Abstract
Glycine is an inhibitory neurotransmitter and is critical for NMDA receptor activation. These roles are dependent on extracellular glycine levels, which are regulated by Na+/Cl−-dependent glycine transporters (GlyTs) in neurones and glia. The glial GlyT subtype GlyT1 is well located to activate NMDA receptors. However, glial GlyTs have not been studied in an intact system thus far. Whole-cell patch-clamp recordings were obtained from Bergmann glia in mice cerebellar slices to determine whether these glia express functional GlyT1 that can mediate both glycine uptake and efflux. In the presence of a glycine receptor blocker, glycine and a substrate agonist for GlyT1, sarcosine, induced voltage-dependent inward currents that were abolished by removing external Na+, identifying them as transport currents. Inhibitors of glycine transport through GlyT1 (sarcosine and (N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS)) reduced glycine currents by ∼85%, consistent with positive immunostaining for GlyT1 in Bergmann glia while inhibitors of glycine transport through GlyT2 (4-benzyloxy-3,5-dimethoxy-N-[1-(dimethylaminocyclopently)methyl]benzamide (ORG 25543) and amoxapine) or through systems A and ASC did not affect glycine transport currents. Following internal glycine perfusion during the recording, outward currents progressively developed at −50 mV and external glycine-induced uptake currents were reduced. Using paired recordings of a Bergmann glial cell and a granule cell in the whole cell and outside-out modes, respectively, depolarizations of Bergmann glia to +20 mV induced a 73% increase in the open probability of glycine receptor channels in membrane patches of granule cells. This increase was prevented when NFPS was included in the bath solution. Overall, these results demonstrate for the first time that Bergmann glia express functional GlyT1 that can work in reverse at near-physiological ionic and internal glycine conditions in brain slices. These glial GlyTs can probably mediate glycine efflux under conditions of metabolic impairments like ischaemia.
Glycine transporters (GlyTs) have been suggested to play an important regulatory role at glycine receptor containing synapses by clearing glycine from the synaptic cleft, and at synapses containing NMDA receptors (NMDARs) by keeping the extracellular glycine levels below saturating concentrations at the glycine site on NMDARs (Wood, 1995; Supplisson & Bergman, 1997; Parsons et al. 1998; Danysz & Parsons, 1998; Gomeza et al. 2003a,b). Glycine as well as d-serine function as obligatory co-agonists by acting on a strychnine-insensitive site of NMDARs (glycineB site) (Johnson & Ascher, 1987; Kleckner & Dingledine, 1988; White et al. 1989; Mayer et al. 1989; Thomson et al. 1989; Schell et al. 1997; Baranano et al. 2001). Glycine binding to the glycineB site has also recently been shown to prime NMDA receptor internalization (Nong et al. 2003). Although there is still some debate whether the glycineB site is saturated in vivo, it is possible that GlyTs can keep locally extracellular glycine levels below saturating concentrations at the glycineB site because GlyTs are colocalized with NMDARs (Smith et al. 1992). This probably depends on regional differences in NMDAR subtype expression and local glycine synthesis/metabolism (Wood, 1995; Danysz & Parsons, 1998). It has also been suggested that reverse glycine transport could result in increased glycine levels at NMDAR-containing synapses (Roux & Supplisson, 2000). Such a Ca2+-independent glycine release has been shown to occur from cultured cerebellar astrocytes under high K+ stimulation (Holopainen & Kontro, 1989) and from hippocampal slices under physiological, high K+ stimulation or various pathological conditions such as ischaemia (Saransaari & Oja, 1994, 2001). Interestingly, an enhancement of ischaemia-induced damage could be obtained in vivo with exogenous glycine, suggesting that the glycineB site was not saturated under these conditions (Dalkara et al. 1990).
Two GlyTs have been cloned, GlyT1 and GlyT2 (Guastella et al. 1992; Smith et al. 1992; Liu et al. 1993; Morrow et al. 1998), that are expressed in astrocytes and in neurones, respectively (Zafra et al. 1995; Adams et al. 1995), and are members of the Na+/Cl−-dependent transporter superfamily (Liu et al. 1993; Aragon et al. 1987). GlyT1 is widely expressed in the CNS whereas GlyT2 has a more limited distribution and is colocalized with inhibitory glycine receptors (Zafra et al. 1995). The glial GlyT1s have been shown to be colocalized with NMDARs in rat brain (Smith et al. 1992) and influence NMDAR function in acute brainstem and hippocampal slices (Supplisson & Bergman, 1997; Bergeron et al. 1998) as well as in the hippocampus and pre-frontal cortex in vivo (Kinney et al. 2003; Chen et al. 2003). Furthermore, GlyT1 can take up or release glycine depending on the Na+ and glycine concentrations, and the membrane potential (Sakata et al. 1997; Roux & Supplisson, 2000) while GlyT2 has a thermodynamic constraint for reverse transport, thus limiting glycine release. Although glia have been shown to contain ∼2 mm glycine (Berger et al. 1977), it is still unknown whether GlyTs in glia can work in reverse.
Bergmann glial processes, which totally ensheath Purkinje neurone soma and dendrites, have been shown to express GlyT1 by in situ hybridization and immunostaining in rats (Guastella et al. 1992; Zafra et al. 1995). However, no functional characterization of GlyT currents has been performed in Bergmann glia in slices thus far. Therefore given the important function of GlyT1 at tightly regulating glycine levels and the potential for control of NMDAR-containing synaptic transmission by GlyT1, we undertook to determine whether Bergmann glia possess functional GlyT1. Furthermore, we questioned whether Bergmann glial GlyTs could mediate glycine efflux. The results of this study will help to elucidate the physiological and pathological functions of glial GlyT1 in the CNS.
Methods
Slice preparation
Cerebellar slices were prepared as previously described (Bordey & Sontheimer, 2000; Barakat & Bordey, 2002). Briefly, 10- to 23-day-old mice were anaesthetized using pentobarbital (50 mg kg−1) and decapitated. A rapid craniotomy allowed the cerebellum to be quickly detached, removed and chilled (0–4°C) in 95% O2–5% CO2 saturated artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 125; KCl 2.5; CaCl2 2; MgCl2 2; NaHCO3 25; glucose 10. Animal anaesthesia with pentobarbital sodium and euthanasia by decapitation were performed in accordance with the Institutional Animal Care and use committee, which is guided by US Government Principles for the utilization and care of Vertebrate Animals used in Testing, Research and Training. Next, the cerebellum was glued to the stage of a vibratome and transversal slices (250 μm thick) were cut in cold oxygenated ACSF. After a recovery period of > 1 h in ACSF, slices were placed in a flow-through chamber, held in position by a nylon mesh glued to a U-shaped platinum wire and continuously superfused with oxygenated ACSF at room temperature. The chamber was mounted on the stage of an upright microscope (Olympus BX50) equipped with a 60 × water immersion objective and infrared optics.
Electrophysiology and drug application
Whole-cell patch-clamp recordings were obtained as previously described (Bordey & Sontheimer, 2000; Barakat & Bordey, 2002). Patch pipettes were pulled from thin-walled borosilicate glass (o.d., 1.55 mm; i.d., 1.2 mm; WPI, TW150F-40) on a PP-83 puller (Narishige, Japan). Pipettes had resistances of 6–8 MΩ when filled with the following solutions (in mm) to study glycine uptake currents in Bergmann glia or glycine receptor currents and channels in granule cells: KCl 140 or KCl 30 + potassium gluconate 110 when noted (for Bergmann glial cells) or CsCl 140 (for granule cells); CaCl2 1.0; MgCl2 2.0; EGTA 10; Hepes 10; MgATP 4; 0.1% lucifer yellow (LY, dilithium salt); pH was adjusted to 7.2 with NaOH for Bergmann glia, which introduced 2.5 mm Na+ in the intracellular solution, or with TrisBase for granule cells. For obtaining simultaneous recordings of a granule cell and a Bergmann glial cell, an outside-out recording of a granule cell was first obtained. Then, after obtaining a simultaneous whole-cell recording from a nearby Bergmann glial cell, the patch from the granule cell was placed just above the recorded Bergmann glial cell but outside the slice. In most cases, the Bergmann glia were pulled up closer to the surface of the slice.
Liquid junction potentials of 2.5 mV and 6–8 mV with a KCl- and potassium gluconate-based solutions, respectively, were not corrected. For experiments lowering extracellular [Cl−], a high molarity agar bridge made out of a bent glass pipette was used. To study the voltage dependence of glycine transporter and GABAA receptor currents, the cells were manually depolarized by adjusting the holding potential. When recorded with a KCl-based solution there was a large change in the holding current when manually depolarizing the recorded cells. In this condition glycine or a GABAA receptor agonist was applied > 3 min after a 20 mV depolarization to allow for the holding currents to stabilize. When voltage steps were applied to the recorded cells 5 mm Cs+ or Ba2+ and 40 mm TEA was added to the extracellular solution in exchange for an equimolar amount of Na+ to suppress K+ currents in Bergmann glia (Bordey & Sontheimer, 2000). To permit reversal of glycine transporters, Hepes sodium salt was used instead of Hepes, 4 mm glycine was added, and 4 mm KCl was removed (26 mm instead of 30 mm). The pH of the internal solution was adjusted with NaOH, which introduced 2.5 mm Na+ and resulted in an internal [Na+] of 12.5 mm, close to reported physiological values in astrocytes (Rose & Ransom, 1996a). Whole-cell recordings were performed using an Axopatch-200B amplifier (Axon Instruments). Current signals were low-pass filtered at 2–5 kHz and digitized on-line at 5–20 kHz using a Digidata 1320 digitizing board (Axon Instruments) interfaced with an IBM-compatible computer system. Data acquisition, storage and analysis performed using pCLAMP version 8.0.2 (Axon Instruments). Settings were determined by compensating the transients of a small (5 mV) 10 ms hyperpolarizing voltage step. The capacitance reading of the amplifier was used as value for the whole-cell capacitance. Bergmann glia exhibiting a Cm < 20 pF were disregarded. The resting potential and input resistance were determined in the first 3 min of whole-cell recordings. Capacitive and leak conductances were not subtracted. Peak currents were determined using Clampfit (Axon Instruments), and statistical values (means ± s.e.m., with n being the number of cells tested) were evaluated with a statistical graphing and curve-fitting program (Origin, MicroCal). To quantify the effect of several drugs on glycine uptake currents, the amplitudes of glycine uptake currents were measured before and then during application of a drug. Statistical comparisons of means were performed with Student's t test (Statview, SAS Institute Inc.). Channel open probability (Po) and amplitude of single channel currents were obtained using pCLAMP (version 9, Axon Instruments). Single channel activity in granule cells was analysed for 60–90 s and 30 s after a 2 s depolarization from −70 to +20 mV of Bergmann glial cells. Po of single channels was obtained from the ratio of the areas under the curves representing open events divided by the sum of the areas under the curves representing open and closed events. Channel activity (NPo) was calculated as a product of the number of channels (N) in the patch and Po.
Receptor and transporter inhibitors were diluted in ACSF and applied by regular bath perfusion or a rapid bath application system composed of a 6-channel-mini-valve perfusion system (Warner Instruments Corp., New Haven, CT, USA). Glycine was occasionally applied by this rapid application system. Transporter substrate agonists were pressure applied by a computer-controlled pressure ejection system and were diluted in ACSF, in which Hepes replaced NaHCO3 and pH was adjusted to 7.4 by NaOH. When NaCl was replaced by choline or another chemical, similar changes were performed in the pressure pipette solution. The pressure ejection pipettes were standard unpolished patch electrodes with resistances of 6–8 MΩ for local agonist application and were positioned just above the slice. The applied pressure was between 3 and 6 p.s.i. For application of two drugs to the same cell, theta glass pipettes with one distinct drug in each compartment were used.
Intracellular perfusion of glycine during the recording
Intracellular perfusion of glycine was performed as we previously reported (Barakat & Bordey, 2002; Barakat et al. 2002). We used a straight pipette holder with a perfusion port (EH-U2, E.W.Wright, CT, USA). Through the perfusion port, a polyethylene tube (i.d. 0.86 mm, o.d. 1.27 mm) was introduced sufficiently far to reach well into the patch pipette solution. A 1 ml syringe containing the LY-filled intracellular solution to be perfused during the recording was connected to the polyethylene tube via an elongated plastic pipette tip. Before adding the patch pipette, positive pressure was manually applied to fill the tube all the way to the end, remove air bubbles, and visualize efflux of solution. Then, after applying negative pressure to prevent any solution leakage but without adding an air bubble to the end of the tube, the patch pipette was inserted into the holder. To perfuse the LY-filled solution containing glycine, a positive pressure was manually applied to add sufficient solution to double the volume in the patch pipette. The concentration of glycine was doubled to obtain the intended final concentration in the cell.
Perforated patch-clamp recordings
The cation-selective ionophore gramicidin was used for perforated patch clamp recordings to prevent interference with the intracellular [Cl−] ([Cl−]i)(Myers & Haydon, 1972; Kyrozis & Reichling, 1995). The gramicidin-containing solution was prepared fresh for each experiment, diluted in pre-filtered intracellular solution to yield a final concentration of 5 μg ml−1. To ensure full efficacy of the gramicidin, the solution was protected from light and renewed every 2 h. The liquid junction potential (4.5 mV) was corrected. Patch pipettes had a resistance of 5–6 MΩ when filled with the intracellular solution. Two precautions were used to minimize gramicidin ejection from the patch pipette when approaching the cells: positive pressure was avoided and the pipette tip was filled with the normal intracellular solution by 15–25 s dipping (the gramicidin-containing solution was added by regular filling). After seal formation, the progress of perforation was followed by applying a ramp protocol every 30 s. Stable perforated recordings (< 45 MΩ series resistances, mean of 30.1 ± 3.9 MΩ before compensation of 60–70%, n = 7) were obtained after > 30 min (mean of 39.6 ± 4.3 min).
GlyT1 and GlyT2 immunostainings
CD1 mice 16–19 days old (n = 3) were anaesthetized with sodium pentobarbital (100 mg kg−1) and then fixed by transcardiac perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 0.1 m phosphate buffer, 9% NaCl) at pH 6.5 for 5 min, followed by 4% PFA in PBS at pH 10.5 for 15 min. The brains were removed and postfixed in the second perfusate for 24 h at 4°C. All tissue was mounted in 2% agarose and sectioned on a vibratome into 20 μm sagittal sections. Slices were washed three times in PBS, permeabilized for 20 min with 0.3% Triton X-100, rinsed three times with PBS, and then blocked overnight at 4°C in PBS containing 1% bovine serum albumin, 10% goat serum, and 0.3% triton. Slices were incubated overnight at 4°C with primary goat and sheep antibody against GlyT1 and GlyT2, respectively (1: 1000, Chemicon) in blocking solution. Slices were subsequently washed three times with PBS and incubated for 90 min at room temperature with secondary antibody donkey anti-goat and anti-sheep IgG conjugated to Alexa flour 488 (1: 1000, Molecular Probes). After three washes in PBS, slices were mounted on glass coverslips with fluorescent mounting medium (Vectashield-DAPI, Vector) and were viewed on an epifluorescence microscope (Olympus microscope BX51) using standard procedures. Control experiments did not include the primary antibodies.
Chemicals were purchased from Sigma (St Louis, MO, USA) unless otherwise noted. The selective non-transportable GlyT1 blocker (N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine (NFPS, also called ALX 5407), was kindly provided by Dr Henk Sipma at Johnson and Johnson Pharmaceuticals, a division of Janssen Pharmaceuticals N.V. (Belgium). A selective non-transportable GlyT2 blocker, 4-benzyloxy-3, 5-dimethoxy-N-[1-(dimethylaminocyclopently)methyl] benzamide (ORG 25543), was kindly provided by Dr Sundaram at Organon Laboratories Ltd (Scotland, UK).
Results
Whole-cell recordings were obtained from 132 visually identified Bergmann glia in cerebellar slices and 8 granule cell patch and Bergmann glial cell simultaneous recordings from 10- to 23-day-old mice. We used 14- to 23-day-old animals to study GlyTs in Bergmann glia because GlyT1 and GlyT2 are already highly expressed in the cerebellum and have almost reached adult values (Zafra et al. 1995). In addition, during this age period NMDA receptor activation is known to significantly influence GABA release at GABAergic interneurone–Purkinje neurone synapses (Glitsch & Marty, 1999; Huang & Bordey, 2004). We performed paired recording experiments in slices from P10–13 mice because more granule cells expressed glycine receptors in slices from P10–13 (∼80%, n = 12) than in slices from older animals (∼55%, data not shown, n = 10). Bergmann glia were identified by the location of their cell body in the Purkinje cell layer and by the small size of their somata (8–12 μm diameter) in comparison to the large cell bodies of Purkinje neurones (Fig. 1A). Every cell recorded in the whole-cell configuration was filled with lucifer yellow and identified as a Bergmann glial cell by a typical morphology characterized by long, parallel processes extending in the molecular layer toward the pial surface (Fig. 1B) (de Blas, 1984; Reichenbach et al. 1995). In addition, recorded cells had a characteristically low mean input resistance of 34.5 ± 1.0 MΩ (n = 109, mean ± s.e.m.), a mean hyperpolarized resting membrane potential (VR) of −79.3 ± 0.6 mV (n = 109), and displayed a passive current profile (data not shown).
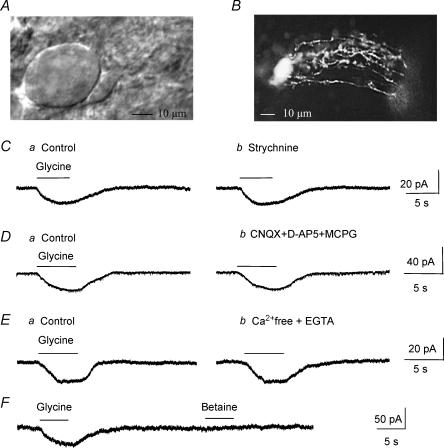
Figure 1. Glycine induces strychnine-insensitive inward currents in Bergmann glia in situ.
A, a DIC photograph of a Purkinje neurone and a Bergmann glial cell somata. B, a fluorescent photograph of a LY-filled Bergmann glial cell. C–E, pressure applications of glycine (500 μm) induced inward currents (C–Ea) that were not affected by bath application of: Cb, 100 μm strychnine, a blocker of glycine receptors; Db, 25 μm CNQX + 50 μm d- APV + 200 μm MCPG, blockers of AMPA/kainate, NMDA and metabotropic glutamate receptors, respectively; and Eb, Ca2+-free + 1 mm EGTA solution. F, glycine and betaine (500 μm each) were pressure applied onto the same cell using a theta glass pipette. All of the recordings were performed from a holding potential of −70 mV with a KCl-based internal solution.
Characterization of glycine uptake currents in Bergmann glia
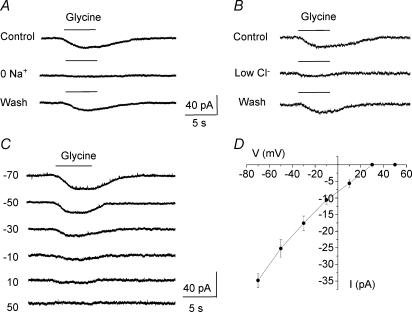
For all of the following experiments, Bergmann glia were recorded at a holding potential of −70 mV in the presence of 1 μm tetrodotoxin (TTX) in the external solution to prevent action potential-induced transmitter release from surrounding neurones. Pressure (5 s) or rapid bath (30 s to 1 min) applications of glycine (500 μm) induced inward currents with mean amplitudes of −39.4 ± 3.8 pA (n = 39/44) and 41.0 ± 4.2 pA (n = 24/27) when recorded with a high and low KCl intracellular solution, respectively. For the study of the glycine uptake currents, cells were recorded with a high KCl solution unless otherwise noted. Glycine-induced currents could be the result of glycine receptor activation and/or Na+-dependent glycine transport systems (Guastella et al. 1992; Smith et al. 1992; Liu et al. 1993; Morrow et al. 1998). Bath application of strychnine (100 μm), a well-known blocker of glycine receptors, had no effect on glycine-induced inward currents (99.4 ± 2.7% of control in the presence of strychnine, n = 6 including 3 cells recorded with a low KCl solution, P > 0.2, Fig. 1C). For all of the following experiments, 100 μm strychnine was added to the TTX-containing extracellular solution. Strychnine-insensitive inward currents were unaffected by bath application of glutamate receptors antagonists, including an AMPA/kainate receptor antagonist (CNQX, 25 μm), a NMDA receptor antagonist (d-APV, 50 μm), and a metabotropic receptor antagonist (MCPG, 200 μm). Glycine-induced currents averaged 97.9 ± 1.0% of control in the presence of glutamate receptor antagonists (n = 4, P > 0.1, Fig. 1D). This result rules out the possibility that glutamate, which could be released following depolarization of presynaptic terminals by glycine uptake induced inward currents by activating glutamate receptors on the recorded Bergmann glial cell. This result also rules out the possibility that glycine-induced currents result from NMDAR activation by glycine. Strychnine-insensitive currents were also unaffected by an extracellular solution containing 0 Ca2+−1 mm EGTA (97.7 ± 1.2% of control in the presence of 0 Ca2+− 1 mm EGTA, n = 3, P > 0.1, Fig. 1E). Glycine uptake currents were also obtained in an extracellular solution containing 0 Ca2+−1 mm EGTA−1 μm TTX when cells were recorded with a low KCl internal solution (n = 6, Fig. 7). In order to ensure the suitability of the pressure application method, we used a theta glass pipette to sequentially pressure apply glycine and betaine onto the same cells. Betaine, which is a substrate agonist of GABA transporter BGT-1/GAT2 (Lopez-Corcuera et al. 1992; Matskevitch et al. 1999), is not transported into Bergmann glia (Barakat & Bordey, 2002). In this condition, pressure applications of glycine induced inward currents while betaine had no effect (Fig. 1F, n = 3). When cells were recorded with a CsCl-based intracellular solution, the mean amplitude of strychnine-insensitive currents averaged −37.4 ± 8.0 pA (n = 5, data not shown), which was not significantly different from that in cells recorded with a KCl-based intracellular solution (mean of −39.4 ± 3.8 pA, n = 39). This result was expected because K+ is not thought to be required for glycine transport as previously shown for the transport of other inhibitory amino acids such as GABA and taurine via Na+/Cl−-dependent transporters (Borden, 1996; Barakat et al. 2002).
Figure 7. Glycine transporters in Bergmann glia can reverse.
A, a solution containing 4 mm glycine was intracellularly perfused during the recording as indicated by the arrow and 500 μm glycine was pressure applied before and after intracellular glycine perfusion. Aa and b, intracellular glycine perfusion induced an outward current in cells held at −50 mV (a) but not in those held at −70 mV (b). In both conditions the inward currents induced by a puff of glycine were diminished by intracellular glycine perfusion. Ac, a solution containing no glycine was intracellularly perfused during the recording (arrow) at −50 mV. B, mean current amplitude against the recording time for cells held at −50 mV and intracellularly perfused with either a solution containing glycine (n = 4, •) or a solution without glycine (n = 4, ○). Baseline currents were considered as the 0 current. C, current traces following 40 mV-increment voltage steps of 150 ms applied from −100 to +60 mV from a holding potential of −70 mV under control conditions (continuous line) and after intracellular perfusion of 4 mm glycine (dashed lines). D, mean I–V curve of glycine efflux currents obtained by subtracting traces after glycine perfusion from control traces (n = 3).
Ion and voltage dependence of glycine-induced currents in Bergmann glia
GlyTs depend on Na+ and Cl− for glycine uptake (Aragon et al. 1987; Guastella et al. 1992; Liu et al. 1993). Figure 2A shows that when Na+ was removed from the extracellular solution (Na+ was replaced by choline or Li+, n = 5, data not shown), strychnine-insensitive glycine currents were reversibly and completely blocked, identifying them as glycine-induced transport currents. Lowering extracellular [Cl−] from 136 to 11 mm (NaCl was replaced with sodium gluconate) significantly (P < 0.03) reduced glycine-induced transport currents by 78.0 ± 3.5% (n = 3, Fig. 2B). When the cell membrane was gradually depolarized from −70 to +50 mV, glycine-induced transport currents display prominent inward rectification and did not reverse for positive membrane potentials up to +50 mV in our recording conditions (Fig. 2C and D, n = 5). Similar current–voltage relationships have been reported for other Na+/Cl−-dependent amino acid transporters (Borden, 1996; Barakat & Bordey, 2002; Barakat et al. 2002). For these experiments and those to determine the voltage dependence of transport reversal, glycine was locally applied onto the cell body and proximal processes to limit any error associated with a poor voltage clamp of distal processes. Such a method has given accurate measurements of the current–voltage (I–V) curve for GABA and taurine transport currents in similar conditions (Barakat & Bordey, 2002; Barakat et al. 2002). Together these data strongly suggest that glycine-induced currents in the presence of strychnine are generated by glycine being taken up into Bergmann glia.
Figure 2. Ion and voltage dependence of strychnine-insensitive glycine-induced currents.
A, glycine-induced currents were abolished by replacing external Na+ with choline, identifying them as glycine transport currents. B, glycine responses were reversibly diminished by reduction of external Cl− (11 mm instead of 136 mm). In A and B recordings were obtained at −70 mV. C, glycine-induced currents recorded at different holding potentials from −70 to +50 mV. These currents inwardly rectified and did not reverse for positive membrane potentials. D, mean current–voltage curve of glycine-induced inward currents (n = 5). All of these experiments were performed in the presence of TTX and a glycine receptor blocker (100 μm strychnine) in the bath.
Immunostaining for GlyT1 and GlyT2
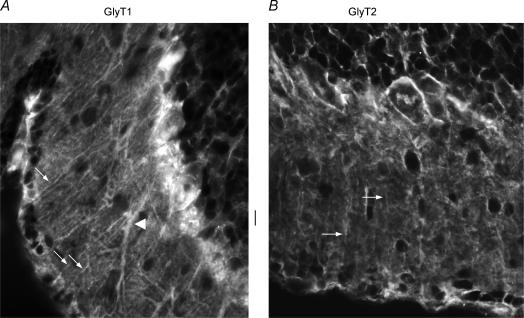
Both GlyT1 and GlyT2 were expressed in the Purkinje cell layer and the molecular layer of the cerebellum. GlyT1 was highly expressed in the cell bodies and processes (arrows in Fig. 3A) of Bergmann glia that displayed endfeet on the pial surface. Weak cytoplasmic GlyT1 was present in Purkinje neurone dendrites (arrowhead, Fig. 3A). GlyT2 stained mostly in the upper part of the molecular layer. GlyT2 weakly stained some presumed glial fibres (arrows in Fig. 3B).
Figure 3. Immunostaining for GlyT1 and GlyT2.
A–B, high power (× 40 objective) photographs of GlyT1 (A) and GlyT2 (B) immunostaining in the cerebellar molecular layer. Arrows and arrowhead in A point to processes from Bergmann glia and a presumed dendrite from Purkinje neurones, respectively, that stain positive for GlyT1. Arrows in B point to a few presumed glial fibres that express GlyT2. Scale bar: 10 μm for both A and B.
Pharmacological characterization of glycine transport currents
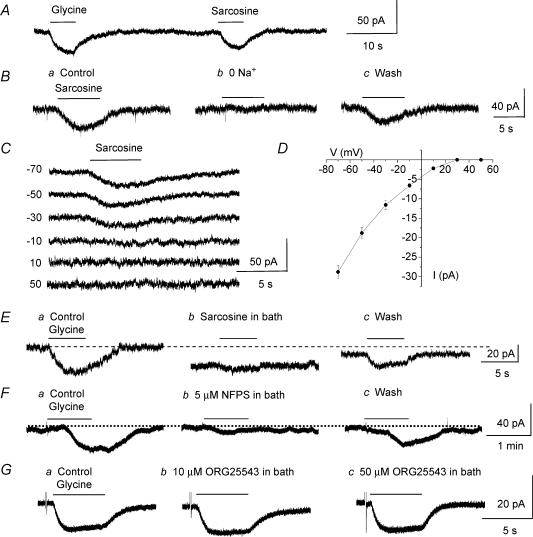
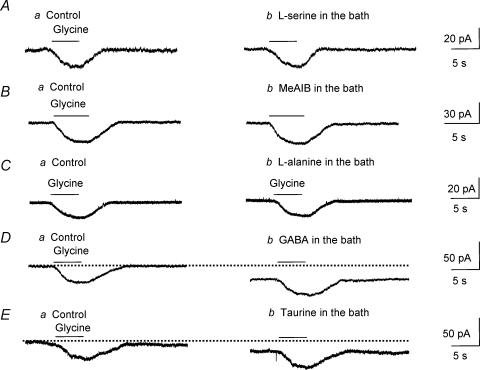
In order to determine the pharmacological profile of glycine transport currents in Bergmann glia, the effects of substrate agonists, several competitive blockers of glycine transport and non-transportable GlyT blockers were tested on strychnine-insensitive glycine-induced currents. Glycine and sarcosine, a substrate agonist of GlyT1, induced inward currents of similar amplitudes when pressure applied onto the same cells by using a theta glass pipette. The means of glycine- and sarcosine-induced currents were −43.7 ± 8.4 pA and −33.7 ± 5.8 pA, respectively (n = 4, Fig. 4A). When Na+ was replaced by choline, sarcosine-induced inward currents were reversibly and completely blocked, identifying these currents as transport currents (n = 5, Fig. 4B). The voltage dependence of sarcosine-induced currents was strictly inwardly rectifying up to +50 mV and did not reverse as observed for glycine-induced transport currents (n = 5, Fig. 4C and D). Bath application of sarcosine (500 μm), which also acts as a competitive blocker of glycine transport through GlyT1 (Smith et al. 1992; Guastella et al. 1992; Liu et al. 1993; Lopez-Corcuera et al. 1998), significantly (P < 0.01) and reversibly reduced glycine-induced transport currents by 83.3 ± 2.4% (n = 11, including 5 cells recorded with a low KCl solution, Fig. 4E). By contrast, 100 μm amoxapine, which blocks glycine transport through GlyT2 by 40% without affecting transport through GlyT1 (Nunez et al. 2000), did not affect glycine-induced currents (99.5 ± 0.9% of control, P > 0.5) and did not induce any inward currents (n = 5, data not shown). Next, we tested the effects of non-transportable blockers of GlyT1, NFPS (Atkinson et al. 2001; Aubrey & Vandenberg, 2001; Mallorga et al. 2003), and of GlyT2, ORG 25543 (Compound 16 in Caulfield et al. 2001), on glycine-induced transport currents. NFPS (5 μm, applied for 3 min prior to glycine application) significantly (P < 0.01) and reversibly reduced glycine-induced transport currents by 87.0 ± 4.9% (from −40.4 ± 11.2 to −6.0 ± 5.1 pA, n = 5 cells recorded with a low KCl solution, Fig. 4F) while ORG 25543 (10–50 μm) had no effect (n = 5, Fig. 4G). NFPS was washed out for a minimum of 20 min. Glycine is also transported by low-affinity transport systems, namely system A and system ASC (Christensen, 1984; Palacin et al. 1998) and both systems have been shown to be expressed in glia (Zafra & Gimenez, 1989; Gadea et al. 1999). Therefore, we tested the effects of competitive blockers of system ASC (l-serine), system A (methylaminoisobutyric acid, MeAIB), and of both systems (l-alanine) (Barker & Ellory, 1990) on glycine-induced transport currents. None of these competitive blockers (each bath applied at 1 mm) affected glycine-induced transport currents (P > 0.1, Fig. 5A, B and C, respectively, n = 3 each). Together these data suggest that glycine is transported through GlyT1 in Bergmann glia. To check for GlyT1 selectivity to glycine, we tested the effects of taurine and GABA on glycine-induced transport currents in the presence of strychnine, picrotoxin and phaclofen to block glycine, GABAA/C and GABAB receptor activations, respectively. Taurine and GABA are known to be taken up by taurine and GABA transporters, respectively, but not by glycine transporters (Guastella et al. 1992; Liu et al. 1992; Smith et al. 1992; Gadea & Lopez-Colome, 2001). Bath application of GABA or taurine each at 500 μm had no effect on glycine-induced transport currents (P > 0.1, Fig. 5D and E, respectively, n = 3 each), suggesting that glycine transport systems in Bergmann glia are selective for the inhibitory amino acid glycine. However, in the presence of GABAA/B/C receptor blockers, GABA and taurine induced inward currents, which probably result from the electrogenic transport of these amino acids by their respective transporters expressed in Bergmann glia (Swan et al. 1994; Morara et al. 1996; Barakat & Bordey, 2002; Barakat et al. 2002).
Figure 4. Pharmacological identification of GlyT1 in Bergmann glia.
A, glycine and sarcosine induced similar inward currents. Both drugs were each applied at 500 μm from a holding potential of −70 mV onto the same cell. B, sarcosine-induced currents (Ba) were reversibly abolished by replacing external Na+ by choline (Bb). C, records of sarcosine-induced currents obtained at different holding potentials from −70 to +50 mV. D, mean I–V curve of sarcosine-induced currents (n = 5). For all of the recordings, sarcosine was pressure applied at 500 μm on cells recorded at a holding potential of −70 mV. E and F, glycine-induced transport currents were markedly and reversibly reduced by bath application of sarcosine (E) and NFPS (F), two blockers of glycine transport through GlyT1. G, bath application of ORG 25543, a GlyT2 inhibitor, did not affect glycine-induced currents.
Figure 5. Pharmacological profile of glycine transport currents in Bergmann glia.
A–C, competitive blockers of system ASC (l-serine), system A (MeAIB), and of both systems (l-alanine) (each applied at 1 mm) had no effect on glycine-induced transport currents. D and E, bath application of substrate agonists of GABA transporters (GABA) or of taurine transporters (taurine, each at 500 μm) had no effect on glycine-induced transport currents. For all of the experiments, glycine-induced currents were recorded in the presence of TTX and 100 μm strychnine in the bath at a holding potential of −70 mV.
Carrier-mediated glycine efflux from Bergmann glia Estimation of the intracellular Cl− concentration in Bergmann glia
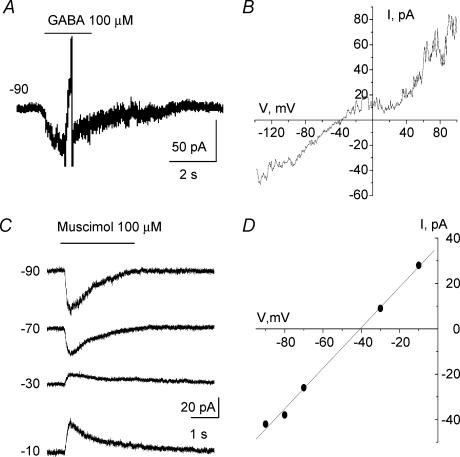
To determine whether glycine transporters could work in reverse at near physiological [Cl−], we first estimated [Cl−]i in Bergmann glia. Gramicidin-perforated patch-clamp recordings were performed to determine the reversal potential (EREV) of GABAA responses induced by GABA or muscimol, a GABAA receptor agonist, and to estimate [Cl−]i, which is known to primarily account for EREV of GABAA receptors (Muller et al. 1994; Kyrozis & Reichling, 1995). Gramicidin is an antibiotic that does not perturb the [Cl−] gradient across the cell membrane (Myers & Haydon, 1972; Kyrozis & Reichling, 1995). Recorded cells had a typical hyperpolarized resting potential of −82.9 ± 6.3 mV and a low input resistance of 39.6 ± 4.3 MΩ (after 35 min of recording). A ramp protocol was applied every 30 s after formation of a gigaseal (data not shown). Once the ramp-induced currents had reached a steady state, 5 mm Ba2+ was applied to block K+ channels and 100 μm GABA or muscimol was pressure applied. EREV of GABAA responses were obtained by either applying a ramp near the peak of GABAA currents and then by subtracting ramps obtained during and before GABA applications (Fig. 6A and B for responses induced by GABA), or by applying GABA or muscimol at different holding potentials (Fig. 6C) and plotting the resulting I–V curves (Fig. 6D). The mean EREV of GABA and muscimol responses was −39.3 ± 4.9 mV (n = 7). Based on the Nernst equation, the calculated [Cl−]i in Bergmann glia would be 30.5 ± 1.3 mm. Considering that GABAA receptors are also permeable to HCO3−, this would give a mean [Cl−]i of 28.4 ± 1.3 mm, using the equation:
Figure 6. Estimation of intracellular [Cl−] using GABAA responses and gramicidin perforated patch-clamp recordings of Bergmann glia.
A, 100 μm GABA-induced current with the gramicidin perforated patch-clamp technique. A ramp protocol was applied near the peak of the current. B, I–V curve of the GABA response shown in A. C, muscimol-induced currents recorded at different holding potentials. Recordings were obtained with the perforated patch-clamp technique. D, I–V curve of the muscimol responses shown in C.
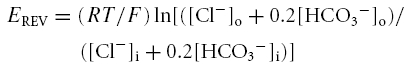 |
with a HCO3−/Cl− permeability ratio of 0.2 (Bormann et al. 1987) and a [HCO3−]i of 16 mm (Staley et al. 1995).
GlyT reversal in Bergmann glia
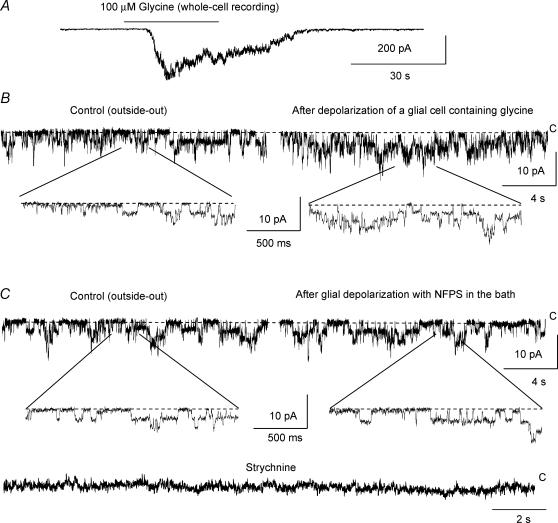
For all of the following experiments whole-cell recordings were initially obtained in a normal external solution, and subsequently in an external solution containing 0 Ca2+/1 mm EGTA/1 μm TTX to block eventual Ca2+-dependent neuronal glycine efflux. Also included in the external solution were 40 mm TEA/5 mm Cs+ or Ba2+/100 μm strychnine to block K+ currents and glycine receptor activation. The intracellular solution contained 32 mm Cl− and 12.5 mm Na+, which are near physiological concentrations of Cl− (see perforated patch-clamp experiments) and Na+ (Rose & Ransom, 1996a). To first test for glycine transport reversal we intracellularly perfused an intracellular solution containing 4 mm glycine + 12.5 mm Na+ during the recording. Intracellular glycine and Na+ concentrations were then about 2 mm and 12.5 mm, respectively (see Methods). This was performed at −50 mV to allow the development of an outward current upon internal glycine perfusion as previously observed for carrier-mediated taurine and GABA efflux currents (Barakat & Bordey, 2002; Barakat et al. 2002). When cells were held at −50 mV, outward currents of +11.2 ± 0.6 pA (n = 3/3) developed upon intracellular perfusion of glycine (Fig. 7Aa). After intracellular glycine perfusion, glycine uptake currents measured at −50 mV were significantly (P < 0.002) decreased by 66 ± 4% (−22.0 ± 1.7 pA before and −7.6 ± 1.4 pA after intracellular glycine perfusion, n = 4). When cells were held at −70 mV, intracellular perfusion of glycine did not induce any outward current but external glycine-induced inward currents were also significantly (P < 0.02) decreased by 54.0 ± 1.1% (−35.0 ± 6.9 pA before and −16.0 ± 3.5 pA after intracellular glycine perfusion, n = 4, Fig. 7Ab). This decrease in the amplitudes of glycine uptake currents was likely due to an intracellular competitive block of glycine uptake by glycine itself. Consistent with this idea, the decrease in the amplitudes of glycine uptake currents was not observed when internal perfusion of a glycine-free intracellular medium was performed (−18.6 ± 1.4 pA before and −18.5 ± 0.7 pA after intracellular glycine-free medium perfusion at a holding potential of −50 mV, n = 3, P > 0.9, Fig. 7Ac). In addition, perfusion of a glycine-free solution did not induce outward currents at −50 mV as can be readily observed on the plot of the mean outward current amplitude as a function of time (Fig. 7B). As a control of glycine intracellular perfusion, lucifer yellow was routinely added in the intracellularly perfused solution but not in the recording solution to confirm that the intracellularly perfused solution was diffusing into the cell during the recording. In all of these experiments, lucifer yellow had diffused into the cell (data not shown). To further observe glycine transport reversal, 40 mV-increment voltage steps of 150 ms were applied from −100 to +60 mV from a holding potential of −70 mV (protocol displayed under the traces in Fig. 7C). In response to voltage steps Bergmann glia generated transient (capacitive) and steady-state currents (Fig. 7C, continuous lines). Intracellular perfusion of 4 mm glycine during the recording induced an increase in the steady-state currents without affecting capacitive currents (Fig. 7C, dotted lines). Point-by-point subtraction of the currents in the presence and absence of intracellular glycine isolated outward currents (data not shown) whose mean I–V curves are displayed in Fig. 7D (n = 3).
In order to further determine whether glycine was released from Bergmann glia via GlyT1 reversal, we obtained outside-out membrane patches from granule cells and used glycine receptor channel activity as a sensor of extracellular glycine. We confirmed that granule cells express glycine receptors (Kaneda et al. 1995) by applying 100 μm glycine onto granule cells recorded in the whole-cell mode. Rapid bath application of glycine induced inward currents in granule cells recorded at −70 mV in the presence of blockers of GABAA receptors (20 μm SR95531), AMPA/kainate (20 μm NBQX) and NMDA receptors (20 μm D-APV) (Fig. 8A). Glycine-induced currents were blocked by a glycine receptor blocker, strychnine (100 μm, data not shown). Sixteen of 25 granule cells displayed single channel activity. The other nine granule cells displayed no or very few channels. Eight simultaneous recordings of a Bergmann glial cell and a granule cell in the whole-cell and outside-out modes, respectively, were obtained. Outside-out membrane patches of granule cells were obtained and placed just above Bergmann glia recorded with a solution containing 4 mm glycine. In most cases, Bergmann glia were pulled up closer to the cell surface. Depolarizations of Bergmann glia from −70 to +20 mV for 2 s induced an increase in single channel activity recorded in membrane patches of granule cells. When the membrane patches were > 50 μm away from the recorded Bergmann glia, no change in the channel activity was detected (data not shown). After Bergmann glial cell depolarization, the channel open probability (NPo) significantly (P < 0.02) increased by 73.1 ± 19.0% in 6 of 8 membrane patches of granule cells. Three minutes after 5 μm NFPS application, depolarizations of Bergmann glia had no significant effect on the open channel probability in granule cell membrane patches (105.0 ± 8.4% of control, n = 5, P > 0.5). The single channels recorded in granule cell membrane patches were identified as glycine receptor channels because they were completely blocked by 100 μm strychnine (Fig. 8C). GlyT1 in Bergmann glia can thus work in reverse and release glycine under near-physiological ionic and internal glycine conditions.
Figure 8. Glycine release through GlyT1 reversal.
A, inward current induced by a rapid bath application of 100 μm glycine in a granule cell recorded at a holding potential of −70 mV in the whole-cell configuration. B, Single channel activity in outside-out patches of a granule cell during control and 30 s after depolarization of an adjacent Bergmann glial cell recorded in the whole-cell configuration. The Bergmann glial cells were depolarized from −70 to +20 mV for 2 s. Bottom, single channel activity on an expanded time scale. C, single channel activity in the membrane patch of a granule cell before and after depolarization of the simultaneously recorded Bergmann glial cell in the presence of the GlyT1 blocker, NFPS (100 μm), and during application of the glycine receptor blocker, strychnine (100 μm, bottom). NFPS was applied for 3 min before the glial cell depolarization. Middle, single channel activity on an expanded time scale. The same cell pair was recorded in B and C. All of the recordings in (A–C) were performed in the presence of blockers of GABAA receptors (20 μm SR95531), AMPA/kainate (20 μm NBQX) and NMDA receptors (20 μm d-APV). C, closed
Discussion
In the present study we report for the first time direct measurements and electrophysiological characterization of glycine transporter currents in glial cells recorded in a near-intact system, the cerebellar slice preparation. More specifically, our data show that: (1) Bergmann glia possess functional glycine transporters, the GlyT1 subtype, and (2) Bergmann glial GlyT1 can mediate electrogenic glycine efflux at and more depolarized than −50 mV.
Glycine transporters GlyT1 mediate glycine influx into Bergmann glia
In ∼90% of the recorded Bergmann glia, glycine induced small inward currents. These glycine-induced currents were not affected by strychnine, an antagonist of glycine receptors, which indicates that glycine receptors are either not present or not functional in Bergmann glia. In addition, strychnine-insensitive glycine-induced currents resembled other Na+/Cl−-dependent transporter currents (Barbour et al. 1990; Borden, 1996; Barakat & Bordey, 2002; Barakat et al. 2002), and were completely blocked by removal of external Na+ and markedly diminished by external [Cl−] reduction, identifying them as transport currents. In the absence of internal glycine the mean I–V curve of glycine transport currents was strictly inwardly rectifying, which differs from the small inward rectification observed for glycine transport currents recorded in occytes (Roux & Supplisson, 2000). It is possible that in our recordings the amplitudes of glycine transport currents are too small for detection at membrane potentials > +20 mV. Nevertheless, no outward currents could be detected at depolarized membrane potentials, as previously reported for GlyT currents recorded in oocytes (Roux & Supplisson, 2000) and other Na+/Cl−-dependent transporter currents recorded in Bergmann glia (Barakat & Bordey, 2002; Barakat et al. 2002). For all of these recordings, Bergmann glia were recorded in conditions to maximize the transport currents with low intracellular Na+ and no glycine. At near-physiological ionic conditions in Bergmann glia (∼30 mm internal [Cl−] found in this study and in hippocampal astrocytes (Bekar & Walz, 2002), and ∼12.5 mm internal [Na+] (Rose & Ransom, 1996a), amplitudes of glycine transport currents were similar to those of transport currents obtained with an intracellular solution containing 145 mm [Cl−] and 2.5 mm[Na+]. However, the presence of 2 mm glycine in the internal solution reduced glycine-induced transport currents, which suggests that intracellular and extracellular glycine competes for transport and supports the conclusion that Na+-dependent transport currents induced by glycine are due to the uptake of glycine plus Na+ into Bergmann glia. Pressure application of sarcosine induced Na+-dependent currents, suggesting the presence of GlyT1 transporters in Bergmann glia. Furthermore, two blockers of glycine transport through GlyT1, sarcosine and NFPS (Smith et al. 1992; Liu et al. 1993; Guastella et al. 1992; Lopez-Corcuera et al. 1998), reduced glycine transport currents by ∼85%, consistent with the presence of positive GlyT1 immunostaining in Bergmann glial processes in this study, as previously reported (Zafra et al. 1995). By contrast, blockers of glycine transport through GlyT2 did not affect glycine transport currents although GlyT2 staining was observed in presumed glial fibres in the molecular layer. GlyT2 immunostaining has not been reported in Bergmann glia but in glial elements of the cerebellar molecular layer (Zafra et al. 1995). Because GlyT2 staining was sparse and essentially absent from glial cell bodies, it is quite possible that GlyT2 currents, if present, are too small for detection. The fact that sarcosine is a competitive inhibitor (Guastella et al. 1992; Lopez-Corcuera et al. 1998) and NFPS inhibition of GlyT1 is concentration and time dependent (Aubrey & Vandenberg, 2001) probably explain the lack of complete inhibition of glycine transport currents by sarcosine and NFPS. NFPS was applied for 3 min, which has been shown to not completely block GlyT1-mediated currents in oocytes (Aubrey & Vandenberg, 2001). The substrate agonists of system A and ASC, which are Na+-dependent transport systems and can mediate glycine uptake (Palacin et al. 1998), did not affect glycine transport currents. This indicates that glycine transport currents are mediated by Na+/Cl−-dependent GlyTs and not by these other glycine transport systems. Altogether, these data suggest that glycine is transported into Bergmann glia essentially through GlyT1 transporters.
GlyTs in Bergmann glia can work in reverse at near-physiological ionic and internal glycine conditions
To study glycine transport reversal, Bergmann glia were recorded at near physiological ionic conditions (32 mm internal [Cl−], and ∼12.5 mm internal [Na+], Rose & Ransom, 1996a). Upon intracellular glycine perfusion during the recording to obtain a final near-physiological intracellular glycine concentration of ∼2 mm (Berger et al. 1977), outward currents progressively developed when the cells were held at −50 mV but not at −70 mV. In addition, glycine uptake currents were significantly reduced in amplitude after intracellular glycine perfusion at −50 mV, which suggests that intracellular glycine influences the uptake of extracellular glycine, as previously reported for GlyT1 injected in oocytes (Roux & Supplisson, 2000). These data suggest that GlyTs can work in reverse upon internal glycine perfusion and glial cell depolarization, which is in good agreement with previous studies on GlyT1 injected in oocytes (Roux & Supplisson, 2000) or expressed in HEK-293 cells (Sakata et al. 1997). These data also suggest that glycine-induced outward currents are not unspecific leakage currents, consistent with a tight coupling between ionic and substrate fluxes (Roux & Supplisson, 2000). The use of paired recordings of a Bergmann glial cell and a granule cell confirmed that glycine was released via GlyT1 when Bergmann glia were depolarized to +20 mV as the increase in the open probability of glycine receptor channels was prevented in the presence of NFPS in the bath. This is consistent with the findings of Roux & Supplisson (2000) that the GlyT1 subtype is well suited to mediate reverse glycine transport. Overall, we identified that at near-physiological internal Na+, Cl− and glycine concentrations glycine can be released via GlyT1 in Bergmann glia at a membrane potential of and more depolarized than −50 mV.
Functional implications of glycine uptake and release in Bergmann glia
GlyTs have been suggested to play an important regulatory role at glycine receptor-containing synapses by clearing glycine from the synaptic cleft and/or at NMDAR-containing synapses by locally keeping extracellular glycine levels below local saturating concentrations at the glycineB site on NMDARs (Wood, 1995; Supplisson & Bergman, 1997; Parsons et al. 1998; Danysz & Parsons, 1998; Gomeza et al. 2003a,b; Kinney et al. 2003; Chen et al. 2003). In the cerebellum, mixed GABA and glycine receptors containing synapses occur onto Golgi cell dendrites in the molecular and granule cell layers (Dumoulin et al. 2001). However, it remains unknown whether Bergmann glial processes, which express GlyT1, encapsulate glycinergic synapses onto Golgi cell dendrites and regulate glycinergic synaptic transmission by removing glycine from the synaptic cleft. At the age studied functional NMDARs are not thought to be present in Purkinje neurones (Llano et al. 1991; Rosenmund et al. 1992). However, they are expressed on presynaptic GABAergic axons and terminals synapsing onto Purkinje neurones and their tonic activation by ambient glutamate enhances spontaneous GABA release from these terminals (Glitsch & Marty, 1999; Huang & Bordey, 2004). Presynaptic NMDARs have also been shown to be involved in long-term depression at synapses between parallel fibres and Purkinje neurones (Casado et al. 2002). Bergmann glia processes closely encapsulate inhibitory and excitatory synapses on Purkinje neurone soma and dendrites (Palay & Chan-Palay, 1974). Thus, since Bergmann glia express functional GlyT1 as shown in our study and in previous immunohistochemical studies (Guastella et al. 1992; Zafra et al. 1995), they are in a prime location to affect the function of NMDARs at both inhibitory and excitatory synapses. Assuming that the glycineB site at these NMDARs is not saturated as shown in vivo in the cerebellum (Wood et al. 1989; Rao et al. 1990; Fedele et al. 1997), in slices and in vivo in other brain regions (Supplisson & Bergman, 1997; Bergeron et al. 1998; Kinney et al. 2003; Chen et al. 2003), and based on the calculations of Roux & Supplisson (2000), glycine uptake into Bergmann glia might decrease tonic NMDAR activation, and reduce GABA release and long-term depression. It is important to consider that d-serine has been suggested to be the endogenous ligand of the glycineB sites in many CNS regions (Schell et al. 1997; Baranano et al. 2001). In the cerebellum, at the age studied, glycine levels progressively increase while d-serine levels decrease (Schell et al. 1997; Miranda-Contreras et al. 1999), suggesting that both glycine and d-serine are important ligands of NMDARs, with an increasing contribution of glycine during neonatal development.
Regarding non-vesicular glycine release, its function remains unclear under physiological conditions. Based on Roux & Supplisson (2000; see Fig. 7), GlyT1 is close to equilibrium for an extracellular glycine concentration of 100 nm and a resting potential of −70 mV. In addition, our data indicate that glycine transporters can reverse at −50 mV upon intracellular glycine perfusion at near-physiological ionic conditions. It is then questionable whether Bergmann glia, whose resting potentials are between −80 and −90 mV, can be sufficiently depolarized by neuronal activity to promote glycine efflux. Non-vesicular glycine release via GlyT1 reversal may in fact occur under high frequency stimulation and probably under ischaemic conditions. During high frequency stimulation (100 Hz), hippocampal astrocytes display synaptically induced currents of ∼1200 pA (Diamond & Jahr, 2000). Depending on the cell resistance (between 5 and 200 MΩ) (Bordey & Sontheimer, 1997; Diamond & Jahr, 2000), astrocytes could be considerably depolarized. In addition, glutamate receptor activation triggers increases in intracellular Na+ (Rose & Ransom, 1996b), which combined with cell depolarizations may be sufficient to induce glycine transport reversal during high frequency stimulation. During ischaemic conditions, glia are depolarized up to −50 mV and intracellular [Na+] is estimated to rise to 39 mm (Attwell et al. 1993). In addition, previous studies have shown Ca2+-independent glycine release in ischaemic conditions in brain slices (Saransaari & Oja, 2001). Assuming that the glycineB site is not saturated in the molecular and Purkinje cell layers, extracellular increased glycine levels could significantly contribute to the development of NMDAR-mediated excitotoxic damages.
Overall, our study of glial Na+/Cl−-dependent glycine transporters represents an important step in understanding the role of theses transporters in the regulation of glycine levels and possibly neurotransmission at NMDAR-containing synapses in the cerebellum and other brain regions.
References
- Adams RH, Sato K, Shimada S, Tohyama M, Puschel AW, Betz H. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon MC, Gimenez C, Mayor F. Stoichiometry of sodium- and chloride-coupled glycine transport in synaptic plasma membrane vesicles derived from rat brain. FEBS Lett. 1987;212:87–90. doi: 10.1016/0014-5793(87)81562-4. [DOI] [PubMed] [Google Scholar]
- Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol Pharmacol. 2001;60:1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ. N[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy) propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport. Br J Pharmacol. 2001;134:1429–1436. doi: 10.1038/sj.bjp.0704381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–1419. doi: 10.1152/jn.2002.88.3.1407. [DOI] [PubMed] [Google Scholar]
- Barakat L, Wang D, Bordey A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J Physiol. 2002;541:753–767. doi: 10.1113/jphysiol.2001.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander retina. J Physiol. 1990;436:169–194. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GA, Ellory JC. The identification of neutral amino acid transport systems. Exp Physiol. 1990;75:3–26. doi: 10.1113/expphysiol.1990.sp003382. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia. 2002;39:207–216. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- Berger SJ, Carter JC, Lowry OH. The distribution of glycine, GABA, glutamate and aspartate in rabbit spinal cord, cerebellum and hippocampus. J Neurochem. 1977;28:149–158. doi: 10.1111/j.1471-4159.1977.tb07720.x. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Caulfield WL, Collie IT, Dickins RS, Epemolu O, McGuire R, Hill DR, McVey G, Morphy JR, Rankovic Z, Sundaram H. The first potent and selective inhibitors of the glycine transporter type 2. J Medicalchem. 2001;44:2679–2682. doi: 10.1021/jm0011272. [DOI] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984;779:255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Tan T, Onur R. Glycine, alanine and serine potentiate glutamate neurotoxicity in cerebral ischemia via NMDA receptors. Eur J Pharmacol. 1990;183:476. [Google Scholar]
- Danysz W, Parsons AC. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- de Blas AL. Monoclonal antibodies to specific astroglial and neuronal antigens reveal the cytoarchitecture of the Bergmann glia fibers in the cerebellum. J Neurosci. 1984;4:265–273. doi: 10.1523/JNEUROSCI.04-01-00265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele E, Bisaglia M, Raiteri M. d-serine modulates the NMDA receptor/nitric oxide/cGMP pathway in the rat cerebellum during in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:43–47. doi: 10.1007/pl00004916. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez E, Lopez-Colome AM. Characterization of glycine transport in cultured Muller glial cells from the retina. Glia. 1999;26:273–279. doi: 10.1002/(sici)1098-1136(199906)26:4<273::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine, and GABA III. Glycine transporters. J Neurosci Res. 2001;64:218–222. doi: 10.1002/jnr.1069. [DOI] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003a;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Ohno K, Hulsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003b;40:797–806. doi: 10.1016/s0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- Guastella J, Brecha N, Weigmann C, Lester HA, Davidson N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc Natl Acad Sci U S A. 1992;89:7189–7193. doi: 10.1073/pnas.89.15.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen I, Kontro P. Uptake and release of glycine in cerebellar granule cells and astrocytes in primary culture: potassium-stimulated release from granule cells is calcium-dependent. J Neurosci Res. 1989;24:374–383. doi: 10.1002/jnr.490240306. [DOI] [PubMed] [Google Scholar]
- Huang H, Bordey A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J Neurosci. 2004;24:5659–5669. doi: 10.1523/JNEUROSCI.1338-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Sur C, Burno M, Mallorga PJ, Williams JB, Figueroa DJ, Wittmann M, Lemaire W, Conn PJ. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy) propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 2003;23:7586–7591. doi: 10.1523/JNEUROSCI.23-20-07586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Meth. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J Biol Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Nelson H, Mandiyan S, Nelson N. Cloning and expression of a cDNA encoding the transporter of taurine and beta-alanine in mouse brain. Proc Natl Acad Sci U S A. 1992;89:12145–12149. doi: 10.1073/pnas.89.24.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. Expression of a mouse brain cDNA encoding novel gamma- aminobutyric acid transporter. J Biol Chem. 1992;267:17491–17493. [PubMed] [Google Scholar]
- Lopez-Corcuera B, Martinez-Maza R, Nunez E, Roux M, Supplisson S, Aragon C. Differential properties of two stably expressed brain-specific glycine transporters. J Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- Mallorga PJ, Williams JB, Jacobson M, Marques R, Chaudhary A, Conn PJ, Pettibone DJ, Sur C. Pharmacology and expression analysis of glycine transporter GlyT1 with [3H]-(N-[3-(4′-fluorophenyl)-3-(4′phenylphenoxy) propyl]) sarcosine. Neuropharmacology. 2003;45:585–593. doi: 10.1016/s0028-3908(03)00227-2. [DOI] [PubMed] [Google Scholar]
- Matskevitch I, Wagner CA, Stegen C, Broer S, Noll B, Risler T, Kwon HM, Handler JS, Waldegger S, Busch AE, Lang F. Functional characterization of the Betaine/gamma-aminobutyric acid transporter BGT-1 expressed in Xenopus oocytes. J Biol Chem. 1999;274:16709–16716. doi: 10.1074/jbc.274.24.16709. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Miranda-Contreras L, Benitez-Diaz PR, Mendoza-Briceno RV, Delgado-Saez MC, Palacios-Pru EL. Levels of amino acid neurotransmitters during mouse cerebellar neurogenesis and in histotypic cerebellar cultures. Dev Neurosci. 1999;21:147–158. doi: 10.1159/000017377. [DOI] [PubMed] [Google Scholar]
- Morara S, Brecha NC, Marcotti W, Provini L, Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. Neuroreport. 1996;7:2993–2996. doi: 10.1097/00001756-199611250-00039. [DOI] [PubMed] [Google Scholar]
- Morrow JA, Collie IT, Dunbar DR, Walker GB, Shahid M, Hill DR. Molecular cloning and functional expression of the human glycine transporter GlyT2 and chromosomal localisation of the gene in the human genome. FEBS Lett. 1998;439:334–340. doi: 10.1016/s0014-5793(98)01390-8. [DOI] [PubMed] [Google Scholar]
- Muller T, Fritschy JM, Grosche J, Pratt GD, Mohler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994;14:2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Nunez E, Lopez-Corcuera B, Vazquez J, Gimenez C, Aragon C. Differential effects of the tricyclic antidepressant amoxapine on glycine uptake mediated by the recombinant GLYT1 and GLYT2 glycine transporters. Br J Pharmacol. 2000;129:200–206. doi: 10.1038/sj.bjp.0703049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex, Cytology and Organization. New York: Springer-Verlag; 1974. p. 236. [Google Scholar]
- Parsons CG, Danysz W, Hesselink M, Hartmann S, Lorenz B, Wollenburg C, Quack G. Modulation of NMDA receptors by glycine – introduction to some basic aspects and recent developments. Amino Acids. 1998;14:207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- Rao TS, Cler JA, Emmett MR, Mick SJ, Iyengar S, Wood PL. Glycine, glycinamide and d-serine act as positive modulators of signal transduction at the N-methyl-D-aspartate (NMDA) receptor in vivo: differential effects on mouse cerebellar cyclic guanosine monophosphate levels. Neuropharmacology. 1990;29:1075–1080. doi: 10.1016/0028-3908(90)90115-8. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Siegel A, Rickmann M, Wolff JR, Noone D, Robinson SR. Distribution of Bergmann glial somata and processes: implications for function. J Hirnforsch. 1995;36:509–517. [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol. 1996a;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and d-aspartate in rat hippocampal astrocytes. J Neurosci. 1996b;16:5393–5404. doi: 10.1523/JNEUROSCI.16-17-05393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Legendre P, Westbrook GL. Expression of NMDA channels on cerebellar Purkinje cells acutely dissociated from newborn rats. J Neurophysiol. 1992;68:1901–1905. doi: 10.1152/jn.1992.68.5.1901. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sakata K, Sato K, Schloss P, Betz H, Shimada S, Tohyama M. Characterization of glycine release mediated by glycine transporter 1 stably expressed in HEK-293 cells. Brain Res Mol Brain Res. 1997;49:89–94. doi: 10.1016/s0169-328x(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Glycine release from hippocampal slices in developing and ageing mice: modulation by glutamatergic receptors. Mech Ageing Dev. 1994;76:113–124. doi: 10.1016/0047-6374(94)91586-5. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characteristics of hippocampal glycine release in cell-damaging conditions in the adult and developing mouse. Neurochem Res. 2001;26:845–852. doi: 10.1023/a:1011624421505. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Brady RO, Jr, Molliver ME, Snyder SH. d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan M, Najlerahim A, Watson RE, Bennett JP. Distribution of mRNA for the GABA transporter GAT-1 in the rat brain: evidence that GABA uptake is not limited to presynaptic neurons. J Anat. 1994;185:315–323. [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Walker VE, Flynn DM. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989;338:422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- White WF, Brown KL, Frank DM. Glycine binding to rat cortex and spinal cord: binding characteristics and pharmacology reveal distinct populations of sites. J Neurochem. 1989;53:503–512. doi: 10.1111/j.1471-4159.1989.tb07362.x. [DOI] [PubMed] [Google Scholar]
- Wood PL. The co-agonist concept: is the NMDA-associated glycine receptor saturated in vivo? Life Sci. 1995;57:301–310. doi: 10.1016/0024-3205(95)00288-h. [DOI] [PubMed] [Google Scholar]
- Wood PL, Emmett MR, Rao TS, Mick S, Cler J, Iyengar S. In vivo modulation of the N-methyl-D-aspartate receptor complex by D-serine: potentiation of ongoing neuronal activity as evidenced by increased cerebellar cyclic GMP. J Neurochem. 1989;53:979–981. doi: 10.1111/j.1471-4159.1989.tb11803.x. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Gimenez C. Characteristics and adaptive regulation of glycine transport in cultured glial cells. Biochem J. 1989;258:403–408. doi: 10.1042/bj2580403. [DOI] [PMC free article] [PubMed] [Google Scholar]