Abstract
SPOC1 airway goblet cells secrete mucin in response to P2Y2 receptor agonists and to secretagogues, phorbol 12-myristate 13-acetate (PMA) and ionomycin, which mobilize elements of the phospholipase C pathway, PKC and Ca2+, respectively. Previous studies demonstrated that mucin secretion from SLO-permeabilized, EGTA-buffered SPOC1 cells was stimulated by PMA at low Ca2+ levels (< 0.1 μm), consistent with the notion that regulated exocytosis may occur by Ca2+-independent pathways. We tested the alternative hypothesis that PMA-induced mucin secretion is, in fact, a Ca2+-dependent process under the conditions of low bulk Ca2+, one that is permitted in the typical SLO-permeabilized cell model by the slow binding kinetics of EGTA. Both IP3 and elevated bulk Ca2+ activated mucin secretion in SPOC1 cells buffered by EGTA, suggesting that IP3 generates a local Ca2+ gradient in the vicinity of the secretory granules to the degree necessary to trigger exocytosis. BAPTA, which binds Ca2+ approximately 100-fold faster than EGTA, diminished IP3-induced mucin release over a range of concentrations by ≥ 69%, yet maintained an essentially normal mucin secretory response to elevated bulk Ca2+ in permeabilized SPOC1 cells. BAPTA also diminished the mucin secretory response of permeabilized cells to PMA, relative to the EGTA-buffered control: at PMA below 30 nm, BAPTA abolished the secretory response, and at higher concentrations it was reduced significantly relative to the EGTA-buffered controls. PMA-induced secretion in EGTA was insensitive to heparin. These results suggest that Ca2+ is released locally during PMA-induced exocytosis, by an IP3-independent mechanism.
Airway goblet cells and submucosal glands secrete the high molecular weight glycoconjugate mucin, the viscoelastic component of mucus. In healthy lungs, mucus plays a principal role in mucociliary clearance (Knowles & Boucher, 2002). However, in the airway obstructive diseases asthma, chronic bronchitis, bronchiectasis, and cystic fibrosis, mucus/mucin hypersecretion is a hallmark characteristic that arises from inflammation-induced goblet cell and submucosal gland hyper- and metaplasia (reviewed by Rogers, 2003). Despite the central role of mucin secretion in health and its importance in airway disease, the regulation of mucin granule exocytosis is poorly understood beyond the level of receptor activation and cellular messenger generation.
SPOC1 cells, derived from rat tracheal epithelium (Randell et al. 1996), secrete mucin in response to purinergic stimulation by ATP or UTP activation of P2Y2 receptors (Abdullah et al. 1996), in agreement with studies on goblet cells in primary cultures from hamster trachea (Kim & Lee, 1991), canine tracheal epithelial explants (Davis et al. 1992), and human bronchial epithelial cells (Chen et al. 2001; Conway et al. 2003). P2Y2 receptors typically couple to PLC (Harden et al. 1995), and mobilization of intracellular Ca2+ by ionomycin and activation of PKC by the DAG mimic, PMA, stimulate mucin secretion in SPOC1 cells (Abdullah et al. 1997; Abdullah et al. 2003). Much of the SPOC1 cell data on the regulation of mucin secretion by Ca2+ and PKC are consistent with the notion that these effectors function independently. Chief among these are the findings that ionomycin and PMA effects are fully additive at maximal concentrations (Abdullah et al. 1997), that the agonist-responsive isoform of PKC, nPKCδ, is a member of the novel, Ca2+-independent PKC subfamily (Abdullah et al. 2003), and that in permeabilized, EGTA-buffered cells PMA stimulates mucin secretion at 10 nm free Ca2+, an order of magnitude below generally accepted basal levels of intracellular Ca2+ (Scott et al. 1998). Ca2+-independent, regulated exocytosis has also been proposed for other secretory cells: (i) non-hydrolysable analogues of GTP stimulate degranulation of permeabilized, EGTA-buffered mast cells (Gomperts et al. 1986), and in mast cells dialysed with EGTA buffer by a whole-cell patch pipette, exocytotic events sensed by changes in membrane capacitance failed to correlate with Ca2+ transients observed by fura-2 fluorescence (Neher & Almers, 1986); (ii) in gonadotropes of the anterior pituitary, PMA stimulates luteinizing hormone (LH) secretion in the absence of observable changes in Ca2+ (Betz et al. 1998); and (iii) in pancreatic duct epithelial cells, exocytosis is stimulated by agents which elevate cAMP, without observable changes in Ca2+ (Koh et al. 2000). Hille et al. (1999) have postulated that such data indicate a Ca2+-independent regulation of exocytotic secretion by protein kinases such as PKC and PKA.
Contrary to the notion of a mechanism for regulated exocytosis that is independent of Ca2+, there is a strong argument that the process is strictly Ca2+ dependent. Ca2+ plays important roles in the regulation of exocytosis, beginning with cortical actin filament disassembly (Trifaro et al. 2000), granule docking to the plasma membrane (Martin, 2002), and fusion of the granule membrane to the plasma membrane during pore formation (Gerber & Sudhof, 2002). In fact, the postulated trigger for regulated exocytosis is synaptotagmin, an obligate accessory protein to the exocytotic SNARE complex. Though the number of studies is at present small, only the Ca2+-dependent synaptotagmin isoforms have been associated with regulated exocytosis (Chapman, 2002; Sudhof, 2002).
Interestingly, experiments yielding the most direct evidence favouring Ca2+-independent exocytosis are typically buffered by EGTA. Because this Ca2+ buffer has relatively slow binding kinetics (Tsien, 1980), one explanation of apparent Ca2+-independent exocytosis is that EGTA permits the generation of local Ca2+ gradients. Hence, we used IP3 and other manoeuvres to test for the existence of Ca2+ gradients in permeabilized, EGTA-buffered cells, and using BAPTA as a probe we tested the hypothesis that regulated SPOC1 cell mucin secretion is, in fact, Ca2+ dependent.
Methods
Materials
Dulbecco's modified Eagle's medium–Ham's nutrient mixture F12 (DMEM–F12) was obtained from Gibco BRL (Gaithersburg, MD, USA) and the supplements from Collaborative Research (Bedford, MD, USA). Bisindolylmaleimide II (BIMII), calphostin C, d-myo-inositol 1,4,5-trisphosphate (IP3; trilithium salt), heparin (sodium salt; low molecular weight), and phorbol-12-myristate-13-acetate (PMA) were purchased from Calbiochem (La Jolla, CA, USA). Ethylene gylcol bis(β-aminoethyl ether)-N,N,N′, N′-tetraacetic acid (EGTA) was purchased from Sigma (St Louis, MO, USA); 1,2-bis(o-aminophenoxy)ethane-N,N,N′ N′-tetraacetic acid (BAPTA; tetrapotassium salt) and TO-PRO were purchased from Molecular Probes (Eugene, OR, USA). Streptolysin-O (SLO) was purchased from Corgenix (Peterborough, UK).
SPOC1 cell culture
SPOC1 cells were seeded in 48-well cluster plates (Costar, Cambridge, MA, USA) at 25 000 cells per well (Scott et al. 1998) and maintained in rat tracheal epithelial cell medium (Randell et al. 1996), as previously described. The cells, passage 7–15, were used for experiments 18–22 days post-confluence.
SLO permeabilization and mucin release
SPOC1 cells were equilibrated before each experiment, as follows, using a Finnpipette multistepper, multichannel pipetter (Needham Heights, MA) and a custom fabricated, multichannel aspirator to quickly remove and replace all solutions from 48-well culture plates. Cells were placed in a 37°C water bath, washed twice with DMEM–F12 (200 μl well−1), and incubated for 30 min; this procedure was repeated three times. Cells were washed twice with phosphate buffered saline (PBS) and then with intracellular buffer (Bufi, composition given below). Immediately following the washes, SPOC1 cells were permeabilized with the bacterial toxin streptolysin-O (SLO) at a final concentration of 1 U ml−1 in Bufi for 30 s (150 μl well−1), as previously described (Scott et al. 1998). Following the permeabilization, cells were washed once in Bufi and incubated with the appropriate solution (200 μl well−1; see Results) for 15 min, at which time the samples were collected for the determination of mucin content. TO-PRO, a membrane-impermeant, DNA-binding fluorescent dye, was used to confirm cell permeability, as previously described (Scott et al. 1998). Notably, TO-PRO and BAPTA are similar in size (relative molecular mass 645 versus 628.8) and EGTA is approximately 50% smaller (relative molecular mass 380.4), making the dye a relevant marker of small molecule permeation. In all of the studies reported below, TO-PRO fluorescence was observed to be uniformly distributed throughout SPOC1 cell cytoplasm indicating that the cells were well permeabilized.
Mucin enzyme-linked lectin assay
Samples collected from each well of the 48-well culture plate were assessed for mucin content using an enzyme-linked lectin assay (ELLA), previously described (Abdullah et al. 1996). Briefly, samples were diluted (100 μl final volume) and bound to 96-well high-binding microtitre plates (Costar), incubated overnight at 4°C or 37°C for 2 h, washed with PBST (0.05% Tween 20 in PBS), and incubated with 2.5 μg ml−1 peroxidase-labelled soybean lectin for 1 h at 37°C. The plates were then washed and developed with O-phenylenediamine in a citrate phosphate buffer (0.0175 m, 0.01% hydrogen peroxide, pH 5.0). H2SO4 (4 m) was used to stop the reaction, and the plates were analysed using optical density at 490 nm (Dynatech microtitre plate reader, model MR5000; Chantilly, VA, USA). Known amounts of purified SPOC1 mucin were used to generate standard curves, on each plate, allowing the results to be expressed as nanograms mucin released per culture.
Intracellular Ca2+ buffer
EGTA stock solutions (50 mm) were prepared as previously described (Gomperts & Tatham, 1992; Scott et al. 1998) after first titrating the EGTA with CaCl2.2H2O to determine true buffer concentrations (Miller & Smith, 1984). BAPTA stock solutions (25 mm) were calculated directly, with allowance made for impurities as determined by HPLC (Molecular Probes, OR, USA). These stocks were stored at −20°C. Free Ca2+ activities were computed with the aid of the computer program Chelator (Schoenmakers et al. 1992) and CaEGTA or CaBAPTA were used to adjust the final free Ca2+ levels. Except where stated, both Ca2+ buffers were used at a final concentration of 3 mm. Intracellular buffer (Bufi) contained 130 mm potassium glutamate, 20 mm Pipes, 1 mm MgATP and MgCl2, and 3 mm EGTA or BAPTA (pH 6.8). Free Ca2+ in Bufi was set to 0.1 μm, or, where stated in Results, was varied from 0.01 to 10 μm.
Ca2+ buffer simulations
Simulations were generated to compare the effectiveness of BAPTA and EGTA to buffer Ca2+ over time and space. For the temporal simulation (Fig. 2A), the free Ca2+ concentration was calculated from a consideration of the rate of change of bound Ca2+ following a step addition of EGTA or BAPTA to a solution containing 10 μm free Ca2+. The rate of change of bound Ca2+, D, is:
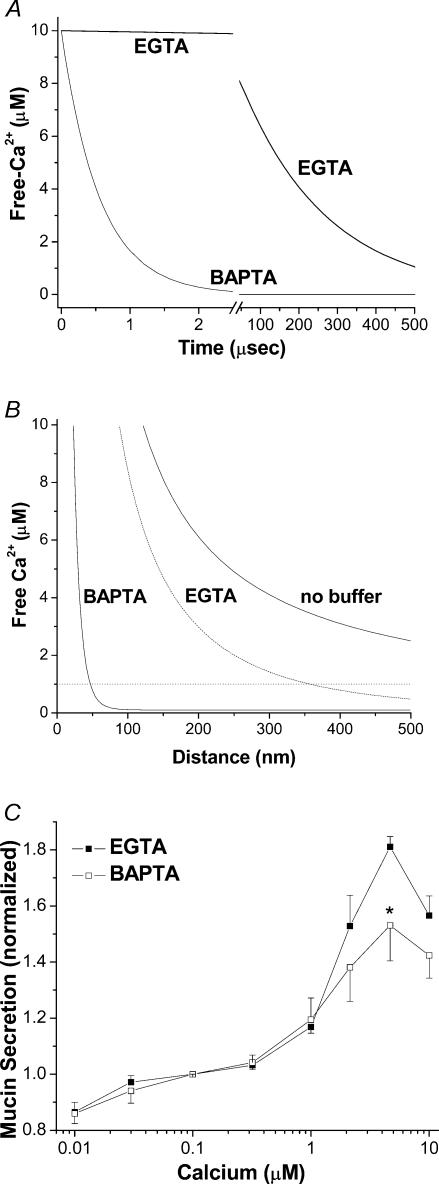
Figure 2. Binding kinetics of EGTA and BAPTA and their relative effectiveness in Ca2+-activated mucin secretion.
A, temporal simulation using eqn (1), illustrating the Ca2+ binding kinetics of EGTA and BAPTA and their effects on free Ca2+ over 500 μs following the step addition of 3 mm buffer to a solution of 10 μm free-Ca2+. B, spatial simulation of steady state free Ca2+ concentration plotted as a function of the distance along a radial extending from a channel opening in a planar membrane. C, comparison of mucin secretion activated in SPOC1 cells by bulk Ca2+ (0.01–10 μm) when buffered by EGTA or BAPTA (n = 6 SPOC1 passages).
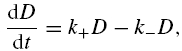 |
with association rate constant k+, dissociation rate constant k–, buffer concentration B, Ca2+ concentration C, complex concentration BC, and time t. This equation can be put into the form:
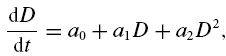 |
which has a solution,
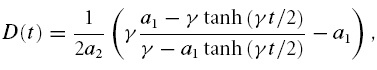 |
where, at D(0) = 0,
The spatial simulation (Fig. 2B) compares the steady state free Ca2+ concentration profiles in the presence of BAPTA, EGTA, or no buffer on one side of a planar boundary with a point Ca2+ source. The profile can be modelled using an approximation to the reaction–diffusion problem first described by Neher (1986) and now known as the excess buffer approximation (for review, see Stern, 1992; Bauer, 2001; Smith et al. 2001). The approximation is valid under conditions of high buffer concentration, low bound buffer to total buffer ratios, and rapid buffer diffusion. In this simulation, Bauer's excess buffer approximation for the case of one buffer was used, with the parameters listed in Table 1, to calculate steady state free Ca2+ concentration as a function of distance from a pore (eqn (9) in, Bauer, 2001). The calculated bound to total ratios for BAPTA and EGTA at the channel were 0.067 and 0.0029, respectively (eqn (12b) in, Bauer, 2001).
Table 1.
Parameters used in temporal and spatial Ca2+ gradient simulations
| Ca2+ total influx (ions s−1)a | 106 | |
| Ca2+ diffusion coefficient (μm2 s−1)a | 220 | |
| Total buffer concentration (mm)c | 3 | |
| Bulk Ca2+ concentration (μm)c | 0.1 | |
| EGTA | BAPTA | |
| Buffer diffusion coefficient (μm2 s−1)b | 113 | 95 |
| Thermodynamic dissociation constant (μm)b | 0.20 | 0.17 |
| Association rate constant (μm−1 s−1)b | 1.5 | 600 |
Statistical analysis
Data collected from the ELLA were normalized to mucin secretion at 0.01 μm Ca2+ under control conditions. Data are presented as the mean ± s.e.m., for a specified number of SPOC1 cell cultures, each culture in a given experiment originating from a different passage. ANOVA and Student's t test were used to determine statistical significance between data sets. * indicates P-values < 0.05.
Results
IP3-induced Ca2+ gradients
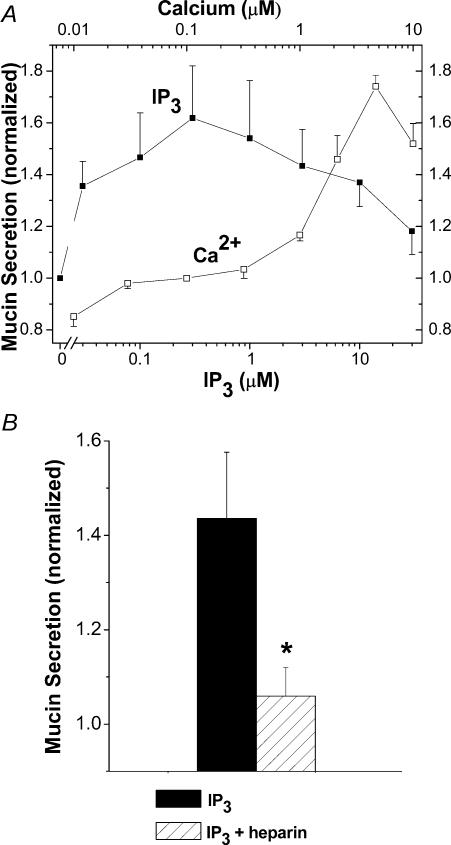
To test whether significant Ca2+ gradients can be generated in SLO-permeabilized, EGTA-buffered SPOC1 cells we used exogenous IP3 to release Ca2+ from internal stores. Cells grown on 48-well culture plates were exposed to IP3 (0.03–30 μm) in 0.1 μm Ca2+ EGTA buffer on one half of the plate. As controls, cells on the other half of the plate were exposed to a series of Ca2+ activities (0.01–10 μm) buffered by EGTA. Mucin secretion induced by increases in bulk Ca2+ was enhanced in a concentration-dependent manner at activities > 1 μm (Fig. 1A), consistent with previous studies in EGTA-buffered SPOC1 cells and many other secretory cells (Scott et al. 1998; and references therein). IP3 also caused a concentration-dependent increase in mucin secretion with a maximum response at 0.3 μm, a value close to its reported EC50 (Wojcikiewicz & Luo, 1998). For reasons that are not clear, IP3 concentrations > 0.3 μm were less effective in stimulating mucin secretion in these cells. Notably, the maximal mucin secretory response to IP3 (0.3 μm) was approximately equal to that induced by 10 μm bulk Ca2+, suggesting that IP3 induces local Ca2+ release either in the vicinity of, or from, mucin secretory granules in EGTA-buffered cells generating a local gradient with a maximal effective concentration of ∼10 μm. Heparin, an IP3 receptor inhibitor, blocked the mucin secretory response to IP3 (Fig. 1B), suggesting that the IP3 effect to release Ca2+ is specific.
Figure 1. Effects of IP3 and bulk Ca2+ on SLO-permeabilized, EGTA-buffered SPOC1 cells.
A, cells grown on one half of a 48-well plate were incubated with IP3 (0.03–30 μm; 0.1 μm Ca2+), whereas the other half received different concentrations of Ca2+ (0.01–10 μm; no IP3). Following permeabilization in this and all other experiments, each treatment was applied to triplicate wells and the mucins released were collected and assessed after a 15 min incubation using a lectin-linked, microtitre plate binding assay. In each case, mucin release was normalized to the secretions at 0.1 μm Ca2+ and is represented as the mean ± s.e.m. (n = 6 SPOC1 passages). B, inhibition of IP3-induced mucin release by heparin. Cells were exposed to 0.3 μm IP3 ± 200 μg ml−1 heparin (n = 6 SPOC1 passages).
Buffer kinetics: relative effects on free Ca2+ and mucin secretion
The two Ca2+ chelators commonly used in biomedical experiments, EGTA and BAPTA, have similar buffer affinities, in contrast to vastly different binding kinetics (see Table 1). To determine whether the faster binding properties of BAPTA might be used to our advantage, we first simulated the time course of Ca2+ binding by the two buffers using starting conditions that mimicked those likely to occur in permeabilized SPOC1 cells under our experimental conditions, i.e. 10 μm free Ca2+ and 3 mm buffer. The simulations indicate that addition of BAPTA, with instantaneous mixing, decreases free Ca2+ to vanishingly low activities in ∼2.5 μs, a time during which EGTA addition has no significant effect (Fig. 2A). As indicated in the figure, EGTA binds only ∼50% of the total free Ca2+ over an extended period of time (150 μs). Therefore, short-lived Ca2+ transients such as those that occur in nerve terminals will be effectively buffered by BAPTA, but not EGTA (e.g. see Adler et al. 1991).
Ca2+ release events associated with G protein-coupled receptor (GPCR) signalling, by comparison to those at synaptic terminals, generally have much longer durations (tens of seconds). In this case, steady-state Ca2+ gradients are likely to be established in the vicinity of Ca2+ release sites. Because small, mobile Ca2+ buffers will determine the Ca2+ concentration profile centred on such sites (see Bauer, 2001; Smith et al. 2001), we simulated Ca2+ gradients generated in the vicinity of a channel opening in the presence of EGTA or BAPTA. Under our experimental conditions, BAPTA is predicted to buffer Ca2+ much closer to the channel than would EGTA (Fig. 2B). At a distance < 50 nm from the mouth of an open channel, with a flux of 106 ions s−1, BAPTA will buffer Ca2+ to levels below 1 μm, whereas a gradient of similar magnitude in EGTA will extend > 350 nm. Hence, BAPTA is an excellent probe for revealing local Ca2+ gradients as well as Ca2+ transients.
As an initial control for potential buffer effects on mucin release, we tested the effects of elevated Ca2+ on mucin secretion from SPOC1 cells permeabilized into EGTA- or BAPTA-based buffers. As shown in Fig. 2C, mucin secretion activated by bulk Ca2+ was similar in both EGTA- and BAPTA-buffered conditions, with the exception of 3 μm Ca2+ where, though mucin secretion was still elevated in BAPTA-buffered cells, it was diminished relative to EGTA.
The findings in Fig. 1 indicate that IP3 releases a significant amount of Ca2+ near sites of mucin granule exocytosis at the apical membrane in EGTA-buffered, permeabilized cells. In experiments conducted with cells of the same passage, we tested whether BAPTA, in the place of EGTA, would diminish mucin secretion elicited by IP3. As shown in Fig. 3, mucin secretion in BAPTA-buffered cells was inhibited by 69–97% at all IP3 concentrations, relative to EGTA.
Figure 3. Effects of rapid Ca+ buffering on IP3-induced mucin secretion from SPOC1 cells.
SLO-permeabilized cells were exposed to IP3 (0.03–30 μm), buffered by EGTA or BAPTA (0.1 μm Ca2+). The EGTA data duplicate those in Fig. 1; the experiments were conducted at the same time, on paired 48-well plates. Two-way ANOVA indicates a significant effect of BAPTA (n = 6 SPOC1 passages).
Lack of buffer effects
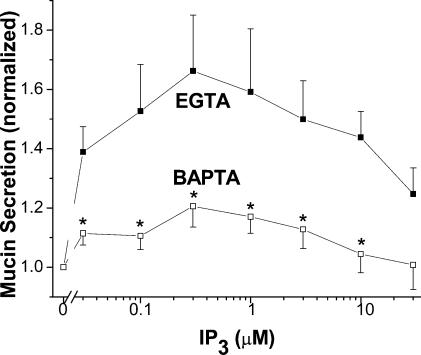
Previous studies suggested that BAPTA might have effects independent of Ca2+ buffering. For instance, in studies with purified IP3 receptors from the cerebellum, BAPTA, EDTA and EGTA were shown to inhibit IP3 binding. The inhibition was competitive between IP3 and the free forms of the buffer (Richardson & Taylor, 1993). Similarly, free-BAPTA was implicated in an inactivation of PKC in liver macrophages (Dieter et al. 1993). Because BAPTA suppressed mucin secretion slightly, but significantly, at high Ca2+ in permeabilized SPOC1 cells (Fig. 2C), we tested for possible direct effects of EGTA and BAPTA on mucin secretion. First, to test whether PKC inactivation might explain the apparent inhibition by BAPTA (Fig. 2C) we used calphostin C and BIMII, in combination, to inhibit PKC (Fig. 4A) during stimulation by high Ca2+. The data indicate no effect of the inhibitors at 3 μm Ca2+ in EGTA- or BAPTA-buffered SPOC1 cells. Mucin secretion was stimulated, though diminished, with BATPA buffering, relative to EGTA, consistent with data of Fig. 2C. Second, as a more general test of Ca2+-independent effects of chelator we measured IP3-induced mucin release at a constant Ca2+ activity of 0.1 μm, over a broad range of EGTA and BAPTA concentrations (0.03–10 mm; Fig. 4B). The results indicate that IP3 elicited mucin secretion independent of EGTA concentration, and that BAPTA generally abolished the stimulatory effects of IP3, again, independent of buffer concentration. Note that in this experiment (cf. Fig. 3), the only point where BAPTA failed to abolish IP3-induced mucin secretion was at 10 μm, a concentration at which Ca2+ buffering by BAPTA is likely to be ineffective. Hence, from these studies we conclude that there were no apparent direct effects of either chelator on the secretory response. Importantly, the lack of buffer concentration effects on IP3-stimulated mucin release in EGTA, or its inhibition by BAPTA, also makes it highly unlikely that the differences observed between the two buffers can be ascribed to differences in permeation of the buffer.
Figure 4. Lack of buffer effects on mucin secretion in SLO-permeabilized SPOC1 cells.
A, cells were exposed to high Ca2+ (3 μm), buffered by EGTA or BAPTA (0.1 μm Ca2+), ± the PKC inhibitors BIMII (1 μm) and calphostin C (1 μm; n = 4 SPOC1 passages). B, cells were exposed to IP3 (0.3 μm) and buffered by either EGTA or BAPTA (0.03–10 mm) at a constant 0.1 μm Ca2+ (n = 4 SPOC1 passages).
Ca2+ dependency of PMA-induced mucin secretion
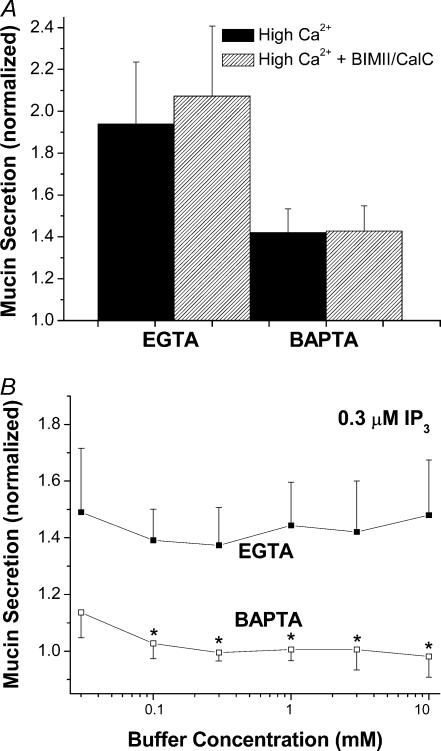
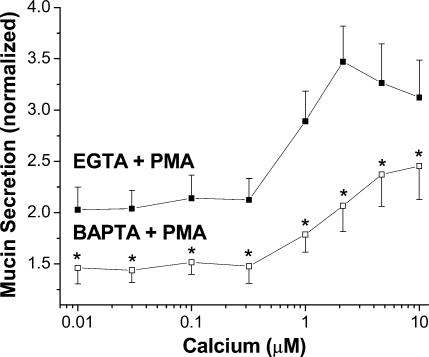
Because BAPTA suppressed IP3-induced Ca2+ gradients, we used it to probe whether the stimulatory effects of PMA on mucin secretion are also Ca2+ dependent. Similar to our previous results (Scott et al. 1998), a concentration of PMA eliciting maximal effects (300 nm) stimulated mucin secretion from EGTA-buffered SPOC1 cells at all Ca2+ activities tested, including those clearly sub-basal to normal resting Ca2+ (Fig. 5). PMA increased mucin release by ∼100% at low Ca2+ activities and up to ∼350% at 3 μm Ca2+. Using BAPTA as the buffer, however, reduced PMA-stimulated mucin secretion by 32–58%, relative to EGTA-buffered cells. Experiments using 10 mm EGTA and BAPTA instead of 3 mm yielded similar results (data not shown).
Figure 5. Effects of rapid Ca2+ buffering on PMA-induced mucin secretion from SPOC1 cells.
SLO-permeabilized cells were exposed to Ca2+ (0.01–10 μm) buffered by EGTA or BAPTA, ± 300 nm PMA. Mucin secretion was normalized to that released by 0.1 μm Ca2+ in the absence of PMA (for clarity, controls not shown; compare with Fig. 2C). Two-way ANOVA indicates a significant reduction by BAPTA, as well as a significant effect of Ca2+ with both buffers (n = 6 SPOC1 passages).
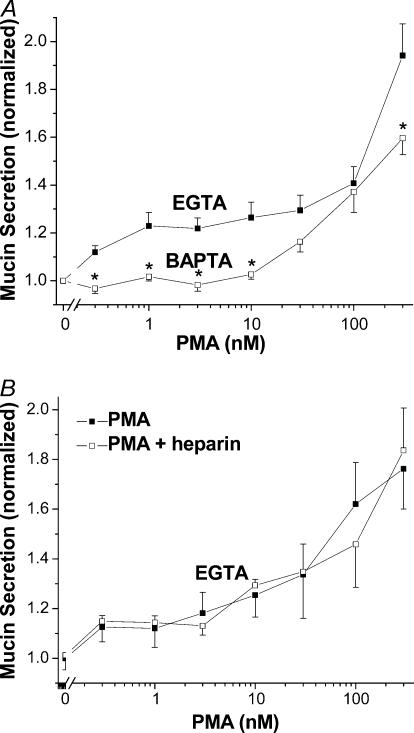
Recent studies from our laboratory identifying the P2Y2 agonist- and PMA-sensitive isoforms of PKC in SPOC1 cells suggested that the effects of PMA to stimulate mucin secretion at concentrations > 30 nm are independent of PKC (Abdullah et al. 2003). Hence, we compared the effects of EGTA and BAPTA buffering on concentration-dependent PMA stimulation of mucin secretion from SLO-permeabilized SPOC1 cells. Cells on 48-well cluster plates were buffered (0.1 μm free Ca2+) by EGTA on one half of a plate and by BAPTA on the other half, and incubated with 0.3–300 nm PMA. As shown in Fig. 6A, there was a biphasic response of EGTA-buffered SPOC1 cells to PMA. Mucin secretion was stimulated significantly over baseline between 1 and 30 nm PMA, the range within which the phorbol activates PKC (Abdullah et al. 2003). A much stronger degree of stimulation was observed for cells exposed to higher PMA concentrations, as previously shown (Abdullah et al. 1997). Mucin secretion elicited by PMA from BAPTA-buffered cells was inhibited significantly: at the lower PMA concentrations BAPTA abolished secretion and at higher concentrations it was diminished relative to EGTA-buffered cells.
Figure 6. Concentration effects of PMA in SLO-permeabilized SPOC1 cells.
A, cells were stimulated with 0.3–300 nm PMA at 0.1 μm Ca2+ in EGTA- or BAPTA-buffered solutions. Two-way ANOVA indicates a significant difference between EGTA and BAPTA (n = 6 SPOC1 passages). B, EGTA-buffered cells were stimulated with 0.3–300 nm PMA at 0.1 μm Ca2+ ± heparin (200 μg ml−1). No significant differences were detected (n = 4 SPOC1 passages).
To test whether IP3-mediated signalling might be responsible for the apparent Ca2+ dependency of PMA-stimulated secretion, we challenged SLO-permeabilized, EGTA-buffered SPOC1 cells with PMA in the presence of heparin. Figure 6B shows that heparin had no effect on the PMA response at any concentration (cf. Fig. 1), suggesting that the resulting secretion of mucin is stimulated by an IP3-independent mechanism.
Discussion
EGTA has been used for nearly a quarter of a century to control Ca2+ in permeabilized secretory cells (Baker & Knight, 1981; Bennett et al. 1981). Remarkably, it continues to be the chelator of choice nearly 20 years after the synthesis of BAPTA (Tsien, 1980), a chelator that binds Ca2+ nearly 2 orders of magnitude faster despite having a similar affinity (Smith et al. 1984; Harrison & Bers, 1987). Theoretically, these kinetics allow BAPTA, but not EGTA, to buffer Ca2+ effectively at the mouths of open Ca2+ channels, up to limits imposed by the diffusivity of the chelator (Neher, 1986; Stern, 1992; Bauer, 2001; Smith et al. 2001). For example, our calculations show that under the conditions of our experiments BAPTA buffers Ca2+ ∼7-fold closer to the channel than does EGTA (Fig. 2B). The faster Ca2+ binding kinetics of BAPTA have been exploited successfully to probe the generation of Ca2+ transients and/or local gradients that occur in exocytosis, using permeable acetoxymethylester analogues to load, or a micropipette or patch pipette to inject/dialyse, the buffer into the target cells. For example, BAPTA markedly attenuated transmitter release at the squid giant synapse (Adler et al. 1991), diminished the amplitude of Ca2+ transients in mouse spinal cord neurones (Tymianski et al. 1994), and inhibited glucose-induced insulin secretion in pancreatic β-cells (Pertusa et al. 1999), while EGTA either had smaller or no significant effect. We took advantage of BAPTA's ability to buffer Ca2+ in near membrane environments to reveal Ca2+ dependency in pathways controlling exocytosis in SPOC1 cells previously thought to be independent of Ca2+ (Scott et al. 1998).
Ca2+ plays a central role in triggering exocytosis in numerous secretory cells; however, the process is particularly poorly defined in airway goblet cells. A useful model is the pancreatic acinar cell where presentation of agonist to the basolateral membrane induces an IP3-dependent Ca2+ release selectively, within the secretory granule region in the apical pole to trigger zymogen granule exocytosis (Ito et al. 1997). Agonist-induced Ca2+ release in acinar cells is sensitive to heparin, indicating an involvement of IP3 (Thorn et al. 1993b), and dialysis of the cell with IP3 induces transient or oscillatory Ca2+ release from IP3-sensitive intracellular Ca2+ stores (Thorn et al. 1993a; reviewed by Berridge, 1993; Petersen, 1992). In goblet cells, IP3 releases Ca2+ from secretory granules (Nguyen et al. 1998).
IP3-generated Ca2+ gradients in SPOC1 cell
IP3 induced mucin secretion from permeabilized, EGTA-buffered SPOC1 cells in a heparin-sensitive manner (Fig. 1). The magnitude of IP3-induced mucin release was similar to that induced by 10 μm bulk Ca2+ levels. Hence, our results suggest that EGTA allows a local elevation in Ca2+ to micromolar levels following its IP3-mediated release from SPOC1 cell internal stores. This elevation is sufficiently robust and long-lived to initiate and trigger the exocytosis of mucin secretory granules. Mucin release elicited by IP3 in the presence of BAPTA was inhibited by 69–100% (Figs 3 and 4), compared to EGTA, suggesting that IP3 releases Ca2+ locally, generating Ca2+ gradients of a magnitude sufficient to trigger exocytosis. The differences in the mucin secretory response to IP3 by cells buffered with EGTA or BAPTA appear to be specific to their binding kinetics, and not to be due to non-specific effects of the buffers (Fig. 4).
Ca2+ dependency of PMA-induced mucin secretion in SPOC1 cells
PMA and PDBu are classical phorbol ester secretagogues used to activate conventional and novel isoforms of PKC. Although they are often used at, or near, micromolar concentrations, maximal activation of PKC typically occurs at 10–30 nm (Liles et al. 1987; Kazanietz et al. 1993). Mucin secretion from SPOC1 cells, however, is stimulated maximally by PMA at 300 nm (Abdullah et al. 1997); hence, this was the concentration used originally to test the Ca2+ independence of PMA effects on mucin release from permeabilized, EGTA-buffered cells (Scott et al. 1998; see Introduction). In the study of Fig. 5, the original observation that 300 nm PMA stimulates mucin release from SPOC1 cells over a range of 0.01–30 μm Ca2+ was duplicated successfully. When BAPTA was used as the Ca2+ buffer in paired experiments, however, mucin secretion was inhibited substantially indicating that, in fact, PMA-induced mucin secretion has a strong Ca2+ dependency. Hence, this result supports the notion that regulated exocytosis from SPOC1 cells is Ca2+ dependent, as suggested by the Ca2+ dependency of regulated exocytotic pathways, in general (see Chapman, 2002; Sudhof, 2002).
In recent years, proteins other than PKC have been shown to possess the C1 domain necessary to be an effective phorbol ester receptor (e.g. see Kazanietz, 2002). Considering that PKC translocation to the membrane saturates between 10 and 30 nm PMA in SPOC1 cells (Abdullah et al. 2003), the effects of higher phorbol ester levels in SPOC1 cells are clearly PKC independent and may be due to the obligate, C1 domain, exocytotic accessory protein, ubMUNC13-2 (Koch et al. 2000; Rhee et al. 2002) that is expressed in these cells. Notably, the PMA concentration–effect studies conducted in BAPTA-based buffers were consistent with this scenario (Fig. 6A): at concentrations below 30 nm the effects of PMA in SPOC1 cells buffered by BAPTA were blocked completely, whereas EGTA proved permissive. PMA concentrations > 30 nm effectively stimulated mucin secretion from BAPTA-buffered cells to levels similar to those elicited in EGTA, except for the highest PMA concentration (300 nm) where mucin release in BAPTA was inhibited slightly. We speculate that the stimulatory effects of low PMA levels (≤ 30 nm) in EGTA-buffered cells correspond to the activation of PKC, which most likely phosphorylates MARCKS to initiate the disruption of actin cortical microfilaments (Trifaro et al. 2000). The stimulation that occurs in both EGTA- and BAPTA-buffered cells at higher PMA concentrations most likely reflects diffusion distances that are very short, a scenario consistent with the activation of ubMUNC13-2 to prime mucin secretory granules docked at the plasma membrane (Brose et al. 2000; Martin, 2002). BAPTA's superior ability to buffer Ca2+ near channel openings might explain the generally diminished secretion in BAPTA-buffered cells, relative to EGTA-buffered cells, at high PMA concentrations (Fig. 6A). As illustrated in Fig. 2B, under the conditions of our experiments BAPTA is expected to buffer Ca2+ to below 1 μm beyond a ∼50 nm radius of channel openings. Within this radius, Ca2+ gradients exceeding 10 μm are expected to develop and are likely to activate Ca2+-dependent proteins. Small distances of this order are appropriate to the plasma and secretory granule membranes as they approach one another during the exocytotic process. Interestingly, these molecular distances may explain why secretion occurred in the face of rapid Ca2+ buffering by BAPTA during stimulation by both IP3 (Fig. 3) and PMA (Figs 5 and 6).
Chief among the unanswered questions in this scenario are the signal initiating Ca2+ release in PMA-treated SPOC1 cells and the source of the Ca2+. PMA activation of PKC in some cells results in a release of Ca2+ from internal stores through an undefined mechanism (e.g. see Xuan et al. 1994), and such could be the case for SPOC1 cells exposed to PMA concentrations ≤ 30 nm. The extra stimulatory effects of PMA at concentrations > 30 nm, however, indicates a PKC-independent mechanism (Abdullah et al. 2003), and the lack of inhibition of PMA-induced secretion by heparin (Fig. 6B) effectively excludes IP3 as the potential signalling molecule. The Ca2+ store could be cisternae of the endoplasmic reticulum located near mucin granules, as it is in gonadotrophs (Tse et al. 1997), or potentially more interestingly, it could be the mucin secretory granule itself (Nguyen et al. 1998; and see Petersen, 1996). A granule-based store raises the intriguing possibility that Ca2+ is released from the granule as it interacts physically with the plasma membrane during the pre-exocytotic events involved in granule docking, priming and formation of the exocytotic pore complex. Precedence for such a mechanism exists in yeast where Ca2+ is released from the lumen of the vacuole as it interacts with its exocytotic docking site to stimulate the final steps of vacuolar secretion (Peters & Mayer, 1998).
In conclusion, by virtue of its slow binding kinetics EGTA is permissive with respect to the generation of local Ca2+ gradients in permeabilized SPOC1 cells, whereas BAPTA is a much more efficient Ca2+ buffer and inhibits mucin secretion. Hence, the SPOC1 cell mucin secretory response to PMA we observed previously in permeabilized, EGTA-buffered cells and proposed to be independent of Ca2+ does, in fact, possesses a definite Ca2+ dependency. What remains to be tested rigorously for Ca2+ dependency in other secretory cells are the cAMP-induced responses proposed to be Ca2+ independent (Koh et al. 2000), as well as other studies in which measurements of whole cell Ca2+ and exocytosis failed to exhibit the correspondence expected for Ca2+-dependent events (e.g. Neher & Almers, 1986). A major problem that needs to be resolved with such studies is the measurement of local Ca2+ – because the studies above were based on the use of fluorescent Ca2+ indicators using wide-field epifluorescence microscopy, the small signal from local Ca2+ gradients that might have occurred could have been buried in the noise of the larger signal being recorded from the whole cell. Ca2+ sparks, for instance, were not observed until the advent of confocal microscopy (Cannell et al. 1994). Clearly, before such data can be accepted as indicating genuine Ca2+-independent exocytosis, the measurements need to be repeated using confocal microscopy and the appropriate controls.
Acknowledgments
The authors are grateful to Dr James Putney for fruitful discussions during the course of these studies, and for the funding of the project by a grant from the National Institutes of Health (HL-63756).
References
- Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol. 2003;285:L149–L160. doi: 10.1152/ajplung.00359.2002. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol. 1997;273:L201–L210. doi: 10.1152/ajplung.1997.273.1.L201. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem J. 1996;316:943–951. doi: 10.1042/bj3160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PF, Knight DE. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981;296:83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. The local Ca concentration profile in the vicinity of a Ca channel. Cell Biochem Biophys. 2001;35:49–61. doi: 10.1385/CBB:35:1:49. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Cockcroft S, Gomperts BD. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhao YH, Wu R. Differential regulation of airway mucin gene expression and mucin secretion by extracellular nucleotide triphosphates. Am J Respir Cell Mol Biol. 2001;25:409–417. doi: 10.1165/ajrcmb.25.4.4413. [DOI] [PubMed] [Google Scholar]
- Conway JD, Bartolotta T, Abdullah LH, Davis CW. Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts. Am J Physiol. 2003;284:L945–L954. doi: 10.1152/ajplung.00410.2002. [DOI] [PubMed] [Google Scholar]
- Davis CW, Dowell ML, Lethem M, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am J Physiol. 1992;262:C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- Dieter P, Fitzke E, Duyster J. BAPTA induces a decrease of intracellular free calcium and a translocation and inactivation of protein kinase C in macrophages. Biol Chem Hoppe Seyler. 1993;374:171–174. doi: 10.1515/bchm3.1993.374.1-6.171. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Sudhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51(Suppl. 1):S3–S11. doi: 10.2337/diabetes.51.2007.s3. [DOI] [PubMed] [Google Scholar]
- Gomperts BD, Barrowman MM, Cockcroft S. Dual role for guanine nucleotides in stimulus-secretion coupling. Fed Proc. 1986;45:2156–2161. [PubMed] [Google Scholar]
- Gomperts BD, Tatham PE. Regulated exocytotic secretion from permeabilized cells. Meth Enzymol. 1992;219:178–189. doi: 10.1016/0076-6879(92)19020-7. [DOI] [PubMed] [Google Scholar]
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Ann Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Bers DM. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987;925:133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Ito K, Miyashita Y, Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz MG. Novel ‘nonkinase’ phorbol ester receptors: the C1 domain connection. Mol Pharmacol. 2002;61:759–767. doi: 10.1124/mol.61.4.759. [DOI] [PubMed] [Google Scholar]
- Kazanietz MG, Areces LB, Bahador A, Mischak H, Goodnight J, Mushinski JF, Blumberg PM. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. Br J Pharmacol. 1991;103:1053–1056. doi: 10.1111/j.1476-5381.1991.tb12299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Moody MW, Nguyen TD, Hille B. Regulation of exocytosis by protein kinases and Ca2+ in pancreatic duct epithelial cells. J General Physiol. 2000;116:507–520. doi: 10.1085/jgp.116.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles WC, Meier KE, Henderson WR. Phorbol myristate acetate and the calcium ionophore A23187 synergistically induce release of LTB4 by human neutrophils: involvement of protein kinase C activation in regulation of the 5-lipoxygenase pathway. J Immunol. 1987;138:3396–3402. [PubMed] [Google Scholar]
- Martin TF. Prime movers of synaptic vesicle exocytosis. Neuron. 2002;34:9–12. doi: 10.1016/s0896-6273(02)00651-7. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Smith GL. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984;246:C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Neher E. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. In: Heinemann U, editor. Calcium Electrogenesis and Neuronal Functioning. Berlin: Springer-Verlag; 1986. pp. 80–96. [Google Scholar]
- Neher E, Almers W. Fast calcium transients in rat peritoneal mast cells are not sufficient to trigger exocytosis. EMBO J. 1986;5:51–53. doi: 10.1002/j.1460-2075.1986.tb04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Chin WC, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+ Nature. 1998;395:908–912. doi: 10.1038/27686. [DOI] [PubMed] [Google Scholar]
- Pertusa JA, Sanchez-Andres JV, Martin F, Soria B. Effects of calcium buffering on glucose-induced insulin release in mouse pancreatic islets: an approximation to the calcium sensor. J Physiol. 1999;520:473–483. doi: 10.1111/j.1469-7793.1999.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Petersen OH. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH. Can Ca2+ be released from secretory granules or synaptic vesicles? Trends Neurosci. 1996;19:411–413. [PubMed] [Google Scholar]
- Randell SH, Liu JY, Ferriola PC, Kaartinen L, Doherty MM, Davis CW, Nettesheim P. Mucin production by SPOC1 cells – an immortalized rat tracheal epithelial cell line. Am J Respir Cell Mol Biol. 1996;14:146–154. doi: 10.1165/ajrcmb.14.2.8630264. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Richardson A, Taylor CW. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J Biol Chem. 1993;268:11528–11533. [PubMed] [Google Scholar]
- Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Scott CE, Abdullah LH, Davis CW. Ca2+ and protein kinase C activation of mucin granule exocytosis in permeabilized SPOC1 cells. Am J Physiol. 1998;275:C285–C292. doi: 10.1152/ajpcell.1998.275.1.C285. [DOI] [PubMed] [Google Scholar]
- Smith GD, Dailey LA, Miyakawa T, Sherman P. Asymptotic analysis of buffered calcium diffusion near a point source. SIAM J Appl Math. 2001;61:1816–1838. [Google Scholar]
- Smith PD, Liesegang GW, Berger RL, Czerlinski G, Podolsky RJ. A stopped-flow investigation of calcium ion binding by ethylene glycol bis (beta-aminoethyl ether)-N,N′-tetraacetic acid. Anal Biochem. 1984;143:188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993a;14:746–757. doi: 10.1016/0143-4160(93)90100-k. [DOI] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993b;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Rose SD, Lejen T, Elzagallaai A. Two pathways control chromaffin cell cortical F-actin dynamics during exocytosis. Biochimie. 2000;82:339–352. doi: 10.1016/s0300-9084(00)00193-0. [DOI] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B, Horstmann H, Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Properties of neuroprotective cell-permeant Ca2+ chelators: effects on [Ca2+]i and glutamate neurotoxicity in vitro. J Neurophysiol. 1994;72:1973–1992. doi: 10.1152/jn.1994.72.4.1973. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Luo SG. Differences among type I, II, and III inositol-1,4,5-trisphosphate receptors in ligand-binding affinity influence the sensitivity of calcium stores to inositol-1,4,5-trisphosphate. Mol Pharmacol. 1998;53:656–662. doi: 10.1124/mol.53.4.656. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Wang OL, Whorton AR. Regulation of endothelin-induced Ca2+ mobilization in smooth muscle cells by protein kinase C. Am J Physiol. 1994;266:C1560–C1567. doi: 10.1152/ajpcell.1994.266.6.C1560. [DOI] [PubMed] [Google Scholar]