Abstract
A plant's ability to produce and respond to ethylene is essential for its vegetative growth. We studied whole-shoot ethylene emission and leaf growth responses to applied ethylene in four Poa spp. that differ inherently in leaf elongation rate and whole-plant relative growth rate. Compared with the fast-growing Poa annua and Poa trivialis, the shoots of the slow-growing species Poa alpina and Poa compressa emitted daily 30% to 50% less ethylene, and their leaf elongation rate was more strongly inhibited when ethylene concentration was increased up to 1 μL L−1. To our surprise, however, low ethylene concentrations (0.02–0.03 μL L−1) promoted leaf growth in the two slow-growing species; at the same concentrations, leaf elongation rate of the two fast-growing species was only slightly inhibited. All responses were observed within 20 min after ethylene applications. Although ethylene generally inhibits growth, our results show that in some species, it may actually stimulate growth. Moreover, in the two slow-growing Poa spp., both growth stimulation and inhibition occurred in a narrow ethylene concentration range, and this effect was associated with a much lower ethylene emission. These findings suggest that the regulation of ethylene production rates and perception of the gas may be more crucial during leaf expansion of these species under non-stressful conditions and that endogenous ethylene concentrations are not large enough to saturate leaf growth responses. In the two fast-growing species, a comparatively higher ethylene endogenous concentration may conversely be present and sufficiently high to saturate leaf elongation responses, invariably leading to growth inhibition.
Ethylene is a plant growth regulator involved in the modulation of a number of processes throughout the life cycle of plants, from seedling development to flowering and senescence (Abeles et al., 1992; Reid, 1995). All plant organs produce and emit ethylene to the surrounding atmosphere with a rate depending on the species and environmental conditions. For example, leaves and shoots of Arabidopsis emit 0.15 to 0.19 nmol g−1 fresh mass h−1 (Bleecker et al., 1988; Knoester et al., 1999) under favorable conditions; a burst of ethylene production (to about 1 nmol g−1 fresh mass h−1) was observed upon pathogen infection (Knoester et al., 1998). In contrast, the aquatic monocot Potamogeton pectinatus produces no ethylene, but it does have the capacity to perceive the gas (Summers and Jackson, 1998). Next to variation in ethylene production, plants also vary considerably in their responsiveness (direction and intensity) to ethylene. Ethylene is classically considered an inhibitor of growth because of its ability to reduce cell elongation (Abeles et al., 1992; Smalle and Van Der Straeten, 1997). However, ethylene can stimulate growth in several tissue types and in different species. For instance, stem and petiole growth is stimulated by ethylene in flooding-tolerant species such as deepwater rice and Rumex palustris, which, therefore, grow vigorously under flooded conditions (Kende et al., 1998; Voesenek and Blom, 1999). Also, some typical terrestrial plants sometimes show a positive growth response when exposed to ethylene. Growth stimulation has been reported for Arabidopsis hypocotyls (Smalle et al., 1997), stems of Stellaria longipes (Emery et al., 1994), mesocotyls of wheat (Triticum aestivum) and oat (Avena sativa; Suge, 1971; Suge et al., 1997), and leaves of sunflower (Helianthus annuus; Lee and Reid, 1997). In some cases, the direction of response to applied ethylene depends on the concentration applied as, for example, in roots of tomato (Lycopersicon esculentum), white mustard (Sinapis alba), and rice (Oryza sativa). Low ethylene concentrations stimulated growth, whereas higher concentrations inhibited growth (Konings and Jackson, 1979).
Fast-growing Poa spp. that occur at low elevations are characterized by an inherently higher leaf elongation rate and final size of individual leaves compared with slow-growing Poa spp. from higher elevations (Körner and Woodward, 1987; Bultynck et al., 1999; Fiorani et al., 2000). It is our aim to unravel the mechanisms that explain inherent differences in leaf growth rate between these species. To this end, we formulated the hypothesis that leaf growth of Poa spp. with a slow-leaf elongation rate is more strongly inhibited by ethylene than that of Poa spp. with a fast-leaf elongation rate. It is expected that the species with slow-elongating leaves produce comparatively more ethylene, thus, reducing leaf elongation rate more strongly. To test this hypothesis, we investigated whole-shoot ethylene emission and its relationship with responsiveness to ethylene of leaf growth of four Poa spp. differing in leaf elongation rate and final leaf length (Fiorani et al., 2000).
It has been suggested that ethylene may induce responses within minutes and that these responses are short-term (Sisler, 1990). This is supported, at the physiological level, by studies on pea (Pisum sativum) seedlings and radish (Raphanus sativus) roots, because ethylene inhibited growth rate in less than 20 min (Warner and Leopold, 1971; Jackson, 1983). In addition, molecular data indicate a rapid activation of components of an ethylene signal transduction cascade (Sessa et al., 1996; Vriezen et al., 1997; Novikova et al., 1999). Nevertheless, a large body of data concerning responsiveness of leaves and roots to applied ethylene has been collected in relatively long time-course experiments (from 1 d to several days of ethylene treatments). Instead, we were interested in the short-term effects (from minutes up to 3–4 h) of exogenously applied ethylene on leaf elongation rate.
Our results indicate that two slow-growing Poa spp. (Poa alpina and Poa compressa) emit smaller, rather than larger, amounts of ethylene compared with two fast-growing Poa spp. (Poa annua and Poa trivialis). The application of exogenous ethylene generally decreased leaf elongation in all of the four species within 20 min. Based on concentration-response curves, the two inherently slow-growing species showed overall a comparatively steeper inhibition response to increases in ethylene concentration. However, when very small concentrations of ethylene were given (0.02–0.03 μL L−1) the higher responsiveness of the latter species was also associated with a significant promotion of leaf elongation rate.
RESULTS
Analysis of Leaf Growth Patterns
First, we analyzed the pattern of growth of leaf 7 of the main stem for at least 48 h. The two subalpine species (P. alpina and P. compressa) showed a slower daily leaf elongation rate compared with the two lowland ones (P. annua and P. trivialis). Leaf elongation rate was invariably higher during the light period and declined at night (Fig. 1). However, no relationship was found between leaf elongation rate during the night and altitude of the natural habitat of these species. The decline in leaf elongation rate during the dark period varied between species ranging from approximately 50% (P. alpina and P. annua) to about 70% (P. compressa and P. trivialis).
Figure 1.
Diurnal growth patterns of main stem leaf 7 of four Poa spp. Mean ± se of four plants are shown. Error bars are shown when larger than the symbols. The same experiment (approximately 48 h recording under the same conditions) was repeated twice, leading to similar results (not shown). The black rectangles on the x axis represent the dark period (8 h) of the diurnal cycle. Leaf elongation rate in the light was calculated as the slope of a linear regression through the data point referring to the interval 8 am to 12 midnight. Similarly, leaf elongation rate in the dark was calculated during the dark period (12 midnight–8 am). The length of the leaves at the beginning of the experiments was set to y = 0 and corresponded to lengths of at least 1 cm of the emerged leaf blades for all the species.
Second, we analyzed the changes in leaf elongation rate during the course of the light period (from 8 am to midnight) on sub-periods of 4 h each (linear regression). Leaf elongation rate decreased slightly from 12 noon to 4 pm with respect to the previous 4 h (1% P. alpina; 5% P. compressa and P. annua; and 4% P. trivialis). For later times toward the end of the 16-h light period, the decline was about 10% in all four species. Based on this analysis, we used the first 4 h of growth in the light as a control growth rate and started ethylene treatments at the end of this period (12 noon) that lasted at least 4 additional h. The inhibition or stimulation effects upon ethylene treatments were expressed as a percentage of the control growth rates, and data were corrected for the declines in leaf growth rate during the light period described above.
Finally, we analyzed the data on control leaf elongation rates from all the experiments for variability between different plant batches. No significant differences (P > 0.05) occurred between plant batches (Table I). Average leaf elongation rates of the two slow-growing species (P. alpina and P. compressa) were significantly lower than those of the two fast-growing species (P. annua and P. trivialis). This result is in agreement with previous data (Fiorani et al., 2000) on the same species. However, the present absolute values of leaf elongation rates are higher than those of the study mentioned above due to the different experimental conditions (i.e. higher temperature, lower light intensity, and higher relative humidity).
Table I.
Leaf elongation rate of four Poa spp.
| Species | Leaf Elongation Rate
|
|
|---|---|---|
| Range | Average | |
| mm h−1 | ||
| P. alpina | 0.46–0.63 | 0.50 ± 0.02 |
| P. compressa | 1.14–1.46 | 1.28 ± 0.04 |
| P. annua | 1.35–1.63 | 1.50 ± 0.03 |
| P. trivialis | 1.31–1.96 | 1.78 ± 0.03 |
Control leaf elongation rate was calculated for all the species during the time interval 8 am to 12 noon of the light period. Depending on the species, the range and average ± se of 10 to 12 independent experiments are shown. A one-way ANOVA was performed to test variation in leaf elongation rate between the different experiments. For all four species P values were >0.05 (not significant). The P values were 0.22 (P. alpina), 0.20 (P. compressa), 0.44 (P. annua), and 0.33 (P. trivialis).
Whole-Shoot Ethylene Emission
The on-line method used to measure ethylene allowed us to discern any diurnal pattern of ethylene emission (Fig. 2). The amount of ethylene emitted by the whole shoot of these Poa spp. at the stage of appearance of leaf 7 of the main stem (or earlier stages) was generally too small to allow a reliable estimation of ethylene emission rates. Therefore, to increase the amounts of ethylene available for analysis, we used plants that were 7 to 10 d older (fresh mass of the shoot ≥0.5 g; 9–10 visible leaves).
Figure 2.
Diurnal patterns of whole-shoot ethylene emission in four Poa spp. In all panels, each data point is the mean of six (P. compressa and P. annua) or eight (P. alpina and P. trivialis) plants. For the sake of clarity, only typical error bars are shown. The black boxes on the x axis represent dark periods of the diurnal cycle. Note that x and y axis have exactly the same scale in all panels. In each panel the name of the species is indicated.
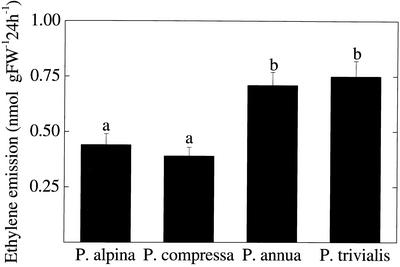
The shoots of all four species tended to emit more ethylene during the light period than during the night. However, P. annua and P. trivialis emitted more ethylene during the first night shortly after transfer of the plants to the cuvettes compared with P. alpina and P. compressa. These relatively high night emission levels were most likely induced by plant manipulation. In addition, an increase during the first hours of the light period followed by a decrease at later times during the day was evident in all four species (Fig. 2). These features prevented the calculation of an average steady-state release over the experimental period; instead, we expressed results as the integrated production over 24-h time intervals for all the species (first night excluded, from 8 am to 8 am next day). The fast-growing P. annua and P. trivialis emitted daily about two times more ethylene than the slow-growing species P. alpina and P. compressa. There were no significant differences between P. alpina and P. compressa or between P. annua and P. trivialis (Fig. 3).
Figure 3.
Whole-shoot ethylene emission of four Poa spp. The data were analyzed for statistically significant differences by means of a one-way ANOVA. Different letters on top of the bars indicate significant differences at P = 0.05 (Tukey-b post hoc test). In each experiment, the ethylene released by the shoots of two plants per each species (one plant per cuvette) was measured, and average values ± se were obtained for a total of eight (P. alpina and P. trivialis) or six (P. compressa and P. annua) plants.
Leaf Growth Responsiveness to Ethylene
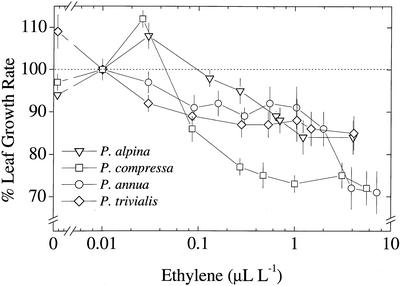
To test the responsiveness of leaf growth of the four Poa spp. to ethylene, we exposed the whole shoot of plants of the main stem to a range of ethylene concentrations and measured leaf length versus time of leaf 7. In all four Poa spp. ethylene concentrations higher than 0.1 μL L−1 inhibited leaf growth rates (Fig. 4). However, the slopes of the concentration-response curves were steeper in the two slow-growing P. alpina and P. compressa than in the fast-growing P. annua and P. trivialis. We tested whether P. alpina and P. compressa differed statistically in their inhibition response (above 0.1 μL L−1). In a two-way ANOVA, we found that the concentration × species linear interaction of P. alpina and P. compressa was significantly different from P. annua and P. trivialis, indicating that the slopes are significantly different (P < 0.05). These results indicate that the two slow-growing species were more responsive to ethylene. A remarkable decrease in leaf elongation rate was observed in P. annua when the concentration was raised from 1 to 7 or 8 μL L−1. These concentrations were also tested on a different batch of plants of this species leading to similar results (not shown). Surprisingly, the direction of response varied between species as a function of the concentration. A promoting effect on leaf elongation rate at low ethylene concentrations (0.02–0.03 μL L−1) was clearly evident in P. alpina and P. compressa. This response was reversed at higher concentrations at which inhibition was observed (Fig. 4).
Figure 4.
Short-term effects of ethylene on leaf growth rate of main stem leaf number 7 of four Poa spp. On the x axis, the applied ethylene concentration is expressed as a log-scale (corresponding range: 0.03–9 μL L−1 for P. annua; 0.03–7 for all the other three species). On the y axis, the responses to ethylene are normalized for control growth rate of plants exposed to humidified air (100%; dotted line), as defined in “Materials and Methods.” Each data point represents the average (n = 4) of an independent experiment. In the bottom left corner, the symbols for all the species are indicated. For the absolute values of control growth rates and the analysis of variability between experiments see Table I. Note that the ethylene concentration in the control air stream was ≤0.01 μL L−1. The data points close to x = 0 were obtained for each species in an experiment in which the traces of ethylene in the air used as control were removed by scrubbing the air with a platinum catalyst. These experiments were done by using the same time frame of those in which ethylene was exogenously applied. Note that the data points close to x = 0 relative to P. annua and P. trivialis exactly overlap.
The control air had an ethylene concentration of 0.01 μL L−1. To further analyze the promoting or inhibiting effects at low concentrations, we performed additional experiments for all of the species in which, instead of adding ethylene to the control air, we removed the traces of this gas using the same platinum catalyst as in our set-up for on-line laser-driven photoacoustic spectroscopy (ethylene concentration reduced from 0.01 to 0.00005 μL L−1). The results were consistent with the responsiveness shown by these species to added low concentrations. P. annua and P. trivialis responded to ethylene removal by equally increasing their leaf elongation rate of about 10% over control (P < 0.05; Fig. 4). In P. alpina and P. compressa, the absence of these trace levels of ethylene in their environment conversely elicited a slight decrease in leaf elongation rate. However, this decline was not significantly different from control (P > 0.05). These findings confirm that low concentrations of ethylene (0.02–0.03 μL L−1) promoted leaf elongation rate in these two slow-growing species. We conclude that, overall, the two slow-growing species were more responsive to applied ethylene than the two fast-growing species and gave a positive, rather than a negative, leaf growth response to small additions of ethylene.
Kinetics of Short-Term Responses
We analyzed our data to estimate the time needed under these experimental conditions to observe a significant response to ethylene application (latent time of response). This was accomplished by plotting leaf elongation rate versus time for each individual plant and by direct examination of the curves. The comparison of the four species and of two different ethylene concentrations applied (0.03 and 7 μL L−1) showed that the latent time of response ranged roughly between 10 and 20 min in all the species (Table II). For each species, no difference was found when ethylene was applied at a low or at a high concentration. An exception was P. trivialis, in that the average latent time of response was higher at 0.03 μL L−1 ethylene than at 7 μL L−1 (Table II). Overall, the time needed to promote (0.03 μL L−1 in P. alpina and P. compressa) and to inhibit (7 μL L−1 in all the species) growth was not significantly different.
Table II.
Latent time of response to applied ethylene of four Poa spp. that naturally occur at different elevations
| Species | Latent Time of Response
|
|||
|---|---|---|---|---|
| Range
|
Average
|
|||
| 0.03 μL L−1 | 7 μL L−1 | 0.03 μL L−1 | 7 μL L−1 | |
| min | ||||
| P. alpina | 7–13 | 10–15 | 10 ± 1 | 12 ± 2 |
| P. compressa | 15–19 | 10–16 | 17 ± 1 | 14 ± 2 |
| P. annua | 11–17 | 10–16 | 14 ± 2 | 14 ± 2 |
| P. trivialis | 11–30 | 10–15 | 20 ± 4 | 13 ± 1 |
Ethylene was applied at 0.03 (promoting effect in P. alpina and P. compressa) and 7 μL L−1 (inhibitory effect in all the species). Range and average ± se are indicated (n = 4).
We also did several experiments (at least one for each species) in which ethylene application was stopped after 4 h of treatment. Within approximately the same time needed to observe a response when ethylene was applied, a recovery of control leaf elongation rates was observed after removal of ethylene (Fig. 5). This result indicates that in our experiments, the continuous presence of ethylene during the 4-h treatments was necessary to maintain the observed responses.
Figure 5.
Representative example of leaf elongation rate responses to 0.28 μL L−1 ethylene application and successive ethylene withdrawal (P. compressa). The values of leaf elongation rate in the graph refer to sub-periods of 4 h in which the first 20 min after ethylene addition and withdrawal were excluded from the calculation (latent time of response). The calculations were accomplished by linear regression. The x axis is the time in hours from the beginning of the experiment. All data points shown were recorded during the light period.
DISCUSSION
Leaf Elongation Rate Responses to Applied Ethylene and Ethylene Emission
The shoots of the two slow-elongating species (P. alpina and P. compressa) are characterized by a low ethylene emission compared with two fast-elongating species (P. annua and P. trivialis). Similarly, Konings and Jackson (1979) found a positive relationship between primary root extension rates and ethylene emission in four species or mutants belonging to three genera. A possible explanation for our findings is related to the effects of applied ethylene on leaf elongation rate. We found that different rates of ethylene emission are related to a different responsiveness of leaf elongation rates to applied ethylene (leaf 7 of the main stem). P. alpina and P. compressa show a steeper kinetics of inhibition of leaf elongation rate when exposed to ethylene concentrations higher than 0.1 μL L−1. In addition, these two species show a stimulation of leaf elongation rate upon treatment at concentration around 0.03 μL L−1 ethylene. Removal of ethylene below the trace levels found in air results in a decrease of leaf elongation rates in these two species. Because of fast ethylene diffusibility, the internal concentration of the gas in the tissue can be adjusted simply by changing the rate of its synthesis or the external concentration (Reid, 1995). We conclude that the natural endogenous ethylene concentration in the two slow-growing species is below the concentration giving the maximum potential leaf elongation rate. In contrast, in the fast-growing P. annua and P. trivialis, the endogenous ethylene concentration is above the concentration required for the maximum leaf elongation rate. In P. alpina and P. compressa, ethylene has opposite effects (promoting or inhibiting) on leaf elongation in a relatively narrow concentration range. In addition, they are also more reactive to ethylene at higher and inhibitory concentrations. These findings indicate that, in the two slow-growing (subalpine) species, regulation of ethylene production, as related to responsiveness of leaf growth rate to applied ethylene, is more crucial in controlling leaf elongation rate compared with that in the two fast-growing (lowland) species. In a study of two S. longipes ecotypes that occur at different elevations, Emery et al. (1994) found that ethylene may determine phenotypic plasticity in stem elongation in response to wind. In that study, ethylene mediated stem growth inhibition caused by wind in the alpine ecotype, whereas low ethylene levels stimulated stem growth in the prairie ecotype independently of wind stress intensity. In addition, the prairie ecotype produced more ethylene under control conditions and did not increase its ethylene production upon wind stress stimulation when compared with the alpine ecotype (Emery et al., 1994). These data support our finding that in alpine plants, ethylene production is comparatively low and that this feature may enable these species to respond to environmental stimuli (e.g. wind speed) by increasing ethylene production and by triggering the appropriate responses.
Can Ethylene Promote Elongation Growth of Terrestrial Plants?
Ethylene acts as a regulator of growth of most vegetative tissues in terrestrial plants, mainly inhibiting the rate of cell expansion (Abeles et al., 1992; Smalle and Van Der Straeten, 1997). However, ethylene can strongly promote fruit peduncule growth in the squirting cucumber (Ecballium elaterium; Jackson et al., 1972). In addition, in elongating roots, a stimulation of growth rate in the presence of low ethylene levels is not uncommon (Konings and Jackson, 1979). Stimulatory effects of low ethylene levels has been reported also for leaf area development of sunflower (Lee and Reid, 1997), stem elongation of S. longipes (Emery et al., 1994), first internode elongation of wheat seedlings (Suge et al., 1997), mesocotyl elongation of oat and wheat (Suge, 1971), and hypocotyl elongation of Arabidopsis seedlings grown in the light (Smalle et al., 1997) or in the dark (Hua and Meyerowitz, 1998). Finally, strong stimulatory effects were reported for leaf sheath extension of Poa pratensis plants exposed to high concentrations of ethephon (2-chloroethylphosphonic acid) for 4 to 6 weeks (Poovaiah and Leopold, 1973). In this study, we found small promoting effects of ethylene on leaf elongation rate only in two of the four species studied; these effects are saturated at around 0.03 μL L−1. In summary, ethylene can promote growth of elongating leaves, stems, hypocotyls, and roots both in certain monocots and dicots of terrestrial environments. The functional significance of these ethylene-mediated growth-promoting effects remains to be clarified.
The Latent Time of Responses to Applied Ethylene
Previous studies of the effects of applied ethylene have almost exclusively investigated long-term growth responses (several hours to days). For instance, a triple response assay is typically performed over several days (Guzman and Ecker, 1990). Only two studies have addressed the topic of latent time necessary to change growth rate of etiolated pea seedlings and radish primary root upon ethylene treatment (Warner and Leopold, 1971; Jackson, 1983). The latent time of responses in pea seedlings (6–approximately 17 min) and radish root (approximately 15 min) are very similar to the present one for all the analyzed species (Table II). In contrast, however, we do not observe a clear difference in the latent time of response at different ethylene levels in the four Poa spp., whereas in pea seedlings, latent time of response was decreased almost 3-fold by doubling the ethylene level (from 5 to 10 μL L−1). The continuous presence of ethylene is needed to sustain these responses, as assessed in removal experiments in which a recovery of control growth rates was observed in pea seedlings (Warner and Leopold, 1971), radish roots (Jackson, 1983), and in the present study. Taken together, these results strongly suggest that a similar mechanism may operate in such different species and tissues.
Which components of the ethylene-response pathway are likely to be involved in such short-term responses? The first event in the ethylene-perception and signal transduction pathway is the binding of the gas with membrane-associated receptors (Johnson and Ecker, 1998). In Arabidopsis, ethylene receptors are encoded by a multigene family and may have a different affinity for ethylene (for review, see Bleecker and Schaller, 1996). Based on ethylene-binding assays, the receptors fall into two main classes; a fast- and a slow-dissociation class with a half-life of 15 to 30 min and more than 2 to 6 h, respectively (Sisler, 1990; Bleecker, 1997). The responses described here (latent time less than 30 min) probably involve the fast-dissociation class of receptors.
The ethylene signal can be transduced rapidly to downstream components involving phosphorylation events. Exposure of different tissues to ethylene increased protein phosphorylation and activated GTP-binding proteins in about 10 (Raz and Fluhr, 1993) or 60 min (Novikova et al., 1997, 1999). In tobacco, mRNA levels of a nuclear protein kinase (PK12) were up-regulated within a few minutes after ethylene treatment (Sessa et al., 1996; Savaldi-Goldstein et al., 2000). These results are consistent with the changes in leaf elongation rate that occurred in less than 30 min in the present study.
CONCLUDING REMARKS
We demonstrated that (a) Poa spp. that occur at different elevations inherently differ in the rate of ethylene production under non-stressful conditions, and that this is related to differential responsiveness of leaf growth to this gas; (b) at low concentrations ethylene promotes leaf growth of the two slow-growing (subalpine) species; and (c) at higher concentrations ethylene inhibits leaf elongation rate in all four Poa spp. and the latent time of response is approximately 20 min.
We investigated changes in leaf elongation rate mediated by ethylene at one developmental stage. Previous experiments showed that leaves of different age may respond differently to ethylene (Banga et al., 1996). Moreover, the effects of ethylene on whole-shoot growth can be addressed in long-term experiments to help to understand the significance of relatively small effects of ethylene on leaf elongation rate of individual leaves. Combining these results would enhance our knowledge of the role of ethylene in vegetative development in terrestrial plants.
MATERIAL AND METHODS
Plant Materials and Growth Conditions
Seeds of the lowland species Poa annua and Poa trivialis were purchased from a local supplier (Kieft bv, Blokker, The Netherlands). Seeds of the subalpine Poa compressa were harvested from a natural population at 1,600 m above sea level and kindly provided by Orto Botanico di Genova (Italy). Seeds of Poa alpina (1,800 m above sea level) were provided by Dr. O.K. Atkin (Faculty of Biology, University of York, UK) and obtained as described previously (Atkin et al., 1996). Only flat-leafed Poa spp. were selected (Atkin et al., 1996; Van Arendonk et al., 1997). P. annua is an annual species, whereas all the other three Poa spp. are perennial. Seeds of all the species were germinated in the dark, at 20°C ± 1°C, for 2 to 3 d on a double layer of filter paper moistened with demineralized water in petri dishes. Seedlings were selected for homogeneity of primary root length (approximately 2 cm), transplanted into sand culture, and watered daily with demineralized water. The sand culture with seedlings was in a growth chamber (20°C ± 1°C, 70% relative humidity [RH], photosynthetic photon flux density 320 ± 20 μmol m−2 s−1, and 16-h photoperiod). After 1 week, the seedlings were washed out of the sand and transferred to an aerated nutrient solution in 33-L containers (for exact composition, see Poorter and Remkes, 1990) under the same conditions as above. The pH was adjusted to 6 every 2nd d, and the solution was renewed weekly. This procedure was repeated for different batches of plants to achieve the desired number of replicates and for the different treatments. In each container, 24 plants of a given species were grown until the appearance of leaf 7 (leaf growth measurements) or 9 and 10 (ethylene emission).
Experimental Setup and Leaf Growth Measurements
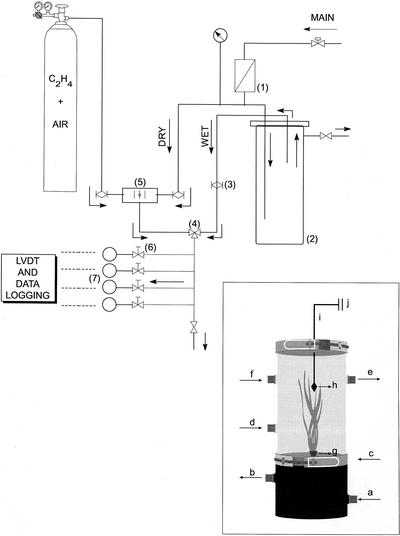
A custom-made flow-through system coupled to linear velocity displacement transducers (LVDTs) was used (Fig. 6). The set-up was used to measure (a) diurnal patterns of leaf growth and (b) short-term leaf growth responses to applied ethylene.
Figure 6.
Schematic representation of the custom-made flow-through system coupled to a LVDT set-up for the measurements of leaf growth rates. To achieve 70% RH in the air stream supplied to the cuvettes, a dry and a wet air stream were combined. For full details see “Materials and Methods.” 1, Air filter; 2, humidifier; 3, mass flow controllers; 4, three-way valve; 5, electronic gas blender; 6, flow meters with needle valves; and 7, cuvettes. All connections were made with air-tight tubes fittings, and the set-up was checked periodically for leakage. Inset, Schematic drawing of a custom-made cuvette is shown. a, Growth solution inlet; b, growth solution outlet; c, closing clamp; d, air flow inlet; e, air flow outlet; f, sensors insertion point; g, rubber stopper; h, leaf clamp + counterweight; i, aluminum thread; and j, to the LVDT arm (not shown). The drawing is not in scale. The cuvettes' internal volumes were 0.7 L (shoot compartment) and 0.3 L (root compartment).
For all the measurements in all species, leaf 7 was used when it was emerged 1 to 2 cm (depending on the species) from the leaf sheath of the previous leaf. First, leaf 7 was chosen because it is representative of an adult-type leaf. Second, to minimize the potential problem of using leaves of a different developmental age at the start of the experiment, we used plants at the 2nd d after the appearance of leaf 7 (duration of leaf elongation is similar in these species; Fiorani et al., 2000). Third, leaf 7 at the start and during the experiment was developing the leaf blade in all species, whereas the leaf sheath was not present.
The set-up was assembled in a temperature-controlled room (20°C ± 1°C). Outdoor compressed air (approximately 30% RH) was used to provide an air stream supply, as described below. The air was filtered and reduced in pressure to approximately 100 kPa. The main line was divided into two separate lines; one line was passed through a humidifier (PVC cylinder with gas-tight lid filled with clay beads and partly filled with demineralized water) to increase the moisture content of the air to near saturation. The flows of the “dry” and “wet” air lines were regulated by mass flow controllers (1 L min−1 capacity; Bronkhorst HIGH-TEC bv, Ruurlo, The Netherlands) and combined in adequate proportions to achieve 70% RH using a three-way valve, as assessed in blank experiments. This constituted the main (control air) inlet line that was divided into five independent lines using a manifold. One line was used as a bleed-off and air sampling point. Four lines were used to deliver a constant air flow (7 L h−1) to each of four correspondent air-tight, two-compartment (root-shoot) custom-made glass cuvettes (Fig. 6, inset). Flows were checked continuously by flow meters equipped with needle valves (Brooks Instrument bv, Veenendaal, The Netherlands).
Plants were sealed at the shoot-root junction in a hollow rubber stopper with polyvinylsiloxilane (addition-type) dental impression material (President light body, Coltène, Switzerland). The stoppers were inserted into a hole of corresponding size in the top lid of the cuvette bottom (root system) compartment. In this compartment, 3 L of pre-aerated, freshly prepared nutrient solution was circulated by water pumps. The tip of leaf 7 (approximately 0.5 cm) was attached to a clamp (fitted with rubber material to avoid mechanical damage of the leaf tip) connected to a 0.1-mm-diameter stainless steel thread to which a 1.6-g weight was attached. The thread was attached to the arm of a custom-made LVDT set-up equipped with LVDT ST 2000 range displacement transducers (sensitivity 9.6 mV V−1 mm−1; Schlumberger Industries, Bognor Regis, West Sussex, UK). The thread was passed through a 0.2-mm-diameter cut syringe tip glued in a hole on the top lid of the cuvette shoot compartment. The plants were then completely enclosed in the cuvettes and immediately supplied with the air stream described above. Each cuvette was provided with a 0.5-mm-diameter syringe tip mounted in an outlet fitting through a septum to avoid excessive pressure build up. The group of LVDTs was energized by a CAH 16 LVDT conditioning PCB interface. The interface was connected to a computer by which data were acquired, displayed on screen every 3 min, and logged by means of a custom software. The zero offset and the gain of the transducers were calibrated against a vernier caliper with a resolution of 0.01 mm over a distance of 50 mm. When total displacement exceeded 50 mm, the LVDTs were repositioned, and the system was reset.
Inside-Cuvette Conditions and Ethylene Treatments
During typical experiments, environmental conditions were measured inside the cuvettes with sensors inserted through septa mounted on custom cuvette fittings. Temperature and RH at the shoot base were measured using a humidity-thermometer (Testo 625, Testo, Almere, The Netherlands) and were 23°C ± 1°C and 75 ± 10, respectively. The overall light intensity at the plant level was 350 ± 20 μmol m−2 s−1 as measured by a photodiode sensor (Ga/ASP; Hamamatsu, Hamamatsu City, Japan). To achieve this light intensity, the cuvettes were placed under 15 fluorescent light tubes (36W/840, Philips, Eindhoven, The Netherlands) and at 50 cm from six vertically oriented lamps (Dulux 38W/11–860, Osram, Milan, Italy). The heat was removed with fans conveying fresh air from air-conditioning units.
Ethylene was supplied 24 h or more after the start of the experiments to allow plants to acclimate to the new conditions and to eliminate possible interference of the ethylene produced as a stress response to manipulation. Ethylene treatments were applied for 4 h by diluting the dry line of the aeration system with a gas stream of 5 or 21 μL L−1 ethylene taken from cylinders (ethylene in balanced air; Praxair N.V., Devel, Belgium), according to the desired ethylene concentration. This was accomplished with a mass flow controller and an electronic gas mixer (Fig. 6). At the time of ethylene application, the ratio between dry and humidified air was kept constant by decreasing proportionally the flow rate of the dry line.
We calculated the time necessary in our set-up to reach a new steady-state ethylene concentration after commencement of the ethylene application. Assuming that the air in the cuvette was homogenously mixed, the time to reach the final gas concentration is given by:
 |
1 |
where T is the time needed to reach 95% or 99% of the final expected concentration, Φ = F/V (F is flow rate in liters per hour; V is cuvette volume in liters), Ct0 and Cf are the initial and the final concentration of ethylene in microliters per liter, respectively. For example, the time needed to reach a new equilibrium concentration estimated with Equation 1 were T95 = 17 min and T99 = 27 min with Ct0 = 0.005 μL L−1 and Cf = 1 μL L−1; F = 7 L h−1; and V = 0.7 L. Leaf growth rates were calculated for each individual plant using linear regression based on 4-h growth registrations when under control conditions defined above. In a similar way, leaf growth rate was calculated during the ethylene treatments (only during steady-state concentrations) and was expressed as a percentage of control growth rate. Ethylene concentration in the inlet air stream was checked at least five times during each experiment. Samples taken from the sampling port were collected in gas-tight bottles sealed with puncture-septa and immediately injected into a gas chromatograph (model 437 A, Chrompack Packard, Bergen op Zoom, The Netherlands; with a 100-cm Hayesep N column and a flame ionization detector; oven temperature = 105°C).
Ethylene Emission
Whole-shoot ethylene emission was measured using on-line infrared CO2-laser driven photoacoustic spectroscopy, as described before (Voesenek et al., 2000 and refs. therein). To remove small hydrocarbons, the air of the flow-through system was passed through a platinum catalyst, consisting of a column with an internal volume of 40 mL filled with platinum-coated silica particles at a temperature of 500°C. CO2 and H2O were removed from the air stream between the cuvettes with the plants and the photoacoustic cell by KOH and CaCl2 scrubbers, respectively. Plants were enclosed in the same cuvette type and were exposed to similar conditions as described above. Whole-shoot ethylene emission was measured for at least 48 h after equilibration (2–5 h) at a flow rate of 1 L h−1 per cuvette. Background signal (measured in an empty cuvette) was subtracted from the experimental data points and the ethylene emission per shoot was calculated (see “Results”). The ethylene recovery was assessed regularly by injecting 1 mL of a 3.08 μL L−1 ethylene standard into the empty cuvette and then integrating the resulting peak. In all experiments, recovery varied between 97% and 101%. The individual data sets were corrected accordingly.
At the end of the experiments, shoot and root were separated, and fresh and dry weights (65°C for at least 48 h) were determined.
ACKNOWLEDGMENTS
We thank Wim Vriezen, Ronald Pierik, Ruud Nabben, and Werner Van Eck for their assistance at the beginning of several experiments, and we are grateful to Prof. Kees Blom for encouragement and support. F.F. thanks Dr. Tim Colmer and Prof. Mike Jackson for critically reading this manuscript before submission. We acknowledge Jos Reulen of the Electronics Department, University of Nijmegen, for designing the software and calibrating the transducers.
Footnotes
This work was funded by a PhD scholarship from Utrecht University (The Netherlands) to F.F.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001198.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. Ed 2. New York: Academic Press; 1992. [Google Scholar]
- Atkin OK, Botman B, Lambers H. The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Funct Ecol. 1996;10:698–707. [Google Scholar]
- Banga M, Slaa EJ, Blom CWPM, Voesenek LACJ. Ethylene biosynthesis and accumulation under drained and submerged conditions: a comparative study of two Rumex species. Plant Physiol. 1996;112:229–237. doi: 10.1104/pp.112.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB. The ethylene binding site of the ETR1 protein. In: Kanellis A, Chang A, Kende H, Grierson D, editors. Biology and Biotechnology of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 63–70. [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Schaller GE. The mechanism of ethylene perception. Plant Physiol. 1996;111:653–660. doi: 10.1104/pp.111.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultynck L, Fiorani F, Lambers H. Control of leaf growth and its role in determining variation in plant growth rate from an ecological perspective. Plant Biol. 1999;1:13–18. [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC. Phenotypic plasticity of stem elongation in two ecotypes of Stellaria longipes: the role of ethylene in response to wind. Plant Cell Environ. 1994;17:691–700. [Google Scholar]
- Fiorani F, Beemster GTS, Bultynck L, Lambers H. Can meristematic activity determine variation in leaf size and elongation rate between four Poa species? A kinematic study. Plant Physiol. 2000;124:845–856. doi: 10.1104/pp.124.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Regulation of root growth and morphology by ethylene and other externally applied growth substances. In: George EC, editor. Growth Regulators in Root Development, Monograph 10. Wantage, UK: British Plant Growth Regulator Group; 1983. pp. 103–116. [Google Scholar]
- Jackson MB, Morrow IB, Osborne DJ. Abscission and dehiscence in the squirting cucumber, Ecballium elaterium: regulation by ethylene. Can J Bot. 1972;50:1465–1471. [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Kende H, van der Knaap H, Hyung Taeg Cho. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, Pieterse CMJ, Bol JF, van Loon LC. Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant-Microbe Interact. 1999;12:720–727. doi: 10.1094/MPMI.1999.12.8.720. [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Henning J, Bol JF. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings H, Jackson MB. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Z Pflanzenphysiol. 1979;92:385–397. [Google Scholar]
- Körner C, Woodward FI. The dynamics of leaf extension in plants with diverse altitudinal ranges: II. Field studies in Poa species between 600 and 3200 m altitude. Oecologia. 1987;72:279–283. doi: 10.1007/BF00379279. [DOI] [PubMed] [Google Scholar]
- Lee SH, Reid DM. The role of endogenous ethylene in the expansion on Helianthus annuus leaves. Can J Bot. 1997;75:501–508. doi: 10.1139/b97-054. [DOI] [PubMed] [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Hall MA. The effect of ethylene on GTP binding in extracts from pea epicotyls. Planta. 1997;201:1–8. [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Kulaeva ON, Hall MA. The effect of ethylene and cytokinin on guanosine 5′-triphosphate binding and protein phosphorylation in leaves of Arabidopsis thaliana. Planta. 1999;208:239–246. doi: 10.1007/s004250050555. [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Leopold AC. Effects of ethephon on growth of grasses. Crop Sci. 1973;13:755–758. [Google Scholar]
- Raz V, Fluhr R. Ethylene signal is transduced via protein phosphorylation events in plants. Plant Cell. 1993;5:523–530. doi: 10.1105/tpc.5.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS. Ethylene in plant growth, development and senescence. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 486–508. [Google Scholar]
- Savaldi-Goldstein S, Sessa G, Fluhr R. The ethylene-inducible PK12 kinase mediates the phosphorylation of SR splicing factors. Plant J. 2000;21:91–96. doi: 10.1046/j.1365-313x.2000.00657.x. [DOI] [PubMed] [Google Scholar]
- Sessa G, Raz V, Savaldi S, Fluhr R. PK12, a plant dual-specificity protein kinase of the LAMMER family, is regulated by the hormone ethylene. Plant Cell. 1996;8:2223–2234. doi: 10.1105/tpc.8.12.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC. Ethylene binding components in plants. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. New York: CRC Press; 1990. pp. 81–99. [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. [Google Scholar]
- Suge H. Stimulation of oat and rice mesocotyl growth by ethylene. Plant Cell Physiol. 1971;12:831–837. [Google Scholar]
- Suge H, Nishizawa T, Takahashi H, Takeda K. Phenotypic plasticity of internode elongation stimulated by deep-seeding and ethylene in wheat seedlings. Plant Cell Environ. 1997;20:961–964. [Google Scholar]
- Summers JE, Jackson MB. Light- and dark-grown Potamogeton pectinatus, an aquatic macrophyte, make no ethylene (ethene) but retain responsiveness to the gas. Aust J Plant Physiol. 1998;25:599–608. [Google Scholar]
- Van Arendonk J, Niemann GJ, Boon JJ, Lambers H. Effects of N-supply on anatomy and chemical composition of leaves of four grass species, belonging to the genus Poa, as determined by image-processing and pyrolysis-mass spectrometry. Plant Cell Environ. 1997;20:881–897. [Google Scholar]
- Voesenek LACJ, Blom CWPM. Stimulated shoot elongation: a mechanism of semiaquatic plants to avoid submergence stress. In: Lerner HR, editor. Plant Responses to Environmental Stresses: From Phytohormones to Genome Reorganization. New York: Marcel Dekker; 1999. pp. 431–448. [Google Scholar]
- Voesenek LACJ, Harren FJM, de Vries HSM, Sikkens CA, te Linkel Hekkert S, Blom CWPM. Photoacoustic and photothermal detection of the plant hormone ethylene. In: Tucker GA, Roberts JA, editors. Methods in Molecular Biology. 141: Plant Hormone Protocols. Totowa, NJ: Humana Press; 2000. pp. 67–91. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Van Rijn CPE, Voesenek LACJ, Mariani C. A homologue of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. Plant J. 1997;11:1265–1271. doi: 10.1046/j.1365-313x.1997.11061265.x. [DOI] [PubMed] [Google Scholar]
- Warner HL, Leopold AC. Timing of growth regulator responses in peas. Biochem Biophys Res Commun. 1971;44:989–994. doi: 10.1016/0006-291x(71)90809-6. [DOI] [PubMed] [Google Scholar]