Abstract
Cardiac tissue expresses several TRP proteins as well as a Mg2+-inhibited, non-selective cation current (IMIC) that bears many characteristics of TRP channel currents. We used the whole-cell voltage clamp technique in pig and rat ventricular myocytes to characterize the permeation, blockage properties and regulation of the cardiac IMIC channels in order to compare them with TRP channels, in particular with Mg2+-sensitive TRPM6 and TRPM7. We show that removing extracellular divalent cations unmasks large inward and outward monovalent currents, which can be inhibited by intracellular Mg2+. Inward currents are suppressed upon replacing extracellular Na+ by NMDG+. Divalent cations block monovalent IMIC and, at 10–20 mm, carry measurable currents. Their efficacy sequence in decreasing outward IMIC (Ni2+= Mg2+ > Ca2+ > Ba2+) and in inducing inward IMIC (Ni2+≫ Mg2+ = Ca2+ ≈ Ba2+), and their permeabilities calculated from reversal potentials are similar to those of TRPM6 and TRPM7 channels. The trivalent cations Gd3+ and Dy3+ also block IMIC in a voltage-dependent manner (δ = 0.4–0.5). In addition they inhibit the inward current carried by divalent cations. IMIC is regulated by pH. Decreasing or increasing extracellular pH decreased and increased IMIC, respectively (pH0.5 = 6.9, nH = 0.98). Qualitatively similar results were obtained on IMIC in rat basophilic leukaemia cells. These effects in cardiac myocytes were absent in the presence of high intracellular buffering by 40 mm Hepes. Our results suggest that IMIC in cardiac cells is due to TRPM channels, most probably to TRPM6 or TRPM7 channels or to their heteromultimeres.
Recent studies have identified TRP channels as major pathways for cation movement in non-excitable cells (Clapham et al. 2001; Montell et al. 2002; Vennekens et al. 2002). In these cells, TRP proteins have been proposed to underlie the Ca2+ entry via Ca2+-release activated (CRAC) or store-operated (SOC) channels following the activation of various receptors coupled to phospholipase C, or the Ca2+ entry in response to various chemical and physical stimuli. Many TRP channels conduct small inward divalent currents at negative potentials and large outward monovalent cation currents at positive potentials under physiological ionic conditions but, as found for voltage-gated Ca2+ channels, they conduct large monovalent cation currents in both directions under conditions where extracellular divalent cations are removed. Among TRP channels, the TRPM subfamily contains channels activated by such various stimuli as intracellular Ca2+ and membrane potential, cold, pH and odourants. Two channels of this subfamily, TRPM6 and TRPM7, are permeable to various divalent cations, including Ca2+, Mg2+ and Zn2+ (Hermosura et al. 2002; Monteilh-Zoller et al. 2003; Schmitz et al. 2003; Voets et al. 2004). The channels may play a critical role in divalent cation transport, and mutations of TRPM6 have been shown to be associated with disturbances of Mg2+ and Ca2+ homeostasis, probably as a result of deficient digestive absorption and renal reabsorption of these ions. These channels are expressed in a wide variety of cells (Minke & Cook, 2002) but have more clearly been characterized in blood cells such as T lymphocytes and rat basophilic leukaemia (RBL) cells (Kozak et al. 2002; Kozak & Cahalan, 2003) and in intestinal and renal cells (Voets et al. 2004). Their function remains unclear in other cell types, including excitable cells, but they are presumed to act as heavy metal transport pathways.
In the cardiovascular system, cardiac tissue and vascular smooth muscle cells express several TRP channel proteins, including TRPM6 and TRPM7 (Nadler et al. 2001; Runnels et al. 2001), but it is still unclear which of the various conductances present in these cells can be attributed to them. We have previously identified a non-selective cation current (called here IMIC), that bears many characteristics of TRP channel currents, including: (1) block by extracellular di-, tri- or polyvalent cations; (2) permeability to the monovalent cations Cs+, K+, Na+ and Li+; and (3) inhibition by intracellular Mg2+ (hence their designation as ‘magnesium inhibited cation’ or ‘MIC’ channels, as for channels found in lymphocytes) (Mubagwa et al. 1997; Bosteels et al. 1999; Zakharov et al. 1999, 2003). A current similar to IMIC has also been identified in neuronal channels (Xiong et al. 1997; Xiong & MacDonald, 1999). The cardiac MIC channels were insensitive to known blockers of gap-junction channels (Macianskiene et al. 2001), their opening was not associated with intracellular Ca2+ store depletion, and their sensitivity to pharmacological modulation was inconsistent with those of CRAC channel currents (Zakharov et al. 2003). The block by intracellular Mg2+ resembles that of certain TRPM channels, which are known to conduct outward-rectifying currents in the presence of external divalent cations (Nadler et al. 2001; Runnels et al. 2001; Hermosura et al. 2002; Monteilh-Zoller et al. 2003; Voets et al. 2004). We therefore wanted to further characterize the permeation and blockage properties of the cardiac channel in order to be able to compare with TRP channels, in particular with TRPM6 and TRPM7. We also wanted to investigate the regulation by pH and compare with that of MIC channels in RBL cells.
Methods
Experimental preparations and electrophysiological techniques
We used pig and rat isolated, single ventricular myocytes. The study was carried out in accordance with the institutional guidelines for the care and use of laboratory animals.
The methods used for cell dissociation and electrophysiological measurements are similar to those described in detail before (Macianskiene et al. 2002). Pigs were initially pre-medicated with 4 mg kg−1 azaperone (intramuscularly) and 0.35 mg kg−1 atropine (intravenously). They were anaesthetized with 5–15 mg kg−1 sodium pentobarbitone (Nembutal; intravenously), supported by assisted respiration and then injected with heparin and a lethal dose of pentobarbitone (Nembutal; 100 mg kg−1) before thoracotomy and tissue extraction. Rats were injected with 150–300 mg kg−1 intraperitoneally, 10 min after injection with heparin. Rats did not receive any pre-medication. The cell dissociation procedure consisted essentially of an enzymatic tissue digestion during a Langendorff perfusion, after which the isolated cells were stored in 0.18 mm Ca2+ Tyrode solution at room temperature (21–22°C). Other myocytes were stored at 4°C and used 24–48 h later. Experiments were also carried out at room temperature.
Voltage clamp and data storage were performed using the pCLAMP software (Axon Instruments). Currents were generated by 4-s symmetrical voltage ramp commands to potentials between −120 and +80 mV, applied every 10 s from a holding potential of −80 mV. Data were filtered at 1 kHz and sampled at 5 kHz. Currents were measured during the descending limb of the voltage ramp. The slow rate of depolarization (0.1 V s−1) during the ascending limb allowed for complete inactivation of the voltage-dependent Na+ currents, while the L-type Ca2+ channels were blocked by 100 μm nifedipine included in all extracellular solutions. Due to difficulties in distinguishing between loss of seal resistance and opening of a non-selective ion channel when perfusing with divalent cation-free solutions in whole-cell mode, two criteria were applied before considering a current change as reflecting a genuine membrane conductance change: (1) the change had to develop progressively and smoothly (without large current jumps) over time; (2) the increase in conductance had to be reversible upon readmission of Ca2+o and/or Mg2+o. In order to minimize junction potential changes during application of divalent cation solutions, we used an agar bridge filled with the pipette solution as reference electrode.
In a few experiments we used RBL cells, in which large MIC currents can be measured, to compare with data obtained in cardiac myocytes. The RBL cells were cultured by incubation in Eagle's minimal essential medium (MEM). Cells were passaged every 3 days, the total number of passages not being more than 12 from one sample. The electrophysiological techniques were essentially similar to those used for cardiac cells: holding potential of −40 mV, ramps of 200–500 ms duration between −100 mV and +60 or +90 mV given every 10 s, at room temperature.
Solutions and drugs
During measurements, the myocytes were superfused with a K+-free Tyrode solution containing (mm): 135 NaCl, 5.4 CsCl, 0.9 MgCl2, 1.8 CaCl2, 0.33 NaH2PO4, 10 Hepes and 10 glucose; pH was adjusted to 7.4 with NaOH. Nominally Ca2+-free or Mg2+-free solutions were prepared by simply omitting these ions from the standard solution. Na+-free external solution was obtained by substituting NaCl with N-methyl-d-glucamine-chloride (NMDG-Cl). External solutions used to study divalent cation permeability contained 10–20 mm of either Ca2+, Mg2+, Ba2+ or Ni2+, added in the NMDG+-based, Na+-free solution, with osmotic compensation achieved by adjusting the NMDG+ concentration. The effect of trivalent cations was studied by adding a lanthanide (Gd3+ or Dy3+) as chloride salt or by adding Ruthenium (Ru3+) Red. NaH2PO4 was omitted from solutions containing the trivalent cations, but was often included in solutions applied subsequently to wash out their effects. The standard internal solution contained (mm): 130 caesium glutamate, 25 CsCl or TEA-Cl, 1 MgCl2, 5 Na2ATP or MgATP, 1 EGTA, 0.1 Na2GTP and 5 Hepes (pH 7.25; adjusted with CsOH). When required, we changed the internal solution to modify the MgCl2 (0–10 mm), ATP (0–5 mm) or EGTA concentrations. Since internal Mg2+ inhibits TRPM6 and TRPM7 currents (see Introduction), a Mg2+-free internal solution was used when testing for divalent cation permeation. In these experiments, 10 mm EDTA was used as Mg2+ chelator (instead of 1 mm EGTA). The free Mg2+ concentration of internal solutions was calculated using the CaBuf software (generously provided by G. Droogmans, Department Physiology, University of Leuven; ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip). Acidic external solutions were buffered with MES. Extracellular solutions containing nifedipine (100 μm; from a 50 mm stock solution prepared in ethanol) were protected from light. Drugs or chemicals were from Sigma-Aldrich or Merck.
The external solutions used for experiments with RBL cells were identical to those for cardiac cells with no glucose. The internal solution contained (mm): 130 caesium glutamate, 25 CsCl or TEA-Cl, 10 BAPTA and 10 Hepes (pH 7.2; adjusted with CsOH).
Solution or drug application was done either by bath perfusion or by a fast solution exchange system (solution change complete within 100 ms) via an array of valve-operated capillary tubes converging into a manifold with the outlet placed 1–5 mm from the cell.
Data analysis
Functions were fitted to data using Clampfit (Axon Instruments) or Origin (Microcal, Northampton, MA, USA). The equation used to obtain the parameters of voltage-dependent block by di- or trivalent cations is given in the Appendix. Ratios of permeability to divalent cations relative to that of Na+ were calculated using the Goldman equation:
where Px is the permeability to the X ion, Di is the divalent cation, [X]i and [X]o are the intracellular or extracellular concentrations of X, α= exp(−FV/RT), with F, R and T having their usual thermodynamic meaning and V the reversal potential of the current carried by Di. PCs/PNa was set at 1.5 as determined in our previous study (Mubagwa et al. 1997). The following modified Hill equation was used to fit the amplitude of IMIC as a function of the proton concentration:
where I is the divalent cation-sensitive current measured at a given pH, Imax is the maximum divalent cation-sensitive current, pH0.5 is the pH level at which half of the maximal current is inhibited, nH is the Hill coefficient of the proton binding, and c is a component persisting at the lowest pH tested.
Average data are expressed as mean ± standard error of the mean (s.e.m.), with n indicating the number of cells studied under each condition. Statistical comparison was made using a two-tailed t test or ANOVA.
Results
Lack of inward-going rectification of IMIC in the absence of internal TEA+
Our previous characterization of IMIC in cardiac and vascular muscle cells as an inward rectifier (Mubagwa et al. 1997; Zakharov et al. 1999) is in apparent contradiction with it being via channels, including many TRP channels, that carry large outward currents. However, the failure to observe large outward currents could have been due to the presence of TEA+ in the internal solution, which was used to block potential ion movement through KV4.x (Ito) channels in our experiments. Since TEA+ is impermeant through TRP-like channels (Bakowski & Parekh, 2002), the presence of TEA+ could cause the apparent inward rectification. We therefore measured IMIC using TEA+-free internal solutions, and included myocytes from pig ventricle, which are known to lack Ito channels (Macianskiene et al. 2002; Li et al. 2003).
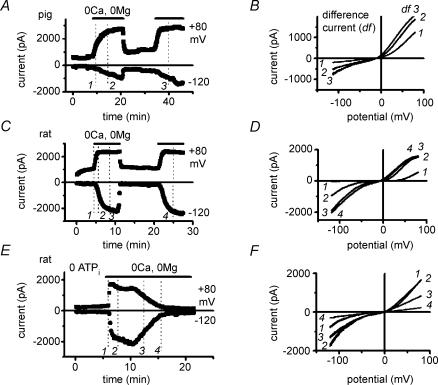
Figure 1A and B illustrates typical changes produced by the removal of extracellular divalent cations on membrane currents in pig and rat myocytes, during cell dialysis with TEA+-free internal solution, with all K+ and voltage-dependent Ca2+ and Na+ channels blocked. The traces of current were obtained using voltage ramps (1) under control conditions, in the presence of Ca2+o and/or Mg2+o, (2) in the absence of divalent cations, and (3) after the readmission of either Ca2+o or Mg2+o. Currents were markedly and reversibly increased in the divalent cation-free solution. Difference currents (not shown; see Fig 2B, D and F), obtained by subtracting currents in the presence from those in the absence of divalent cations, show that removal of Ca2+o and Mg2+o unmasked large outward and inward currents with a reversal potential close to 0 mV. The characteristic shape of the current–voltage relationship (with a low slope conductance region near 0 mV), the reversal potential of the induced current (Erev: −2.1 ± 1.3 mV in pigs, n = 21; −5.8 ± 1.8 mV in rats, n = 16; P = 0.10) and the current density (at −120 mV with 20 μm free [Mg2+]i: −9.4 ± 1.0 pA pF−1 in pigs; −8.0 ± 0.9 pA pF−1 in rats; P = 0.32) were similar under the same experimental conditions in both species. Figure 1C and D illustrates that including TEA+ (25 mm) in the internal solution caused a flattening of the current–voltage relation at the most positive potentials, while the shape at negative potentials remained unchanged. This resulted in an apparent inward rectification in both species (e.g. in pigs, ratio between currents at +80 mV and at −80 mV: 0.5 ± 0.1 with TEA+, n = 11, versus 2.1 ± 0.4 without TEA+, n = 21; P = 0.007). Since pig cells, in contrast to rat cells, do not express Ito channels (Macianskiene et al. 2002; Li et al. 2003), these results indicate that the large outward currents in the absence of extracellular divalent cations are unlikely to be contaminations by currents through endogenous KV4.x channels but probably represent unblocked IMIC.
Figure 1. Removal of extracellular divalent cations (Cao2+, Mgo2+,) unmasks large outward and inward monovalent cation current (IMIC) in myocytes of pig (A and C) and rat (B and D).
Original traces of currents obtained using voltage ramps in the presence of divalent cations (1.8 mm Ca2+o, 0.9 Mg2+o; •), during their depletion (nominally 0 mm Ca2+o and 0 mm Mg2+o; ^) and after their re-addition (▪). Internal free [Mg2+]i: 20 μm. A and B, cells dialysed without TEA+. No inward-going rectification of IMIC in the absence of internal TEA+. C and D, cells dialysed with internal solution containing 25 mm TEA+. Notice a flattening of the current–voltage relationship of IMIC (causing apparent inward-going rectification).
Figure 2. Accounting for the effect of removing extracellular divalent cations by one single membrane conductance.
A,C and E, time course of changes in membrane currents during extracellular divalent cation depletion and re-addition. Periods of exposure to solutions of divalent cation-free solution are indicated by horizontal bars. B, D and F, MIC current, obtained as difference between traces in the absence and in the presence of Ca2+o and Mg2+o. Currents were taken at different times, and their labels indicate the corresponding sampling times in panels A, C and E. Notice constancy of reversal potential during different time courses of inward and outward currents, and during run-down. Internal free [Mg2+]i: 20 μm in A and C, 0 mm in E. External solution change with bath perfusion in A and C, with fast perfusion system in E.
Time course and run-down
Typical time courses of the changes induced by removing extracellular divalent cations are shown in Fig. 2A and C, and illustrate that the effects develop progressively, are reversible and qualitatively reproducible in the same cell. The tendency for larger currents to develop during a second exposure to divalent-free solution (see Figs 4B and 5A) has been attributed to progressive dilution of intracellular Mg2+ by the pipette solution (Zakharov et al. 2003). However, even when 10 mm EDTA was included in the pipette solution (hence causing rapid chelation of internal Mg2+), IMIC induced 5 min after patch rupture was smaller than the current obtained later during cell dialysis, indicating that removal of internal Mg2+ per se was not sufficient, but needed an additional, slow step, to allow full desinhibition of IMIC. We also noticed that outward currents develop earlier and reach steady state faster than inward currents during bath solution change to the Ca2+o- and Mg2+o-free medium (Fig. 2A and C). Equally, inward currents were the first to be blocked (before outward currents) upon reapplication of the divalent cations (not illustrated). Using the fast solution exchange system, we were able to elicit more rapid development of IMIC, probably due to faster washout of blocking divalent cations. However, even in this case, inward current development lagged behind that of outward current (see Figs 2E, 4A, 5A and 9A). This difference in the time course of development of inward and outward currents does not necessarily imply that the outward and inward components are carried by different types of channels. This is supported by the finding that during the whole course of the experiments, Erev of the induced current remained unchanged (Fig. 2B and D). Rather than reflecting the participation of different channel types, the different time course is likely to be the result of a faster unblock at positive than at negative potentials (as a result of the voltage-dependent affinity for block) during progressive changes of extracellular divalent cation concentration.
Figure 4. Voltage-dependent block of MIC current by trivalent cations.
A and B, time course of changes in membrane currents at −120, −80 and +80 mV during extracellular divalent cation depletion, in the absence and in the presence of either 10 μm (A) or 100 μm Gd3+ (B). Currents measured using voltage ramps. Periods of exposure to divalent cation-free solutions and to trivalent cations are indicated by horizontal bars. Phosphate included in the divalent cation-free solution in B but not A during wash-out of Gd3+. Notice marked block caused by Gd3+ at negative but not at positive potentials. C and D, relative IMIC remaining in the presence of the trivalent ions Gd3+ (C) or Dy3+ (D). Values near reversal potential were removed because of large scatter due to divisions by small numbers. Notice also incomplete block at extreme negative potentials. Line through data points drawn by fitting with of eqn (A5) Appendix. Internal free [Mg2+]i: 20 μm. Extracellular solution change with either fast perfusion system (A) or bath perfusion (B). Slow onset of Gd3+-induced block in B is probably related to the slow concentration change during bath solution change. Pig (A–C) and rat (D) ventricular myocytes.
Figure 5. Spontaneous run-up of IMIC during wash-out of internal Mg2+, but absence of measurable divalent cation entry with physiological extracellular divalent cation concentrations.
A and B, time evolution of currents measured at −120, −80 and +80 mV using voltage ramps. Periods of exposure to solution without Ca2+o and Mg2+o indicated by horizontal bars. C, effect of internal Mg2+ on the total inward current at −120 mV in the presence of extracellular divalent cations. Currents (means ±s.e.m.) were measured at the beginning of patch rupture and after 20 min. Internal free Mg2+ concentration calculated using CaBuf (see Methods). Difference between values in the various columns were not significant (paired t test used for comparing data of same [Mg2+]i at the beginning of patch rupture versus after 20 min; ANOVA used for all data of different [Mg2+]i). D, effect of internal Mg2+ on the current induced at −120 mV upon removal of the divalent cations. *P < 0.001 when comparing (by ANOVA with post hoct test) with data at 20 μm free [Mg2+]i. NS, non-significant difference (P > 0.05). Extracellular solution change with fast perfusion system. Pig ventricular myocytes. Notice (1) run-up of outward current but absence of significant increase of inward current during prolonged dialysis with 1.8 mm Ca2+o and 0.9 mm Mg2+o, in the external solution, and (2) suppression of IMIC by internal Mg2+.
Figure 9. High intracellular pH buffering prevents changes of IMIC upon extracellular acidification.
A, time course of changes in membrane currents during extracellular divalent cation depletion and repletion. Extracellular acidosis was applied in divalent-free solutions. Cell dialysed with pipette solution containing 40 mm Hepes measured at +80, −80 and −120 mV. Periods of exposure to divalent cation-free and acidic solutions are indicated by horizontal bars. B, mean values of relative IMIC (divalent cation-sensitive current) in cells dialysed with high Hepes at normal and acidic pH values. Current at pH 7.4 taken as 100%. Internal free [Mg2+]i: 20 μm. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
In cells dialysed with ATP-free solution we observed a run-down of IMIC during prolonged cell dialysis (Fig. 2E). Even in some cells dialysed with 5 mm Na2ATP, run-down could be observed and typically started 50–60 min after patch rupture (not illustrated). The run-down is qualitatively similar to that observed in RBL cells (Kozak & Cahalan, 2003), but in our case it was slower for inward than for outward currents. Since Erev of the induced current remained unchanged (Fig. 2F), the differential time course of run-down of outward and inward currents is also not in contradiction with one single type of channel being involved since the slower run-up of inward current could mask its initial phase of run-down. The mechanism of run-down was not investigated, but given that it occurred earlier in the absence of internal ATP, it may be related to a loss of phosphorylation.
Voltage-dependent block by di- and trivalent cations
We have previously reported that divalent and trivalent cations block the inward component of IMIC in rat myocytes (Mubagwa et al. 1997; Bosteels et al. 1999). No clear analysis was made then of the effect on outward currents, which were largely blocked by internal TEA+. In the present study, we re-examined this effect in the presence of large outward currents. Figure 3A and B illustrates the effect of Ca2+ added at 10 and 20 μm after the development of monovalent IMIC in the absence of extracellular divalent cations. Both concentrations reduced the inward currents but had no or less effect on the outward currents. To determine the voltage dependence of the Ca2+o-induced block, we took the difference between currents in the nominal absence and in the presence of divalents as reference IMIC. IMIC remaining in the presence of the micromolar Ca2+o concentrations (Fig. 3C) was then expressed relative to this reference level (Fig. 3D). The data show that channel blockage was nearly absent at the most positive potentials but increased as potentials became less positive. Block was more pronounced at negative potentials, but a voltage-dependent relief from block with hyperpolarization was noticeable. Figure 4A and B illustrates the effect of 10 and 100 μm Gd3+. While the trivalent cation suppressed the inward component of IMIC induced in the absence of Ca2+o and Mg2+o, there was also little or no effect on outward currents. A similar effect was obtained with 100 μm Dy3+ (not illustrated). In addition to these classic trivalent cations, ruthenium red also blocked IMIC, and at 10 μm only inward current was markedly suppressed (not illustrated). We also examined the voltage dependence of the block induced by trivalent cations by plotting the ratio of IMIC (divalent cation-sensitive or difference current) in the presence and absence of trivalent cations in the same cell. Figure 4C and D illustrates this analysis for the block induced by Gd3+ and Dy3+, respectively. Although the magnitude of block increased at more negative potentials, a saturation of the extent of the effect, with incomplete current block (see data with 10 μm Gd3+ in Fig. 4A and C), was present at negative potentials. This behaviour is somewhat similar to that of divalent cations, for which the block ceased to increase with hyperpolarization and actually appeared to decrease at very negative potentials (see Fig. 3D). A recent study indicates that the same occurs with organic polycations such as spermine and spermidine on similar currents in RBL cells (Kerschbaum et al. 2003). This suggests that, in addition to an increase of block with hyperpolarization, another process is taking place and causes a relief from block at negative potentials. Such a relief from block can be accounted for by either an entry of the blocker into the cell (‘punch-through’; Kerschbaum et al. 2003) at negative potentials or a competition between the trivalent blocker and an extracellular cation entering the channel (see Discussion). In the absence of any evidence that trivalent cations pass through the channel, we fitted our data by an equation (see Appendix: eqn (A5)) incorporating a competition in addition to the trivalent-induced channel block. The fitting of the data generated an electrical distance (δ; from outside) for block at 0.64 ± 0.12 for Ca2+, 0.46 ± 0.01 for Gd3+ and 0.43 ± 0.09 for Dy3+.
Figure 3. Voltage-dependent block of MIC current by divalent cations.
A, time course of changes in membrane currents at −120, −80 and +80 mV during extracellular divalent cation depletion, and following the addition of 10 μm or 20 μm Ca2+. Currents measured using voltage ramps. Periods of exposure to divalent cation-free solutions and to 10 μm or 20 μm Ca2+ are indicated by horizontal bars. B, current–voltage relationships in control conditions (in the presence of extracellular divalent cations; ▪), during extracellular divalent cation depletion (^), and following the the addition of 10 μm (•) or 20 μm Ca2+ (□). C, MIC current–voltage relationship. IMIC obtained as difference current between traces in the nominal absence of divalent cations and in the presence of Ca2+. Reference IMIC in nominal absence of Ca2+o (^) is partly blocked by 10 μm (•) or 20 μm Ca2+ (□). Notice marked block at negative but not at positive potentials. D, relative IMIC remaining in the presence of 10 μm or 20 μm Ca2+. Values near reversal potential were removed because of large scatter due to divisions by small numbers. Notice also relief from block at extreme negative potentials. Line through data points drawn by fitting with of eqn (A5) Appendix. Internal free [Mg2+]i: 20 μm. Extracellular solution change with bath perfusion. Rat ventricular myocyte.
As noticed previously, washing out the trivalent cations in the absence of PO43− did not allow full recovery of IMIC (see Fig. 4A). However, recovery was obtained when the trivalent cation application was very short or, after long applications, when PO43− was included in the solution during wash-out (see Fig. 4B).
Inhibition by intracellular Mg2+ and permeability to divalent cations
A characteristic of cloned TRPM6/7-channels heterologously expressed in HEK cells (Nadler et al. 2001; Hermosura et al. 2002) and of similar channels natively present in RBL cells (Kozak & Cahalan, 2003) is their inhibition by high intracellular Mg2+ concentrations ([Mg2+]i). We reported previously that cell dialysis with micromolar or nominally zero Mg2+ pipette concentrations promoted IMIC, and that inclusion of millimolar MgCl2 or MgATP concentrations in the pipette solution inhibited the current in rat myocytes (Zakharov et al. 2002, 2003). We used the sensitivity to [Mg2+]i to examine whether the cardiac channels conduct significant current in the presence of physiological extracellular Na+, Ca2+ and Mg2+ concentrations. Cells were dialysed with internal solutions containing either 0 mm MgCl2+10 mm EDTA (∼0 μm free [Mg2+]i), 1 mm MgCl2+ 5 mm Na2ATP + 1 mm EGTA (∼20 μm free [Mg2+]i), 0 mm MgCl2+ 5 mm MgATP + 1 mm EGTA (∼0.6 mm free [Mg2+]i) or 10 mm MgCl2+ 0 mm ATP (∼10 mm free [Mg2+]i). In addition to omitting ATP from the pipette solution containing 10 mm MgCl2, endogenous ATP was further depleted by superfusing with glucose-free solution. Figure 5A and B illustrates the time course of currents measured at −120, −80, and +80 mV in pig cells dialysed with 0 mm MgCl2+ 10 mm EDTA (Fig. 5A) or 10 mm MgCl2 (Fig. 5B) while superfusing with Na+, Ca2+ and Mg2+ at physiological concentrations for at least 20 min. In cells dialysed with Mg2+-free or low [Mg2+]i solutions there was a spontaneous increase of outward current in the presence of extracellular divalent cations, which probably corresponds to an initial run-up of unblocked outward component of IMIC associated with the wash-out of internal Mg2+ (Fig. 5A; see also Fig. 3A). This result is similar to that obtained for TRPM6/7 channels. However, there was no significant change of total inward current with time during the 20 min superfusion with either low [Mg2+]i or high [Mg2+]i (Fig. 5C). After about 20 min exposure to external divalent cations, these were removed. Both outward and inward components of IMIC induced in divalent cation-free solution were largely suppressed by high [Mg2+]i (Fig. 5D). Similar inhibitory effects were obtained with either 10 mm MgCl2+ 0 ATP (∼0 μm free [Mg2+]i) or 5 mm MgATP (free [Mg2+]i= 0.6 mm) (Fig. 5D), hence supporting the view that ATP is not necessary for (Kozak & Cahalan, 2003), but may modulate the inhibition by Mg2+. These data confirm the magnesium sensitivity of the channels studied here, and also suggest that the channels do not carry substantial inward current under resting conditions in the presence of physiological concentrations.
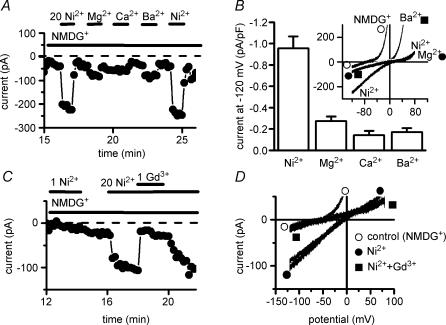
The properties described above, including the shape of the IMIC–voltage relationship, the inhibition by internal Mg2+ and the run-down with time, resemble those of currents carried by TRPM6/7 channels (Hermosura et al. 2002; Monteilh-Zoller et al. 2003; Schmitz et al. 2003; Voets et al. 2004). Since these channels are reported to constitute pathways for the entry of divalent cations such as Ca2+, Mg2+, Ba2+, Ni2+ or Zn2+, we tested the possibility that the cardiac conductance is also permeable to divalent cations. Figure 6 shows experiments in which 10–20 mm of various divalent cations were applied for brief periods (1–2 min) using fast solution change. The presence of IMIC was first confirmed by superfusing with divalent-free solution. Then Na+o was replaced by NMDG+ in the continued absence of divalent cations, which resulted in a suppression of the inward monovalent cation current (at −120 mV, from −18.5 ± 2.72 to −0.9 ± 0.26 pA pF−1; P < 0.001; paired t test; n = 29) and an increase of the outward current (at +80 mV, from +16.3 ± 2.27 to +30.8 ± 3.19 pA pF−1; P < 0.01; paired t test; n = 29). Upon switching to an NMDG+-based solution containing 10 mm of the divalent cations, the large outward currents were decreased (Fig. 6A and B). The efficacy sequence in decreasing outward IMIC was Ni2+≥ Mg2+ > Ca2+ > Ba2+ (Fig. 6C). At the same time that outward currents were suppressed, inward currents increased. This is illustrated in Fig. 7A, which displays the inward currents on a larger current scale because of the small magnitude of basal currents and of the changes induced by divalent cations. Increases in inward current were largest with Ni2+ and reversible. Figure 7B shows the magnitude of inward currents induced at −120 mV with the various divalent cations tested. While relatively large currents were obtained with Ni2+ in all cells, with Ca2+ or Ba2+ inward current increase was less obvious in some cells. Current–voltage relationships (see inset of Fig. 7B) show that in addition to an inward shift in the presence of the divalent cations, the zero-current (reversal) potential was also changed from −59.8 ± 4.7 mV (n = 27) in the presence of NMDG+ alone to less negative values (Ni2+: −23.5 ± 3.9 mV, n = 20; Mg2+: −26.7 ± 4.3 mV, n = 12; Ca2+: −37.1 ± 3.1 mV, n = 10; Ba2+: −26.7 ± 4.3 mV, n = 12).
Figure 6. Block of outward currents by divalent cations.
A, time course of changes in membrane currents in Na+-free extracellular solution (Na+ replaced by NMDG+), in the absence of extracellular divalent cations and during addition of various divalent cations. Currents measured at +80 mV. Periods of exposure to the divalent cations are indicated by horizontal bars. B, original traces of currents obtained using voltage ramps in the various conditions: in NMDG+ in the absence of divalent cations, and during addition of 10 mm Ni2+o, Mg2+o, Ca2+o or Ba2+o. C, means and s.e.m. of the outward current (expressed as percentage relative to the current in NMDG+ alone) remaining in the presence of various divalent cations. Internal free [Mg2+]i: 0 mm. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
Figure 7. Permeability to divalent cations.
A and C, time course of changes in membrane currents in Na+-free extracellular solution (Na+ replaced by NMDG+), in the absence of extracellular divalent cations and during addition of various divalent cations at 20 mm. Currents measured at −120 mV. Periods of exposure to the divalent cations are indicated by horizontal bars. In C, 1 mm Gd3+ was added in the presence of 20 mm Ni2+. B, means and s.e.m. of the inward current density (in pA pF−1) induced upon addition of the various divalent cations. Inset: current–voltage relations. D, original traces of currents obtained using voltage ramps in the various conditions: in NMDG+ in the absence of divalent cations (^), in the presence of 20 mm Ni2+ (•), and in the presence of 1 mm Gd3+ added in addition to Ni2+ (▪). Internal free [Mg2+]i: 0 mm. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
Figure 7C and D shows that the currents carried by Ni2+ could be effectively and reversibly suppressed by adding Gd3+ (0.1–1 mm). In the presence of both Ni2+ and Gd3+ added to NMDG+, the inward current returned to the level obtained with NMDG+ alone.
Regulation of IMIC by pH
A Ca2+o-sensing non-selective current similar to IMIC has been identified in neuronal channels (Xiong et al. 1997; Xiong & MacDonald, 1999) and shown to be sensitive to changes in the extracellular pH (Chu et al. 2003). A recent study suggests that this neuronal current is carried by TRPM7 (Aarts et al. 2003). We therefore tested the effect of decreasing and increasing pHo on the cardiac IMIC. Figure 8A and B shows typical effects obtained upon acidifying or alkalinizing the extracellular medium. After IMIC had reached steady state in divalent-free solution under normal conditions (pHo 7.4), decreasing or increasing pHo caused a reversible decrease or increase, respectively, of IMIC. The magnitude of IMIC change depended on pH as illustrated in Fig. 8C, which presents average data from 3 to 25 pig cells. There was an apparent saturation of the effect of acidosis below pH 5.5. The relationship between IMIC amplitude (expressed relative to the current at normal pHo, taken as 100%) and proton concentration could be fitted by the Hill equation (see Methods), with half-effective pHo (pH0.5) of 6.9 and nH of 0.98. The IMIC–voltage relationships in Fig. 8D show that the relative change caused by a given pH was the same at all potentials, indicating a lack of voltage dependence in the effect of protons. Similar results were obtained in rat mycoytes (n = 3).
Figure 8. Regulation by pH.
A and B, time course of changes in membrane currents during extracellular divalent cation depletion and repletion. Extracellular acidosis and alkalosis were applied in divalent-free solutions. C, relative divalent-sensitive current at different pH values. Current at pH 7.4 taken as 100%. Currents were measured at +80 mV (▪), −80 mV (^) and −120 mV (•). D, current–voltage relationships of IMIC at different pH values. Internal free [Mg2+]i: 20 μm. Extracellular solution change with bath perfusion. Pig ventricular myocytes.
Since extracellular pH changes are known to cause indirect changes of intracellular pH (pHi), we investigated the role of pHi by increasing the buffering capacity of the intracellular medium. Figure 9 shows the effect of removing extracellular divalent cations in a cell dialysed with 40 mm Hepes. While under such conditions IMIC was preserved, acidic pHo failed to suppress IMIC.
Our data show that, like neuronal Ca2+o-sensing non-selective current probably carried by TRPM7, cardiac IMIC is regulated by pH. In order to compare with another cell types previously reported to natively express large MIC currents attributed to TRPM7 (Kozak et al. 2002; Kozak & Cahalan, 2003) or eventually TRPM6 channels, we also examined the pH dependence of IMIC in rat basophilic leukaemia cells (RBL) in culture. Figure 10A and B shows that Ca2+o and Mg2+o removal induced currents with characteristics similar to those of cardiac cells. In the divalent cation-free solution, outward currents developed much faster that inward currents (see traces labelled with numbers in Fig. 10A), Erev was close to 0 mV, and inward rectification was induced by including TEA+ in the internal medium (Fig. 1B). The effect of changing pHo while superfusing with the divalent cation-free solution is illustrated in Fig. 10C, and average results are presented in Fig. 10D. The effects of acidosis (decrease of IMIC) or alkalosis (small increase) in RBL cells are qualitatively similar to those obtained in cardiac (see above) or neuronal cells (Chu et al. 2003). The dependence on pHo could be described by a half-effective pH 0.5 of 6.2 and nH of 1.3.
Figure 10. MIC current in rat basophilic leukaemia cells (RBL) and its regulation by pH.
A and B, unmasking of IMIC by the removal of extracellular divalent cations (Ca2+o, Mg2+o). Original traces of currents obtained using voltage ramps in the presence of divalent cations (1.8 mm Ca2+o, 0.9 Mg2+o; •) and during their depletion (nominally 0 mm Ca2+o and 0 mm Mg2+o; ^). A, cell dialysed without TEA+; no inward-going rectification of IMIC. B, cell dialysed with internal solution containing 25 mm TEA+. Notice IMIC apparent inward-going rectification. C, time course of changes in membrane currents measured at −90 mV during extracellular divalent cation depletion and re-addition. Extracellular pH was changed while superfusing with the divalent-free solutions. D, pHo dependence of MIC current. Relative divalent-sensitive current (with current at pH 7.4 taken as 100%) at different pH values. Internal free [Mg2+]i: 0 mm. Extracellular solution change with fast perfusion system.
Discussion
In the present study we show that cardiac cells express currents with properties largely resembling those carried by TRPM6 and TRPM7 channels expressed in cell cultures. Large monovalent cation currents (IMIC) carried by cardiac channels in the absence of extracellular divalent cations are inhibited by internal Mg2+ and are blocked voltage dependently by divalent and trivalent cations. Outward currents are also decreased with internal TEA+. Although no divalent cation currents can be detected with physiological extracellular concentrations of Ca2+ and Mg2+, measurable permeability is present in the presence of high (10–20 mm) concentrations. In addition, we show that the channels are regulated by pH.
Different types of TRP proteins, with a particularly marked expression of TRPM6, TRPM7 and TRPP1/2, have been detected in cardiac tissue (Chen et al. 1999; Nadler et al. 2001; Runnels et al. 2001). Since several TRPs expressed in other cells form channels that conduct large monovalent cation currents in the absence of extracellular divalent cations, the corresponding proteins present in cardiac cells are among the candidate molecular structures underlying the large outward and inward currents unmasked by external divalent-cation free solutions (IMIC). Many TRP channels are Ca2+ selective and conduct large Ca2+ current in the presence of extracellular Ca2+. Given the absence of detectable Ca2+ current in basal conditions, the channels described in the present study do not seem to belong to this group. Other TRP channels are less selective but conduct such small divalent currents that it is necessary to use high extracellular divalent cation concentrations in order to obtain measurable currents. This is the case for TRPM6 and TRPM7 channels. In addition to the low resting permeability to divalent cations of cardiac myocytes under physiological conditions, both the shape of the IMIC–voltage relationship and its sensitivity to inhibition by internal Mg2+ are arguments to suggest that the underlying channels involve TRPM6- or TRPM7-like channels or their heteromultimeres. Like currents due to these channels, IMIC carries large outward currents. Inward rectification of IMIC reported in our previous experiments is not an inherent property of the channels but is shown to be caused by internal impermeant cations such as TEA+. Other potential molecular candidates for IMIC expressed in cardiac cells include connexin hemichannels, polycystin (Chen et al. 1999) and NSC1 (Suzuki et al. 1998). Arguments against the involvement of connexins have been presented elsewhere (Bosteels et al. 1999). TRPP1/2 (polycystin or autosomal dominant polycystic kidney disease, ADPKD) channels are highly Ca2+ permeable and show a different pharmacological profile (e.g. insensitivity to block by ruthenium red, inhibition by amiloride (Volk et al. 2003), in contrast to IMIC).
IMIC can be blocked by divalent and trivalent cations. The efficacy of divalent cations in blocking outward currents follows a sequence (Ni2+≥ Mg2+ > Ca2+ > Ba2+) similar to the one reported for the block of outward currents through cloned TRPM6 (Voets et al. 2004) and TRPM7 channels (Monteilh-Zoller et al. 2003). A similar sequence has been obtained for the ion selectivity sequence as measured by changes in the reversal potential of TRPM6 currents (Voets et al. 2004). Our data are also consistent with those obtained for TRPM6 and TRPM7 channels in that Ni2+ carries larger current than the other divalent cations tested. However, whereas in the case of both TRPM6 and TRPM7 channels Ba2+ appeared to carry the second largest divalent current after Ni2+, in our study Ba2+ current was not larger compared with Mg2+ current. The sequence of divalent inward current amplitudes (Ni2+≫ Mg2+≥ Ca2+≈ Ba2+) did not exactly match that for block of outward currents. Using the reversal potentials and the Goldman equation, we obtained the following permeability ratios: PNi/PNa= 1.82 ± 0.28 (n = 18), PMg/PNa= 1.80 ± 0.36 (n = 12), PCa/PNa= 0.86 ± 0.14 (n = 10), PBa/PNa= 1.81 ± 0.36 (n = 12). Thus, calculated permeability ratios suggest a large permeability to Ba2+ among the tested divalent cations, despite the fact that Ba2+ caused the least marked block of outward current and carried little inward current. Also in cloned TRPM6/7, Ba2+, which appeared to be the least effective in blocking outward current (consistent with our results in native cardiac channels), was nevertheless more permeant compared with both Mg2+ and Ca2+, as assessed by current amplitude (in contrast to endogenous cardiac channels). At least in our cells, a possible reason to account for the very positive reversal potential with Ba2+ is a contamination by divalent cation current through L-type channels that could remain unblocked despite the presence of the high (100 μm) nifedipine concentration used in our external solutions. However, the differences could also be related to properties inherent to cardiac IMIC channels. In a single file model of ion movement within the channel, permeation follows penetration of a cation molecule through one channel mouth, the binding of the cation onto a site within the channel, and the subsequent unbinding and movement across the inner part of the channel. The affinity for the cation binding to the site within the channel largely determines ion selectivity, whereas the rate of permeation (i.e. the current flow) depends both on the ability to dissociate from the binding site (hence on the affinity) and on the energy barrier for crossing from the binding site to the other side of the channel. Ion throughput may thus be larger for an ion with less affinity for binding. This partly accounts for the larger monovalent cation current through many Ca2+-selective channels observed in the absence of extracellular divalent cations, despite the lower binding affinity of the monovalent cations compared with that of Ca2+. This may also be the case for Ba2+ relative to Ni2+ and Mg2+: the lower effect on outward current suggests lower affinity than that of Mg2+ or Ca2+ for binding within the channel, but the lower affinity could allow easier ion throughput. Given the small magnitude of currents carried by cloned TRPM6 channels or by the endogenous cardiac channels, and the difficulties in accurately measuring reversal potential, it is not possible to exactly determine the ion selectivity sequence. Thus more data are needed before concluding that the permeability sequences are indeed different.
Trivalent cations (Dy3+, Gd3+ and Ru3+) also blocked IMIC. This block was fully reversible when the precaution was taken to wash with phosphate-containing solution. The high sensitivity to trivalent cations distinguishes IMIC from the Ca2+o-sensitive non-selective current of pulmonary endothelial cells, which was not affected by 300 μm Gd3+ (Voets et al. 1996), but is similar to that of basal non-selective channels present in endocardial endothelial cells, which are themselves permeable to Ca2+ and Ba2+ (Manabe et al. 1995). We found that the block by trivalent cations was voltage dependent in pig and rat myocytes. Although block was higher at negative than at positive potentials, there was partial relief from block at more negative potentials. Such a relief from block has generally been accounted for by an entry of the blocker through the channel (see Kerschbaum et al. 2003). However, this may not always be compatible with the large molecular size of some blockers. Rather than considering a relief from block due to permeation of the blocker, one may also simply consider a reversible binding of the blocker to a site within the channel pore in the presence of a competitor for the same site. Such a competition may be exerted by other extracellular cations, including the permeant monovalent cations or other blockers with lower affinity for the channel (e.g. contaminating divalent cations). This model (see equations in Appendix) fits the data with trivalent cations satisfactorily. Previously, we also noticed saturation of block by divalent cations at negative potentials (Mubagwa et al. 1997). While the K0.5 for block by Ca2+o decreased steeply with positive potentials, a constant K0.5 was obtained at potentials negative to −50 mV. This finding is also inconsistent with a simple voltage-dependent block (Woodhull, 1973) induced by the divalent cation but can also be accounted for by a relief from block either due to entry of the divalent cation in the cell, or due to competition between the divalent and another extracellular cation.
In addition to being regulated by internal Mg2+, the channels underlying IMIC are also regulated by pH. Acidosis in the extracellular medium decreases, whereas alkalosis increases IMIC. A similar regulation by pH is also found in neuronal (Chu et al. 2003) and RBL cells (this study; see Fig. 10). Since TRPM7 channels have been shown or proposed to underlie IMIC in the neuronal and RBL cells, respectively, the similarity in pH dependence between these different cell types is an additional argument to suggest that a common ion channel protein underlies IMIC. The fact that the effects of external pH were absent when intracellular buffer capacity was increased (by including higher Hepes concentrations) indicates that pHo effects were mediated by their known secondary changes in intracellular pH (Deitmer & Ellis, 1980). Differences between different cell types in the effects of pHo on pHi might account for the observed difference in pH0.5. The voltage-independent regulation of IMIC by changes of external pH, as also found for a similar current in neuronal cells (Chu et al. 2003), suggests that the effect of protons is not due to a competition for permeation with the charge carrier.
The physiological role of the channels underlying IMIC in cardiac cells remains elusive. Channels underlying IMIC are conductive at very positive potentials in the presence of physiological concentrations of Na+ and divalent cations in the extracellular medium. The outward rectification of IMIC in the presence of extracellular divalent cations resembles that of the background current found in cardiac cells after blockade of ion-selective and other voltage-dependent or exchanger currents. Ca2+o-sensitive non-selective channels have been proposed to contribute to the basal background channels that influence the resting and action potentials of atrial cells, since Gd3+ could hyperpolarize the resting membrane and shorten the action potential (Zhang et al. 2000). However, given the non-specific action of trivalent cations, such effects could be unrelated to IMIC. A significant IMIC contribution to the background inward current is also not expected since resting membrane potential needs to be maintained at a high level in extra-nodal cardiac tissue, to allow high availability of TTX-sensitive channels and rapidly conducting action potentials. However, it is not known whether IMIC might contribute larger currents in nodal cells where fast conduction is not necessary. A divalent cation-sensitive current partly resembling IMIC has been identified in the sino-atrial nodal cells (Hagiwara et al. 1992). Besides cardiac cells, currents similar to IMIC could contribute to the resting membrane potential in vascular smooth muscle (Bae et al. 1999; Zakharov et al. 1999, 2003). Another study in arterial muscle cells identified a Na+ entry pathway that is inhibited by Mg2+o and La3+o, hence resembling IMIC (although tentatively identified as a store-operated pathway), and may participate in cell signalling via changes in Na+i (Arnon et al. 2000).
It is difficult to predict the magnitude of divalent cation current under physiological conditions, with Ca2+ and Mg2+ concentrations one order of magnitude lower than those (10–20 mm) needed to elicit significant current under experimental conditions. The experimentally measured current amplitudes are uppermost limits, which may not be approached in physiological conditions. However, it should be noted that high influx rates are not needed for a sustained transport mechanism to have substantial effect on intracellular ion homeostasis. If, as a gross approximation, we estimate that the total current carried by the divalent cations under physiological conditions is one order of magnitude less than the current measured in the present study, then currents due to Mg2+ or Ca2+ influx would amount to 1–2 pA. For an average cell (100–150 pF, 50 pl; Rodrigo & Chapman, 1991), this will cause a molar influx of 0.75 μmol l−1 s−1 or ∼45 μmol l−1 min−1. For Ca2+ most of the entering cation may not be expected to lead to a substantial rise in cytosolic Ca2+ levels, because Ca2+ will easily be either bound to intracellular Ca2+ buffers, sequestrated by intracellular compartments, or extruded via transporters such as the Na+–Ca2+ exchanger. Slow refilling of the sarcoplasmic reticulum after caffeine-induced depletion in the absence of stimulation could be via a background Ca2+ influx at resting membrane potentials (Shannon et al. 2003). For Mg2+, such an influx, if not corrected by transport systems or intracellular buffers similar to those involved in Ca2+ handling, could have a substantial effect on the cytosolic divalent cation concentration. For example, if uncorrected, the influx rate estimated above could double the normal intracellular Mg2+ concentration (0.5–1 mm) within 10–20 min. Subsequently, the inhibition of IMIC by intracellular Mg2+ may act as a feedback loop, leading to self-limitation of Mg2+ inflow, thus contributing to the intracellular Mg2+ homeostasis.
In conclusion, we propose that the extracellular divalent cation-sensitive non-selective current in cardiac cells is due to TRPM channels, most probably to TRPM6, TRPM7 or their heteromultimeres. Although no measurable divalent current is obtained at physiological concentrations, permeation of divalent cations can be demonstrated at high concentrations. Small but continuous inflow of ions through these channels may contribute to Mg2+ and Ca2+ homeostasis. The channels may also constitute pathways for the entry of heavy metals. Additional work is needed to determine the role played by these channels in physiological and pathological conditions.
Acknowledgments
This work was supported by grants from FWO, the Flemish Foundation for Science (to J.V., K.M. and K.R.S.), by a grant from the NIH (RO1 HL54150, to V.B. and S.I.Z.), and by a scholarship from the Belgian Technical Cooperation (to A.G.). We would like to thank Virginie Bito and Elke Detre for supplying ventricular tissue and some of the isolated myocytes, Dr Arne P. Neyrinck for supplying ventricular tissue, and Professor G. Droogmans for generously allowing us to use the CaBuf software.
Appendix
We assume (1) a reversible binding of a blocker to a site within the channel pore and (2) the possibility of a competitor occupying the same site.
In the absence of the competitor, the relative unblocked current in the presence of a given concentration of blocker (B) is given by the following modified Michaelis-Menten-like equation:
| (A1) |
where KB is the dissociation constant.
Voltage-dependent increase of block is brought into the equation by assuming that blocker concentration at the binding site within the channel is given by:
| (A2) |
where Bo is the (di- or trivalent cation) blocker concentration in the extracellular solution, ▵GB is the change in free energy upon binding of the blocker to its site within the channel, δB is the electrical distance (from outside) of the binding site, zB is the valency of the blocker, Vm the transmembrane potential, and F (Faraday constant), R (gas constant) and T (absolute temperature) have their usual thermodynamic meanings.
The assumption of a competition between the blocker and another cation (C) implies that we should replace the dissociation constant KB with:
| (A3) |
where C, the competitor concentration at the binding site, is given by an equation of the same general form as equation (A2) above:
| (A4) |
and nH is the Hill coefficient of the competitor binding.
The resulting general equation is:
| (A5) |
Competition at the same binding site implies equal electrical distance for blocker and competitor, i.e.
| (A6) |
Equation (A5) could be further reduced into a simpler form by assuming that the binding of the competitor involves the same total number of electrical charges as that of the blocker, i.e. zB=nHzC, but was used as it stands above.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- Bae YM, Park MK, Lee SH, Ho WK, Earm YE. Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J Physiol. 1999;514:747–758. doi: 10.1111/j.1469-7793.1999.747ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflugers Arch. 2002;443:892–902. doi: 10.1007/s00424-001-0775-8. [DOI] [PubMed] [Google Scholar]
- Bosteels S, Matejovic P, Flameng W, Mubagwa K. Sodium influx via a non-selective pathway activated by the removal of extracellular divalent cations: possible role in the calcium paradox. Cardiovasc Res. 1999;43:417–425. doi: 10.1016/s0008-6363(99)00098-x. [DOI] [PubMed] [Google Scholar]
- Chen XZ, Vassilev PM, Basora N, Peng JB, Nomura H, Segal Y, Brown EM, Reeders ST, Hediger MA, Zhou J. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature. 1999;401:383–386. doi: 10.1038/43907. [DOI] [PubMed] [Google Scholar]
- Chu XP, Zhu XM, Wei WL, Li GH, Simon RP, MacDonald JF, Xiong ZG. Acidosis decreases low Ca2+-induced neuronal excitation by inhibiting the activity of calcium-sensing cation channels in cultured mouse hippocampal neurons. J Physiol. 2003;550:385–399. doi: 10.1113/jphysiol.2003.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kasanuki H, Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J. 2003;84:2293–2305. doi: 10.1016/S0006-3495(03)75035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J. 2003;84:922–927. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J General Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GR, Du XL, Siow YL, O K, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res. 2003;58:89–98. doi: 10.1016/s0008-6363(02)00859-3. [DOI] [PubMed] [Google Scholar]
- Macianskiene R, Matejovic P, Sipido K, Flameng W, Mubagwa K. Modulation of the extracellular divalent cation-inhibited non-selective conductance in cardiac cells by metabolic inhibition and by oxidants. J Mol Cell Cardiol. 2001;33:1371–1385. doi: 10.1006/jmcc.2001.1401. [DOI] [PubMed] [Google Scholar]
- Macianskiene R, Moccia F, Sipido KR, Flameng W, Mubagwa K. Channels involved in transient currents unmasked by removal of extracellular calcium in cardiac cells. Am J Physiol Heart Circ Physiol. 2002;282:H1879–1888. doi: 10.1152/ajpheart.00952.2001. [DOI] [PubMed] [Google Scholar]
- Manabe K, Takano M, Noma A. Non-selective cation current of guinea-pig endocardial endothelial cells. J Physiol. 1995;487:407–419. doi: 10.1113/jphysiol.1995.sp020889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J General Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. J Physiol. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Chapman RA. The calcium paradox in isolated guinea-pig ventricular myocytes: effects of membrane potential and intracellular sodium. J Physiol. 1991;434:627–645. doi: 10.1113/jphysiol.1991.sp018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Murata M, Ikeda M, Miyoshi T, Imai M. Primary structure and functional expression of a novel non-selective cation channel. Biochem Biophys Res Commun. 1998;242:191–196. doi: 10.1006/bbrc.1997.7931. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J Physiol. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Volk T, Schwoerer AP, Thiessen S, Schultz JH, Ehmke H. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc Res. 2003;58:76–88. doi: 10.1016/s0008-6363(02)00858-1. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J General Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Lu W, MacDonald JF. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94:7012–7017. doi: 10.1073/pnas.94.13.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, MacDonald JF. Sensing of extracellular calcium by neurones. Can J Physiol Pharmacol. 1999;77:715–721. [PubMed] [Google Scholar]
- Zakharov SI, Mongayt DA, Cohen RA, Bolotina VM. Monovalent cation and L-type Ca2+ channels participate in calcium paradox-like phenomenon in rabbit aortic smooth muscle cells. J Physiol. 1999;514:71–81. doi: 10.1111/j.1469-7793.1999.071af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Smani T, Leno E, Macianskiene R, Mubagwa K, Bolotina VM. Monovalent cation (MC) current in cardiac and smooth muscle cells: regulation by intracellular Mg2+ and inhibition by polycations. Br J Pharmacol. 2003;138:234–244. doi: 10.1038/sj.bjp.0705074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Smani T, Macianskiene R, Mubagwa K, Bolotina VM. Regulation of the hidden monovalent cation (MC) current by intracellular Mg2+, extracellular polyamines and 2-APB. Biophys J. 2002;82:624a. [Google Scholar]
- Zhang YH, Youm JB, Sung HK, Lee SH, Ryu SY, Ho WK, Earm YE. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J Physiol. 2000;523:607–619. doi: 10.1111/j.1469-7793.2000.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]