Abstract
The inner pore of the voltage-gated Na+ channel is predicted by the structure of bacterial potassium channels to be lined with the four S6 α-helical segments. Our previously published model of the closed pore based on the KcsA structure, and our new model of the open pore based on the MthK structure predict which residues in the mid-portion of S6 face the pore. We produced cysteine mutants of the mid-portion of domain IV-S6 (Ile-1575–Leu-1591) in NaV 1.4 and tested their accessibility to intracellularly and extracellularly placed positively charged methanethiosulfonate (MTS) reagents. We found that only two mutants, F1579C and V1583C, were accessible to both outside and inside 2-(aminoethyl)-methanethiosulfonate hydrobromide (MTSEA) Further study of those mutants showed that efficient closure of the fast inactivation gate prevented block by inside [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET) at slow stimulation rates. When fast inactivation was inhibited by exposure to anthropleurin B (ApB), increasing channel open time, both mutants were blocked by inside MTSET at a rate that depended on the amount of time the channel was open. Consistent with the fast inactivation gate limiting access to the pore, in the absence of ApB, inside MTSET produced block when the cells were stimulated at 5 or 20 Hz. We therefore suggest that the middle of IV-S6 is an α-helix, and we propose a model of the open channel, based on MthK, in which Phe-1579 and Val-1583 face the pore.
The inner pore of the voltage-gated Na+ channel is generally assumed to be lined by the S6 segments of its domains I–IV arranged as α-helices (Fozzard & Hanck, 1996; Marban et al. 1998; Catterall, 2000). Several lines of evidence support this proposal. First, X-ray crystallographic studies of bacterial K+ channels (Doyle et al. 1998; Jiang et al. 2002; Jiang et al. 2003) show that M2 or S6 segments in those channels line the inner pore, and these are analogous to the S6 segments of voltage-gated Na+ channels. Secondly, mutagenesis studies using a variety of local anaesthetic/antiarrythmic drugs, which are generally thought to block current by binding in the inner pore, implicate several residues in multiple S6 segments. In domain IV-S6 alanine scanning experiments have identified two residues in NaV 1.2, Phe-1764 and Tyr-1771, that participate in local anaesthetic drug binding. The third line of evidence involves bulky mutants of the phenylalanine site. Phe-1710 in NaV 1.3, which is equivalent to Phe-1764 in NaV 1.2, tolerated replacement by tryptophan (Li et al. 1999) and the equivalent residue in NaV 1.4 (Phe-1579) tolerated a change to lysine (Wright et al. 1998), consistent with the proposal that this residue faces open space in the pore rather than into a densely packed protein. These observations all support the idea that IV-S6 lines the pore and suggest the side of the helix that faces the pore.
However, there are experimental data that conflict with the above proposal. The two domain IV-S6 residues that participate in local anaesthetic drug binding are predicted to be two turns apart on the same side of an α-helical structure (Ragsdale et al. 1994). Located halfway between them on the same side is a valine, which would also be predicted by the α-helix to face the pore. However, Vedantham & Cannon (2000) studied the cysteine mutant of this residue (V1583C) in NaV 1.4 and concluded that it faced into the protein, not into the pore. Their interesting experimental findings pose a major problem in defining the inner pore structure. If their conclusion is correct, then the domain IV-S6 segment is not an α-helix in this region, so that the residues that interact with local anaesthetic drugs, Phe-1579 and Tyr-1586, do face the pore but the valine lying between them does not. Alternatively, domain IV-S6 is an α-helix and the two residues in the local anaesthetic drug binding site are also buried in the protein, producing their effect on local anaesthetic binding indirectly. While rotation of the S6 helix during activation might alter the location of these residues and expose or hide them, such conformational changes should not lead to a discrepancy between access of local anaesthetics and MTS reagents. Because this region is critical for local anaesthetic drug binding, and because it probably participates in conformational changes during gating, it is important to define its orientation relative to the central permeation path.
In an attempt to establish orientation of residues in the mid-portion of domain IV-S6 that face the pore, we substituted cysteine for the inner pore domain IV-S6 residues of NaV 1.4 that are located according to our model (Lipkind & Fozzard, 2000) to be below the selectivity filter (Ile-1575 to Leu-1591) and determined their accessibility using the methanethiosulfonate reagents 2-(aminoethyl)-methanethiosulfonate hydrobromide (MTSEA) and [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET). This method has been very useful for defining residues that line the pore of the acetylcholine receptor (Akabas et al. 1992) and the Shaker K+ channel (Liu et al. 1997), and MTSEA accessibility was critical to the suggestion by Vedantham & Cannon (2000) that Val-1583 faces into the protein. Our experiments agreed with those of Vedantham & Cannon that V1583C interacts with MTSEA applied from both outside and inside. We further found that cysteine-substituted Phe-1579, one of the residues critical for local anaesthetic binding, also interacted with internal and external MTSEA. Similarly to the mutant V1583C, MTSEA modification of F1579C was unaffected by free cysteine in the contralateral solution or by the presence of partial TEA block. Neither of these two mutants that interacted with MTSEA interacted with MTSET during trains of voltage steps at 1 Hz when fast inactivation gating was permitted. However, F1579C and V1583C could be blocked by MTSETin when the channels were activated at 5 and 20 Hz. When currents were prolonged by anthropleurin-B toxin (ApB), which interferes with inactivation from the open state (Sheets & Hanck, 1999), MTSET blocked both mutants with a rate that was dependent on the duration and frequency of depolarization. These data provide direct evidence that both Phe-1579 and Val-1583 do indeed face the pore, since MTSETin could not interact if these residues were buried in the protein. Furthermore, access to these sites appears to require channel activation and/or opening. Because these residues are predicted to be located on the same side of an α-helix, these data also support the idea that the inner pore IV-S6 is α-helical.
Methods
Site-directed mutagenesis
The rat skeletal muscle (Nav 1.4) cDNA flanked by the Xenopus globulin 5′- and 3′ -untranslated regions (provided by J. R. Moorman, University of Virginia) was cloned into the Bluescript SK vector (Stratagene, La Jolla, CA, USA). Cysteine scanning mutagenesis was performed at selected positions between 1575 and 1591. All mutations were introduced using the Quikchange Site-Directed Mutagensis Kit (Stratagene), according to the manufacturer's protocols, or PCR in a four-primer strategy. Mutagenic oligonucleotide primers included changes in silent restriction sites as well as the desired mutation. Mutations identified as positive through qualitative restriction mapping were confirmed by DNA sequencing of the regions contained between unique restriction sites used in the subsequent reconstruction of the full-length plasmids. The wild-type μ1 cDNA was shuttled into the mammalian expression vector pCI-neo (Promega, Madison, WI, USA). Regions between unique restriction sites containing the individual mutations were subcloned into this construct for expression in cells. Transfections were by exposure to Ca phosphate (Invitrogen, Carlsbad, CA, USA).
Electrophysiology and data analyses
Wild-type (WT) and mutant channels were stably expressed in HEK293 cells. Selection was made in Dulbecco's modified Eagle's medium (DMEM) containing 600 μg ml−1 G418. In some cases, channels were also transiently expressed in HEK293 cells, and these cells were cotransfected with pHook1 and transfected cells were identified with the Capture-Tec bead system (Invitrogen). Na+ currents were recorded with the whole-cell configuration of patch clamp using an Axopatch 1D, 200 or 200A amplifier (Axon Instruments, Union City, CA, USA). Currents were filtered at 5 or 10 kHz through a 4-pole Bessel filter and digitized at 100 kHz at 12- or 16-bit resolution with a Digidata 1200A or 1322A data acquisition system and pCLAMP 7 or 8 (Axon Instruments). Pipettes had typical resistances between 0.8 and 1.3 MΩ. Recordings were made at room temperature (20–22°C) in a bathing solution containing (mm): 140 or 10 NaCl, 0 or 140 CsCl, 0 or 2.5 KCl, 2 CaCl2, 0 or 1 MgCl2, and 10 Hepes (pH 7.4 with CsOH). The low-Na+ solution was used if the expressed Na+ currents were too large for adequate voltage clamp control. Control studies showed that MTS reagent interaction was not affected by this change in Na+ concentration. The pipette solution was (mm): 100 CsF, 40 or 5 NaCl, 0 or 40 CsCl, 10 EGTA, and 10 Hepes (pH 7.2–7.4 with CsOH). MTSEA and MTSET were obtained from Toronto Research Chemicals (North York, ON, Canada), prepared just before use, and applied internally or externally at concentrations of 2.5 mm (MTSEA) or 1 or 2 mm (MTSET). Anthropleurin B (ApB, a kind gift of K. Blumenthal of SUNY Buffalo, NY) was used at 0.5–1.0 μm and was present in the bath prior to sealing of the patch pipette. For experiments with extracellular application of MTS reagents, currents were allowed to equilibrate over 5 min before they were applied. Modification was monitored with 20-ms test pulses applied to −20 mV from a holding potential of −100 to −120 mV every 2 s, except where indicated. For experiments with higher stimulations rates with and without ApB the holding potential was −140 mV.
Data were analysed using locally written programs in Matlab (Mathworks, Natick, MA, USA), SigmaPlot (SPSS, Chicago, IL, USA), and Origin (OriginLab, Northampton, MA, USA). Pooled data are presented as the mean ± s.e.m. Statistical comparisons were made by using Student's t test for paired comparisons or one- or two-way ANOVA followed by Tukey's test for multiple comparisons, and P < 0.05 was considered significant.
Molecular modelling
Molecular models were generated using the Insight and Discover graphical environment (Molecular Simulations Inc., San Diego, CA, USA) as previously described (Lipkind & Fozzard, 1994, 2000). Energy calculations utilized the consistent valence force field approximation. For minimization procedures, the steepest descents and conjugate gradients were used. For consideration of the inner pore of the Na+ channel formed by the S6 α-helices of domains I–IV, we have used the arrangement of the main chains of the M2 α-helices in the open state of the MthK K+ channel (Jiang et al. 2002), populated them with the corresponding amino acids of the NaV 1.4 channel, and energy minimized the predicted structure. Other aspects of the structure, including the alignment of the amino acid sequences, were as previously proposed for the closed channel (Lipkind & Fozzard, 2000).
Results
Accessibility to MTSEA
We have previously suggested a model for the Na+ channel pore based on the KcsA channel and the extensive mutagenesis and toxin interaction data available for the vestibule of the Na+ channel (Lipkind & Fozzard, 2000). The channel outer pore is formed by an α-helix-turn-β-strand P loop motif from each domain (Lipkind & Fozzard, 2000; Yamagishi et al. 2001; Struyk & Cannon, 2002), which together create a shallow funnel-shaped channel entrance and selectivity filter surrounded by S6 helices from each domain. The inner pore is lined by the C-terminal portions of the homologous S6 α-helices. In this model the selectivity filter is located at the level of Ile-1575 from domain IV. Consequently, amino acids downstream (C-terminal) to Ile-1575 are predicted to form the inner pore.
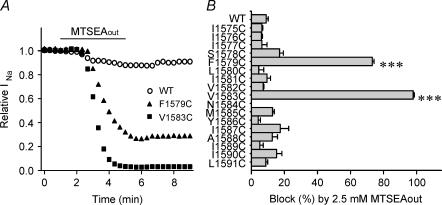
In order to test the model of the inner pore, cysteine mutations were made in 17 positions in the middle and lower parts of domain IV-S6 (1575 through 1591). All cysteine mutants except N1584C expressed currents well. Currents were allowed to stablize over 5 min and then accessibility was tested by voltage steps at 0.5 Hz. MTSEA (2.5 mm) was added to the outside solution (MTSEAout). The wild-type NaV 1.4 channel has cysteines in positions 1569 and 1572, but there was little effect of MTSEA exposure on the WT channel (9.0 ± 1.2% block, n = 5). This is in agreement with previous reports (Chiamvimonvat et al. 1996; Vedantham & Cannon, 2000; Sunami et al. 2000). Only two of the IV-S6 cysteine mutants (F1579C and V1583C) were affected by 2.5 mm MTSEAout (Fig. 1A and B, and Table 1). Block of V1583C (99 ± 1.6% block, t½ < 30 s; n = 3) was similar to the previous report of Vedantham & Cannon (2000). In addition, 2.5 mm MTSEAout rapidly blocked the F1579C channel (73.2 ± 1.0% block, t½= 36 ± 5 s; n = 4). Reduction of F1579C current by MTSEAout was less complete and somewhat slower than that for V1583C (Fig. 1A), and it was accompanied by a negative shift of voltage dependence of inactivation and accelerated current decay of the residual current (not shown). It is possible therefore that binding of MTSEA changed current indirectly by altering gating rather than by producing physical block. On the other hand, for V1583C the current reduction by 2.5 mm MTSEAout was almost complete, so that no suggestion could be made about the effect of MTSEA modification of V1583C on gating.
Figure 1. Effects of MTSEAout on IV-S6 cysteine mutants.
A, representative data showing the effects of externally applied 2.5 mm MTSEA on WT, F1579C and V1583C. The bar indicates the period of exposure to MTSEA in the bath solution. B, block of WT and mutant channels by externally applied 2.5 mm MTSEA. Data represent the mean and s.e.m. from 2–6 cells for each mutant. Stimulation frequency was 0.5 Hz from a holding potential of −100 or −120 mV to −20 mV for 20 ms. ***P < 0.001 compared with WT.
Table 1.
Experiments with F1579C
| Treatment | Block | t½ | n |
|---|---|---|---|
| 2.5 mm MTSEAout | 60 ± 5.1% | 36 ± 5 s | 4 |
| 40 mm TEAin | 48 ± 4.2% | 25 ± 5 s | 6 |
| 2.5 mm MTSEAout+ 40 mm TEAin | 56 ± 6.4% | 45 ± 18 s | 3 |
| 2.5 mm MTSEAout+ 20 mm cysteinein | 71 ± 8.3% | 36 ± 11 s | 4 |
| 2.5 mm MTSEAin+ 20 mm cysteineout | 68 ± 12.6% | 70 ± 18 s | 3 |
| 2.5 mm MTSEAin+ 40 mm TEAin | 62 ± 9.6% | 125 ± 23 s | 4 |
| 2.5 mm MTSEAin | 52 ± 6.5% | 120 ± 22 s | 8 |
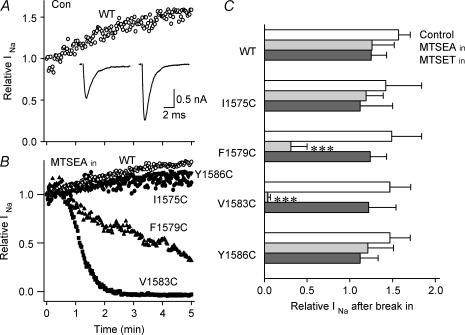
This pattern of modification and available data from the literature led us to examine mutant cysteinyl side-chain accessibility from the cytoplasmic side of the channel for the four positions expected to be orientated on the same side of an α-helix. For I1575C, F1579C, V1583C and Y1586C we applied MTSEA to the intracellular side of the cell through the patch pipette, monitoring Na+ currents continuously after the cell membrane was ruptured at a holding potential between −100 and −140 mV. This method of MTS exposure was complicated by a change in currents that occurs in the first few minutes after membrane rupture. Figure 2A shows an example of this time course. When the cell membrane was ruptured at a holding potential of −100 mV and test pulses were applied to −20 mV every 2 s, peak currents of the WT channel increased with time and the current decay became faster. On average, peak current of WT channels increased by 57 ± 14% (n = 5) during 5 min after break-in. Consequently, the effects of internal MTSEA must be interpreted in the context of a background increase in peak Na+ current.
Figure 2. Effects of internal MTS reagents on cysteine mutants at Ile-1575, Phe-1579, Val-1583, and Tyr-1586.
A and B, the time course of the peak currents elicited by voltage steps to −20 mV from a holding potential of −100 to −120 mV after establishment of the whole-cell configuration (time 0) in WT without MTS reagents (control) (A) and in WT, I1575C, F1579C, V1583C and Y1586C with 2.5 mm MTSEA in the pipette (B). Stimulation frequency was 0.5 Hz. Current traces in A were recorded 0 and 5 min after break-in. C, effects of internally applied MTS reagents on WT, I1575C, F1579C, V1583C and Y1586C. Saturating concentrations of MTS reagents (2.5 mm MTSEA, 2 mm MTSET, 10 mm MTSES) were applied in the pipette and the effects were determined 5 min after break-in. The columns from top to bottom in each channel correspond to those for control (con), MTSEA and MTSET, respectively. Data represent the mean ± s.e.m. from 2 to 5 cells. ***P < 0.001 compared with WT.
MTSEAin blocked currents only of V1583C and F1579C (Fig. 2B and C). Block of V1583C by MTSEAin was complete and rapid, similar to that of MTSEAout block of this mutant. Block of F1579C by MTSEAin was less complete (52 ± 6.5%, t½ = 120 ± 22 s, n = 8) and was somewhat slower, as we also observed for block of this mutant by MTSEAout (see above). Consequently, both F1579C and V1573C were accessible to MTSEA from both sides of the membrane. The extent of block was less and rate of block was somewhat slower for F1579C, perhaps reflecting lesser accessibility of this mutant for MTSEA modification.
Vedantham & Cannon (2000) showed that MTSEA block of V1583C was not affected by free cysteine on the side opposite to the exposure to MTSEA. To determine whether transcysteine also did not interfere with the MTSEA interaction for F1579C, we placed 20 mm cysteine on the opposite sides from MTSEA. MTSEAout block of F1579C with 20 mm free cysteine inside was 71 ± 8.3% (t½ of 36 ± 11 s, n = 4) and MTSEAin block with 20 mm free cysteine outside was 68 ± 12.6% (t½ of 70 ± 18 s, n = 3). Consequently, as for V1583C, transcysteine failed to prevent MTSEA block from either side for the F1579C mutant.
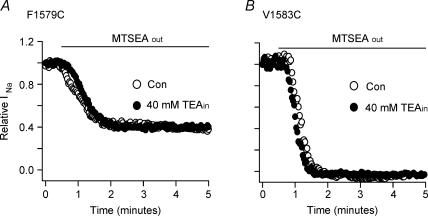
Vedantham & Cannon (2000) also reported that the pore blocker tetraethylammonium (TEA) failed to affect the rate of MTSEA modification of V1583C. When we included 40 mm TEA in the pipette solution, the current of F1579C was blocked by 48.5 ± 4.2% (n = 6). TEA block developed with a t ½= 25 ± 8.5 s (n = 3) after a brief and variable delay probably related to the time for the pipette to establish an adequate opening (not shown). The remaining currents after 40 mm TEA intracellular treatment were then examined for MTSEAout modification. In the example of Fig. 3A, when TEA-treated F1579C channel was exposed to 2.5 mm MTSEAout for 4.5 min, the current was blocked by 60%. On average, TEA-treated F1579C showed 56 ± 6.4% block (n = 3) by 2.5 mm MTSEAout, with a t½ = 45 ± 18 s, which was not different from that for the MTSEAout block of F1579C without TEA (t½ = 36 ± 5 s, n = 4). Consistent with the previous report of Vedantham & Cannon (2000) about V1583C, there was no difference in the amount or rate of MTSEAout block between non-treated and TEA-treated V1583C (Fig. 3B). Testing the effect of TEA on inside MTSEA block was more difficult, because the reagents were applied through the pipette and both effects developed concurrently. When 40 mm TEA and 2.5 mm MTSEA were both included in the pipette solution the F1579C current was blocked 62 ± 9.6% with t½ = 125 ± 23 s (n = 4), and the rate was not different from the rate of block for 2.5 mm MTSEAin alone (t½ = 120 ± 22 s, n = 8). TEA block alone by diffusion from the pipette was much faster than MTSEAin block, and MTSEAin block was faster in smaller cells.
Figure 3. Internal TEA does not affect MTSEA modification of F1579C (A) and V1583C (B) in HEK293 cells.
In A and B, representative data are shown and the bar indicates the period of exposure to 2.5 mm MTSEA in the bath solution. MTSEA perfusion was started at least 10 min after rupture of the cell membrane. In case of intracellular application of TEA (TEAin), the cell was dialysed with a pipette solution containing 40 mm TEA. Currents were elicited by 20-ms pulses to −20 mV from a holding potential of −100 mV every 2 s.
Accessibility to MTSET at slow stimulation rates
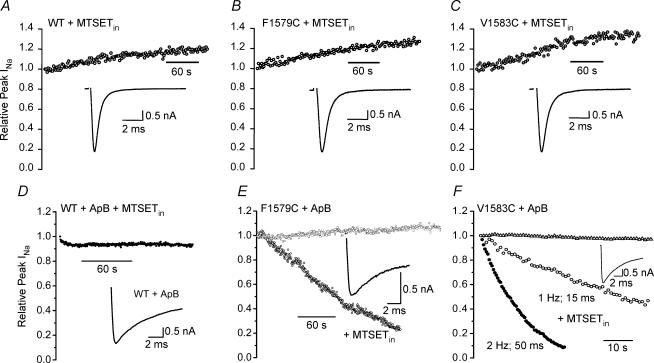
MTSET interaction should be a more reliable indicator of location of cysteine at a hydrophilic interface because it does not cross intact membranes (Holmgren et al. 1996). It is, however, larger than MTSEA, which might interfere with access to an inner pore site. External application of 1 mm MTSET to the two mutants that were sensitive to MTSEAout (F1579C, V1583C), using the same stimulation protocol as with MTSEAout, showed no block (not shown). In addition, in contrast to MTSEAin, MTSETin with 0.5 Hz stimulation rate did not modify F1579C or V1583C (Figs 2C, and 4B and C), nor did it modify I1575C or Y1586C, the other two positions predicted to be on the same side of an α-helix (Fig. 2C).
Figure 4. Ability of MTSETin to modify currents with and without prolongation of open times by ApB toxin.
In each case inset shows currents in depolarizations to −20 mV at the beginning of the train. A, B and C, MTSETin has no effect on the time course of current during a train of 20 ms steps at 0.5 Hz in the WT, F1579C or V1583C. D, prior treatment with ApB has little effect on the effect of MTSETin on WT current. E, prior treatment of F1579C with ApB showed no accumulation of block or inactivation when stimulated with 15 ms steps at 1 Hz (○), but addition of 2 mm MTSETin resulted in dramatic block that was not complete at 4 min (•). F, prior treatment of V1583C with ApB showed no block or inactivation when stimulated with 15 ms steps at 1 Hz (Δ), but addition of 2 mm MTSETin led to rapid block during 1 min (○). Addition of 2 mm MTSETin and stimulation at 2 Hz with 50 ms steps produced rapid and almost complete block (•). See text for additional details.
Accessibility to MTSETin with anthropleurin B
We considered the possibility that the failure of MTSETin to interact with V1583C in the Vedantham & Cannon (2000) experiments, and with F1579C and V1583C in our experiments using 0.5 Hz stimulation rate, was because diffusion of MTSET into the inner pore was limited by the short time during which the pore was open between activation and the normal inactivation process. Under the conditions of both experiments the average channel was open less than 1 ms during each depolarization. This possibility could be tested by increasing the time available for diffusion of the MTS reagents into the pore between activation and fast inactivation.
Anthropleurin B (ApB) is a site 3 toxin that binds to extracellular residues of domain IV (Rogers et al. 1996; Benzinger et al. 1998) and specifically inhibits inactivation from the open state (Sheets & Hanck, 1999). Accordingly, we treated the cells expressing WT, F1579C and V1583C channels with a high concentration of ApB (0.5–1.0 μm), which slowed current decay by 20-fold. The toxin produced slowing of the decay phase of the activated current to time constants of 6.5 ± 0.5 ms (n = 3). For comparison, the time constant for decay of the mutant V1583C without ApB treatment was 0.36 ± 0.02 ms (n = 3). If ApB also induced some component of slow inactivation, then interpretation of the effects of MTSET would be difficult because inactivation would accumulate during the stimulus protocol. However, for WT, F1579C and V1583C stimulation at 1 or 2 Hz with 15 ms or 50 ms steps for several minutes did not reduce peak Na+ current (Fig. 4D–F).
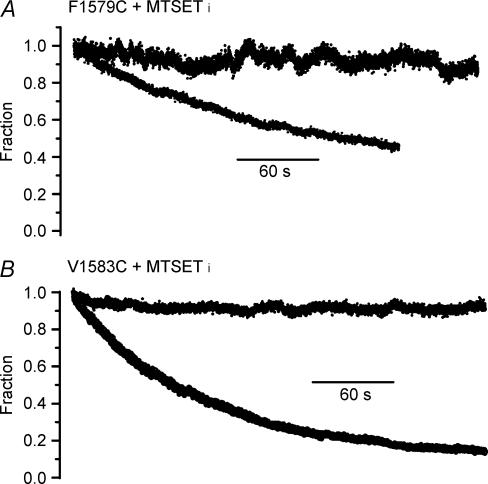
When 2 mm MTSETin was included in the pipette, stimulation of ApB modified V1583C at 1 Hz with 15 ms steps to −10 mV resulted in marked reduction in peak current within 1 min (Fig. 4F). In four cells currents were blocked to 43 ± 8% of their initial value at 1 min. In a separate experiment, stimulation at 2 Hz with 50 ms steps to −10 mV resulted in complete loss of current within 30 s. (Fig. 4E). Thus the total time the channel was open was critical to development of block. No reduction in current was evident in WT channels with this stimulation protocol when they were modified with ApB and MTSET was included in the pipette, which supports the idea that ApB treatment did not expose a cysteine elsewhere in the channel protein with which MTSET could interact to produce the block (n = 3, Fig. 4D). Similar to the effects of MTSEA, modification by MTSET took longer for F1579C. Similar MTSETin experiments with ApB-modified F1579C using 2 Hz trains with 50 ms steps to −10 mV showed > 90% block in 4 min (Fig. 4E, n = 4). It seemed therefore that diffusional access of the modifier to the inner pore influenced modification extent and rate.
Accessibility to MTSETin at fast stimulation rates
In order to rule out the possibility that ApB modification produced a change in secondary structure that allowed modification by MTSETin, we increased diffusional access in the absence of toxin by rapid pulsing, using brief depolarizations, which would open the channel, but not promote slow inactivation. If successful, this would strengthen our conclusion that the dramatic use-dependent block of F1579C and V1583C was not somehow artifactually induced by ApB in a way not detected by our control experiments. We tested F1579C and V1583C for accumulation of inactivation with 5-ms steps at 5 or 20 Hz. For F1579C without MTSETin at 20 Hz, peak current decreased 8 ± 1.4% (n = 3) at 4 min (Fig. 5A). For V1583C without MTSETin (Fig. 5B), two cells showed no change in peak current although in a third there was a 30% decline. In all cases a pause after the stimulus train produced no increase in peak current, as would have been expected if the reduction in peak current had been because inactivation had developed. Two additional V1583C control cells were studied at 5 Hz, and no decrease in peak current or increase after the train occurred in either cell. It therefore appeared that the rapid pulsing protocol produced little or no cumulative inactivation.
Figure 5. MTSETin effects at rapid stimulation rates.
A, stimulation at 20 Hz for the mutant F1579C alone (flat trace) and with MTSETin (declining trace). B, stimulation at 20 Hz for the mutant V1583C (flat trace) and with MTSETin (declining trace).
When 10 mm MTSETin was included in the pipette, peak current with rapid stimulation always decreased. For F1579C studied at 20 Hz the current fell 31 ± 4.0%(n = 6) in 4 min (Fig. 5A). In two cells, the current was monitored for several minutes after termination of the 20 Hz trains that had produced 33% and 40% fall. In neither instance was any recovery of current evident, consistent with MTSET irreversibly interacting with the channel and blocking current. In two additional cells MTSEA was added to the outside solution after seal with pipettes containing MTSETin. The current was blocked 70% rapidly during the first minute of MTSEA exposure, as previously seen with MTSEAout and no further reduction in current occurred during rapid pulsing. This is consistent with binding of MTSEA to F1579C, thereby preventing interaction with MTSETin. For V1583C in three cells with MTSETin and 20 Hz stimulation, current fell to < 15% of its initial value in 2–3 min (Fig. 5B). The extent of block was comparable to that seen with ApB at a slower stimulation rate. One cell studied at 5 Hz also showed an exponential fall in current magnitude, reaching 33% of initial value by 4 min.
Discussion
The purpose of these experiments was to identify the mid-segment residues of domain IV-S6 of the NaV 1.4 channel that line the inner pore. Cysteine mutants of Phe-1579 and Val-1583 were accessible to MTSEA from both sides of the membrane, and to MTSET from the cytoplasm if channel opening was enhanced. Although when normal inactivation was permitted and stimulation was slow, MTSET failed to block, the mutants were blocked by MTSET when stimulation with short steps was at 5 or 20 Hz or at 1 Hz when inactivation was delayed by prior exposure to the toxin ApB. This suggests that the failure of Vedantham & Cannon (2000) to see interaction of V1583C with MTSET was because the channel was not open for sufficient time for adequate diffusion of the reagents into the pore. In support of that interpretation, Alekov et al. (2003) have reported in abstract form that MTSES can modify F1579C when it is accompanied by a second IV-S6 mutation (I1589C) that disrupted inactivation. These data are consistent with molecular modelling that places Phe-1579 and Val-1583 side chains in the pore and with the direct role that has been proposed for Phe-1579 in the local anaesthetic binding site.
It remains puzzling that MTSEA can interact with cysteine mutants of these sites from both sides of the membrane. However, the failure of MTSEA to show functional changes with other cysteine mutants of the S6 α-helix that surround Phe-1579 and Val-1583 suggests that close packing of those side chains within the protein may prevent MTSEA interaction sterically. We cannot rule out the possibility that MTSEA does reach and interact with the other mutants without producing a functional effect, but the selective functional effect of MTSEA on F1579C and V1583C shows that these mutants are special. Because they face the pore, F1579C and V1583C would have sufficient space around them that the MTSEA interaction can develop.
Alternatively, there may be a hydrophilic path that allows MTSEA access to the pore directly, but without permitting access of hydrated cations. Such a parallel path has been proposed for the access of extracellular local anaesthetic drugs to their binding site, which includes Phe-1579, i.e. in NaV 1.5, this path can strongly influence use dependence of local anaesthetic block (Lee et al. 2001). Permanently charged QX analogues can reach their binding site from the outside in NaV 1.5 (Alpert et al. 1989; Ragsdale et al. 1994; Qu et al. 1995; Sunami et al. 2000). Several residues in domain IV-S6 behind the outer vestibule and P loop residues appear to be involved in the QX access path. Such an access path can be created for QX in NaV 1.4 by the single mutation C1572T in domain IV-S6 (Sunami et al. 2000). In addition, MTSEA is able to block the QX access path in Nav 1.4 that is produced by the IV-S6 mutation I1575A, suggesting that MTSEA may indeed have access to this path (Sunami et al. 2000).
MTSEA block of V1583C was prevented if channels were slow-inactivated during reagent exposure (Vedantham & Cannon, 2000), suggesting that a gating-related conformational change may move the mutant side chain from the pore face into the protein. Those investigators also found that MTSEA block was also prevented by prior treatment with batrachotoxin (BTX). Because both BTX and ApB are known to interfere with fast inactivation, it might at first seem surprising that BTX protected the channel while ApB facilitated MTSET modification. However, BTX requires the presence of several residue side chains in domains I and IV, including Phe-1579, so it binds in the same region as local anaesthetic drugs (Linford et al. 1998; Wang et al. 1998; Wang & Wang, 1999) and therefore it probably acted to protect the channel from MTS modification. ApB, on the other hand, binds to the outside of the Na+ channel (Rogers et al. 1996; Benzinger et al. 1998) and so prolonged channel opening without interfering with the MTSET binding site. The relationship of BTX to both Phe-1579 and Val-1583, combined with our demonstration that Phe-1579 faces the pore, further supports the proposal that Val-1583 is near Phe-1579 and also faces the pore.
If MTSEAout does enter the pore via the protein, then why did 20 mm internal free cysteine not interfere? Perhaps cysteine fails to enter the pore. And why did internal TEA fail to interfere with the interaction? It seems possible that TEA binds higher in the pore, which is consistent with our model of the relationship of the vestibule and S6, although it may also be that the rapid on–off blocking interaction of TEA did not compete successfully with the rate of irreversible MTS reaction. We did see some difference in the rate of MTSEA block between V1583C and F1579C. Block of V1583C was rapid from both sides and was complete, while block from both sides was slower for F1579C and it was incomplete.
Sources of error
Delivery of MTS reagents via the pipette poses several problems. The pipette must be prepared and used quickly before loss of reactive reagent, break-in with the patch pipette should be clean, and reagent must diffuse out of the tip of the pipette. Although the brief delay after break-in and establishment of the holding potential complicated measurement of the time course of effect, use of small cells and those with small leak led to consistent measurements. The interaction of MTS reagents with some mutants implies that the reagents were delivered successfully to the channel pore via the pipette and that failure to show functional effect with other cysteine mutants was not because of failure of the MTS reagents to reach the channel. The other problem with delivery of reagent via the pipette solution was that stabilization of the currents after break-in could not be established before onset of reagent effects. The run-up of peak current seen in most experiments with WT channels and with those mutants showing no interaction provided a reasonable and consistent baseline for determining the occurrence of interaction with F1579C and V1583C, although quantification of the effect may have been less reliable.
It is possible that ApB treatment not only prolonged the time course of fast inactivation, but also exposed native cysteine residues unrelated to the mutant being studied, resulting in spurious results. The failure to show any effect of inside MTS reagent when the WT NaV 1.4 channels were treated with ApB reassures us that exposure of cysteines unrelated to our mutations was unlikely. Even though ApB is known to bind to the channel extracellularly, one might worry that ApB modification altered the orientation of pore residues. Experiments with ApB and lidocaine together indicated effects on the channel were additive, consistent with no gross alteration in the lidocaine binding site (Hanck et al. 2000). In addition, block by MTSETin could also be achieved by rapid stimulation with short depolarizations that produced little or no cumulative inactivation. The effects of stimulation on MTSETin block of V1583C were as dramatic as those with inactivation delayed by ApB, and the effect was graded by rate of stimulation. The most plausible interpretation of our data is that the Phe-1579 and Val-1583 sites are located above the point where the activation gate occludes the pore, although our experiments cannot exclude that side chain accessibility changes when the channel transitions from the closed to activated conformation, i.e. the gating step could have rotated the S6 helix to expose the mutants to the pore. However, MTS interaction required that the channel not be occluded by the inactivation gate. Lastly, MTSETin block of F1579C was different in character from block of V1583C. Block was slower and less complete for this site with both inside and outside MTSEA and inside MTSET. The differences between MTS interactions with F1579C and V1583C require further study.
The pore lining
Prior to beginning these experiments there were three reasons to think that the IV-S6 residues facing the pore included Phe-1579 and Val-1583. (1) Phe-1579 is implicated in the binding of local anaesthetic drugs that access their binding site via the pore. (2) Homology modelling of the Na+ channel from crystal structures of K+ channels favours the orientation of Phe-1579 and Val-1583 toward the pore. (3) They both tolerate bulky and charged side chain substitutions, as if they face an open space. Evidence against Val-1583 facing the pore from the experiments of Vedantham & Cannon (2000) is that its cysteine mutant is blocked by both outside and inside MTSEA, and intracellular free cysteine and TEA fail to alter the effect of outside MTSEA.
In these experiments we present three new observations regarding the orientation of IV-S6 residues in the inner pore. (1) The cysteine mutant of Phe-1579 was also blocked by outside and inside MTSEA. (2) Both F1579C and V1583C were blocked by inside MTSET, but only if the channels were activated at rapid rates by steps that produced minimal cumulative inactivation, and faster stimulation caused faster block. Again, V1583C was blocked faster and more completely at each rate than was F1579C. (3) Delay of inactivation by exposure of the channels to the toxin ApB also facilitated MTSET block of F1579C and V1583C. The preponderance of evidence supports the idea that these two residues face the inner pore after activation.
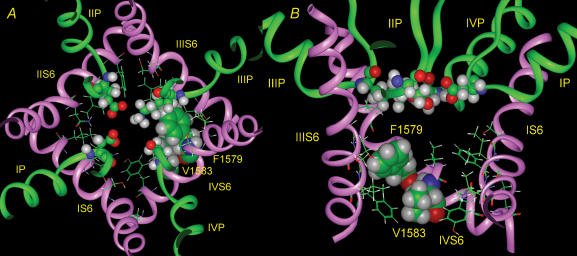
The two reactive sites identified in the region tested are located on adjacent positions on the same face of an α-helix. It is plausible to suppose that domain IV S6 is an α-helix and that it remains so during the gating process. Moreover, MTSET was able to modify F1579C and V1583C only when the channel was open, so they are probably above the activation gate. The crystal structure of the bacterial potassium channel KcsA represents the closed state of the pore (Doyle et al. 1998), and this structure has been used to model the Na+ channel pore (Lipkind & Fozzard, 2000; Yarov-Yarovoy et al. 2002). There is also a crystal structure of a related calcium-gated potassium channel MthK, which represents the open state (Jiang et al. 2002). In the MthK structure the lower half of the M2 helix (analogous to the S6 helices of voltage-gated channels) is bent outward because of a flexible hinge formed by a highly conserved glycine residue (Gly-83 in MthK, Gly-99 in KcsA), which produces an open vestibule of diameter 12 Å. A similar glycine also controls gating for a bacterial Na+ channel (NaChBac; Zhao et al. 2004), where Gly-219 serves as the hinge. Alignment of amino acid sequences of voltage-gated Na+ channels from eukaryotes shows that for these channels glycine is conserved in the homologous positions in S6 segments in domains I through III, but domain IV contains a serine. It is likely that this serine is also a hinge for IV-S6. Serine also belongs to the α-helix breaking set of amino acids (Chou & Fasman, 1978) since its low conformational preference Pα to be in the middle of a helix (0.44) is about the same as for glycine (0.47) (Creighton, 1993). Serine could therefore produce a bend in the domain IV S6 segment in voltage-gated Na+ channels. We therefore, constructed a model of the open Na+ channel pore, based on MthK. The best proposal for the pore lining would be a 100 deg arc that includes Ile-1575, Phe-1579, and Val-1583 (Fig. 6), which allows a bend in the S6 helix to tilt it away from the pore centre. Importantly, the side chain hydroxyl of Ser-1578 compensates for the broken hydrogen bond with the main chain carbonyl of Tyr-1574 that is present in the previous closed state model. In both models Ile-1575, Phe-1579, and Val-1583 face the pore, although in the new open state model cysteine substituted residues would be predicted to be further from the centre of the pore and closer to the cytoplasm as would be expected from the state dependence of MTSET modification. In the closed state model, Tyr-1586 is part of a densely packed interaction with other S6 segments at the helix cross-over (Cα is 5.4 Å from pore centre). In the open state model it is folded away from the pore (Cα moves 5 Å farther away from the pore centre). Because of its distance from the centre of the pore, interaction of MTS reagents may not cause block of current.
Figure 6. Proposed model of the open Na+ channel pore for NaV 1.4.
A, top view. The backbones for P-loops and S6 helices of domains I–IV are shown as green and pink ribbons, respectively. Only the side chains of residues of S6 segments, facing the pore are shown. Residues of domain IV S6 Ile-1575, Phe-1579, and Val-1583 are distinguished as space-filled, while ball and stick representations are shown for the four selectivity filter residues Asp-400, Glu-755, Lys-1237 and Ala-1529 of the domain I–IV P loops. B, side view of the same model with the ribbon of domain II S6 removed. The pore lining residues are shown space-filled as in A.
Acknowledgments
We thank Connie Mlecko and J. R. Liu for their excellent technical assistance. This work was supported by N.I.H. RO1 HL-65661 (H.A.F).
References
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Alekov A, Popa O, Derra E, Lehmann-Horn F, Lerche H. A key role of segment D4/S6 in Na channel gating. Biophys J. 2003;84:67a. (abstract) [Google Scholar]
- Alpert LA, Fozzard HA, Hanck DA, Makielski JC. Is there a second external lidocaine binding site on mammalian cardiac cells. Am J Physiol. 1989;257:H79–H84. doi: 10.1152/ajpheart.1989.257.1.H79. [DOI] [PubMed] [Google Scholar]
- Benzinger GR, Kyle JW, Blumenthal KM, Hanck DA. A specific interaction between the cardiac sodium channel and site-3 toxin Anthopleurin B. J Biol Chem. 1998;273:80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms, the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, Pérez-García MT, Ranjan R, Marban E, Tomaselli GF. Depth asymmetries of the pore-lining segments of the Na channel revealed by cysteine mutagenesis. Neuron. 1996;16:1037–1047. doi: 10.1016/s0896-6273(00)80127-0. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Empirical predications of protein conformation. Ann Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Creighton EE. Proteins, Structures and Molecular Properties. 2. New York: W. H. Freeman; 1993. [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel, molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Fozzard HA, Hanck DA. Structure and function of voltage-dependent sodium channels, comparison of brain II and cardiac isoforms. Physiol Rev. 1996;76:887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- Hanck DA, Makielski JC, Sheets MF. Altered gating of lidocaine-bound cardiac Na channels. Pflugers Arch. 2000;439:814–821. doi: 10.1007/s004249900217. [DOI] [PubMed] [Google Scholar]
- Holmgren M, Liu Y, Xu Y, Yellen G. On the use of thiol-modifying agents to determine channel topology. Neuropharm. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadone M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Sunami A, Fozzard HA. Cardiac-specific external paths for lidocaine, defined by isoform-specific residues, accelerate recovery from use-dependent block. Circ Res. 2001;89:1014–1021. doi: 10.1161/hh2301.100002. [DOI] [PubMed] [Google Scholar]
- Li H-L, Galue A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Molec Pharm. 1999;55:134–141. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci U S A. 1998;95:13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. A structural model of the tetrodotoxin and saxitoxin binding site of the Na channel. Biophys J. 1994;66:1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. KcsA crystal structure as framework for a molecular model of the Na channel pore. Biochem. 2000;39:8161–8170. doi: 10.1021/bi000486w. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Marban E, Yamagishi T, Tomaselli GF. Structure and function of voltage-gated sodium channels. J Physiol. 1998;508:647–657. doi: 10.1111/j.1469-7793.1998.647bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Rogers J, Tanaka T, Scheuer T, Catterall WA. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na channel. Proc Natl Acad Sci U S A. 1995;92:11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na channel by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Gating of skeletal and cardiac muscle sodium channels. J Physiol. 1999;514:425–436. doi: 10.1111/j.1469-7793.1999.425ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. Slow inactivation does not block the aqueous accessibility to the outer pore of voltage-gated Na channels. J General Physiol. 2002;120:509–516. doi: 10.1085/jgp.20028672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A, Glaaser IW, Fozzard HA. A critical residue for isoform differences in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel. Proc Natl Acad Sci U S A. 2000;97:2326–2331. doi: 10.1073/pnas.030438797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham V, Cannon SC. Rapid and slow voltage-dependent conformational changes in segment IVS6 of voltage-gated Na channels. Biophys J. 2000;78:2943–2958. doi: 10.1016/S0006-3495(00)76834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang S-Y. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated μ1 Na channels. Pfluegers Arch. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- Wang S-Y, Wang GK. Batrachotoxin-resistant Na channels derived from point mutations in transmembrane segment D4–S6. Biophys J. 1999;76:3141–3149. doi: 10.1016/S0006-3495(99)77465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SN, Wang S-Y, Wang GK. Lysine point mutations in Na channel D4–S6 reduce inactivated channel block by local anesthetics. Mol Pharmacol. 1998;54:733–739. [PubMed] [Google Scholar]
- Yamagishi T, Li RA, Hsu K, Marban E, Tomaselli GW. Molecular architecture of the voltage-dependent Na channel, Functional evidence for α-helices in the pore. J General Physiol. 2001;118:171–181. doi: 10.1085/jgp.118.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acids residues in transmembrane segments IS6 and IIS6 of the Na channel α-subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yarov-Yarovoy V, Scheuer T, Catterall WA. A gating hinge in Na+ channels, A molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]