Abstract
Kainate receptors (KARs) are abundantly expressed in the central nervous system at a period of intense synaptogenesis and might participate in the maturation of neural networks. We have described the postnatal development of mossy fibre excitatory synaptic transmission in CA3 pyramidal cells and we have explored the potential role of KARs in synaptic maturation. In CA3 pyramidal cells, mossy fibre stimulation evokes EPSCs as early as postnatal day 3 (P3). At this early stage, mossy fibre (MF)-EPSCs are fully blocked by GYKI 53655, an AMPA receptor (AMPAR) antagonist. A postsynaptic KAR component can only be detected from P6. Thus, AMPAR-EPSCs precede KAR-EPSCs during postnatal maturation at this synapse. All MF-EPSCs display a KAR component after P10. A key issue of the present work is that between P6 and P9, the presence of a postsynaptic KAR component tightly coincides with AMPAR-mediated EPSCs of large amplitude, and with the onset of low frequency facilitation (from 0.1 Hz to 1 Hz), a presynaptic form of short-term synaptic plasticity. In addition, mice lacking functional KARs throughout postnatal development display MF-EPSCs of significantly smaller amplitude at stages of maturation where synaptic KARs are normally present, due to both pre- and postsynaptic impairment of synaptic transmission. These data suggest a role for KARs in the maturation of mossy fibre synapses.
Kainate receptors (KARs) are ionotropic glutamate receptors assembled from different combinations of GluR5, GluR6, GluR7, KA1 and KA2 subunits. Much of our knowledge on the synaptic function of KARs arises from the analysis of their role at synapses made by dentate gyrus mossy fibres onto hippocampal CA3 pyramidal cells. AMPARs are the main receptors involved in mossy fibre synaptic transmission, with a minor participation of NMDA receptors (Henze et al. 2000). KARs mediate a postsynaptic component of small amplitude and slow kinetics with summation properties that might be important for the temporal integration of excitatory inputs (Castillo et al. 1997; Vignes & Collingridge, 1997; Mulle et al. 1998; Cossart et al. 2002). Upon repeated mossy fibre stimulation, activation of KARs probably localized on mossy fibre axon terminals leads to different forms of short-term synaptic plasticity (Schmitz et al. 2000; Contractor et al. 2001; Kullmann, 2001; Lerma et al. 2001; Schmitz et al. 2001). Electrophysiological comparison of wild-type mice and mice deficient in the GluR6 subunit, as well as the use of antagonists selective for presynaptic KARs, have implicated KARs in the induction of the presynaptic and NMDA-independent form of LTP at this synapse (Bortolotto et al. 1999; Contractor et al. 2001; Lauri et al. 2001).
Several lines of evidence suggest a role for KARs in neuronal development but nothing is known about the developmental maturation of KARs in CA3 pyramidal cells. Early reports detected KAR subunit mRNAs in the brain at early developmental stages (Bettler et al. 1990; Bahn et al. 1994). In the somatosensory cortex, developing thalamocortical synapses express postsynaptic KARs. Moreover, during a critical period for experience-dependent plasticity, the KAR contribution to synaptic transmission decreases while the AMPAR contribution increases (Kidd & Isaac, 1999). Finally, synaptic stimulation of KARs enhances the motility of mossy fibre axonal filopodia in immature hippocampal slices (Tashiro et al. 2003).
Given the extensively studied function of KARs at the mature mossy fibre synapse and the suggested role that KARs could play in the formation of synaptic circuits, we sought to describe the postnatal development of mossy fibre synaptic responses mediated by AMPARs and KARs in CA3 pyramidal cells during the first three postnatal weeks. We show that AMPAR-EPSCs precede KAR-EPSCs at mossy fibre synapses. The appearance of a synaptic KAR component tightly coincides with a large increase in the amplitude of the AMPA-component of MF-EPSCs, as well as with the onset of frequency facilitation, a characteristic form of short-term plasticity at this synapse. We finally examine the maturation of pre- and postsynaptic features of MF-EPSCs in mice devoid of both GluR5 and GluR6 subunits. Altogether, our data indicate a role for KARs in the maturation of mossy fibre synapses.
Methods
Experimental procedures followed the recommendations of the CNRS ethics committee and the French Ministry of Agriculture and Forestry concerning animal care (authorization number, A33093). Parasagittal brain slices (350 μm thick) were prepared from P0 to P21 mice (P0 being the day of birth). Both control and mutant mice had a mixed genetic backround (129SvJ × C576Bl6). Pups and young mice were killed by cervical dislocation and the brains were dissected in ice-cold saline solution. Slices were stored in an oxygenated chamber at room temperature for 1 h prior to transfer to a submersion recording chamber. Whole-cell voltage-clamp recordings (2.6–3.4 MΩ electrodes, −60 mV holding potential) were made from hippocampal CA3 pyramidal cells visualized by infrared video-microscopy. Experiments were performed at room temperature (22–25°C). Slices were superfused with extracellular solution composed of: 125 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 26 mm NaHCO3, 2.3 mm CaCl2, 1.3 mm MgCl2, 25 mm glucose and saturated with 95% O2–5% CO2. Bicuculline (10 μm) and d-aminophosphonovalerate (d-AP5; 25 μm) were added to the bath to, respectively, block GABAA and NMDA receptors. The intracellular solution was composed of: 140 mm CsCl, 10 mm Hepes, 10 mm EGTA, 2.3 mm MgCl2, 1.3 mm CaCl2, 2 mm ATP-Na (pH 7.30).
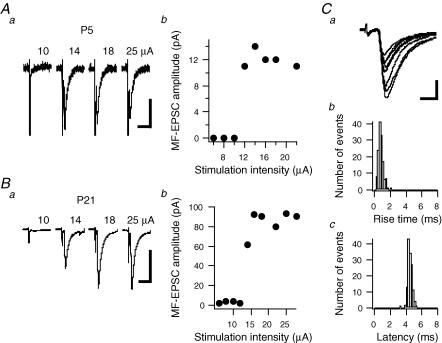
Patch pipettes filled with Hepes-buffered external solution were placed at the inner border of the dentate gyrus to stimulate mossy fibres. While recording from a pyramidal cell, the stimulating electrode was moved to a position where a distinct EPSC with fixed latency was evoked (see Fig. 1). Stimulation intensity (15–20 μA for a 200 μs pulse) was adjusted just above the sharp threshold for activation of a synaptic response which was observed at all ages between P3 and P21 (see Fig. 1) and in both mouse genotypes. Using low stimulation intensity, no prominent polysynaptic activation was observed. The group II mGluR agonist LCCG-1 (10 μm) was routinely applied for 3 min to confirm that mossy fibres were stimulated. Recordings were analysed using IGOR Pro (WaveMetrics, Inc.).
Figure 1. Methods used for mossy fibre stimulation.
Representative recordings (average of 60 traces including failures) of a CA3 pyamidal cell from a P5 (Aa) and from a P21 wild-type mouse (Ba). A patch pipette filled with an NaCl-based solution was placed at the inner border of the dentate granule cell body layer. A 200 μs stimulation pulse was delivered at variable intensities. While recording, the stimulating pipette was moved until a distinct EPSC was evoked. Scale bars: Aa, 20 ms and 5 pA; Ba, 20 ms and 50 pA. Ab and Bb, MF-EPSC average amplitude as a function of stimulation intensity. For the present study, stimulation intensity was set just above the sharp threshold for triggering a synaptic response. Note that further increasing stimulation intensity within our working range did not increase average MF-EPSC amplitude. Ca, 8 consecutive traces recorded in a CA3 pyramidal cell from a P21 wild-type mouse (at a frequency of 0.5 Hz). MF-EPSCs display variable amplitude but fixed synaptic latency. Note that no prominent polysynaptic activity is observed. Scale bars: 5 ms, 50 pA. Cb and Cc, histograms of the distribution of MF-EPSC rise times and latencies, respectively, for the pyramidal cell exemplified in Ca.
MF-EPSCs were obtained by averaging 30–100 responses. KAR-EPSCs were recorded in the presence of the AMPAR antagonist, GYKI 53655 (50 μm). ‘Low frequency-facilitation’ was evoked by stimulating mossy fibres at frequencies of 0.1, 0.2, 0.5 and 1 Hz. Each test period was preceded by 18 stimuli at 0.1 Hz, allowing the EPSC to return to the initial level. For ‘train facilitation’ (5 stimuli at 50 Hz), the amplitude of the fifth EPSC was normalized relative to the first EPSC in the train. For Sr2+ experiments, slices were bathed in an extracellular solution containing SrCl2 (2 mm) and a low concentration of CaCl2 (0.03 mm). Asynchronous EPSCs were recorded during a 350 ms interval, begining 50 ms after a train of five stimuli at 50 Hz. The following statistical tests were used where appropriate: Mann-Whitney U test, Kolmogorov-Smirnov test, two-way ANOVA.
Results
Properties of mossy fibre EPSCs at early developmental stages
MF-EPSCs were recorded in CA3 pyramidal cells during the first three postnatal weeks in mice. Stimulation was performed with a patch pipette placed in the dentate gyrus and intensity was adjusted to just above the sharp threshold for activation of a synaptic response (see Methods and Fig. 1). Under these experimental conditions synaptic responses displayed fixed latency and no prominent polysynaptic activity. To further ascertain that pure MF-EPSCs were studied, we routinely checked the selective inhibition of these synaptic responses by the group II mGluR agonist LCCG-1 (10 μm) (for review see Henze et al. 2000; Fig. 2A). MF-EPSCs were detected as early as P3. In some instances, MF-EPSCs displayed synaptic fatigue at a stimulation frequency of 0.1 Hz, as previously described (Gasparini et al. 2000; data not shown). In most instances, MF-EPSCs displaying no synaptic fatigue at a rate of 0.1 Hz were detected from P3. We tested whether the rate of MF-EPSC failures changed with postnatal maturation by comparing four age groups representing different morphological stages of mossy fibre development (see Discussion) (P3–5; P6–9; P10–14; P15–21). No significant difference was observed in failure rates between the age groups when a low stimulation frequency was used (0.1 Hz) (Fig. 2B). We examined whether low frequency facilitation (with frequencies ranging from 0.1 Hz to 1 Hz), a presynaptic feature of mossy fibre synapses (Salin et al. 1996), was present at the earliest developmental stages (Fig. 2A). The extent of low frequency facilitation was measured as the relative MF-EPSC amplitude averaged at 1 Hz versus at 0.1 Hz (Fig. 2C). No significant facilitation was observed at P3–5 (P > 0.5). Clear frequency facilitation was observed from P6 (P < 0.005). The level of frequency facilitation significantly increased at the latest stage tested (P15–21) (P10–14 versus P15–21, P < 0.01). Low frequency facilitation clearly correlated with a decrease in failure rate (Fig. 2B).
Figure 2. Properties of MF-EPSCs at early developmental stages.
A, at P5, MF-EPSCs are of small amplitude and do not show low frequency facilitation when stimulation frequency is increased from 0.1 to 1 Hz. At later stages, MF-EPSCs display facilitation. Five consecutive sample traces are superimposed for each age and for each frequency. Right traces (average), for P5 and P21, superimposed average traces at two frequencies of stimulation (0.1 and 1 Hz). LCCG-1 (10 μm) was bath applied for 3 min at the end of the experiment. B, proportion of failures as a function of stimulation frequency, for different age groups (P3–5, n = 6; P6–9, n = 23; P10–14, n = 15; P15–21, n = 10). At low stimulation frequency (0.1 Hz), the proportion of failures does not significantly differ between the age groups. The rate of failures decreases when frequency of stimulation is increased in all age groups, except at P3–5. C, low frequency facilitation expressed as the normalized EPSC amplitude at 1 Hz compared to 0.1 Hz (×100), in the various age groups, in wild-type mice and in GluR5−/− × GluR6−/− mice. No facilitation is observed at P3–5 for both genotypes. Marked facilitation is observed after P6 in wild-type mice and in GluR5−/− × GluR6−/− mice. In wild-type mice, but not in GluR5−/− × GluR6−/− mice, facilitation further increases after P15 (* P < 0.05). D and E, the level of synaptic facilitation during high frequency trains (5 pulses, 50 Hz) does not change significantly with age and with genotype. D, sample traces of average synaptic responses at P3 and P21 in wild-type mice. E, to quantify train facilitation, the fifth MF-EPSC was normalized to the amplitude of the first EPSC. Amplitudes of individual EPSCs were measured from a baseline current immediately preceding the stimulus artefact for each EPSC. The error bars represent ± s.e.m.
After the second postnatal week, low frequency facilitation was impaired in mice lacking functional KARs, consistent with previous observations (Contractor et al. 2001) (Fig. 2C). The level of facilitation was 716 ± 137% (n = 18) in wild-type mice and 397 ± 87% (n = 9) in GluR5−/− × GluR6−/− mice (significant difference with P < 0.05). Interestingly, at earlier developmental stages, no difference was observed when comparing the extent of low frequency facilitation at mossy fibre synapses in both genotypes (Fig. 2C). These data point to a biphasic maturation of low frequency facilitation at mossy fibre synapses. KARs appear to be involved in the upregulation of low frequency facilitation only after the second postnatal week.
Using a pharmacological approach, KARs have also been involved as facilitatory presynaptic receptors during high-frequency trains (50–100 Hz) (Lauri et al. 2001; Schmitz et al. 2001). We examined ‘train facilitation’ of MF-EPSCs following a 50 Hz train (5 stimuli) at different stages of maturation (Fig. 2D). No significant difference in the normalized amplitude of the first versus the fifth EPSC was observed between the different age groups (P > 0.5) (Fig. 2E). In addition, a facilitatory effect of KARs could not be revealed at any developmental stage by comparing wild-type and mutant mice (Fig. 2E). Previous observations on GluR6−/− mice resulted in the same conclusion using a 100 Hz train of 10 stimuli (Contractor et al. 2001). However, in this study, a difference in paired-pulse ratio was observed between the two genotypes at 20 and 40 ms interstimulus intervals, in contrast to our results on GluR5−/− × GluR6−/− mice (see also Mulle et al. 1998). The reason for a discordance between the pharmacological results (Lauri et al. 2001; Schmitz et al. 2001) and the data obtained with mutant mice is unclear. The discrepancy might be due to species differences (rat versus mouse), or to compensatory changes in double mutant mice.
AMPAR-EPSCs precede KAR-EPSCs at mossy fibre synapses
We evaluated the relative contribution of AMPARs and KARs to MF-EPSCs at different stages of postnatal development. GYKI 53655 (50 μm) fully blocked all MF-EPSCs at early developmental stages: we found no evidence for a synaptic KAR component before P6 (Fig. 3). We systematically checked that no compound KAR-EPSC was evoked by a train of stimuli (Fig. 3), which can unmask small KAR-EPSCs (Castillo et al. 1997; Vignes & Collingridge, 1997). Between P6 and P9, MF-EPSCs displaying a KAR component were detected in an increasing proportion of cells (42% at P6, n = 12; 55% at P7, n = 11; 80% at P8, n = 5; 75% at P9, n = 4) (Figs 3 and 4B). After P10, all MF-EPSCs displayed a KAR component (15 out of 15 cells, P10–14). The peak amplitude of the synaptic KAR component, measured in the presence of GYKI 53655 at a stimulation rate of 0.5 Hz (100 traces averaged) ranged from 1 to 18 pA and did not clearly increase with age between P6 and P21. No significant difference was found in the decay time constant of KAR-EPSCs at any stage of maturation (P6–9: 62.7 ± 8 ms, n = 18; P10–14: 54.8 ± 7 ms, n = 15; P15–22: 75.6 ± 6 ms, n = 11) (P > 0.2). Due to the small relative amplitude of KAR-EPSCs and slower onset kinetics as compared to control MF-EPSCs, the peak amplitude of MF-EPSCs closely corresponds to that of the AMPAR component. MF-EPSC amplitude will thus be considered as AMPAR-EPSC amplitude for the rest of the study. The relative peak amplitude of KAR versus AMPAR synaptic currents reached a maximum at the end of the second week (Fig. 4C).
Figure 3. AMPAR EPSCs precede KAR EPSCs at mossy fibre synapses.
MF-EPSCs recorded in CA3 pyramidal cells at different ages, in control conditions (25 μm d-AP5, and 10 μm bicuculline) and in the presence of GYKI 53655, to isolate KAR-mediated synaptic responses. Left traces, each trace represents the average of 100 traces recorded at a stimulation frequency of 0.5 Hz. Right traces represent average synaptic compound EPSCs in response to a train of 5 stimuli at 50 Hz, at a rate of 0.2 Hz. At P4, and in a proportion of cells at P6, GYKI 53655 abolishes synaptic responses, indicating that MF-EPSC is mediated by AMPAR but not by KARs at early stages of maturation, even in response to a high frequency train of stimuli (5 stimuli at 50 Hz). In some cells between P6 and P9, and in all cells after P10, a small KAR-mediated synaptic component can be detected, with slow kinetics. KAR-EPSCs summate in response to a train of stimuli. Example traces at P6 and at P21.
Figure 4. Age-dependent changes in AMPAR and KAR-EPSC characteristics.
A and B, plots of the average amplitude of AMPAR- (left) and of KAR-mediated EPSCs (right) as a function of age. Peak EPSC amplitude was measured for the average of 100 consecutive traces recorded at a frequency of 0.5 Hz. Each symbol represents an individual CA3 pyramidal cell. Left, open circles represent cells in which MF-EPSCs display a KAR component and filled circles those with no KAR synaptic component. Note the difference in amplitude between the two categories of MF-EPSCs at ages P6–9. Appearance of a KAR component at P6 correlates with a large increase in the amplitude of some EPSCs. C, histogram of the ratio of the amplitude of KAR-EPSCs versus AMPAR-EPSCs in the different age groups. This ratio reaches a maximum at P10–14 (P10–14 versus P15–21, P < 0.005). At P6–9, the shaded columns correspond to all cells, the open column corresponds to cells displaying KAR-EPSCs. The error bars represent ± s.e.m.
Evidence for a role of postsynaptic KARs in the maturation of MF-EPSCs
The average amplitude of MF-EPSCs evoked with a minimal stimulation intensity (at a frequency of 0.5 Hz), increased with age over the first two postnatal weeks (Figs 2 and 4A). Between P6 and P9, two categories of MF-EPSCs were observed, those displaying and those devoid of a synaptic KAR component. MF-EPSCs displaying a KAR component were of larger amplitude than MF-EPSCs lacking a KAR component (Fig. 5A and B). The average amplitude of AMPAR-mediated MF-EPSCs was 24 ± 6 pA (n = 11) for MF-EPSCs without a KAR component, and 98 ± 17 pA (n = 18) for MF-EPSCs with a KAR component (significant difference with P < 0.0005). Interestingly, MF-EPSCs with a KAR component exhibited clear low frequency facilitation (366 ± 54% at 1 Hz, n = 15), whereas MF-EPSCs without a KAR component only displayed a low level of facilitation (162 ± 45% at 1 Hz, n = 5) (Fig. 5C and D) (the difference between the two groups is significant with P < 0.02). The presence of a KAR component at individual mossy fibre synapses thus clearly coincides with signs of maturation of MF-EPSCs, i.e. large AMPA-mediated MF-EPSC amplitude and clear low frequency facilitation.
Figure 5. At individual mossy fibre synapses, the presence of a KAR component is correlated with AMPAR EPSCs of large amplitude and with low frequency facilitation.
A, plot of the peak amplitude of KAR-EPSCs as a function of the corresponding value for AMPAR-EPSCs, at ages P6 to P9 when only a proportion of MF-EPSCs display a KAR-mediated component. B, sample traces of MF-EPSCs displaying a KAR component (KAR+ at P6) or devoid of a KAR component (KAR–) recorded at two stimulation frequencies. C and D, comparison of the average amplitude of AMPAR-EPSCs and of the level of low frequency facilitation at ages P6 to P9 for MF-EPSCs displaying a KAR component (KAR+, n = 18) and for those devoid of a KAR synaptic component (KAR–, n = 11 for amplitude, n = 5 for frequency facilitation; *** P < 0.0005; * P < 0.02). The error bars represent ± s.e.m.
Either the presence of synaptic KARs is merely a consequence of maturation of MF-EPSCs, or synaptic KARs play a role in the maturation of MF-EPSCs. This latter possibility implies that the absence of KARs could impair the maturation of MF-EPSCs. We thus tested whether the absence of the major KAR subunits GluR5 and GluR6, at all developmental stages, affected the maturation of MF-EPSCs. Significant differences were found in the average amplitude of AMPAR-mediated MF-EPSCs (recorded at a stimulation rate of 0.1 Hz), after P6 between wild-type and GluR5−/− × GluR6−/− mice (P < 0.005; Fig. 6A). This difference might be due to genotype-dependent changes in presynaptic parameters such as release probability or mossy fibre excitability. At a stimulation frequency of 0.1 Hz, no change was found in paired-pulse ratio (with a 20 ms interval), as would be expected with a change in release probability between the genotypes (wild-type: 162 ± 9%, n = 11; GluR5−/− × GluR6−/−, 159 ± 19%, n = 7). However, GluR5−/− × GluR6−/− mice showed a larger rate of failures at mossy fibre synapses (wild-type: P3–5, 39 ± 16%, n = 4; P6–9, 51 ± 10%, n = 22; P10–14, 30 ± 4%, n = 15; P15–21, 30 ± 6%, n = 9; GluR5−/− × GluR6−/−: P3–5, 75 ± 10%, n = 3; P6–9, 54 ± 10%, n = 7; P10–14, 50 ± 8%, n = 9; P15–21, 50 ± 9%, n = 8). This indicates that mossy fibre excitability differs in wild-type mice as compared with GluR5−/− × GluR6−/− mice. In order to take into account this parameter we compared mossy fibre synaptic potency (i.e. average amplitude excluding failure). A significant difference in mossy fibre synaptic potency was observed between the two genotypes at P15–P21 (Fig. 6B) (P < 0.03). This difference is in favour of changes also occurring at a postsynaptic level.
Figure 6. Comparison of MF-EPSCs in wild-type mice and in mice lacking kainate receptor subunits.
A, comparison of the average amplitude of MF-EPSCs recorded at 0.1 Hz in wild-type mice (filled columns, n = 6, 19, 15 and 10, respectively) and in GluR5−/− × GluR6−/− mice (open columns, n = 3, 7, 9 and 8, respectively). Average MF-EPSC amplitude is significantly smaller in GluR5−/− × GluR6−/− mice (** P < 0.005). B, comparison of the average MF-EPSC potency (i.e. average amplitude excluding failures) recorded at 0.1 Hz in wild-type mice (filled columns, n = 6, 19, 15 and 10, respectively) and GluR5−/–× GluR6−/− mice (open columns, n = 3, 7, 9 and 8, respectively). MF-EPSC potency is significantly smaller in GluR5−/− × GluR6−/− mice (* P < 0.05). The error bars represent ±s.e.m.
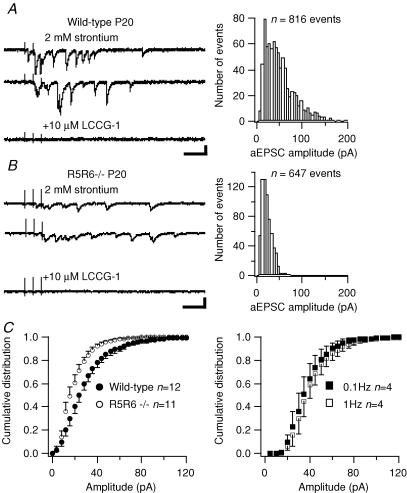
A more direct assessment of quantal events arising from a stimulated synapse has made use of Sr2+ (Bekkers & Clements, 1999). Replacing Ca2+ by 2 mm Sr2+ in the external solution produced asynchronous EPSCs (aEPSCs) in response to a short burst of mossy fibre stimuli (5 stimuli at 50 Hz), at variable latencies lasting for several hundred milliseconds (Fig. 7Ba and Ca). At other synapses, it was demonstrated that asynchronous EPSCs arose from the same synapses that generated the evoked EPSCs (Bekkers & Clements, 1999). We think that under our experimental conditions asynchronous EPSCs originate from mossy fibre synapses and not from other glutamatergic afferents to CA3 pyramidal cells. Indeed, we stimulated the dentate gyrus with a patch pipette at minimal intensities and we routinely checked that both synchronously evoked EPSCs and aEPSCs were inhibited by the mGluR agonist LCCG-1 (Fig. 8A and B). It could be argued that the synchronously evoked MF-EPSCs trigger commissural EPSCs by activation of the CA3 neuronal network. This is unlikely since these putative recurrent EPSCs were not prominent in control conditions (Fig. 7A), in which stimulation is much more efficient in evoking an EPSC than in the presence of Sr2+. Indeed, Sr2+ largely decreases release probability and the amplitude of synchronously evoked EPSCs (Fig. 7A). In addition, we observed little variability in the rise time of aEPSCs within a given cell, as would be expected if EPSCs arising from various distal synapses were also collected (Fig. 7Bb and Cb). Thus, aEPSCs recorded in the presence of Sr2+ in our experimental conditions probably originate from mossy fibre synapses. We compared the amplitude of mossy fibre aEPSCs evoked in CA3 pyramidal cells from wild-type and from GluR5−/− × GluR6−/− mice, at ages ranging from P11 to P20. In both genotypes, we observed a large variability in the amplitude of aEPSCs in a given CA3 pyramidal cell, ranging from less than 10 pA to 200 pA. We observed no clear difference in the average frequency of aEPSCs between the genotypes (aEPSC frequency during a 350 ms period, 50 ms after the last stimulation; wild-type, 11.6 ± 2.3 Hz, n = 10; GluR5−/− × GluR6−/−, 11.9 ± 1.6 Hz, n = 9; P > 0.5). However, in most cells from wild-type mice, there was an excess of large amplitude aEPSCs in comparison with GluR5−/− × GluR6−/− (Fig. 8). On average, mossy fibre aEPSCs in GluR5−/− × GluR6−/− mice were of significantly smaller amplitude (P < 0.05, Kolmogorov-Smirnov test) (Fig. 8C). To verify that aEPSC amplitude was not correlated with release probability, we compared the amplitudes of aEPSCs recorded in conditions where trains were given at two different rates (0.1 versus 1 Hz). In spite of a change in aEPSC frequency (11.6 ± 0.8 Hz at 0.1 Hz; 18.5 ± 4.7 Hz at 1 Hz, wild-type mice, n = 4), there was no significant difference in aEPSC amplitude (P > 0.8, Kolmogorov-Smirnov test) (Fig. 8C). Thus changes in release probability could be related to changes in aEPSC frequency, but not to any change in aEPSC amplitude. These data indicate a postsynaptic difference in the amplitude of AMPA-mediated MF-EPSCs in mice lacking KARs.
Figure 7. Recording of asynchronous MF-EPSCs in the presence of strontium.
A, representative traces in a P21 wild-type mouse of MF synaptic responses to a train of 5 stimuli (50 Hz) in control conditions (2.3 mm Ca2+) and in the presence of strontium (2 mm Sr2+ and 0.03 mm Ca2+). Using minimal intensity stimulation, no prominent polysynaptic activity is observed in control conditions. In the presence of Sr2+, a large decrease in release probability is observed for the synchronous synaptic response. A large barrage of asynchronous EPSCs is, however, triggered by the train of stimuli. Ba, asynchronous EPSCs were detected and plotted as a function of latency after the last stimuli for 50 consecutive traces (5 s intervals). Bb, histogram distribution of the rise time of aEPSCs for the same cell as in A and Ba. Ca, plot of aEPSC amplitudes as a function of latency after the last stimuli for 50 consecutive traces (5 s intervals) in a GluR5−/− × GluR6−/− mouse. Note the overall comparable frequency of aEPSCs as compared to Ba. Cb, histogram distribution of the rise time of aEPSCs for the same cell as in Ca.
Figure 8. Comparison of the amplitude of asynchronous MF-EPSCs in wild-type and in GluR5−/− × GluR6−/− mice.
A and B, left, sample consecutive traces of MF-evoked asynchronous EPSCs (aEPSCs), recorded in a CA3 pyramidal cell, in an extracellular medium where Ca2+ is replaced by Sr2+ (2 mm). In response to a train of 3 mossy fibre stimuli (50 Hz, every 5 s), a barrage of aEPSCs is recorded for several hundreds of milliseconds. Last trace, addition of LCCG-1 (10 μm) blocks all aEPSCs, confirming their MF origin (scale bars: 50 ms, 50 pA). Right, histograms of the amplitudes of aEPSCs for the corresponding cells; aEPSCs with a latency ranging from 50 to 400 ms after the last pulse were taken into account. A, wild-type mice; B, GluR5−/− × GluR6−/− mice. C, left, average cumulative amplitude histogram of aEPSCs showing a significant difference between wild-type (•, n = 7) and GluR5−/− × GluR6−/− (○, n = 6) mice (P < 0.05, Kolmogorov-Smirnov test). C, right, average cumulative amplitude histogram of aEPSCs showing no significant difference in wild-type mice between aEPSCs recorded at a rate of 0.1 Hz (▪, n = 4) and 1 Hz (□, n = 4) (P > 0.8, Kolmogorov-Smirnov test). The error bars represent ± s.e.m.
Discussion
In this paper we describe the maturation of mossy fibre synaptic transmission in CA3 pyramidal cells during the first three postnatal weeks and we evaluate the role of KARs in this process. One important aspect of this study is the finding that the emergence of a KAR component in MF-EPSCs tightly coincides with a shift to MF-EPSCs of large amplitude displaying a high degree of low frequency facilitation. In keeping with this finding, we present evidence that the lack of KARs during the whole postnatal development perturbs the maturation of AMPAR-mediated MF-EPSCs. We propose that these changes occur both at a pre- and postsynaptic level. These data thus suggest that KARs play a critical role in the maturation of mossy fibre synapses.
What are the different stages of the functional maturation of MF-EPSCs? In a first stage, before P3, no MF-EPSCs could be evoked. In a second stage (P3–P5), stable MF-EPSCs mediated by AMPARs but devoid of a synaptic KAR component were recorded. At this stage, MF-EPSCs did not display low frequency facilitation when stimulation frequency was increased from 0.1 Hz to 1 Hz, and had an average amplitude in the order of 20 pA. A third stage (P6–P9) was characterized by the appearance of large amplitude AMPAR-mediated MF-EPSCs in an increasing number of cells tested. There was a strong correlation between the amplitude of AMPAR-mediated MF-EPSCs and the presence of a synaptic KAR component. At this stage, MF-EPSCs displayed low frequency facilitation. However, the extent of facilitation was strikingly higher for synapses containing a synaptic KAR component. The period when KARs begin to be detected at a postsynaptic level also corresponds to the period where the action of GABAA receptors switch from depolarizing to hyperpolarizing (Ben-Ari, 2002). In a fourth stage (P10–P14), all MF-EPSCs display a KAR component. Finally, during the third postnatal week, MF-EPSCs demonstrate mature characteristics, i.e. the presence of a KAR component and a high level of low frequency facilitation.
KARs facilitate glutamate release at MF synapses in CA3 pyramidal cells in short-term synaptic plasticity protocols (Schmitz et al. 2000, 2001; Contractor et al. 2001; Lauri et al. 2001). We examined how the implication of presynaptic KARs correlated with the maturation of MF-EPSCs. By comparing wild-type mice and mice devoid of the principal KAR subunits GluR5 and GluR6 (Mulle et al. 2000), we found no indication of an involvement of KARs in synaptic facilitation during high frequency trains (50 Hz), in keeping with previous results (with 100 Hz trains) (Contractor et al. 2001). In the study by Contractor et al. (2001), a slight difference was, however, found in paired pulse facilitation (with two stimuli given at a 20 ms interval, i.e at 50 Hz; but not with two stimuli given at a 10 ms interval) between wild-type and GluR6−/−, which we have not found in the present study using GluR5−/− × GluR6−/− (see also Mulle et al. 1998). Both sets of data using mutant mice (Contractor et al. 2001; the present study) are contradictory to those obtained using a pharmacological approach, which indicate upregulation of synaptic transmission by presynaptic KARs during the train of high frequency stimuli train of high frequency stimuli (Lauri et al. 2001; Schmitz et al. 2001). The reason for this discrepancy is not understood. It is not linked to the proposed presence of presynaptic GluR5 receptors (Lauri et al. 2001), but might be due to species differences (rat versus mouse), or to compensatory mechanisms. The level of facilitation during high frequency trains did not change during maturation. In contrast, the extent of low frequency facilitation, a characteristic feature of MF-EPSCs (Salin et al. 1996), sharply increased during maturation. Low frequency facilitation was not observed at the early P3–P5 stage. At later stages (P6–P14), low frequency facilitation was observed both in wild-type and in GluR5−/− × GluR6−/− mice. Finally, during the third postnatal week, the level of low frequency facilitation was significantly higher in wild-type mice than in GluR5−/− × GluR6−/− mice. We thus confirmed that the lack of KARs impaired low frequency facilitation (Contractor et al. 2001) at late stages of maturation. However, low frequency facilitation is not impaired at earlier stages, strongly suggesting that KARs are not necessary for the induction of low frequency facilitation. Moreover, the implication of presynaptic KARs in facilitating synaptic transmission is an index of late maturation stages of MF-EPSCs. The involvement of KARs in low frequency facilitation at late stages of maturation does not preclude the presence of presynaptic KARs earlier during development. However, we have no evidence that these putative presynaptic KARs might be activated by glutamate released during mossy fibre stimulation.
How do these stages compare with the morphological maturation of mossy fibre synapses? Synapses between mossy fibre terminals and dendrites of CA3 pyramidal cells in rodents are formed postnatally, and progressively mature following several steps (Amaral & Dent, 1981). Early in the postnatal period, there is no sign of spine development: small immature mossy fibre expansions directly contact proximal dendrites. Between P3 and P9, the size of mossy fibre terminals and the number of postsynaptic densities increase, in parallel with the morphological segregation of synaptic and puncta adherentia junctions (Mizoguchi et al. 2002). The postsynaptic component of the mossy fibre synapses in CA3 pyramidal cells, the ‘thorny excrescence’, only emerges from the proximal portion of the pyramidal cell dendrite after P9. The mossy fibre synapses are fully matured at P21 with, however, a more subtle protracted development of the system long into adulthood. The amplitude of MF-EPSCs greatly increases at stages where the size of the mossy fibre terminal and the number of active zones increase. A typical unitary MF-EPSC has been reported to be composed of between 2 and 16 quanta (Jonas et al. 1993). It is likely that the number of quanta per MF-EPSC increases with maturation, in parallel with an increase in the number of active zones and their associated postsynaptic densities. It would be interesting to correlate this amplitude of MF-EPSCs with the number of release sites and active zones. Postsynaptic KARs can already be observed at a developmental stage where the indented spine in the mossy fibre terminal is not present, suggesting that the slow kinetics of postsynaptic KARs do not depend on the mature architecture of the mossy fibres and the postsynaptic thorny excrescences. However, it is interesting to note that presynaptic KARs only become implicated in low frequency facilitation at a stage where the ensemble of mossy fibre terminal and complex dendritic spine (Henze et al. 2000) creates a wide but confined synaptic space. Thus, a possibility is that presynaptic KARs can only be activated by synaptically released glutamate at developmental stages when the architecture of the mature mossy fibre synapse is in place, allowing glutamate to diffuse in the restricted synaptic cleft to activate presynaptic KARs.
Thus, in contrast with what has been observed at thalamocortical synapses (Kidd & Isaac, 1999), AMPARs precede KARs in mossy fibre synapses. Moreover, the amplitude of MF-EPSCs, as well as the development of low frequency facilitation characterizing mature synapses, tightly correlates with the presence of a postsynaptic KAR component. This prompted us to examine if the absence of KARs during the first postnatal weeks impaired the maturation of MF-EPSCs. In GluR5−/− × GluR6−/− mice, in which functional KARs cannot be detected at any postnatal developmental stage, we found that the average amplitude of MF-EPSCs mediated by AMPARs was significantly smaller than in wild-type mice, at stages where a KAR component is normally present (i.e, after P6). This difference could be explained in part by changes in presynaptic parameters. Indeed, we found a reduction in mossy fibre excitability in GluR5−/− × GluR6−/− mice, as judged by an increase in failure rate. However, comparison of paired-pulse ratios and of the frequency of asynchronous EPSCs did not provide evidence in favour of a difference in release probability between the two genotypes. The reduction in synaptic potency and in the amplitude of asynchronous MF-EPSCs in the absence of KARs is in favour of changes of the postsynaptic characteristics of MF-EPSCs. At the postsynaptic level, a likely possibility is a decrease in the number of synaptic AMPARs. A decrease in the number of release sites per mossy fibre terminal might also explain the difference. Asynchronous EPSCs originating from mossy fibres might not be uniquantal events. Thus, a reduction in the number of release sites might explain a decrease in the amplitude of aEPSCs, provided that multiple release sites can operate synchronously in the presence of Sr2+. We have no indication yet as to the mechanism explaining both these presynaptic and postsynaptic differences. A detailed ultrastructural study would be necessary to test if the lack of KARs also impairs the morphological maturation of mossy fibre synapses, and the number. From our data, we suggest that KARs are implicated in the maturation of mossy fibre synapses. Although we found no evidence for a synaptic activation of presynaptic KARs at early maturation stages, we do not exclude the possibility that KARs present on axonal afferents early in development may play an important role in the maturation process. In keeping with this, KARs have recently been shown to be involved in the bidirectional regulation of mossy fibre filopodial motility which could promote the establishment of initial contacts and help in the stabilization of newly formed synapses (Tashiro et al. 2003).
We have recently demonstrated an interaction between the GluR6 subunit of KARs and cell adhesion proteins of the cadherin–catenin complex (Coussen et al. 2002), which are involved in the formation of synapses together with the nectin–afadin system (Yagi & Takeichi, 2000; Mizoguchi et al. 2002). It is thus tempting to speculate that KARs could play a role in the formation and maturation of mossy fibre synapses through their interaction with cell-adhesion proteins. Finally, it would also be important to analyse how KARs are involved in the developmental regulation of the transient GABAergic phenotype displayed by a population of mossy fibre synapses (Walker et al. 2001; Gutierrez et al. 2003).
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, the Ministère de la Recherche of France, the Conseil Régional d'Aquitaine and the Centre Hospitalier Universitaire de Bordeaux. We thank Steve Heinemann and the Salk Institute for making mutant mice available, and Guillaume Casassus, Laurent Groc, David Perrais and Arnaud Ruiz for helpful discussions on the manuscript.
References
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Clements JD. Quantal amplitude and quantal variance of strontium-induced asynchronous EPSCs in rat dentate granule neurons. J Physiol. 1999;516:227–248. doi: 10.1111/j.1469-7793.1999.227aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O'shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crepel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mege RM, Mulle C. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the ‘glutamatergic’ granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. 10.1016/S0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. 10.1016/S0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. 10.1016/S0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, Tsutsumi T, Sonoda S, Ide C, Takai Y. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Andreas SP, Perez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate induced seizures in GluR6-deficient mice. Nature. 1998;392:601–604. doi: 10.1038/33408. 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. 10.1016/S0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. 10.1016/S0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors. A two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/s0896-6273(03)00299-x. 10.1016/S0896-6273(03)00299-X. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. 10.1016/S0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]