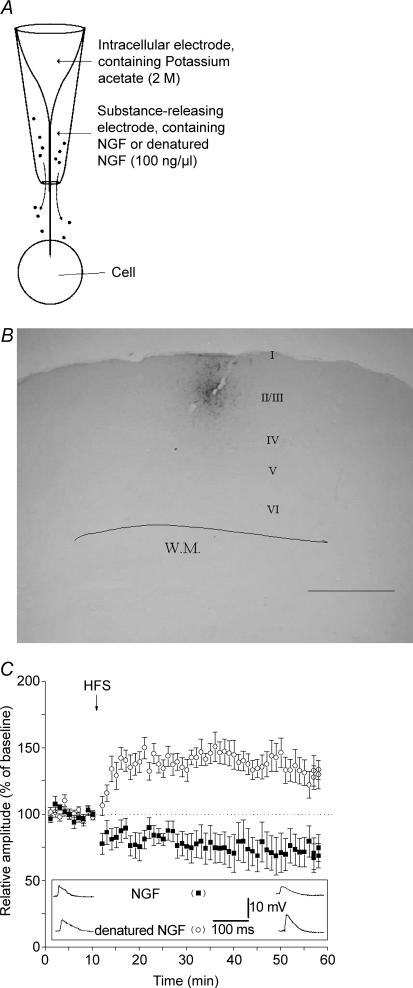
In a previous paper, it was shown that HFS caused a persistent potentiation of the evoked field potentials that was impaired when NGF was locally applied through the recording electrode (Pesavento et al. 2000). To test the action of locally delivered NGF on synaptic plasticity of regular spiking neurones in layer II–III, we designed a special double coaxial electrode made of an inner sharp electrode used for intracellular recording surrounded by an outer pipette (extracellular pipette) used for local drug delivery (Fig. 2A). The solution contained in the outer pipette diffused locally by capillarity and influenced cells in the close proximity of the recording site. To assess the maximal spreading of delivered compounds we filled the outer pipette with biocytin (0.5% in ACSF). Biocytin applied extracellularly has been shown to be taken up by neurones (reviewed by McDonald, 1992) and thus it represents a useful tool for labelling individual neurones and for tracing neuronal connections. Figure 2B shows that a small portion of the cortex presents a spot of labelling surrounded by a few labelled neurones. This supports the idea that compounds delivered in such way have a spatially restricted action.
Figure 2. NGF depresses LTP and generates LTD after high frequency stimulation.
A, concentric electrode used to record from the cell and to locally deliver NGF (or denatured NGF). The inner portion is the sharp electrode used for electrophysiological recording. The outer electrode has an opening of about 50 μm and contains the drug to be delivered. B, neurones labelled by uptake of 0.5% biocytin released from the outer electrode (scale bar = 800 μm). C, comparison between the effects of NGF and denatured NGF. Local application of NGF (100 ng ml−1, ▪) induces LTD instead of LTP following HFS. Heat-inactivated NGF (100 ng ml−1, ○) does not affect LTP. Values indicate mean EPSP amplitudes ± s.e.m. Inset depicts EPSPs recorded from two representative neurones during local application of NGF (top raw) and denatured NGF (bottom raw). EPSPs were recorded before HFS (baseline, left) and at the 50th minute following HFS (right).
When NGF (100 ng ml−1) was delivered through the outer pipette, HFS no longer induced LTP in RS cells; instead, HFS induced a stable LTD (Fig. 2C; n = 9, mean change of EPSP amplitude = 72 ± 11%).
To control the specificity, NGF was denatured by heating (80°C for 10 min) and tested for its activity on PC12 cells immediately before using it. In particular, PC12 cells did not differentiate when treated with heat-inactivated instead of active NGF (20 ng ml−1), as previously reported (Pesavento et al. 2000). When the outer pipette was filled with denatured NGF, HFS induced a normal LTP (Fig. 2C; n = 7, mean change of EPSP amplitude = 133 ± 9%). Statistical comparisons among different groups showed that LTP magnitude in denatured NGF-treated slices was not significantly different with respect to that in control slices (Fig. 5).
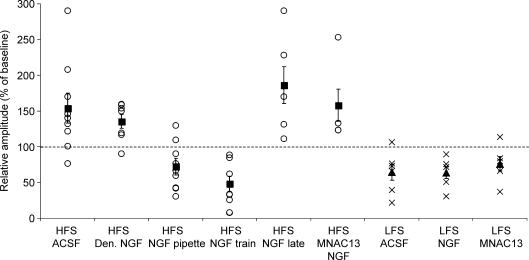
Figure 5. Summary of averaged EPSPs amplitudes of recorded neurones grouped for each kind of treatment.
○, the relative mean EPSP amplitude of each cell recorded from 40 to 50 min after the HFS, compared with baseline. ×, the relative mean EPSP amplitude of each cell recorded from 45 to 55 min after LFS, compared with baseline. ▪ and ▴, the mean change in EPSP amplitude for each treatment. Denatured and pipette NGF were locally applied using coaxial electrode. NGF train, NGF treatment during HFS; NGF late, NGF treatment 10 min after HFS; HFS MNCA13, NGF treatment during HFS in slices incubated in ACSF containing anti-TrkA antibody; HFS and LFS ACSF, HFS and LFS in control slices perfused with ACSF; LFS NGF, NGF treatment during LFS; LFS MNCA13, LFS elicited in slices incubated in ACSF containing anti-TrkA antibody. Statistical comparisons among different groups: (HFS stimulation: ACSF versus pipette NGF, P = 0.003; ACSF versus denatured NGF, P > 0.05; ACSF versus NGF train, P = 0.0008; pipette NGF versus NGF train, P > 0.05; LFS-induced LTD was not significantly different among all groups).
NGF affects synaptic plasticity during the induction phase
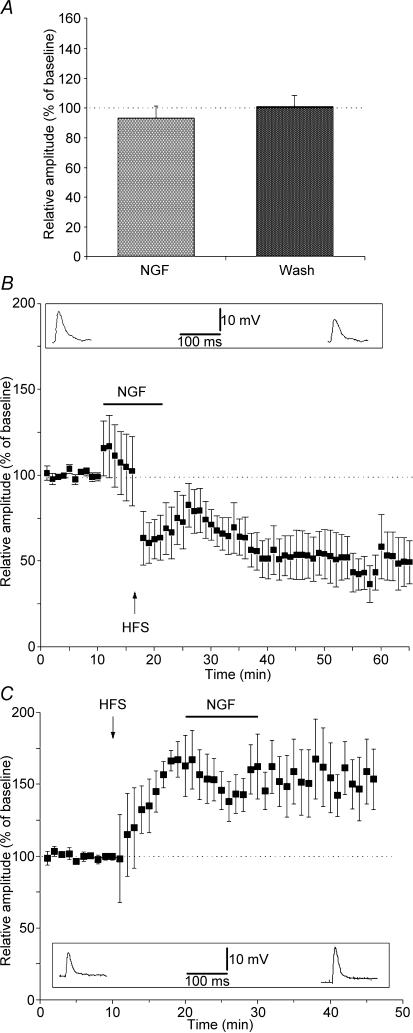
Using the coaxial electrode, NGF was delivered for the whole period of recording, making it impossible to judge whether NGF acts on basal synaptic transmission. In addition, from the previous data it was difficult to understand whether NGF was required during the HFS and/or the maintenance phase of the induced long-term synaptic change. To deal with these issues, in additional groups of slices, NGF (100 ng ml−1) was dissolved in ACFS and supplied by general perfusion for 10 min only. To test if NGF alone has any influence on basal synaptic transmission we supplied NGF (100 ng ml−1) for 10 min without HFS and using baseline stimulation (0.05 Hz); no significant change of the mean EPSP amplitude was observed in RS cells during NGF treatment (Fig. 3A; n = 7, mean change of EPSP amplitude during NGF application = 93 ± 8%, mean change of EPSP amplitude after NGF wash out = 100 ± 8%). NGF application did not affect either resting membrane potential (Vm = −67 ± 2 mV before NGF; Vm = −66 ± 3 mV during NGF treatment) or input resistance (Rm = 76 ± 5 MΩ before NGF; Rm = 74 ± 5 MΩ during NGF). When HFS was delivered during the application of NGF in another group of slices a stable LTD was obtained (Fig. 3B; n = 7, mean change of EPSP amplitude = 46 ± 12%). The difference between control and NGF-treated slices was significant (Fig. 5). The higher magnitude of depression observed with bath application of NGF, compared with that observed during local delivery (Fig. 5), could be due to bath dilution of the neurotrophin released from the outer pipette in the latter case (Pesavento et al. 2000).
Figure 3. NGF affects LTP during the induction phase.
A, NGF does not directly affect EPSP amplitude. Columns represent the average of 10 min of EPSPs, pooling together all cells recorded during bath supply of NGF (NGF) and after NGF washout (wash). Baseline stimulation (0.05 Hz) was used during NGF treatment and washout. B, NGF delivered in the bath during HFS is sufficient to generate LTD after HFS. Bath supply of NGF 5 min before HFS induces LTD instead of LTP following HFS. C, NGF applied 10 min after HFS does not affect LTP. Values indicate mean EPSP amplitudes ± s.e.m. Insets depict EPSPs recorded from representative neurones during baseline stimulation before HFS and at the 50th minute after HFS.
The next question was whether NGF could modulate LTP when supplied after induction of LTP by HFS. To test this, NGF was bath-applied 10 min after HFS, i.e. after induction of LTP. We found that amplitude of LTP was unaffected by NGF (Fig. 3C; n = 5, mean change of EPSP amplitude = 154 ± 21%). These results indicate that NGF is required only during the induction phase of long-term change of synaptic efficacy.
To find out whether NGF exerted its action through the TrkA receptor we made use of the monoclonal antibody MNAC13 that blocks the extracellular portion of human and rat TrkA receptors (Cattaneo et al. 1999). This IgG1A mouse monoclonal antibody, besides being highly specific and selective for the extracellular domain of TrkA receptor, does not bind to any other closely related members of the Trk receptor family. By surface plasmon resonance experiments it was possible to show that the binding affinity towards its antigen is in the nanomolar range (Covaceuszach et al. 2001). MNAC13 was able to inhibit NGF effects on cultured PC12 cells at a concentration of 10 μg ml−1 (data not shown). Thus, to block TrkA we incubated slices in ACSF solution containing 10 μg ml−1 of MNAC13 for at least 2 h before recording. Following this method we have previously shown that antibodies penetrate into slices without interfering with basal responses of field potentials (Sermasi et al. 2000). Pretreatment with MNAC13 did not affect membrane potential (Vm = −73 ± 2 mV) nor input resistance (Rm = 75 ± 5 MΩ). In the cells recorded after this treatment, normal LTP was obtained when HFS was delivered during bath application of NGF (n = 5, mean change of EPSP amplitude = 157 ± 31%, Fig. 5). We conclude that NGF signalling through TrkA induces a shift from LTP to LTD in RS neurones of layer II–III in rat visual cortex.
Long-term depression induced by LFS is not affected by NGF
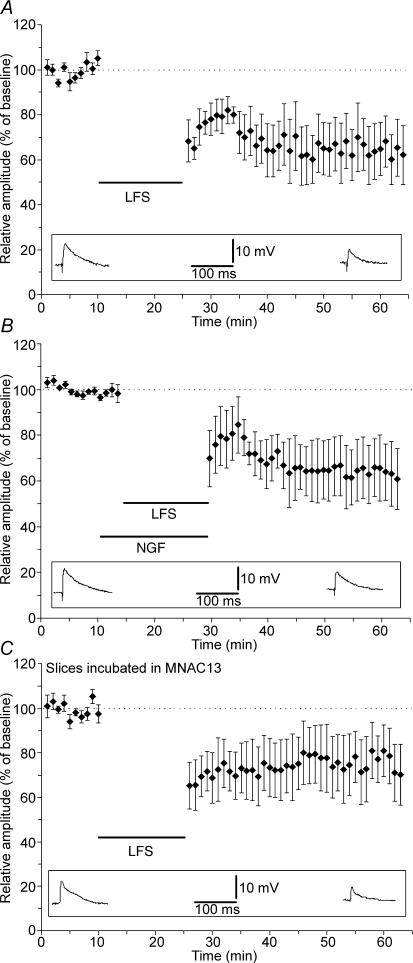
A series of experiments were performed to assess if NGF effect is selective for LTP induced by HFS. We focused our attention on LTD elicited by LFS. The LFS protocol (900 pulses at 1 Hz) induced a stable long-term depression in RS cells (Fig. 4A, control slices, mean EPSP amplitude = 64 ± 12%, n = 6). Bath application of 100 ng ml−1 NGF during LFS stimulation produced LTD whose amplitude was not statistically different from that observed in control slices (Fig. 4B; mean EPSP amplitude = 59 ± 13%, n = 5).
Figure 4. NGF does not affect LTD.
A, long-term depression of EPSP following LFS of WM. B, bath application of NGF during LFS does not modify LTD. C, incubation of the slices in a solution containing the anti-TrkA monoclonal antibody MNAC13 does not change LTD expression. Values indicate mean EPSP amplitudes ± s.e.m. Insets depict EPSPs recorded from representative neurones recorded before LFS (baseline, left) and at the 40th minute following LFS (right).
Having verified that LTD was not modified by exogenous application of NGF, we asked if endogenous NGF could be involved in LTD induced by LFS. In order to test this hypothesis we incubated the slices in ACSF solution containing 10 μg ml−1 of MNAC13 with the aim of preventing TrkA activation by endogenous NGF. Even in these conditions, LFS induced a stable LTD whose amplitude was not statistically different from that obtained in RS cells from control slices (Fig. 4C; mean EPSP amplitude = 75 ± 12%, n = 5). These results indicate that NGF is not involved in mechanisms underlying LFS-induced synaptic depression.
Discussion
The major results reported in the present paper may be summarized as follows. First, treating visual cortical slices with NGF converts LTP into LTD in RS cells of layer II–III following HFS of white matter. Second, the results obtained using blocking antibody indicate that the NGF-dependent shift from LTP to LTD is mediated by TrKA. Third, slices treated with NGF or blocking antibodies against TrkA show a normal LTD in response to LFS of white matter.
NGF has been extensively investigated as a regulator of membrane excitability (Rudy et al. 1987; Toledo-Aral et al. 1995; Levine et al. 1995; Lesser et al. 1999; Grumolato et al. 2003) but, paradoxically, data concerning its possible role as a neuromodulator of synaptic plasticity are scarce. It has been reported that in hippocampal slices, perfusion of NGF blocks the expression of LTP (Tancredi et al. 1993). In contrast, several other groups have reported no effects of NGF in modulating synaptic plasticity both in hippocampus and visual cortex (Kang & Schuman, 1995; Figurov et al. 1996; Akaneya et al. 1997).
NGF is the first neurotrophic factor whose effect on the visual cortical plasticity has been examined. During the critical period, NGF injection into the lateral ventricle of rats (Domenici et al. 1991; Maffei et al. 1992; Domenici et al. 1993) or local TrkA activation in primary visual cortex (Pizzorusso et al. 1999) largely prevents the dramatic change in ocular preference of visual cortical neurones in response to monocular deprivation. Recent data obtained by recording field potentials showed that local supply of NGF delivered through the recording pipette inhibits LTP in layer II–III of developing visual cortex (Pesavento et al. 2000). These data suggested the idea that NGF blocks plasticity when the cortical network is highly plastic.
In the present study, by recording from single neurones, namely RS neurones of layer II–III, we found that high frequency stimulation of white matter that would normally induce LTP instead elicited a stable LTD when NGF was supplied. The first possibility is that the NGF effects on synaptic plasticity are non-specific due to generalized depression of synaptic transmission. This hypothesis is unlike since we have shown that (i) denatured NGF used as a control does not affect synaptic plasticity and (ii) NGF does not change the amplitude of EPSPs during basal stimulation before HFS.
Part of the results reported in the present paper were obtained using a new electrode for local release of compounds with the aim of reproducing the experimental conditions adopted by Pesavento et al. (2000), where NGF was shown to interact with LTP when locally applied through the recording pipette. This approach presents some limitations such as the impossibility of controlling the time of compound release. However, it overcame the difficulty of obtaining a release restricted to the neighbourhood of the recorded cell when using sharp electrode technique. We showed that only the area surrounding the electrode was reached by released compounds, thus avoiding generalized action on a broad cortical area. We found that local and continuous release of NGF converts LTP into LTD following HFS stimulation. Thus, NGF may modify the dynamics of neuronal plasticity in cortical neurones of developing visual cortex acting on local cortical circuitry.
Pesavento et al. (2000) described a reduction of LTP amplitude instead of a shift to LTD following HFS in the presence of NGF. The discrepancy may arise from the field potential recordings used by Pesavento et al. (2000), thus reflecting the contribution of different types of cells, versus the single cell recordings used in the present study. It may well be that NGF induces LTD in pyramidal-like neurones and no action and/or LTP in other types of cortical neurones, thus resulting in null effect on field potentials.
Our experiments using a general perfusion supply method permitted us to apply NGF for a limited time period and to differentiate NGF effects on synaptic plasticity from the direct effect on synaptic transmission. We showed that NGF did not directly depress synaptic transmission. Delivery of NGF simultaneously with of HFS induced the shift from LTP to LTD, similarly to what has been reported with NGF local treatment. Furthermore, delayed application of NGF with respect to HFS did not change the amplitude of LTP and therefore did not influence the potentiated synapses. Thus, to convert LTP to LTD, NGF must be present when one cell is receiving the afferent inputs inducing specific synaptic modifications. The temporal relationship between NGF and afferent input together with the failure to directly modulate synaptic transmission suggests that NGF action in developing visual cortex involves a direct change in the properties of synaptic plasticity in selected populations of cells.
Treatment with blocking TrkA antibodies (Cattaneo et al. 1999; Covaceuszach et al. 2001; Tropea et al. 2002;), at concentrations capable of inhibiting NGF effects on PC12 cells, abolished HFS-induced LTD, indicating that NGF action is mediated through binding and activation of TrkA. The amplitude of LTP was not changed by the presence of antibodies against TrkA, suggesting that basal endogenous NGF in visual cortex allows for maximal expression of LTP.
To examine whether NGF or TrkA antibody changes frequency dependence of long-term synaptic plasticity, we investigated the expression of LTD elicited by LFS. In this experimental condition, NGF did not change the amplitude of LTD, indicating that its effect is selective for synaptic modification induced by HFS. Also, the blockade of endogenous NGF signalling by treatment with TrkA antibodies did not influence LTD elicited by LFS. Thus, at the cellular level, NGF could act by shifting the postsynaptic modification threshold (τm) for the induction of LTP towards higher levels of activity (Bienenstock et al. 1982, Benuskova et al. 1994, 1999; reviewed by Bear, 2003) so that our tetanic stimulation, which under normal conditions exceeds τm and therefore induces LTP, would instead remain below threshold, thus resulting in LTD. In agreement with this hypothesis, stimulation frequency that normally is below τm, such as LFS, induced normal LTD in the presence of NGF. The present data suggest that increasing NGF in developing visual cortex changes the property of synaptic plasticity, favouring synaptic weakening over synaptic strengthening.
Monocular deprivation during the critical period causes an ocular dominance shift towards the open eye with a reduction in the activity of cortical neurones driven by the closed eye (Wiesel & Hubel, 1963). This form of visual cortical plasticity is clearly based on different patterns of afferent activity in the visual cortex driven by open and closed eyes. It has been proposed that cortical synapses are depressed or strengthened depending on the relationship between afferent and postsynaptic neuronal activity (for reviews see Shatz, 1990; Katz, 1999). When the neuronal activity is reduced or unrelated to postsynaptic activity, as occurs when the eye is closed, cortical synapses are depressed. Conversely, cortical connections driven by the open eye are characterized by correlated activities and therefore are strengthened. The present in vitro results suggest that cortical connections with pyramidal cells of layer II–III remain depressed in the presence of NGF, even when they receive an input that otherwise would cause an increase in synaptic efficacy. We raise the hypothesis that NGF-induced LTD may counteract synaptic strengthening normally favouring cortical connections driven by the open eye in monocularly deprived animals. Consistent with this idea, NGF treatment in monocularly deprived rats results in a decrease of activity-dependent competition between the two eyes and an absence of ocular dominance shift towards the open eye (Domenici et al. 1991; Maffei et al. 1992; Lodovichi et al. 2000).
Blocking TrkA by using monoclonal antibodies abolishes NGF effects on synaptic plasticity. In the rat, TrkA receptors are expressed by cholinergic neurones in the basal forebrain projecting to several areas, including visual cortex (Steininger et al. 1993; Sobreviela et al. 1994; Li et al. 1995; Molnar et al. 1998). Thus, exogenously supplied NGF can be targeted to cholinergic neurones expressing TrkA receptors. The bulk of available evidence suggests that NGF acts on these neurones, increasing ACh release (Maysinger et al. 1992; Knipper et al. 1994; Sala et al. 1998). Recently, it has been reported that carbachol, an agonist of muscarinic receptors, induces LTD in visual cortical slices (Kirkwood et al. 1999). These data are in agreement with the hypothesis that the NGF-dependent shift from LTP to LDT in visual cortical neurones is mediated by acetylcholine. However, it is difficult to definitively prove this hypothesis since the classical pharmachological tools used to interfere with acetylcholine release and/or cholinergic receptors also affect the machinery underlying LTP induction and maintenance. We cannot exclude the alternative possibility that cortical interneurones could be involved in the NGF-dependent shift from LTP to LTD. Indeed, it has been shown that a few interneurones expressing calbindin and neuropeptide Y, present TrkA immunoreactivity in rat visual cortex, especially at late postnatal ages (Tropea et al. 2002). Several reports have documented that recruitment of GABAergic neurones reduces LTP and favours LTD (Artola & Singer, 1987; Kirkwood & Bear, 1994; Rozas et al. 2001).
Acknowledgments
We thank Emanuele Pesavento for designing the coaxial electrode used in the present paper, Elisa Margotti for biocytin staining. We are also grateful to Enrico Cherubini, Alessandro Cellerino, Matteo Caleo and Mathew Diamond for critical reading of the manuscript and helpful suggestions. We acknowledge W. Anderson for online data acquisition system.
References
- Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Meth. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benuskova L, Diamond ME, Ebner FF. Dynamic synaptic modification threshold: Computational model of experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1994;91:4791–4795. doi: 10.1073/pnas.91.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benuskova L, Ebner FF, Diamond ME, Armstrong-James M. Computational study of experience-dependent plasticity in adult rat cortical barrel-column. Network: Computation Neural Systems. 1999;10:303–323. [PubMed] [Google Scholar]
- Berardi N, Cellerino A, Domenici L, Fagiolini M, Pizzorusso T, Cattaneo A, Maffei L. Monoclonal antibodies to nerve growth factor affect the postnatal development of the visual system. Proc Natl Acad Sci U S A. 1994;91:684–688. doi: 10.1073/pnas.91.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Capsoni S, Margotti E, Righi M, Kontsekova E, Pavlik P, Filipcik P, Novak M. Functional blockade of tyrosine kinase A in the rat basal forebrain by a novel antagonistic anti-receptor monoclonal antibody. J Neurosci. 1999;19:9687–9697. doi: 10.1523/JNEUROSCI.19-22-09687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48:1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Covaceuszach S, Cattaneo A, Lamba D. Purification, crystallization and preliminary X-ray analysis of the Fab fragment from MNAC13, a novel antagonistic anti-tyrosine kinase A receptor monoclonal antibody. Acta Crystallogr D Biol Crystallogr. 2001;57:1307–1309. doi: 10.1107/s0907444901010666. [DOI] [PubMed] [Google Scholar]
- Domenici L, Berardi N, Carmignoto G, Vantini G, Maffei L. Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc Natl Acad Sci U S A. 1991;88:8811–8815. doi: 10.1073/pnas.88.19.8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Cellerino A, Berardi N, Cattaneo A, Maffei L. Antibodies to nerve growth factor (NGF) prolong the sensitive period for monocular deprivation in the rat. Neuroreport. 1994;5:2041–2044. doi: 10.1097/00001756-199410270-00013. [DOI] [PubMed] [Google Scholar]
- Domenici L, Cellerino A, Maffei L. Monocular deprivation effects in the rat visual cortex and lateral geniculate nucleus are prevented by nerve growth factor (NGF). II. Lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1993;251:25–31. doi: 10.1098/rspb.1993.0004. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fox K. The critical period for long-term potentiation in primary sensory cortex. Neuron. 1995;5:485–488. doi: 10.1016/0896-6273(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Louiset E, Alexandre D, Ait-Ali D, Turquier V, Fournier A, Fasolo A, Vaudry H, Anouar Y. PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur J Neurosci. 2003;17:71–82. doi: 10.1046/j.1460-9568.2003.02426.x. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Katz LC. What's critical for the critical period in visual cortex? Cell. 1999;99:673–676. doi: 10.1016/s0092-8674(00)81665-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Lesser SS, Holmes TM, Pittman AJ, Lo DC. Induction of electrical excitability by NGF requires autocrine action of a CNTF-like factor. Mol Cell Neurosci. 1999;14:169–179. doi: 10.1006/mcne.1999.0778. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–347. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The saga of the nerve growth factor. Neuroreport. 1998;9:71–83. [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Differential effects of NGF and BDNF on voltage-gated calcium currents in embryonic basal forebrain neurons. J Neurosci. 1995;15:3084–3091. doi: 10.1523/JNEUROSCI.15-04-03084.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knusel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neuroanatomical labeling with biocytin: a review. Neuroreport. 1992;3:821–827. doi: 10.1097/00001756-199210000-00001. [DOI] [PubMed] [Google Scholar]
- Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysinger D, Herrera-Marschitz M, Goiny M, Ungerstedt U, Cuello AC. Effects of nerve growth factor on cortical and striatal acetylcholine and dopamine release in rats with cortical devascularizing lesions. Brain Res. 1992;577:300–305. doi: 10.1016/0006-8993(92)90287-j. [DOI] [PubMed] [Google Scholar]
- Molnar M, Tongiorgi E, Avignone E, Gonfloni S, Ruberti F, Domenici L, Cattaneo A. The effects of anti-nerve growth factor monoclonal antibodies on developing basal forebrain neurons are transient and reversible. Eur J Neurosci. 1998;10:3127–3140. doi: 10.1046/j.1460-9568.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- Pesavento E, Margotti E, Righi M, Cattaneo A, Domenici L. Blocking the NGF–TrkA interaction rescues the developmental loss of LTP in the rat visual cortex: role of the cholinergic system. Neuron. 2000;25:165–175. doi: 10.1016/s0896-6273(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Berardi N, Rossi FM, Viegi A, Venstrom K, Reichardt LF, Maffei L. TrkA activation in the rat visual cortex by antirat trkA IgG prevents the effect of monocular deprivation. Eur J Neurosci. 1999;1:204–212. doi: 10.1046/j.1460-9568.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Kirschenbaum B, Rukenstein A, Greene LA. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987;7:1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala R, Viegi A, Rossi FM, Pizzorusso T, Bonanno G, Raiteri M, Maffei L. Nerve growth factor and brain-derived neurotrophic factor increase neurotransmitter release in the rat visual cortex. Eur J Neurosci. 1998;10:2185–2191. doi: 10.1046/j.1460-9568.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill W. Links Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. J Neurophysiol. 1999;81:1341–1354. doi: 10.1152/jn.1999.81.3.1341. [DOI] [PubMed] [Google Scholar]
- Sermasi E, Margotti E, Cattaneo A, Domenici L. Trk B signalling controls LTP but not LTD expression in the developing rat visual cortex. Eur J Neurosci. 2000;4:1411–1419. doi: 10.1046/j.1460-9568.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Klein R, Barbacid M, Palfrey HC. High-affinity nerve growth factor receptor (Trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Res. 1993;612:330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D'Arcangelo G, Mercanti D, Calissano P. Nerve growth factor inhibits the expression of long-term potentiation in hippocampal slices. Neuroreport. 1993;4:147–150. doi: 10.1097/00001756-199302000-00008. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Tropea D, Capsoni S, Covaceuszach S, Domenici L, Cattaneo A. Rat visual cortical neurones express TrkA NGF receptor. Neuroreport. 2002;13:1369–1373. doi: 10.1097/00001756-200207190-00031. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Voronin LL, Christiakova M, Artola A, Singer W. All-or-none excitatory postsynaptic potentials in the rat visual cortex. Eur J Neurosci. 1995;7:1751–1760. doi: 10.1111/j.1460-9568.1995.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]