Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) are widely distributed within the brain where they contribute to the regulation of higher cognitive functions. The loss of the cholinergic function in Alzheimer's disease patients, along with the well-known memory enhancing effect of nicotine, emphasizes the role of cholinergic signalling in memory functions. The hippocampus, a key structure in learning and memory, is endowed with nAChRs localized at pre- and postsynaptic levels. In previous work on the immature hippocampus we have shown that, at low probability (P) synapses, activation of α7 nAChRs by nicotine or by endogenously released acetylcholine persistently enhanced glutamate release and converted ‘presynaptically silent’ synapses into functional ones. Here we show that in the same preparation, at high P synapses, nicotine induces long-term depression of AMPA- and NMDA-mediated synaptic currents. This effect was mediated by presynaptic α7- and β2-containing receptors and was associated with an increase in the paired pulse ratio and in the coefficient of variation. High P synapses could be converted into low P and vice versa by changing the extracellular Ca2+/Mg2+ ratio. In these conditions nicotine was able to persistently potentiate or depress synaptic responses depending on the initial P-values. A bi-directional control of synaptic plasticity by nicotine would considerably enhance the computational properties of the network during a critical period of postnatal development thus contributing to sculpt the neuronal circuit.
Exposure to nicotine, the neuroactive component of tobacco, improves attention, learning and memory processes in both experimental animals and humans (Rezvani & Levin, 2001). These effects are mediated by selective nicotine acetylcholine receptors (nAChRs) which are widely expressed in the CNS (Tribollet et al. 2004). The loss of cholinergic signalling in individuals affected by Alzheimer's disease is associated with a loss of high cognitive functions including memory (Paterson & Nordberg, 2000).
The hippocampus, a key structure in learning and memory processes, receives a large cholinergic innervation from the septo-hippocampal pathway (Kasa, 1986). Moreover, this structure is enriched in nAChRs, in particular of homomeric α7 and heteromeric α4β2 subtypes (Alkondon & Albuquerque, 1993; Sudweeks & Yakel, 2000; Fabian-Fine et al. 2001; Tribollet et al. 2004). Postsynaptic nAChRs localized mainly on GABAergic interneurones mediate rapidly desensitizing nicotine currents (Jones & Yakel, 1997; Frazier et al. 1998). Presynaptic nAChRs present on both GABAergic and glutamatergic terminals regulate transmitter release (Gray et al. 1996; Alkondon et al. 1997; Radcliffe & Dani, 1998; Alkondon et al. 1999; Alkondon & Albuquerque, 2001; Maggi et al. 2001). Modulation of transmitter release is ensured by calcium entry through nAChRs (Gray et al. 1996; Lena & Changeux, 1997) and/or voltage-dependent calcium channels activated by the depolarizing action of nicotine (Rathouz & Berg, 1994). Calcium may in turn trigger a variety of signal transduction processes leading to changes in neuronal excitability and synaptic efficacy.
In previous work on hippocampal slices from newborn rats, focused on silent or low probability (P) Schaffer collateral–CA1 synapses, we have shown that a brief application of nicotine enhanced synaptic efficacy and converted ‘presynaptically silent’ synapses into conductive ones in a persistent way. This effect was mediated by presynaptic α7 nAChRs and was mimicked by endogenous acetylcholine released from cholinergic fibres (Maggi et al. 2003).
Here we show that, in the same preparation from newborn animals, application of nicotine to high P synapses induces a persistent reduction of synaptic efficacy, an effect that is mediated by homomeric α7- and heteromeric β2-containing nAChRs localized on glutamatergic terminals.
Methods
Slice preparation
Transverse hippocampal slices (300–400 μm thick) from P1–P7 Wistar rats were prepared as previously described (Maggi et al. 2003). The procedure was in accordance with the regulations of the Italian Animal Welfare Act and was approved by the local authority veterinary service. Briefly, animals were decapitated after being anaesthetized with an i.p. injection of urethane (2 g kg−1). The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 130, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, MgCl2 1.3, CaCl2 2, glucose 25, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). After 1 h, an individual slice was transferred to the recording chamber where it was continuously superfused at 33°C with oxygenated ACSF at a rate of 2–3 ml min−1.
Electrophysiological recordings
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated excitatory post synaptic currents (EPSCs) evoked by minimal stimulation of the Schaffer collateral were recorded at −60 mV from individual CA1 pyramidal neurones using the patch clamp technique in the whole cell configuration. We routinely started our recordings at least 5 min after breaking into the whole cell configuration when synaptic responses were stable. Patch electrodes, formed from thin borosilicate glass (Hilgenberg, Malsfeld, Germany) had a resistance of 4−6 MΩ when filled with an intracellular solution containing (mm): caesium methanesulphonate 125, CsCl 10, Hepes 10, EGTA 0.6–2, MgATP 2, NaGTP 0.3, QX-314. Bicuculline methiodide (5 μm) was routinely added to the bath solution to block GABAA receptors. In some experiments (n = 28) a low concentration of tetrodotoxin (TTX, 10 nm) was added to reduce polysynaptic activity. Schaffer collaterals were stimulated with bipolar twisted NiCr-insulated electrodes placed in stratum radiatum. The stimulus intensity was adjusted to evoke minimal EPSCs that were often intermingled with transmission failures. Transmitter failures were estimated by visual discrimination. Usually paired pulses (50 ms interval, duration 100 μs) were used to test for paired-pulse facilitation or depression. In some experiments, two converging inputs to the same pyramidal cell were alternately activated (every 2 s). The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiments. Cells exhibiting 15–20% changes were excluded from the analysis.
N-Methyl-d-aspartate (NMDA)-mediated EPSCs were recorded at +40 mV in the presence of DNQX (10 μm) and bicuculline (5 μm) to block AMPA and GABAA receptors, respectively. Additional experiments were performed with patch pipettes containing the calcium chelators 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 20 mm, purchased from Sigma, Milan, Italy).
In another set of experiments, the effect of nicotine was tested on field EPSPs evoked by pair of stimuli (50 ms interval, 0.1 Hz frequency) delivered to the Schaffer collaterals in the stratum radiatum. Field EPSPs were recorded with a glass microelectrode filled with the extracellular solution in the presence of α-3-(2-carboxy-piperazin-4-yl)-propyl-1-phosphonic acid (CPP) and bicuculline to block NMDA and GABAA receptors, respectively.
Drugs were applied to the bath via a three-way tap system. Drugs used were: tetrodotoxin (TTX, Affinity Research Products, Exeter, UK); bicuculline methiodide (RS)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), 6,7-dinitroquinoxaline-2,3-dione (DNQX) purchased from Tocris Cookson, Bristol, UK; nicotine, α-bungarotoxin (α-BGT), dihydro-β-erythtroidine (DHβE), purchased from Sigma, Milan, Italy; QX-314 purchased from Alomone Labs (Jerusalem, Israel).
If not otherwise stated, data are expressed as means ±s.e.m. Statistical comparisons were made with the use of paired t test (P < 0.05 was taken as significant).
Data acquisition and analysis
Data were acquired with LTP114 software package (courtesy of W. W. Anderson, Bristol University, UK). Current signals were transferred to a computer after digitization with an A/D converter (Digidata 1200, Axon Instruments, Union City, CA, USA). Data were sampled at 10 kHz, filtered with a cut-off frequency of 2 kHz and analysed off-line either with LTP114 and/or Clampfit 8.1 Program (Axon Instruments).
Results
Nicotine increases or decreases glutamate release in low and high probability synapses, respectively
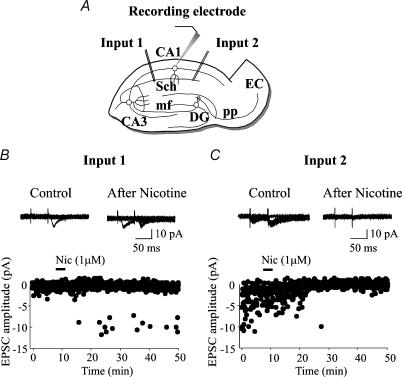
EPSCs were elicited in CA1 pyramidal cells at −60 mV by minimal stimulation of the Schaffer collaterals (at 0.25 Hz). In the majority of the experiments two pulses delivered at 50 ms interval were used to determine paired-pulse facilitation or depression. EPSCs were mediated by glutamate acting on AMPA receptor types since they were completely suppressed by DNQX (10 μm, n = 4; data not shown). As shown in the schematic diagram of Fig. 1A, two different synaptic inputs (input 1 and input 2) converging into the same neurone, were alternately stimulated with paired stimuli. Representative samples of EPSCs evoked by input 1 and input 2 before and after application of nicotine are represented in the insets of Fig. 1B andC. In control conditions, stimulation of input 1 (Fig. 1B) failed to induce any synaptic response over 100 consecutive trials. The probability (P) of obtaining a success was close to 0. However, occasional responses occurred to the second stimulus suggesting that this synapse was ‘presynaptically silent’ (Gasparini et al. 2000; Maggi et al. 2003). The presence of successes to the second pulse largely depends on the presynaptic increase in release probability. Nicotine (1 μm, a concentration close to the value present in the smoker's blood immediately after smoking a cigarette; Dani & Heinemann, 1996) applied in the bath for 3 min, induced the appearance of responses to the first stimulus and increased the number of successes to the second one (Fig. 1B). Interestingly, this effect persisted for at least 40 min after nicotine application. In contrast to input 1, input 2 was not silent, but exhibited several successes intermingled with failures. In this case, nicotine (1 μm for 3 min) produced a progressive depression of the amplitude of synaptic currents (Fig. 1C). After 40 min, only small amplitude currents could be detected. The depressant effects of nicotine on synaptic responses were not due to run down of the currents with time. In fact, in contrast with the present results, a run down should have affected both inputs in the same way. No changes in the holding current or input conductance were observed during or after nicotine-induced potentiation or depression. Similar observations were made in five cells.
Figure 1. Nicotine enhances or decreases synaptic efficacy at connections with different initial release probability.
Two inputs (input 1 and input 2) converging into the same CA1 pyramidal cell were alternately activated (pair of stimuli at 50 ms interval) at 0.25 Hz. AMPA-mediated EPSCs were recorded at −60 mV. A, schematic diagram showing localization of stimulating and recording electrodes. Sch, Schaffer collaterals; mf, mossy fibre; pp, perforant path; DG, dendate gyrus; EC, entorhinal cortex. B, time course of the amplitude of the EPSCs evoked before and after nicotine application (bar). Input 1: synapse presynaptically silent; in control conditions only response failures were detected to the first stimulus and few responses to the second one. Application of nicotine (bar) induced the appearance of synaptic currents that persisted for at least 30 min after nicotine application. Insets represent 10 consecutive traces recorded in control and 20 min after nicotine. C, input 2: synapse with high initial release probability. Nicotine (bar) strongly increased the number of transmitter failures and reduced the amplitude of the first and second EPSC. Insets represent 10 consecutive traces recorded in control and 20 min after nicotine.
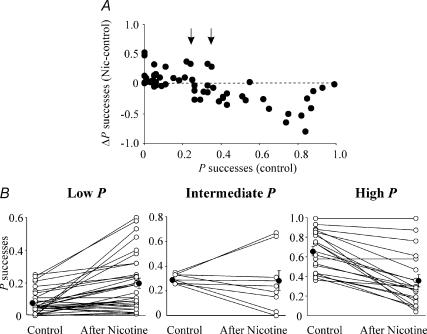
On 59 synaptic inputs (from 46 cells), nicotine differently affected the probability of glutamate release. In some neurones it increased P whereas in others it decreased P. In Fig. 2A, the difference between the probability of successes obtained after nicotine application and that obtained in control (ΔP successes (Nic-control); ordinate), is plotted against the initial probability of successes (P successes (control); abscissa). It is clear from the graph that synaptic responses with initial P below 0.25 exhibited ΔP successes (Nic-control) equal to or above 0 while synaptic responses with initial P above 0.35 exhibited ΔP successes (Nic-control) below 0. Therefore, data points falling above the dashed line refer to EPSCs in which P increased after nicotine whereas those below refer to EPSCs in which P decreased after nicotine. Responses falling between the two arrows refer to those in which nicotine produced either a potentiation or a depression. The correlation coefficient for the plot of Fig. 2A was 0.66 (p < 0.00001). The three groups (arbitrarily divided into low, intermediate and high P) are reported separately on Fig. 2B in which open symbols represent individual P-values obtained before and after nicotine and filled symbols respective average values. While previous work has been focused on the effects of nicotine on low P synapses (Maggi et al. 2003), the present one concerns high P synapses (P > 0.35).
Figure 2. Nicotine-induced changes in success rate vary according to the initial release probability of the synapses under examination.
A, the difference between the probability of successes obtained 20 min after nicotine application and that obtained in control (ΔP successes (Nic-control); ordinate; n = 59) is plotted against the initial probability of successes (P successes (control); abscissa). It is clear from the graph that synaptic responses with initial P below 0.25 exhibit ΔP successes (Nic-control) equal to or above 0 while synaptic responses with initial P above 0.35 exhibited ΔP successes (Nic-control) below 0. An intermediate group with initial P between 0.25 and 0.35 (marked by two arrows) exhibit ΔP successes (Nic-control) above and below 0. The correlation coefficient for the plot is 0.66 (p < 0.00001). B, the three groups in A are represented separately. Open symbols represent individual P-values before (control) and 20 min after nicotine application while filled symbols represent average P-values. In this and the following figures error bars refer to s.e.m.
Nicotine persistently depresses synapses with high P
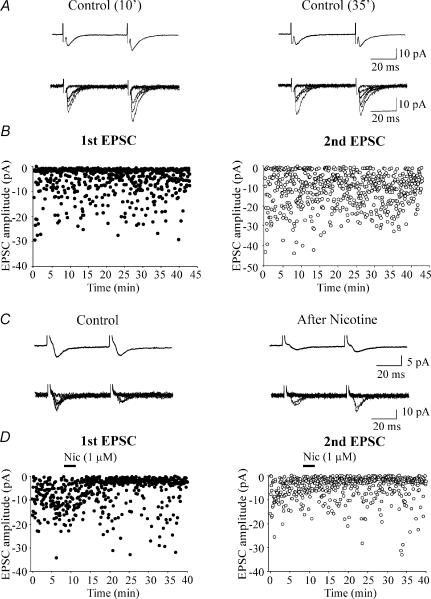
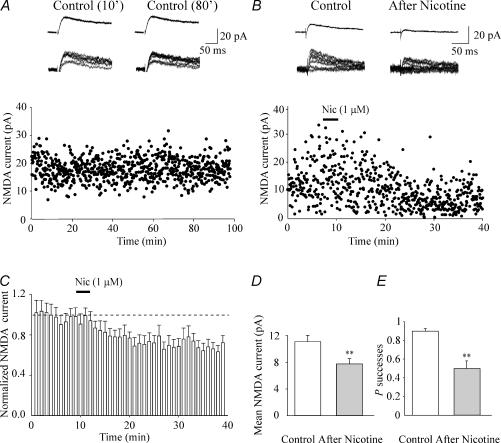
Traces of Fig. 3C represent average responses (upper) and superimposed individual examples of successes and failures (lower) recorded in control conditions and after nicotine application from a cell with high P (initial P = 0.74). It is clear from Fig. 3C and D that nicotine increased the number of transmitter failures to the first and the second pulse in a paired-pulse protocol. This led to a mean reduction of EPSC amplitude (from 7.8 ± 0.8 pA to 1.9 ± 0.5 pA and from 4.2 ± 0.8 pA to 2.1 ± 0.4 pA, for the first and second EPSC, respectively). As expected, nicotine-induced reduction in P was associated with an increase in the paired pulse ratio (see average traces of Fig. 3C). The long-lasting effects of nicotine on synaptic efficacy were not due to run down of synaptic responses with time since in the absence of the drug, EPSCs remained stable without decrement in their amplitude for long period of time (30–40 min; n = 4, Fig. 3Aand B). Summary data for 13 cells in which nicotine effects were followed for at least 20 min are represented in Fig. 4A. Here the mean peak amplitude of the first EPSCs, recorded every minute (columns) and normalized to control values, is plotted against time. The dashed line represents the mean amplitude of EPSCs in control before nicotine application. In all cases, the depression induced by nicotine was long lasting without changes in holding current or input conductance. Overall, in 20 cells nicotine produced a significant reduction in the amplitude of the first and second EPSCs (the amplitude of the first and second EPSC, measured 20 min after nicotine application was 57.1 ± 5.7% and 54.5 ± 4.7% of control values, respectively; p < 0.001). This was associated with a significant increase in failure rate (from 0.35 ± 0.05 and 0.34 ± 0.05 to 0.64 ± 0.06 and 0.62 ± 0.05 for the first and second EPSC, respectively; p < 0.01).
Figure 3. At high probability synapses nicotine persistently reduces synaptic efficacy.
A, average (upper) and six superimposed individual traces (lower) evoked by a pair of stimuli (50 ms a part) delivered to the Schaffer collateral 10 min (left) and 35 min (right) after the beginning of the recording. In this and the following figures, average traces include successes and failures. B, time course of the amplitude of the first (filled circles) and second (open circles) EPSCs evoked in control conditions (in the absence of nicotine application). Note the stability of synaptic currents without decrement throughout the experiment. C, average (upper) and eight superimposed individual traces (lower) evoked before (left) and 20 min after nicotine (right). Note increase in failure rate and decrease in EPSC amplitude after nicotine. D, time course of the amplitude of the first (filled circles) and second (open circles) EPSCs evoked before and after nicotine application (bar).
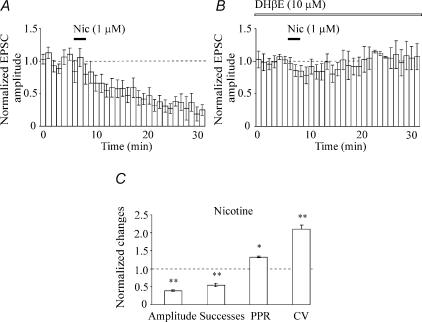
Figure 4. The broad-spectrum nicotine receptor antagonist DHβE prevents nicotine-induced long-term depression.
A, average time course (13 cells) of the amplitude of AMPA-mediated EPSCs normalized to their control (dashed line) before and after nicotine (bar). B, bath application of DHβE (10 μm; open bar) prevents the action of nicotine (filled bar) on AMPA-mediated EPSCs (normalized to their controls, dashed line; n = 6). In A and B, each bin is 1 min. C, each column represents nicotine-induced changes (normalized to control values, dotted line) in the amplitude, success rate, paired pulse ratio (PPR) and coefficient of variation (CV) for 20 cells. The increase in CV and PPR suggest a presynaptic site of action of nicotine-induced synaptic depression. *p < 0.05, **p < 0.01.
The depressant effect of nicotine was mediated by nAChRs since it was prevented by DHβE (10 μm) known to block at these concentrations most of nAChR subtypes (n = 8; Bertrand et al. 1992; Fig. 4B). These data further suggest that nicotine-induced long-lasting depression of the EPSCs was not due to run down of synaptic currents.
Nicotine depresses AMPA-mediated EPSCs by acting on a presynaptic site
Additional experiments were performed to elucidate the locus of expression of nicotine-induced long-term depression in high P synapses. Firstly, we measured changes in paired pulse ratio (PPR) and in coefficient of variation (CV) of response amplitude. Nicotine induced a significant increase in PPR (from 1.20 ± 0.13 to 1.57 ± 0.19; p < 0.05; n = 19) and in the CV (from 0.93 ± 0.07 to 1.95 ± 0.25; p < 0.001; n = 19), suggesting a presynaptic site of action (Fig. 4C). It is worth noting that control data include cells either with initial paired-pulse facilitation (n = 11) or depression (n = 8).
Secondly, we examined the effects of nicotine on NMDA-mediated EPSCs. If nicotine reduces glutamate release by acting on presynaptic nAChRs, it should affect both AMPA and NMDA receptor-mediated responses. In the hippocampus these two receptors are colocalized (Bekkers & Stevens, 1989). Therefore in the following experiments the effects of nicotine were tested on NMDA receptor-mediated EPSCs. Individual NMDA-mediated EPSCs were elicited at +40 mV by stimulation of the Schaffer collateral in the presence of DNQX (20 μm) and bicuculline (5 μm) to block AMPA and GABAA receptors, respectively. As in the case of AMPA, nicotine (1 μm for 3 min) induced a persistent depression of NMDA-mediated EPSCs. As shown in the example of Fig. 5B, application of nicotine induced a long-lasting change in the amplitude of synaptic responses associated with an increase in failure rate. Insets above the graph show superimposed individual traces and average responses (including failures). On average, nicotine significantly reduced the peak amplitude of NMDA-mediated synaptic currents from 11.1 ± 0.9 pA to 7.8 ± 0.8 pA; p < 0.01 Fig. 5D. This effect was associated with a significant reduction of successes rate (from 0.89 ± 0.03 to 0.54 ± 0.09; p < 0.01; Fig. 5E). Nicotine-induced depression of NMDA receptor-mediated EPSCs was long lasting as shown in the summary graph of Fig. 5C. However, in comparison with AMPA-mediated responses the effect of nicotine on the amplitude NMDA-mediated EPSCs was less pronounced (30% in the case of NMDA while 57% in the case of AMPA responses). The higher affinity of glutamate for NMDA receptors may account for this difference (Patneau & Mayer, 1990). However, we cannot exclude that at +40 mV, the membrane potential at which NMDA receptor-mediated currents have been studied, a potentiation may occur through calcium entry via NMDA receptor channels (Voronin et al. 2004). This would partially counterbalance nicotine-induced synaptic depression. To test for this possibility additional experiments were performed using BAPTA (20 mm) in the patch pipette. In the presence of intracellular BAPTA, nicotine was still able to persistently depress NMDA receptor-mediated responses (on average from 17.6 ± 1.8 pA to 13.3 ± 1.8 pA; n = 4; data not shown). Similarly, nicotine was still able to depress AMPA receptor-mediated responses in cells dialysed with intracellular BAPTA (20 mm; from 14.5 ± 6.1 pA to 6.3 ± 3.3 pA; n = 4). This suggests that the entry of calcium into the postsynaptic cell does not contribute to nicotine-induced long-term depression. As for AMPA-mediated EPSCs, in the absence of nicotine, NMDA-mediated synaptic currents remained stable without decrement in their amplitude with time, suggesting that run down was not involved in the observed effects (n = 3; Fig. 5A).
Figure 5. Nicotine induces a persistent reduction in amplitude of NMDA-mediated EPSCs.
A, NMDA-mediated EPSCs evoked at +40 mV by minimal stimulation of the Schaffer collaterals, in the presence of DNQX (20 μm) and bicuculline (5 μm). Average traces (upper) and 10 consecutive superimposed traces (lower) obtained 10 min (left) and 80 min (right) after the beginning of the recording are shown in the insets. Note the stability of the recordings without decrement of NMDA-mediated synaptic currents with time. B, NMDA-mediated EPSCs evoked at +40 mV by minimal stimulation of the Schaffer collaterals, before, during (filled bar) and after nicotine. Note that nicotine induced a persistent depression of synaptic currents (in the insets average traces and 10 consecutive superimposed traces obtained in control and 20 min after nicotine). C, average time course of the amplitude of NMDA-mediated EPSCs normalized to their control (dashed line) before and after nicotine (bar). Each bin is 1 min (n = 11). D, mean amplitude of NMDA-mediated EPSCs (successes plus failures) before and after nicotine (n = 11). E, average success rate of NMDA-mediated EPSCs in control and after nicotine application (n = 11). **p < 0.01.
Both α7- and β2-containing nAChRs contribute to nicotine-induced depression of the EPSCs
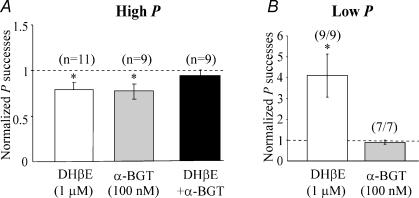
In order to clarify nAChR subtypes involved in nicotine-induced persistent depression of glutamate release, additional experiments were performed using selective nAChRs antagonists. In line with previous data (Maggi et al. 2003), in low P synapses α-BGT (100 nm), a selective α7 antagonist (Couturier et al. 1990), blocked nicotine-induced potentiation (n = 7/7) while DHβE (1 μm), known at this concentration to selectively block β2-containing receptors (Zoli et al. 1998), was ineffective (n = 9/9; see Fig. 6B).
Figure 6. The depressant effect of nicotine on EPSCs is mediated by α7 and β2-containing nAChRs.
A, in high P (> 0.35) synapses nicotine-induced changes in the number of successes (to the first pulse) obtained in the presence DHβE (1 μm, white column), α-BGT (100 nm, grey column) or DHβE (1 μm) plus α-BGT (100 nm, black column) are normalized to the control values (dashed line). Note that in the presence of DHβE plus α-BGT nicotine did not modify the probability of successes. B, in low P (< 0.25) synapses nicotine-induced changes in the number of successes (to the first pulse) obtained in the presence DHβE (1 μm, white column) or α-BGT (100 nm, grey column) are normalized to the control values (dashed line). Note that in low P synapses the potentiating effect of nicotine was mediated by α7 receptor subtypes. *p < 0.05.
In high P synapses, DHβE (1 μm) prevented the depressant effects of nicotine in 7 out of 11 cells while in the remaining 4 was ineffective. Overall, in the presence of DHβE, nicotine induced a significant (p < 0.05) reduction in the probability of successes (of 21.8 ± 8.6%) associated with a depression of EPSC amplitude (of 33.9 ± 9.7%, Fig. 6A; n = 11). α-BGT (100 nm) failed to block nicotine-induce depression in 6 out of 9 neurones (P of successes changed from 0.58 ± 0.08 to 0.38 ± 0.08; p < 0.01) while in the remaining three it prevented nicotine action. Overall, in the presence of α-BGT nicotine induced a significant (p < 0.05) reduction in the probability of successes (of 23.5 ± 8.4%) accompanied by a reduction of EPSC amplitude (of 31.5 ± 10%, Fig. 6A; n = 9). Bath application of DHβE (1 μm) together with α-BGT (100 nm) completely prevented nicotine effects on high P synapses (n = 9; Fig. 5A). These data suggest that both α7- and β2-nACh-containing receptors contribute to nicotine action. Therefore, while in agreement with a previous study (Maggi et al. 2003) at low P synapses the potentiating effect of nicotine was clearly mediated by α7 nAChRs, the depressant effect observed at high P synapses was mediated by β2 and α7 nAChRs.
Nicotine-induced persistent potentiation or depression of EPSCs depends on the initial release probability
To evaluate the dependence of nicotine effect on the initial release probability, we manipulated P by changing the extracellular Ca2+/Mg2+ concentration ([Ca2+/Mg2+]o).
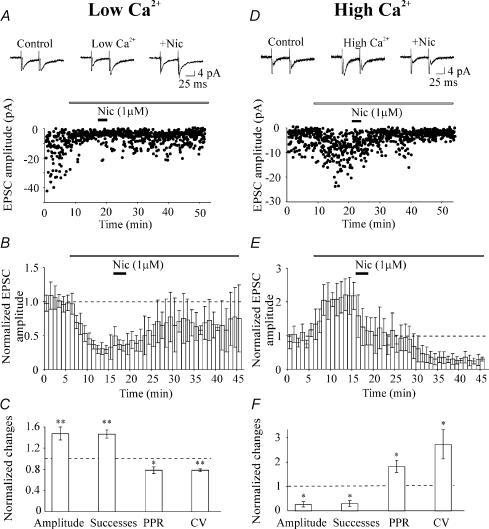
In a first set of experiments on high P synapses, nicotine was applied after P was reduced by diminishing the [Ca2+/Mg2+]o from 2/1.3 mm to 1/2 mm. This produced a change in P from 0.65 ± 0.10 to 0.26 ± 0.05 (p < 0.01; n = 7; see Fig. 7A and B). This effect was associated with an increase in PPR (from 1.43 ± 0.23 to 2.20 ± 0.12; p < 0.05; n = 7) and in the CV (from 1.07 ± 0.16 to 2.12 ± 0.29; p < 0.01; n = 7). Subsequent application of nicotine produced a significant (p < 0.01) and persistent increase in successes rate (of 46.7 ± 7.7%) and in EPSC amplitude (of 47.6 ± 12.4%) that was associated with a significant decrease in PPR (from 2.20 ± 0.12 to 1.69 ± 0.12; p < 0.05; n = 7) and CV (from 2.12 ± 0.29 to 1.66 ± 0.24; p < 0.01; n = 7; Fig. 7C).
Figure 7. Nicotine action depends on the initial P.
Time course of the amplitude of the first EPSC (filled circles) evoked in normal (2 mm calcium, 1.3 mm magnesium) and after switching to a low calcium containing solution (1 mm calcium, 2 mm magnesium, open bar). Note increase in failure rate and depression of EPSC amplitude in low Ca2+ (see average traces in the insets). Further application of nicotine (filled bar) produced a persistent increase in success rate and in EPSC amplitude (see insets). B, average time course of the effects of nicotine (filled bar) on EPSCs amplitude (normalized to their controls, dashed line) before and after switching to a low calcium-containing solution (open bar). Each bin is 1 min (n = 7). C, each column represents nicotine-induced changes in amplitude (including failures), successes rate, PPR, and CV, normalized to the pre-nicotine low extracellular Ca2+ concentration condition (dashed line; n = 7). D, time course of the amplitude of the first EPSC (filled circles) evoked in normal calcium and after switching to a high calcium-containing solution (4 mm calcium, 1 mm magnesium, open bar). Note decrease in failure rate and increase in EPSC amplitude in high Ca2+ (see average traces in the insets). Further application of nicotine (filled bar) produced a persistent decrease in success rate and in EPSC amplitude (see insets). E, average time course of the effects of nicotine (filled bar) on EPSCs amplitude (normalized to their controls, dashed line) before and after switching to a high calcium-containing solution (open bar). Each bin is 1 min (n = 5). F, each column represents nicotine-induced changes in amplitude (including failures), successes rate, PPR, and CV, normalized to pre-nicotine high extracellular Ca2+ concentration condition (dashed line; n = 5). *p < 0.05; **p < 0.01.
In a second series of experiments on low P synapses, P was increased by raising the [Ca2+/Mg2+]o from 2/1.3 mm to 4/1 mm. In this condition, the percentage of successes and the EPSC amplitude increased from 0.28 and 2.8 pA to 0.82 and 10.2 pA, respectively (see example of Fig. 7D). Overall in high calcium–low magnesium solution, P changed from 0.28 ± 0.04 to 0.56 ± 0.12 (p < 0.05; n = 5; Fig. 7D and E). This effect was associated with a reduction in the PPR and in the CV (from 1.48 ± 0.13 to 0.92 ± 0.18; p < 0.05; n = 5). Subsequent application of nicotine caused a reduction of successes rate (of 70.5 ± 12.5%; p < 0.05; n = 5) and a depression of EPSC amplitude (of 73.8 ± 10.9%; p < 0.05; n = 5; Fig. 7F). These were accompanied with an increase in both PPR and CV (from 0.92 ± 0.18 and 1.16 ± 0.32 to 1.63 ± 0.25 and 2.28 ± 0.49, respectively; p < 0.05 in both cases; n = 5; Fig. 7F). These experiments indicate that the direction of nicotine action strictly depends on the initial value of P.
In the presence of α-BGT, nicotine persistently depresses the amplitude of the field EPSPs
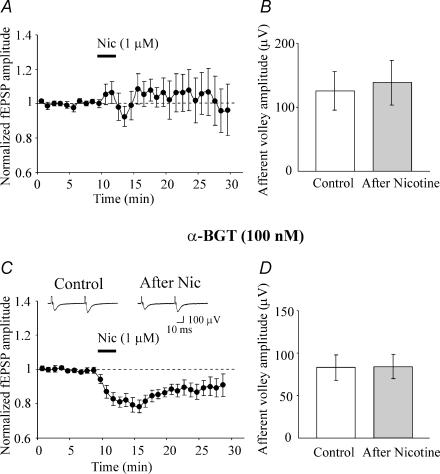
In order to see whether nicotine effects were still present on neurones non-dialysed with the intracellular solution (as in whole cell recordings), the effects of nicotine were tested on field EPSPs. These were recorded in the presence of bicuculline (5 μm) and CPP (50 μm) to block GABAA and NMDA receptors, respectively. Application of nicotine (1 μm for 3 min) produced either a potentiation (40.9 ± 9.7%; n = 4/10 slices) or a depression (44.9 ± 17.9%; n = 4/10) of the field EPSPs. In two slices, the amplitude of the field EPSPs was unaffected by nicotine. Summary data for all 10 slices are represented in Fig. 8A. On average nicotine did not change the amplitude of the field EPSPs but increased the variability of the responses as indicated by the large fluctuations in the error bars. Nicotine application did not modify presynaptic cell excitability since the amplitude of the afferent volleys was not affected (Fig. 8B). In a second set of experiments the effects of nicotine were tested on field EPSPs recorded in the presence of α-BGT (100 nm) which is known to prevent the potentiating effect of nicotine (Maggi et al. 2003). In these experimental conditions nicotine induced a depression of the field EPSPs in 12/14 slices. In the remaining two slices nicotine had no effect. As shown in the summary data from the 12 slices in which nicotine was effective, a significant (p < 0.01) depression of 25.2 ± 2.3% was observed that persisted for more than 30 min (Fig. 8C). In the presence of α-BGT, the amplitude of the afferent volley was similar before and after nicotine application (Fig. 8D) indicating that changes in presynaptic excitability do not account for nicotine-induced short or long-term depression.
Figure 8. In the presence of α-BGT, nicotine depresses the field EPSPs.
A, mean amplitude of field EPSPs (normalized to control, dashed line) recorded before and after nicotine application (bar). Each point represents the average of 6 responses evoked every 10 s in 10 different slices. B, mean amplitude of the afferent volley of the first field EPSP, recorded before (control, white column) and after nicotine application (grey column; n = 3). C, mean amplitude of field EPSPs (normalized to control, dashed line) recorded before and after nicotine application (bar) in the presence of α-BGT (100 nm) from the 12 slices in which nicotine was effective. Note that nicotine induced a significant depression in the amplitude of the field EPSP (p < 0.01). Insets above the graphs represent average traces (n = 100) from a representative slice recorded before (left) and 20 min after nicotine application (right). D, mean amplitude of the afferent volley of the first field EPSP, recorded before and after nicotine application (n = 5) in the presence of α-BGT.
Discussion
The present experiments clearly show that at immature Schaffer collateral–CA1 synapses, a brief exposure to nicotine can produce either a potentiation or a depression of synaptic strength depending on the initial state of the synapse. Both these effects are persistent. In particular, at high P synapses nicotine induces a long-term depression, an effect that involves the activation of both α7- and β2-containing receptors.
Nicotine-induced depression of synaptic currents was not due to run down of the EPSCs following wash out of intracellular factors since: (i) in the absence of nicotine, synaptic currents could be recorded for long period of time without decrement in their amplitude; (ii) when two converging inputs to the same neurone were alternately activated, the depression of one input was often associated with the potentiation or no change of the other; (iii) the depressant action of nicotine was prevented by DHβE at concentrations known to block both α7- and β2-containing nAChRs (Bertrand et al. 1992); and (iv) nicotine applied in the presence of αBGT was still able to depress extracellular field EPSPs, in conditions in which the intracellular milieu of the neurones remained intact.
A depressant effect of nicotine on glutamatergic transmission may result from the indirect excitation of inhibitory interneurones as shown by Alkondon et al. (1999) and Ji et al. (2001). Thus, activation of nAChRs by acetylcholine applied locally to GABAergic interneurones, has been shown to shift long-term into short-term potentiation if the administration of the drug precedes the stimulation of the Schaffer collateral (Ji et al. 2001). In this case synaptic modulation is dependent on the location and timing of nicotine activity. Furthermore, an indirect action of nicotine on GABAergic interneurones may account for the long-term depression observed in the CA1 region of the adult rat hippocampus (Fujii & Sumikawa, 2001). This effect is probably mediated by fast desensitizing α7 nAChRs receptors.
In the immature hippocampus, GABA depolarizes and excites principal cells though an outward flux of chloride (Cherubini et al. 1991). Therefore, nicotine-induced enhancement of GABA release should facilitate and not depress principal cells. In spite of this concern, the present data allow the exclusion of an indirect effect mediated through GABAA receptors because our experiments were routinely performed in the presence of bicuculline, a GABAA receptor antagonist. However, the depression of glutamate release may result also from the activation of GABAB receptors. Thus, nicotine may enhance the release of GABA that would activate GABAB receptors localized on glutamatergic terminals. However, this hypothesis is difficult to reconcile with the observation that nicotine affects differently low and high P inputs converging into the same neurone.
A direct inhibitory role of nicotine on NMDA receptors has been reported in cultured hippocampal neurones (Fisher & Dani, 2000). This effect was postsynaptic and required extracellular calcium and activation of calmodulin since it was prevented by the calmodulin antagonist fluphenazine. However, in the present experiments nicotine-induced depression of NMDA-mediated synaptic currents persisted in neurones loaded with BAPTA suggesting that calcium entry into the postsynaptic cell through nAChRs was not involved in calcium–calmodulin-dependent inhibition of NMDA receptors.
In the hippocampus nAChRs are widely expressed on both presynaptic and postsynaptic membranes (Alkondon et al. 1997; Zoli et al. 1998; Sudweeks & Yakel, 2000; Ji et al. 2001; Fabian-Fine et al. 2001). Therefore, the observed effect could result from the activation of pre- or postsynaptic receptors. Several lines of evidence suggest that nicotine-induced long-term depression depends on the activation of presynaptic nAChRs. Thus, the increase in the number of transmitter failures, the increase in PPR and in the CV favour a presynaptic site of action. This was further substantiated by the observation that nicotine affects both AMPA- and NMDA-mediated synaptic currents.
In previous work (Maggi et al. 2003) nicotine effects were selectively studied on low P or silent synapses. In these synapses the alkaloid produced a long-lasting facilitating effect that was mediated by α7 nAChRs. The present study has been focused mainly on high P synapses. Here nicotine induced a clear depressant effect, which involved both homomeric α7- and heteromeric β2-containing nAChRs. The latter could per se account for nicotine-induced long-term depression as suggested by the field experiments in which, in the presence of α-BGT, only a depression was observed. It should be stressed that a population response (field EPSP) results from the sum of several individual inputs. In the absence of nAChR antagonists, each of them could be either facilitated or depressed by nicotine. This explains why in control conditions the amplitude of the field EPSP was unaffected by nicotine in spite of a large variability of individual responses. Moreover, field experiments allow exclusion of the participation of NMDA receptors in nicotine-induced long-term depression.
Both α7 and α4 β2 nAChRs are permeable to calcium (Tsuneki et al. 2000). However, the amount of current carried by Ca2+ varies between 10% and 3% in α7 and α4 β2 nAChRs, respectively (Fucile, 2004). Calcium may arise also through the activation of voltage-dependent calcium channels following nicotine-induced depolarization of synaptic terminals (Rathouz & Berg, 1994) and/or calcium-induced calcium release mechanisms (Sharma & Vijayaraghavan, 2003). Therefore it is likely that activation of presynaptic α7- and/or β2-containing nAChRs may differentially modify intracellular calcium concentration leading to an increase or a decrease in synaptic efficacy according to the different transduction pathway which would be activated. Different intracellular calcium concentrations in the postsynaptic neurone have been proposed to explain the induction mechanisms underlying long-term potentiation (LTP) or long-term depression (LTD) in the CA1 hippocampal area. A large increase in calcium would result in LTP while a modest increase in LTD (Lisman, 1989).
Interestingly, the same neurone could be differentially affected by nicotine, according to the initial P. This is in some way intuitive, because for instance synapses with very low P cannot be further depressed while synapses with very large P cannot be further potentiated. However, the same synapse may undergo different types of modulation according to the previous history. The possibility for nicotine to enhance synaptic efficacy in originally high P synapses converted into low P (using a different calcium/magnesium ratio in the extracellular solution) and vice versa strongly supports this view. To our knowledge, the idea that a neurotransmitter such as acetylcholine acting on nicotine receptors can either increase or decrease release probability depending on the initial P is novel and opens new perspectives in the study of developmental plasticity. Although this concept cannot be generalized, it appears to be particularly relevant for nicotine receptors given their high permeability to calcium (Tsuneki et al. 2000).
In conclusion it is clear from the present experiments that in the immature hippocampus, the bi-directional regulation of transmitter release by activation of nAChRs might have important physiological significance in information processing and can play an important role in fine-tuning neuronal connectivity before the establishment of the adult neuronal circuit.
Acknowledgments
This work was supported in part by a grant from Ministero Istruzione Universita' e Ricerca (MIUR & COFI 2002) and from Telethon (GGP030416) to E.C. E.S. was the recipient of a Novartis fellowship. We are grateful to R. Giniatullin and L. L. Voronin for their comments and suggestions.
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Devillers-Thiery A, Revah F, Galzi JL, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux JP. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci U S A. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben Ari Y. GABA: an excitatory transmitter in early postnatal life. Review. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Dani JA. Nicotinic receptors on hippocampal cultures can increase synaptic glutamate currents while decreasing the NMDA-receptor component. Neuropharmacology. 2000;39:2756–2769. doi: 10.1016/s0028-3908(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acethylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sumikawa K. Nicotine accelerates reversal of long-term potentiation and enhances long-term depression in the rat hippocampal CA1 region. Brain Res. 2001;894:340–346. doi: 10.1016/s0006-8993(01)02058-3. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release. Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasa P. The cholinergic systems in brain and spinal cord. Prog Neurobiol Rev. 1986;26:211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Klink R, Lena C, Korn H, Changeux JP. Calcium mobilization elicited by two types of nicotinic acetylcholine receptors in mouse substantia nigra pars compacta. Eur. J Neurosci. 2000;12:2475–2485. doi: 10.1046/j.1460-9568.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- Voronin LL, Altinbaev RS, Bayazitov IT, Gasparini S, Kasyanov AV, Saviane C, Savtchenko L, Cherubini E. Postsynaptic depolarisation enhances transmitter release and causes the appearance of responses at ‘silent’ synapses in rat hippocampus. Neuroscience. 2004;126:45–59. doi: 10.1016/j.neuroscience.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]