Abstract
Two muscle insulin-like growth factor-I (IGF-I) mRNA splice variants (IGF-IEa and IGF-IEb) have been identified in rodents. IGF-IEb, also called mechano growth factor (MGF) has been found to be upregulated by exercise or muscle damage. Growth hormone (GH) is the principal regulator of IGF-I expression in several tissues including skeletal muscle. Therefore, we investigated the effect of chronic GH excess or disruption of GH receptor (GHR) signalling, and the acute effect of GH administration on expression of muscle IGF-I isoforms using transgenic mice that express bovine GH (bGH), GHR gene-disrupted (GHR−/−) mice and GH-deficient lit/lit mice before and after exogenous GH administration. MGF mRNA in skeletal muscle was increased in bGH mice whereas it was decreased in GHR−/− mice compared with control animals. Exogenous GH administration to dwarf lit/lit mice significantly increased muscle MGF but not IGF-IEa mRNA 4 h after treatment. Twelve hours after GH treatment, both MGF and IGF-IEa mRNAs in muscle were increased compared with vehicle-treated lit/lit mice. In contrast in GH-sufficient lit/+ mice, both MGF and IGF-IEa mRNAs were increased 4 h after and returned to the basal level 12 h after GH treatment. Hepatic IGF-I isoforms were regulated in parallel by GH. Thus, our results demonstrated that: (1) MGF mRNA in skeletal muscle is expressed in parallel with GH action; (2) MGF mRNA in muscle is produced preferentially in the situation of GH deficiency in contrast to the pattern in the GH-sufficient state; and (3) the induction of IGF-I isoforms by GH is tissue-specific.
Insulin-like growth factor-I (IGF-I) is a ubiquitous peptide that stimulates skeletal growth and cell differentiation in an autocrine/paracrine as well as endocrine fashion (D'Ercole et al. 1984; Stewart & Rotwein, 1996). Previous work indicates that IGF-I is involved in the maintenance of skeletal muscle tissue and also in prevention of cell death and is an important regulator of protein synthesis (Adams, 1998; Rotwein, 2003). IGF-I has a critical role to activate the muscle satellite (stem) cells that are required for local muscle repair (Adams, 1998). Alternative splicing of the IGF-I gene is known to generate several different variants depending on their exon sequences (Stewart & Rotwein, 1996). Yang et al. (1996) cloned the cDNAs of IGF-I splice variants that are expressed in muscle of rabbits and humans. These include IGF-IEa and mechano growth factor (MGF). The former is the same as the circulating IGF-IEa produced by the liver, and the latter is reported to be detected in normal muscle and is markedly upregulated after mechanical stimulation (Yang et al. 1996; McKoy et al. 1999). MGF in rodents is structurally identical to IGF-IEb, originally identified as an isoform of hepatic IGF-I (Roberts et al. 1987). MGF mRNA is derived from the IGF-I gene by alternative precursor mRNA splicing, the sequence of which has a 52 base pair insert in rodents within the E domain. This results in a translational frame-shift that results in a different carboxy terminal sequence to that of IGF-IEa. Previous reports suggest that the E domain of pro-IGF-I precursor may function as a unique growth factor independent of IGF-1 (Siegfried et al. 1992; Tian et al. 1999; Yang & Goldspink, 2002). IGF-IEa peptide transfected into C2C12 myoblast cells caused an increase in cell density and the myoblasts fused to form myotubes whereas MGF peptide caused the mononucleated myoblasts to increase in number but prevented differentiation (Yang & Goldspink, 2002). Hill & Goldspink (2003) demonstrated that MGF was rapidly expressed after muscle damage following the activation of muscle satellite cell and upregulation of IGF-IEa, suggesting that the initial pulse of MGF, not the IGF-IEa, was responsible for satellite cell activation in rat skeletal muscle. In addition, Aperghis et al. (2004) demonstrated that MGF is markedly more effective than IGF-Ia for motoneurone survival, suggesting that MGF may play a role in neuronal maintenance and survival as well as muscle satellite cell activation.
Growth hormone (GH) is the principal regulator of IGF-I expression in tissues (Clemmons et al. 1981; D'Ercole et al. 1984). Transgenic mice that express bovine GH have high serum IGF-I levels as well as high hepatic IGF-I gene expression compared with wild-type mice (Chen et al. 1997; Iida et al. 2004). In contrast, GH-deficient lit/lit mice or GH receptor (GHR) gene-disrupted (GHR−/−) mice have extremely reduced levels of serum IGF-I as well as low hepatic IGF-I mRNA levels (Donahue & Beamer, 1993; Zhou et al. 1997). There is in vitro evidence for the regulation of IGF-I by GH in a mouse C2C12 myoblast cell line (Sadowski et al. 2001; Frost et al. 2002). In vivo, hypophysectomized rats showed reduced expression of IGF-I in muscle that was restored by GH administration (Lemmey et al. 1997; Wilson et al. 1998). Therefore, we hypothesized that MGF, derived from IGF-I gene by alternative splicing, might be regulated not only by exercise or damage as reported previously, but also by GH in skeletal muscle. Ageing in humans is characterized by a loss of muscle strength and mass, and also by a gradual decline in circulating GH (Rudman et al. 1990). It is possible that sarcopenia may be linked to impairment of satellite cell activation by IGF-I, especially by MGF (Chakravarthy et al. 2000). If a chronic state of GH deficiency is associated with reduced levels of MGF, it is compatible with the phenotype of reduced muscle mass observed in elderly people or lit/lit mice. Recently, Hameed et al. (2004) reported that MGF mRNA was significantly increased in healthy elderly men after treatment for 12 weeks with recombinant GH. The same study demonstrated that MGF mRNA was not changed whereas IGF-IEa mRNA was significantly increased after treatment for 5 weeks with recombinant GH, suggesting that IGF-I isoforms in muscle were differentially regulated by GH. However, the effects of chronic GH excess or deficiency on regulation of MGF mRNA, or acute effects of GH on expression of different splice variants of the IGF-I gene remain to be characterized.
The aim of this study was to: (1) determine the effect of chronic GH excess or disruption of GHR signalling on expression of IGF-I isoforms in skeletal muscle; (2) determine the acute effect of a physiological or slightly supraphysiological dose of GH on expression of IGF-I isoforms in muscle of GH-deficient lit/lit mice or GH-sufficient lit/+ mice; and (3) examine the differences in regulation by GH of expression of IGF-I gene isoforms in muscle compared to liver.
Methods
Animals and tissues
All studies were performed using 3-month-old male mice. Two different strains of mice, referred to as bGH (giant transgenic mice that express bovine GH) and GHR−/− (dwarf GH receptor/binding protein gene-disrupted mice) were used to examine the chronic effect of excessive or deficient GH action. Non-transgenic or non-disrupted littermates were used as controls, respectively. These mouse colonies were generated and maintained at Ohio University. The production and characterization of bGH transgenic and GHR−/− mice were described in detail previously (Chen et al. 1997; Zhou et al. 1997). The GH-deficient lit/lit dwarf mice with an inactivating mutation of GH-releasing hormone receptor (Godfrey et al. 1993) were used to examine the effect of chronic isolated GH deficiency and the acute response to GH treatment. The lit/+ mice (GH-sufficient) were used as controls. C57BL/6 J lit/lit and lit/+ mice were produced at a customized colony at the Jackson Laboratory (Bar Harbour, ME, USA). A multiple control experimental design was used, that included: (1) vehicle-treated lit/lit mice, reflecting profound chronic GH deficiency; (2) GH-treated lit/lit mice, reflecting acute changes in response to a single dose of GH; (3) vehicle-treated lit/+ mice, reflecting the normal pattern of GH in terms of both magnitude and pulsatility (Donahue & Beamer, 1993); and (4) GH-treated lit/+ mice, reflecting at least physiological, if not supraphysiological effects of GH. Mice (n = 5 in each group) received a s.c. bolus (120 ng (g body weight)−1) of rat recombinant GH (rrGH) (Genentech Inc., South San Francisco, CA, USA) or vehicle (0.9% saline, 100 μl) 4 h and 12 h before anaesthesia with halothane and were killed for tissue harvesting. All mice were allowed to move freely. Food and water were supplied ad libitum. Liver and skeletal muscle (gastrocnemius) were collected and flash-frozen in liquid nitrogen and stored at −80°C for subsequent mRNA analysis. All animal experiments were approved by the Animal Care and Use Committees of the University of Virginia or Ohio.
Total RNA preparations
The RNA extraction was performed using Tri Reagent (Molecular Research Center, Inc. Cincinnati, OH, USA) followed by RNeasy Mini Kit (Qiagen, Inc. Valencia, CA, USA) according to the manufactures' instructions. The quantity of extracted total RNA was determined using the RiboGreen RNA Quantification Kit (Molecular Probes, Eugene, OR, USA) with a Genios multidetection reader (Phenix Research Product, Hayward, CA, USA).
Real-time RT-PCR
Primers for murine IGF-IEa and IGF-IEb were designed as previously described (Hill & Goldspink, 2003) with modifications (Fig. 1A). The 18S rRNA primers were as previously described (Chen & Hughes-Fulford, 2001). The PCR primers used were as follows: IGF-IEa, forward, 5′-GCTTGCTCACCTTCACCAGC-3′, reverse, 5′-AATGTACTTCCTTCTGAGTCT-3′; IGF-IEb, forward, 5′-GCTTGCTCACCTTCACCAGC-3′, reverse, 5′-AAATGTACTTCCTTTCCTTCTC-3′; 18S rRNA, forward, 5′-TCAAGAACGAAAGTCGGAGG-3′, reverse, 5′-GGACATCTAAGGGCATCACA-3′. The reaction of RT-PCR was previously described (Iida et al. 2004). Briefly, 500 ng total RNA from liver and skeletal muscle was reverse transcribed in a total volume of 10 μl using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacture's instruction. A 1 : 20 dilution of the resultant cDNA was prepared and 4 μl of this template was used in the real-time PCR protocol. The iCycler iQ Real-Time PCR detection system (Bio-Rad Laboratories, Inc. Hercules, CA, USA) was used for sample cDNA quantification. Real-time quantitative PCR was performed in a volume of 20 μl using JumpStart Taq DNA Polymerase (Sigma, St Louis, MO, USA) and SYBR Green I (Molecular Probes) was used for detection of PCR products. The PCR protocol consisted of 5 min at 95°C followed by 40 cycles of 15 s at 94°C, 40 s at 55°C, and 45 s at 72°C. To assess PCR specificity, melting curves from 55 to 95°C in 0.5°C steps of 10 s with measurement of fluorescence were generated at the end of each PCR. A single melting peak and a single band on 2% agarose gel electrophoresis were confirmed for each gene product. The nucleotides of PCR products were confirmed by sequencing using a DNA sequencer (model 3100, Applied Biosystems, Foster City, CA, USA).
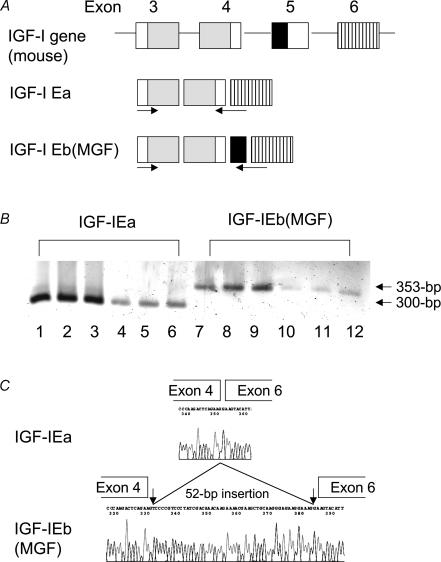
Figure 1. Detection of IGF-IEa and MGF mRNA in skeletal muscle.
A, alternative splicing process producing murine IGF-I isoforms and the position of primers used in this study. The grey box indicates the coding region of mature IGF-I protein. The black box indicates the 52-base pair nucleotides that are inserted in IGF-IEb protein. B, detection of both IGF-IEa and IGF-IEb (MGF) in skeletal muscle of wild-type mice (lane: 1–3 and 7–9) and GHR−/− mice (lane: 4–6 and 10–12). Amplified products by RT-PCR were separated on 2% agarose gel and visualized with ethidium bromide staining. C, sequence analysis of the band shown in B confirmed that the amplified products were IGF-IEa and IGF-IEb (MGF), respectively.
Quantification
Plasmids including the PCR product of murine IGF-IEa, IGF-IEb or 18S rRNA were constructed, and the DNA concentration of each plasmid was determined using a Biomate Spectrophotometer (260 nm/280 nm) (Thermo Spectronic, Rochester, NY, USA). These plasmids were used as standards for quantification. A standard curve was generated by amplifying serial dilutions of a known quantity of plasmid DNA. The standards and cDNA samples were then co-amplified in the same reaction plate. The standard curve displayed a linear relationship between cycle threshold (Ct) values and the logarithm of input plasmid copy number. The amount of product in a particular sample was determined by interpolation from a standard curve of Ct values generated from the plasmid dilution series. All measurements were performed in triplicate in individual assays and the mean value of the triplicate was used for analysis.
Data analysis
Results of the expression of isoforms of the IGF-I gene were adjusted to18S rRNA amplified, and were expressed as mean ± s.e.m. Differences were determined by unpaired t test or one-way ANOVA, as appropriate. P < 0.05 was considered significant.
Results
Mice were weighed before injection of GH or vehicle. The bGH mice weighed 42.7 ± 3.9 g (mean ± s.d.) whereas the control mice weighed 29.9 ± 2.4 g (P < 0.0005). The GHR−/− mice weighed 19.4 ± 3.3 g whereas the control mice weighed 32.1 ± 2.7 g (P < 0.001). The lit/lit mice weighed 17.1 ± 1.8 g whereas lit/+ mice weighed 30.4 ± 1.2 g (P < 0.001).
Using the specific primer for IGF-IEa and MGF (IGF-IEb), the RT-PCR products from skeletal muscle of GHR−/− as well as wild-type mice were detected (Fig. 1B). These products were confirmed to be IGF-IEa and MGF, respectively, by sequencing (Fig. 1C).
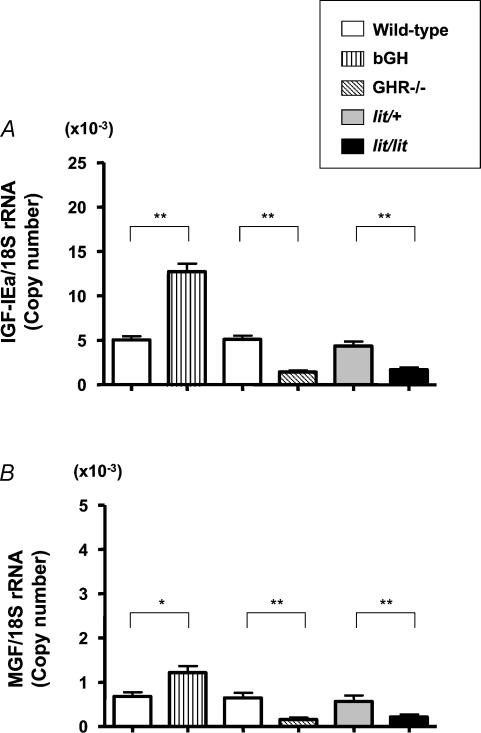
In this study, 18S rRNA was used as a housekeeping gene as our preliminary study showed the consistency of 18S rRNA irrespective of altered GH action or treatment by GH whereas the amount of other housekeeping genes such as GAPDH or β-actin was changed by GH treatment (data not shown). As shown in Fig. 2A, IGF-IEa mRNA in skeletal muscle in bGH mice was 266% and that in GHR−/− mice was 30% of that in control mice. IGF-IEa mRNA in skeletal muscle in lit/lit mice was 39% of that in lit/+ mice. On the other hand, MGF mRNA in skeletal muscle in bGH mice was 152% and that in GHR−/− mice was 19% of that in control mice. MGF mRNA in skeletal muscle in lit/lit mice was 25% of that in lit/+ mice (Fig. 2B).
Figure 2. Expression of IGF-IEa and MGF mRNA in skeletal muscle.
Expression of IGF-IEa (A) and MGF (B) mRNA in skeletal muscle in bGH, GHR−/− and lit/lit mice. Non-transgenic or non-knockout littermates were used as controls for bGH or GHR−/−, respectively. lit/+ mice were used as controls for lit/lit mice; n = 6 in each group. *P < 0.05; **P < 0.01.
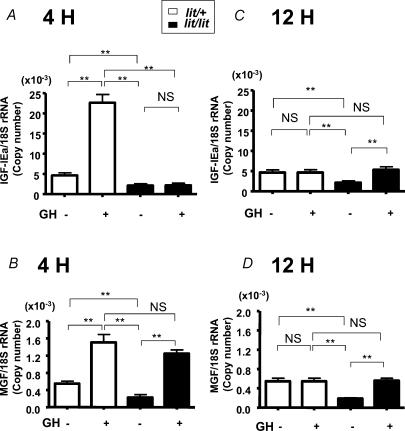
We then examined the changes of IGF-I isoforms expressed in muscle after a single injection of GH using GH-deficient (lit/lit) or GH-sufficient (lit/+) mice. Both IGF-IEa and MGF mRNAs were significantly increased (490% and 275% of control mice, respectively) 4 h after GH injection in lit/+ mice. On the other hand, IGF-IEa mRNA was not increased whereas MGF was significantly increased (553% of control mice) 4 h after GH injection in lit/lit mice (Fig. 3A and B). In lit/+ mice, both IGF-IEa and MGF mRNAs returned to the basal level 12 h after GH injection whereas both IGF-IEa and MGF mRNAs were significantly increased (252% and 301% of control mice, respectively) 12 h after GH injection in lit/lit mice (Fig. 3C and D).
Figure 3. Expression of IGF-IEa and MGF mRNA in skeletal muscle.
Expression of IGF-IEa (A and C) and MGF (B and D) mRNA in skeletal muscle of lit/+ mice or lit/lit mice 4 h (A and B) or 12 h (C and D) after GH- or vehicle-treatment; n = 5 in each group. **P < 0.01; NS, not significant.
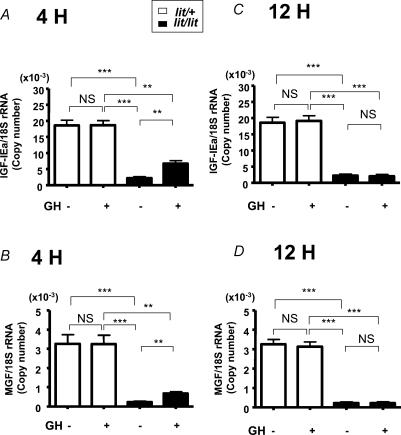
Finally in the same studies we examined liver, where IGF-I mRNA is abundantly expressed, to verify the tissue-specificity of regulation of MGF expression. We confirmed that both IGF-I isoforms were expressed in liver of not only wild-type mice but also bGH and GHR−/− mice (data not shown). In contrast to the skeletal muscle, in lit/+ mice neither IGF-IEa nor MGF mRNAs were increased whereas in lit/lit mice, both were significantly increased (303% and 299% of control mice, respectively) 4 h after GH treatment, and returned to basal level 12 h after GH injection (Fig. 4).
Figure 4. Expression of IGF-IEa and MGF mRNA in liver.
Expression of IGF-IEa (A and C) and MGF (B and D) mRNA in liver of lit/+ mice or lit/lit mice 4 h (A and B) or 12 h (C and D) after GH- or vehicle-treatment; n = 5 in each group. **P < 0.01; ***P < 0.001; NS, not significant.
Discussion
The main finding of the present study is that in skeletal muscle, in addition to IGF-IEa mRNA, MGF mRNA is regulated by GH. Previous reports have emphasized the role of exercise in regulation of MGF mRNA expression in skeletal muscle (Yang et al. 1996; McKoy et al. 1999). Of more importance, we found that a single injection of GH increased MGF mRNA more rapidly than IGF-IEa mRNA in skeletal muscle of GH-deficient mice. Furthermore, the kinetics of MGF and IGF-IEa in skeletal muscle after GH treatment were different depending on whether the mouse was GH-deficient or not.
There is some evidence that IGF-IEb is regulated by GH (Roberts et al. 1987; Lowe et al. 1988). However, the previous report focused on hepatic IGF-IEb regulation by GH using hypophysectomized rats with four daily high dose injections of purified rat GH (150 μg, i.p) (Lowe et al. 1988). The advantages and differences of our study from previous reports are that: (1) mouse models with isolated GH deficiency, completely disrupted GHR signalling, or chronic GH excess were used; (2) we focused on skeletal muscle to elucidate the role of IGF-IEb as ‘mechano growth factor’; (3) we examined the acute effect of GH treatment in GH-deficient and GH-sufficient mice; and (4) we used a lower, more physiological dose of rrGH (120 ng (g body weight)−1, i.e. < 4 μg per injection for control animals) than that used in the previous study (150 μg per injection, i.e > 500 ng (g body weight)−1) (Lowe et al. 1988) in order to mimic as closely as possible the physiological state (Waxman et al. 1991; Kalu et al. 1998). That the dose was close to physiological levels is supported by the lack of effect in liver of the GH-sufficient lit/+ mice to this dose of rrGH in contrast to the large effect observed in the GH-deficient lit/lit mice (Fig. 4).
Our results using bGH and GHR−/− mice suggest that MGF as well as IGF-IEa mRNA levels in skeletal muscle paralleled GH action in the chronic state (Fig. 2). On the other hand, detection of MGF and IGF-IEa mRNA in skeletal muscle of GHR−/− mice indicates that GH is not the exclusive regulator of MGF and IGF-IEa in skeletal muscle as previously suggested (DeVol et al. 1990). Both MGF and IGF-IEa mRNAs were detected in GHR−/− mice in liver as well (data not shown). It is well known that ageing is associated with reduced serum GH levels and with reduced muscle mass. If MGF plays a relevant role to activate muscle satellite cells as demonstrated by Yang & Goldspink (2002), our results suggest that reduced serum GH levels associated with ageing might play a role in this reduction of muscle mass. We speculate that low MGF mRNA expression in the basal state in GH deficiency may be associated with a relative resistance to exercise-induced MGF expression. GH may be crucial to facilitate the increase of MGF by exercise as discussed by Hameed et al. (2004). Supporting our present data, lower expression of MGF mRNA in old rats was observed compared with that in young rats before and after muscle overload (Owino et al. 2001). However, no difference was observed in the basal expression level of MGF in muscle between young and older people although there was a significant difference between these groups after exercise (Hameed et al. 2003). The reason for the discrepancy between rodents and humans is unclear. However one possible explanation is that muscle strength varies in older people depending on life-style, and non-GH factors (i.e. habitual exercise) may compensate for the reduced GH-associated MGF mRNA expression in some older people.
A relevant finding in this study is that MGF mRNA in skeletal muscle increased more rapidly than IGF-IEa after GH treatment in GH-deficient lit/lit mice (Fig. 3). On the other hand, IGF-IEa mRNA was already increased 4 h after GH treatment in GH-sufficient lit/+ mice (Fig. 3A and B). Taken together, MGF mRNA is preferentially produced after GH treatment in skeletal muscle in the chronic GH-deficient state. As mice used in this study were physically active, we are unable to exclude the possibility that activity status plays a role in regulating the splicing of the IGF-I gene after upregulation of primary IGF-I transcript by GH administration. However, the significant differences of MGF mRNA induction between GH-treated and vehicle-treated lit/lit mice despite all mice being physically active suggest that GH may have a potential role to regulate pre-mRNA splicing of the IGF-I gene in skeletal muscle. There is evidence that MGF has a different physiological role in skeletal muscle from IGF-IEa. Yang & Goldspink (2002) reported that the distinct E domain of MGF inhibits terminal differentiation while increasing myoblast proliferation, which contrasts with the role of mature IGF-I peptide. Furthermore, Shavlakadze et al. (2004) demonstrated that transgenic mice with skeletal muscle-specific overexpression of IGF-IEa did not stimulate the early events associated with myogenesis, suggesting that other IGF-I isoforms such as MGF rather than IGF-IEa may play a relevant role in the early events of tissue repair in skeletal muscle. Thus, preferential induction of MGF mRNA by GH replacement in the situation of GH deficiency may be favourable for the efficient activation of muscle satellite cells although we did not measure the protein level of MGF.
Another interesting finding in this study is that the timing of IGF-I mRNA expression was different between liver and skeletal muscle despite the fact that a s.c injection was not expected to affect preferentially any of these tissues. Four hours after GH administration in lit/lit mice, both IGF-IEa and MGF were increased in liver (Fig. 4A and B) whereas only MGF was increased in skeletal muscle (Fig. 3A and B). On the other hand 12 h after GH treatment, both hepatic IGF-IEa and MGF mRNA levels returned to the basal level (Fig. 4C and D) whereas both IGF-IEa and MGF mRNA levels were increased in skeletal muscle (Fig. 3C and D). This suggests a faster mRNA turnover in liver, that might be related to a tighter regulation of IGF-I production and secretion, as the liver is the main source of the IGF-I protein in serum that regulates pituitary GH secretion (Yakar et al. 1999; Sjogren et al. 1999; Wallenius et al. 2001). Alternatively, it might reflect an overall faster mRNA turnover in liver than in skeletal muscle, affecting a variety of genes besides IGF-I. There were no differences in hepatic IGF-IEa or MGF mRNA levels between GH-treated and vehicle-treated lit/+ mice either 4 h or 12 h after treatment (Fig. 4) whereas in skeletal muscle, both IGF-IEa and MGF mRNA levels were significantly increased in lit/+ mice 4 h after GH treatment (Fig. 3A and B) and returned to the basal level 12 h after treatment (Fig. 3C and D). These results show a different timing of induction of IGF-I mRNA by GH between liver and skeletal muscle; muscle is a slow responder to GH in vivo. Sadowski et al. (2001) demonstrated that the activation of mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-phosphate kinase (PI3K) induced by GH inhibited, rather than stimulated, IGF-I mRNA expression in C2C12 myoblast cell lines. Frost et al. (2002) demonstrated that signal transducer and activator of transcription (Stat) 3 played a critical role in GH-induced IGF-I mRNA induction in C2C12 myoblast cells, in contrast to the results observed in hepatocytes (Ram et al. 1996). These results suggest that the difference of post receptor pathways involved in GHR signalling in a given tissue may explain the tissue-specific difference of induction of IGF-I mRNA. We did not find that hepatic MGF was increased more rapidly than hepatic IGF-IEa. The rrGH dose used in this studies might have been low enough to induce detectable changes in liver in GH-sufficient lit/+ mice. Alternatively, an increase in IGF-IEa earlier than 4 h and/or MGF mRNA in liver returning quickly to the basal level may have been missed. Considering that physiologically hepatic IGF-I is secreted in the circulation and that it accounts for > 75% of serum IGF-I (Yakar et al. 1999; Sjogren et al. 1999), the physiological significance of hepatic MGF is unclear but may be different from that of skeletal muscle.
In conclusion, we have demonstrated that MGF expression in skeletal muscle is associated with GH action in vivo. In a GH-deficient state, MGF mRNA is increased by GH administration more rapidly than IGF-IEa whereas this is not observed in the GH-sufficient state. The induction of IGF-I mRNA expression by GH is tissue-specific.
Acknowledgments
We are grateful to Pattie Hellmann and Amy Holland for excellent technical assistance. This work was supported in part by a grant from the Foundation for Growth Science in Japan, a grant from the Uehara Memorial Foundation (to K.I.), and by a grant from Pharmacia Corporation division of Pfizer, Inc., by an unrestricted grant from Bristol-Myers Squibb Foundation (to M.O.T.) and a gift to the laboratory by Mr Sal Ranieri. J.J.K. is supported, in part, by the state of Ohio's Eminent Scholar Program that includes a gift by Milton and Lawrence Goll, by NIH Grant RO1 AG19899-03 and by a grant from DiAthegen, LLC.
References
- Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60. [PubMed] [Google Scholar]
- Aperghis M, Johnson IP, Cannon J, Yang SY, Goldspink G. Different levels of neuroprotection by two insulin-like growth factor-I splice variants. Brain Res. 2004;1009:213–218. doi: 10.1016/j.brainres.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Chen NY, Chen WY, Kopchick JJ. Liver and kidney growth hormone (GH) receptors are regulated differently in diabetic GH and GH antagonist transgenic mice. Endocrinology. 1997;138:1988–1994. doi: 10.1210/endo.138.5.5123. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Underwood LE, Van Wyk JJ. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981;67:10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259:E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2-1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology. 2002;143:492–503. doi: 10.1210/endo.143.2.8641. [DOI] [PubMed] [Google Scholar]
- Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol. 2004;555:231–240. doi: 10.1113/jphysiol.2003.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol. 2003;549:409–418. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Del Rincon JP, Kim DS, Itoh E, Nass R, Coschigano KT, Kopchick JJ, Thorner MO. Tissue-specific regulation of growth hormone (GH) receptor and insulin-like growth factor-I gene expression in the pituitary and liver of GH deficient (lit/lit) mice and transgenic mice that overexpress bGH or a bGH antagonist. Endocrinology. 2004;145:1564–1570. doi: 10.1210/en.2003-1486. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Orhii PB, Chen C, Lee DY, Hubbard GB, Lee S, Olatunji-Bello Y. Aged-rodent models of long-term growth hormone therapy: lack of deleterious effect on longevity. J Gerontol A Biol Sci Med Sci. 1998;53:B452–B463. doi: 10.1093/gerona/53a.6.b452. [DOI] [PubMed] [Google Scholar]
- Lemmey AB, Glassford J, Flick-Smith HC, Holly JM, Pell JM. Differential regulation of tissue insulin-like growth factor-binding protein (IGFBP) -3, IGF-I and IGF type 1 receptor mRNA levels, and serum IGF-I and IGFBP concentrations by growth hormone and IGF-I. J Endocrinol. 1997;154:319–328. doi: 10.1677/joe.0.1540319. [DOI] [PubMed] [Google Scholar]
- Lowe WL, Jr, Lasky SR, LeRoith D, Roberts CT., Jr Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. 1988;2:528–535. doi: 10.1210/mend-2-6-528. [DOI] [PubMed] [Google Scholar]
- McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol. 1999;516:583–592. doi: 10.1111/j.1469-7793.1999.0583v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- Ram PA, Park SH, Choi HK, Waxman DJ. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem. 1996;271:5929–5940. doi: 10.1074/jbc.271.10.5929. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Jr, Lasky SR, Lowe WL, Jr, Seaman WT, LeRoith D. Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol. 1987;1:243–248. doi: 10.1210/mend-1-3-243. [DOI] [PubMed] [Google Scholar]
- Rotwein P. Insulin-like growth factor action and skeletal muscle growth, an in vivo perspective. Growth Horm IGF Res. 2003;13:303–305. doi: 10.1016/j.ghir.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Sadowski CL, Wheeler TT, Wang LH, Sadowski HB. GH regulation of IGF-I and suppressor of cytokine signaling gene expression in C2C12 skeletal muscle cells. Endocrinology. 2001;142:3890–3900. doi: 10.1210/endo.142.9.8365. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Davies M, White JD, Grounds MD. Early regeneration of whole skeletal muscle grafts is unaffected by overexpression of IGF-1 in MLC/mIGF-1 transgenic mice. J Histochem Cytochem. 2004;52:873–883. doi: 10.1369/jhc.3A6177.2004. [DOI] [PubMed] [Google Scholar]
- Siegfried JM, Kasprzyk PG, Treston AM, Mulshine JL, Quinn KA, Cuttitta F. A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc Natl Acad Sci U S A. 1992;89:8107–8111. doi: 10.1073/pnas.89.17.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- Tian XC, Chen MJ, Pantschenko AG, Yang TJ, Chen TT. Recombinant E-peptides of pro-IGF-I have mitogenic activity. Endocrinology. 1999;140:3387–3390. doi: 10.1210/endo.140.7.7044. [DOI] [PubMed] [Google Scholar]
- Wallenius K, Sjogren K, Peng XD, Park S, Wallenius V, Liu JL, Umaerus M, Wennbo H, Isaksson O, Frohman L, Ohlsson C, Jansson JO. Liver-derived IGF-I regulates GH secretion at the pituitary level in mice. Endocrinology. 2001;142:4762–4770. doi: 10.1210/endo.142.11.8478. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Rattray M, Thomas CR, Moreland BH, Schulster D. Effects of hypophysectomy and growth hormone administration on the mRNA levels of collagen I, III and insulin-like growth factor-I in rat skeletal muscle. Growth Horm IGF Res. 1998;8:431–438. doi: 10.1016/s1096-6374(98)80295-5. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B & LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]