Abstract
We investigated, by using the patch clamp technique, Ca2+-mediated regulation of heterologously expressed TRPC6 and TRPC7 proteins in HEK293 cells, two closely related homologues of the transient receptor potential (TRP) family and molecular candidates for native receptor-operated Ca2+ entry channels. With nystatin-perforated recording, the magnitude and time courses of activation and inactivation of carbachol (CCh; 100 μm)-activated TRPC6 currents (ITRPC6) were enhanced and accelerated, respectively, by extracellular Ca2+ (Ca2o+) whether it was continuously present or applied after receptor stimulation. In contrast, Ca2o+ solely inhibited TRPC7 currents (ITRPC7). Vigorous buffering of intracellular Ca2+ (Ca2i+) under conventional whole-cell clamp abolished the slow potentiating (i.e. accelerated activation) and inactivating effects of Ca2o+, disclosing fast potentiation (EC50: ∼0.4 mm) and inhibition (IC50: ∼4 mm) of ITRPC6 and fast inhibition (IC50: ∼0.4 mm) of ITRPC7. This inhibition of ITRPC6 and ITRPC7 seems to be associated with voltage-dependent reductions of unitary conductance and open probability at the single channel level, whereas the potentiation of ITRPC6 showed little voltage dependence and was mimicked by Sr2+ but not Ba2+. The activation process of ITRPC6 or its acceleration by Ca2o+ probably involves phosphorylation by calmodulin (CaM)-dependent kinase II (CaMKII), as pretreatment with calmidazolium (3 μm), coexpression of Ca2+-insesentive mutant CaM, and intracellular perfusion of the non-hydrolysable ATP analogue AMP-PNP and a CaMKII-specific inhibitory peptide all effectively prevented channel activation. However, this was not observed for TRPC7. Instead, single CCh-activated TRPC7 channel activity was concentration-dependently suppressed by nanomolar Ca2i+ via CaM and conversely enhanced by IP3. In addition, the inactivation time course of ITRPC6 was significantly retarded by pharmacological inhibition of protein kinase C (PKC). These results collectively suggest that TRPC6 and 7 channels are multiply regulated by Ca2+ from both sides of the membrane through differential Ca2+−CaM-dependent and -independent mechanisms.
Ca2+-dependent regulation is a ubiquitous mechanism by which the activity of various types of Ca2+-permeable cation channels is finely tuned (Levitan, 1999; Saimi & Kung, 2002). Examples are not restricted to small conductance Ca2+-activated K+ channels, cyclic nucleotide-gated cation channel, NMDA receptor, ryanodine/inositol 1,4,5,-trisphospate (IP3) receptors, and L- and P/Q-type voltage-dependent Ca2+ channels, but also include a variety of native receptor-operated cation (ROC) channels with less well elucidated properties (e.g. Siemen, 1993) and their molecular candidates, the mammalian homologues of Drosophila transient receptor potential (TRP/TRPL) protein (Montell, 2001; Clapham et al. 2001; Minke & Cook, 2002).
In photoreceptor cells of the wild-type Drosophila's eye, it was originally found that light-induced currents which reflect the opening of TRP and TRPL channels are dually modulated by photolytically released Ca2+ with facilitation and inactivation in rising and plateau phases of the response, respectively (Hardie, 1995). Later, a similar reciprocal regulation by Ca2+ was also found for the canonical members of the TRP superfamily (TRPC). Earlier studies demonstrated that manoeuvres to increase Ca2i+ concentration ([Ca2+]i) such as application of ionomycin and direct Ca2+ infusion into the cell enhanced the activity of heterologously expressed TRPC3 channels (Zitt et al. 1997), while sustained elevation in [Ca2+]i by use of Ca2+/EGTA or BAPTA failed to stimulate or even inhibited them (Zitt et al. 1997; Kamouchi et al. 1999). It has subsequently been shown that a CaM binding site (CIRB), which also exhibits binding affinity for the IP3 receptor (IP3R), is present on the carboxy tail of TRPC3 channels (Zhang et al. 2001), and CaM binding sites highly homologous to this are identified in all other members of the TRPC family (Tang et al. 2001). It has experimentally been shown that application of CaM antagonists or IP3R peptides relieves the tonic inhibitory effects of CaM via CIRB thereby increasing the basal TRPC3 channel activity. This observation has been interpreted to represent the molecular mechanism underlying store depletion-activated Ca2+ entry (SOC) during receptor stimulation (Kiselyov et al. 1998; Boulay et al. 1999; Zhang et al. 2001). However, there is also good evidence to suggest that members of the TRPC3/6/7 subfamily are activated by diacylglycerol in a store-independent fashion (e.g. Hofmann et al. 1999; Trebak et al. 2003). Inhibitory actions of CaM have also been suggested for TRPC4 (Tang et al. 2001) and TRPC1 (Singh et al. 2002; Vaca & Sampieri, 2002). In the latter, the role of CaM has been assigned to Ca2+-dependent feedback inhibition of endogenous SOC via a C-terminal site more distal to CIRB (Singh et al. 2002) as well as prolongation of delay of SOC activation via a common binding site for CaM and IP3R, mostly likely CIRB (Vaca & Sampieri, 2002). As for other TRPC members, both spontaneous and agonist-induced activities of the TRPC5 channel have been shown to be enhanced by Ca2+ entering through the channel itself (Okada et al. 1998; Yamada et al. 2000), which are also potentiated directly by extracellular Ca2+ (and lanthanides; Jung et al. 2003), as has been found in several native ROC channels (e.g. Inoue, 1991; Helliwell & Large, 1998; Aromolaran & Large, 1999). Furthermore, preliminary results have indicated that the magnitude of agonist-induced TRPC6 currents dramatically changes in response to extracellularly applied Ca2+ with a complex time course (Inoue et al. 2001). These findings strongly suggest that Ca2+-mediated regulation from both sides of the cell membrane may be a powerful and common means to modulate TRPC channel activity.
There is now a growing body of evidence that TRPC6 is broadly distributed in extra-brain tissues, especially enriched in vascular smooth muscles, and may function as an integral subunit of native ROC channels activated via sympathetic nerve excitation, intravascular pressure increase and vasoactive peptides and growth hormones (Inoue et al. 2001, 2004; Jung et al. 2002; Welsh et al. 2002). Despite this potential importance, little detailed information is yet available as to how Ca2+ modulates TRPC6 channel activity, although a recent Ca2+ fluorometric study has reported that a CaM-mediated mechanism is involved in the positive modulation of this channel (Boulay, 2002). The present study was thus initiated to gain more insight for the complex actions of extra- and intracellular Ca2+ on TRPC6 channels in comparison with TRPC7, another member of the same subfamily which exhibits contrasting responses to Ca2+, in terms of whole-cell and single channel recordings. As the results, we have found that TRPC6 and TRPC7 channels undergo effective but differential regulation by extra- and intracellular Ca2+ in CaM-dependent and -independent manners. Part of this study has been communicated to the 76th annual meeting of the Japanese Pharmacological Society (Shi et al. 2003).
Methods
Cell culture and transfection
Human embryonic kidney 293 (HEK293) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. For transfection, the cells were reseeded in a 35 mm culture dish and allowed to grow to 40–60% confluency, and then transfected with a mixture of 2 μg plasmid vector (pCI-neo) incorporating TRPC DNAs (murine TRPC6, murine TRPC7 or their six chimeras; see below) and 0.4 μg pCI-neo-πH3-CD8 (cDNA of the T-cell antigen CD8), with the aid of 20 μl of the transfection reagent SuperFect™ (Qiagen, Germany). In some experiments, 2 μg of plasmid DNA for mutant calmodulin (mutCaM; see below) was cotransfected. About 24 h after transfection, cells were reseeded onto coverslips pre-coated with 100 μm poly-l-lysine. Electrophysiological measurements were performed within 48–72 h after transfection.
Construction of TRPC6/7 chimeras and mutant calmodulin (mutCaM)
The TRPC6/7 chimeras and the calmodulin mutant (mutCaM) were constructed by using PCR. In T667, the amino acid sequence 1–726 containing the N-terminus (1–402) and the hydrophobic core H1–H8 (403–726) of murine TRPC6 (Mori et al. 1998) was linked to the C-terminal sequence 673–862 of murine TRPC7 (Okada et al. 1999). In T776, the TRPC7 sequence 1–672 containing the N-terminus (1–348) and H1–H8 (349–672) was linked to the TRPC6 C-terminus 727–930. For the design of other chimeras, see Supplementary Fig. 1. In mutCaM, aspartate residues 21, 57, 94 and 130 in 4 E-F hands were replaced with alanine to ablate the Ca2+-binding ability of calmodulin.
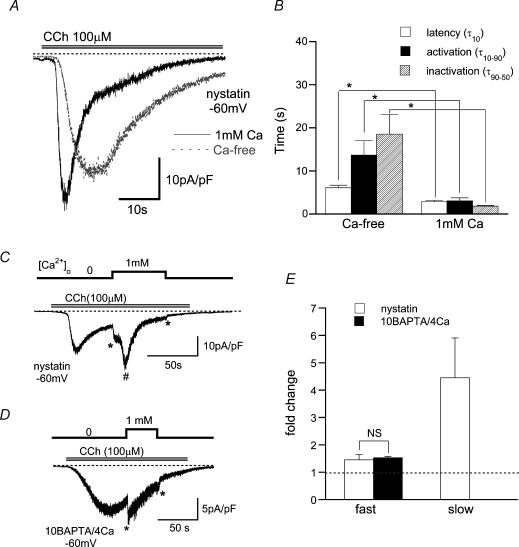
Figure 1. Acceleration of activation and inactivation time courses of murine TRPC6 currents by extracellular Ca2+ (Ca2+o).
Holding potential: −60 mV. A, typical traces for CCh (100 μm)-evoked TRPC6 currents (ITRPC6) in the presence (black, continuous line) and absence (grey, dotted line) of 1 mm Ca2+ in the bath, recorded from the same cell with nystatin-perforated recording. B, summary of latency (τ10; time for 10% activation of the peak from the onset of CCh application), activation time (τ10–90; time for 10–90% activation of the peak), and inactivation time (τ90–50; time for 90–50% of the peak in inactivation phase) of ITRPC6 with 0 and 1 mm Ca2+ evaluated from the experiments as shown in A(n = 12). Repeated application of CCh at short intervals (< 5min) usually resulted in a rundown and slowed activation and deactivation of ITRPC6. To minimize the errors arising from this problem, ITRPC6 was activated at an interval of 10 min, which led to recovery of the second response to CCh to 0.82 ± 0.09 of the first one (n = 9; evaluated in Ca2+-free external solution), and the response in the presence of Ca2+o was taken on the second application of CCh. C and D, ITRPC6 was first activated in the absence of Ca2+o and then exposed to 1 mm Ca2+o, under nystatin-perforated and conventional whole-cell (10 BAPTA/4 Ca internal solution) voltage clamp, respectively. E; summary of the effects of Ca2+o (1 mm) on ITRPC6 such as illustrated in C and D. Relative ITRPC6 amplitude (fold change) is calculated as the ratio of ITRPC6 amplitude just after to that before (no Ca2+ present) application of Ca2+o. For slow Ca2+o-induced potentiation (#), ITRPC6 amplitude at the peak potentiation was normalized to that just before application of Ca2+o. Symbols ‘*–*’ and ‘#’ stand for the fast potentiation and slow potentiation, respectively. n = 5–8. ‘*’ in B; P < 0.05 with paired t test. ‘NS’ in E denotes no statistical significance.
Electrophysiology
The details of the three variants of the patch clamp technique employed in this study (i.e. nystatin-perforated, conventional whole-cell, and single channel recordings) are essentially the same as described elsewhere (see supplementary information in Inoue et al. 2001). Leak currents were estimated by constructing the current–voltage relationship in non-transfected cells. However, in successful recordings, the leak current estimated in this way was as small as 0.14 ± 0.05 pA pF−1 at −60 mV (n = 15), and thus not corrected in the present study. For single channel recordings, pipette electrodes having a resistance of 5–10, 4–5 and 1.5–2 MΩ (with pipette solution described below) were used in cell-attached (C/A), outside-out (O/O) and inside-out (I/O) configurations, respectively, while for whole-cell recordings, the pipette input resistance was 2.5–4 MΩ. Voltage generation and current signal acquisition were implemented through a high-impedance low-noise patch clamp amplifier (EPC9; HEKA Elektronik, Germany). Sampled data were low-pass filtered at 1 kHz (I/O, O/O) or 2 kHz (C/A) and stored on a computer hard disc after digitization at 2–5 kHz (I/O, O/O) or 20 kHz (C/A). Long-term recordings (> 30 s, e.g. whole-cell currents in Fig. 1) were performed in conjunction with an A/D, D/A converter, Powerlab/400 (ADInstruments, Australia; sampling rate: 100 Hz), and data evaluation was made with the associated software, Chart v3.6. Concentration–response relationships were fitted by a non-linear least square routine, using two types of Hill equation. For single Hill fitting:
where I, C, IC50, and n denote the normalized current amplitude (the ratio of currents after to before drug application), drug concentration, half-maximal inhibitory concentration and the cooperativity factor, respectively.
For double Hill fitting (Fig. 2):
where I, C, IC50, and n have the same meaning as described above, while ΔI, EC50 and n′ denote the normalized current increase, half-maximal effective concentration and the cooperativity factor, respectively. It should, however, be mentioned that the fitting using this equation could provide only approximate values for EC50 and IC50, since the results of double non-linear fitting were prone to small changes in data points of a limited number.
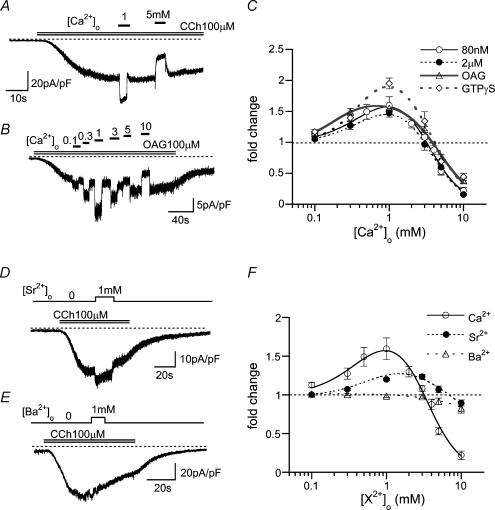
Figure 2. Biphasic effects of Ca2o+ on ITRPC6 under strongly intracellular Ca2+ (Ca2i+)-buffering conditions.
Recording conditions used were the same as in Fig. 1D. A and B, typical examples of ITRPC6 at varying [Ca2+]o evoked by 100 μm CCh (A) or OAG (B). C, relationships between [Ca2+]o and relative ITRPC6 amplitude (fold change) under various conditions; 100 μm CCh with [Ca2+]i= 80 nm (10 mm BAPTA/4 mM Ca2+, open circles) or 2 μm (10 mm BAPTA/9.5 mm Ca2+, filled circles); 100 μm OAG ([Ca2+]i: 80 nm, open triangles); 100 μm GTPγS ([Ca2+]i: 80 nm, open diamonds). n = 5–12. D and E, typical examples of the effects of 1 mm extracellular Sr2+ (D) and Ba2+ (E) on ITRPC6 evoked by 100 μm CCh ([Ca2+]i: 80 nm). F; relationships between relative ITRPC6 amplitude (fold change) and extracellular Ca2+, Sr2+ or Ba2+ concentration. n = 5–14. Curves in C and F are drawn according to the results of double Hill fitting (see Methods), which gave the EC50 and IC50 values (mm), respectively, in C of 0.44 and 3.56 (80 nm), 0.42 and 3.61 (2 μm), 0.38 and 4.27 (OAG), and 0.41 and 3.54 (GTPγS), and in F, 0.44 and 3.56 (Ca2+), and 0.84 and 7.44 (Sr2+).
Single channel analysis was made using the software Pulsefit and/or a freely distributed web software Win/EDR v.2.3 (Dr J. Dempster, University of Strathclyde, UK). To evaluate the unitary current amplitude, all-points or all-points-in-open-state amplitude histograms were constructed. To calculate the relative open probability (NPo), single channel currents were averaged for each 3–5 s time interval and then converted to the NPo value according to I = iNPo where I, i, N and Po denote the averaged single channel current, unitary amplitude, the number of open channels and their open probability, respectively. The baseline for channel opening was normally determined by constructing all-point histograms, but when this was unfeasible due to baseline drifts, the records were divided into several segments, each of which was corrected for its linear trend by visual inspection. In the case of large I/O or O/O patch experiments where precise evaluation of unitary amplitude (i) was often difficult due to multiple channel openings, we adopted the averaged ‘i’ value determined by C/A recordings.
Solutions
Solutions with the following composition were used. Pipette solution for nystatin-perforated recording (mm): 140 CsCl, 2 MgCl2, 10 Hepes, 10 glucose (adjusted to pH 7.2 with Tris base). Internal solution for conventional whole-cell recording and O/O recording (mm): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgCl2, 10 BAPTA, 4 CaCl2, 10 Hepes, 2 ATP, 0.1 GTP, 10 glucose (adjusted to pH 7.2 with Tris base; [Ca2+]=ca 80 nm; ‘10 BAPTA/4 Ca internal solution’). In some experiments in which the effects of a broader range of [Ca2+]i were investigated (e.g. Figs 5A and 6A), [Ca2+] was adjusted by using 10 mm BAPTA and appropriate concentrations of Ca2+ as performed in I/O recordings (see below). In Figs 8 and 9, in order to only weakly buffer [Ca2+]i, 10 mm BAPTA was replaced by 0.1 mm EGTA and 0.04 mm Ca2+ added; adjusted to pH 7.2 with Tris base; ‘0.1 EGTA-internal solution’). Ca2+-free external solution (mm): 140 NaCl, 5 KCl, 1.2 MgCl2, 1 EGTA, 10 Hepes, 10 glucose (pH 7.4, adjusted with Tris base). For Ca2+-, Sr2+-, or Ba2+-containing external solutions, various concentrations of CaCl2, SrCl2 or BaCl2 were added to the Ca2+-free solution with the omission of EGTA. Unless otherwise stated, pipette solution for C/A and I/O recordings contained (mm): 140 NaCl, 5 tetraethylammonium-Cl, 1.2 MgCl2, 0.1 CaCl2, 10 Hepes, 10 glucose, 0.1 carbacol (CCh) (pH 7.4, adjusted with Tris base). Ca2+ at 0.1 mm was usually necessary to successfully obtain the ‘giga’ seal. For C/A recording, cells were bathed in high potassium solution with the following composition to null the transmembrane potential (mm): 140 KCl, 2 EGTA, 2 MgCl2, 10 Hepes (pH 7.2, adjusted with Tris base). The bathing solution for I/O recording had the composition (mm): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgSO4, 2 EGTA, 10 Hepes, 2 ATP, 0.1 GTP (pH 7.2, adjusted with Tris base). To obtain the micromolar range [Ca2+], a low affinity buffer, HEDTA, rather than EGTA was used. The amount of Ca2+ required to obtain the desired [Ca2+] was calculated using Fabiato and Fabiato's program with enthalpic and ionic strength corrections (Brooks & Storey, 1992), as performed previously (Inoue & Ito, 2000).
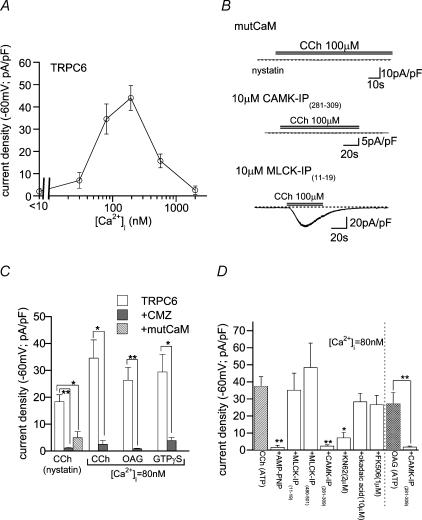
Figure 5. Essential requirement of CaM-kinase II-mediated phosphorylation for TRPC6 channel activation.
Bath: 1 mm Ca2+-containing external solution. A, relationship between [Ca2+]i and CCh (100 μm)-evoked ITRPC6 density. n = 5–19. B, typical examples of ITRPC6 recorded from cells coexpressing Ca2+-insensitive mutant calmodulin (mutCaM; nystatin-perforated recording; top trace) and those intracellularly perfused (> 5min; whole-cell; [Ca2+]i: 80 nm; 10 mm BAPTA/4 mm Ca2+) with 10 μm CaM-kinase inhibitory peptide (middle trace) or MLCK inhibitory peptide (bottom trace). C; effects of calmidazolium (CMZ; 3 μm) pretreatment or mutCaM coexpression on ITRPC6 evoked by 100 μm CCh, OAG or GTPγS. n = 5–15. D; effects of kinase and phosphatase inhibitors on ITRPC6 evoked by 100 μm CCh or OAG. Whole-cell recording ([Ca2+]i: 80 nm). n = 7–17. *P < 0.05, **P < 0.01 with unpaired t test (C: rightmost two columns in D) and with ANOVA and pooled variance t test (the other columns in D: control is hatched).
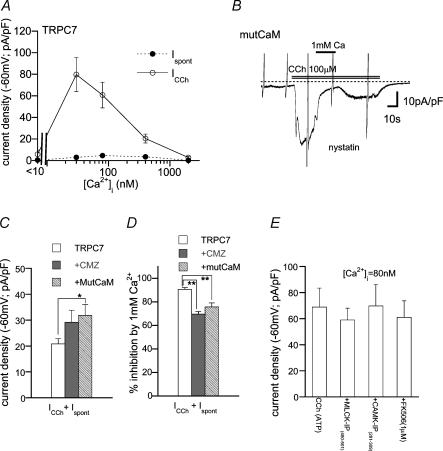
Figure 6. CaM-mediated inhibition of I.
TRPC7Bath: Ca2+-free external solution. A, relationship between [Ca2+]i and spontaneously activated (Ispont) or CCh (100 μm)-evoked (ICCh) ITRPC7. n = 8–14. Whole-cell recording. B, a typical example of ITRPC7 recorded from a mutCaM-coexpressing cell under nystatin-perforated voltage clamp. C and D, CMZ pretreatment (3 μm) or mutCaM coexpression enhances the density (C) and relieves Ca2+o (1 mm)-induced inhibition (D) of ITRPC7. Nystatin-perforated recording. n = 7–20. E, ineffectiveness of inhibitors for MLCK, CaMKII and calcineurin for ITRPC7. n = 5–8. *P < 0.05, **P < 0.01 with ANOVA and pooled variance t test.
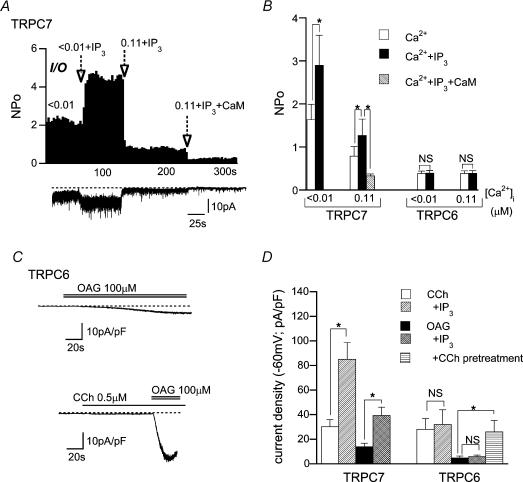
Figure 8. Intracellular IP3 differentially affects single TRPC7 and TRPC6 channel activity.
A, typical trace (lower panel) and corresponding NPoversus time plot (upper panel) for CCh-activated TRPC7 channel activity at two different [Ca2+]i values (< 10 nm and 0.11 μm) with IP3 (10 μm) or wild-type CaM (1 μm). I/O recording at −60 mV. B, summary of the effects of Ca2+i, IP3 (10 μm) or CaM (1 μm) on single TRPC7 and TRPC6 channel activity evaluated from experiments such as shown in A. n = 5–8. C, typical examples of OAG (100 μm)-induced ITRPC6 at −60 mV with (lower panel) or without (upper panel) a subthreshold activating concentration of CCh (0.5 μm). D, summary of the effects of intracellular IP3 (10 μm; added in the pipette) or a subthreshold concentration of CCh (0.5 μm) on ITRPC6 and ITRPC7. n = 5–7. In C and D, bath and pipette contained 1 mm Ca2+-containing external and 0.1 EGTA internal solutions, respectively. *P < 0.05 with unpaired t test or ANOVA and pooled variance t test.
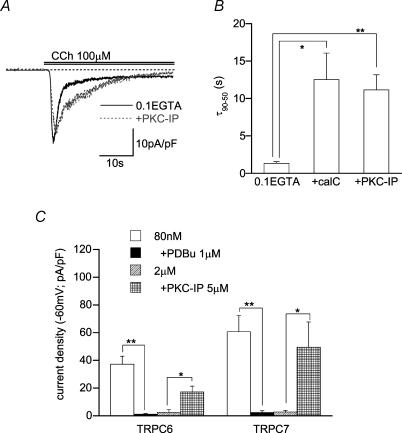
Figure 9. PKC mediates inactivation of TRPC6 and TRPC7 channels.
A, representative records of CCh-evoked /TRPC6 with or without PKC inhibitory peptide (PKC-IP(19–36), 5 μm) in the pipette. B, effects of PKC inhibitors on the inactivation time of /TRPC6 (τ90–50; see Fig. 1 legend); control (0.1 EGTA internal solution): pretreatment with calphostin C (500 nm, 5min, middle): intracellular perfusion of PKC-IP(19–36) (5 μm, 5min, right). n = 7–12. Recording conditions in A and B were the same as in Fig. 8C and D. C, effects of PKC inhibitors and activators on /TRPC6 or /TRPC7 density at two different [Ca2+]i levels. n = 5–12. *P < 0.05, **P < 0.01 with unpaired t test (in C) or ANOVA and pooled variance t test (in B).
In whole-cell, O/O and I/O single channel recordings, solutions were rapidly applied through a solenoid-driven fast solution change device ‘Y-tube’ (Inoue et al. 2001).
Chemicals
Calmidazolium (chloride salt), 1-oleoyl-2-acetyl-sn-glycerol (OAG), 5′-adenylylimidodiphosphate (AMP-PNP), GTPγS, calmodulin, KN-62, okadaic acid, FK506, CaM-kinase II inhibitory peptide (CAMK-IP(281–309)), myosin light chain kinase (MLCK) inhibitory peptide (MLCK-IP(480–501)), and protein kinase C inhibitory peptide (PKC-IP(19–36)) were purchased from Calbiochem (La Jolla, CA, USA), carbachol, Hepes, SrCl2, BaCl2 and PDBu from Sigma (St Louis, MO, USA), ATP, GTP, BAPTA and EGTA from Dojindo (Kumamoto, Japan), and MLCK inhibitory peptide (MLCK(11–19) amide) from Alexis (Nottingham, UK). HEDTA was kindly provided by BASF Japan, Ltd.
Statistics
All data are expressed as means ±s.e.m. To evaluate statistical significance of the difference between a given set of data, Student's paired and unpaired t test, and one-way ANOVA with pooled variance t test (Bonferroni's correction) were employed for single and multiple comparisons, respectively.
Results
Multiple effects of Ca2+ on heterologously expressed TRPC6 currents
Under quasi-physiological conditions with nystatin-perforated voltage-clamp (Horn & Marty, 1988; −60 mV), human embryonic kidney 293 (HEK293) cells expressing murine TRPC6 differentially responded to muscarinic receptor stimulation (carbachol; CCh 100 μm) at different levels of external Ca2+ (Ca2+o) (Fig. 1A). The time courses of activation and inactivation of CCh-induced current (ITRPC6) evaluated in the same cell were clearly faster in the presence of 1 mm Ca2+o as compared with its absence. Paired data from 12 cells indicate that three parameters representing the rates of activation and inactivation, t10, t10–90 and t90−50, are significantly shorter in the presence of Ca2+o (Fig. 1B). The extent of maximum activation (i.e. peak amplitude) was also greater with 1 mm Ca2+ in the bath (25.7 ± 2.8 versus 37.8 ± 4.9 pA pF−1 at −60 mV, n = 12; P < 0.05 with paired t test). These results show that the presence of Ca2+o enhances the extent of activation and accelerates the activation and inactivation processes of ITRPC6. Similar enhancement and acceleration of activation (or potentiation) and of inactivation were observed, when Ca2+o was added after ITRPC6 had already reached the peak activation (Fig. 1C). In this case, however, in addition to slower potentiating and inactivating phases (#), an immediate increase in ITRPC6 amplitude (*–*), which was not discernible in the continued presence of Ca2+o (Fig. 1A), was also visualized.
The potentiating and inactivating effects of Ca2+o on ITRPC6 were still observed when conventional whole-cell recording with weak Ca2+ buffering capacity (0.1 EGTA internal solution) was used (data not shown). However, inclusion of 10 mm BAPTA in the pipette (10 BAPTA/4 Ca internal solution; calculated [Ca2+]i = 80 nm; conventional whole-cell clamp mode) selectively abolished the slow potentiating and inactivating effects of Ca2+o on ITRPC6 without affecting the fast potentiating actions (Fig. 1D and E), suggesting that the slow effects of Ca2+o are mediated by elevation in [Ca2+]i. Under these conditions, the effects of Ca2+o on ITRPC6 occurred as a biphasic function of [Ca2+]o characterized by potentiation in the submilli- to millimolar range (< 1–3 mm; apparent EC50: ∼0.4 mm) and inhibition in a higher concentration range (> 1–3 mm; apparent IC50: ∼4 mm) (Fig. 2A and C). These Ca2+o effects most likely result from the actions on TRPC6 channel per se, since comparable [Ca2+]o dependence was observed when ITRPC6 was more directly activated by GTPγS or OAG, by bypassing the receptor, G-protein or phospholipase C (PLC) (Fig. 2B and C). Similar biphasic (but less pronounced) effects on ITRPC6 were also observed for Sr2+, but were virtually absent for another Ca2+-mimetic, Ba2+ (Fig. 2D–F).
The rapidity and resistance to vigorous [Ca2+]i buffering of fast potentiation and inhibition by Ca2+ and Sr2+ suggest that their site(s) of actions may reside at the extracellular side of the TRPC6 channel. In strong support of this idea, the extent of potentiation and inhibition were not changed by clamping [Ca2+]i to a more elevated level (2 μm; filled circles in Fig. 2C), and quantitatively comparable effects of Ca2+o could still be obtained on single TRPC6 channel activity recorded in the cell-free, outside-out (O/O) patch configuration (Fig. 3A and B).
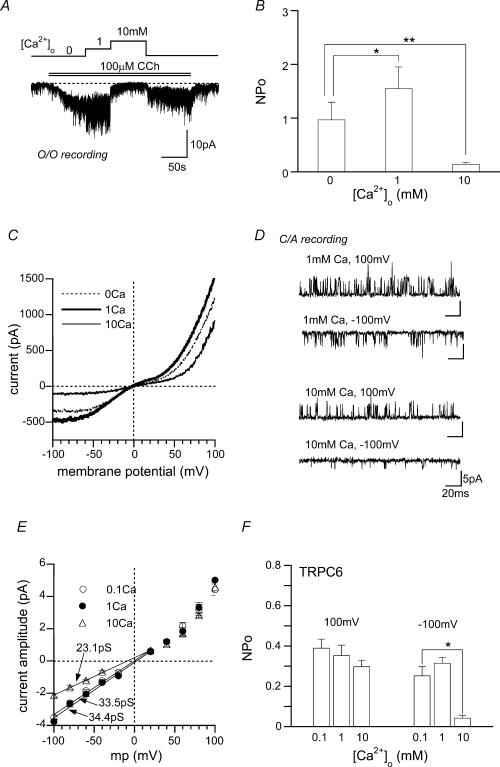
Figure 3. Potentiating and inhibitory effects of Ca2o+ on single TRPC6 channels.
A and B, O/O recording at −60 mV. O/O membranes were sequentially exposed to CCh (100 μm) and three different [Ca2+]o. A representative record (A) and the summary of five separate O/O experiments (B). Averaged NPo is plotted against [Ca2+]o. C, typical current–voltage (I–V) relationships of ITRPC6 at different [Ca2+]o (0, 1 or 10 mm Ca2+ in the bath) evaluated from the same cell. D–F, C/A recording. Transmembrane potential was zeroed by high K+ external solution. Typical examples of single CCh-activated TRPC6 channels at two different Ca2+ concentrations in the pipette (i.e. [Ca2+]o= 1 or 10 mm) and membrane potentials (−100 or 100 mV) (D), and I–V relationships (E) and [Ca2+]o dependence of NPo (F) of CCh-activated TRPC6 channels. n = 5. In E, numerals indicate the unitary conductances evaluated by linear data fitting between −100 and 20 mV (continuous lines). *P < 0.05, **P < 0.01 with paired t test (in B) or ANOVA and pooled variance t test (in F).
The current–voltage (I–V) relationships for macroscopic ITRPC6 show that the fast inhibition by high millimolar Ca2+o occurs via a voltage-dependent mechanism; the extent of the inhibition was significantly decreased at strongly depolarized membrane potentials (dotted versus thin continuous curves in Fig. 3C; percentage inhibition by 10 mm Ca2+o relative to 0 mm: 75 ± 5% at −100 mV, 42 ± 6% at 100 mV, n = 7; P < 0.05). This voltage dependence can be accounted for at least in part by voltage-dependent reductions of the unitary conductance and degree of activation (NPo) of single TRPC6 channels, since both reductions were relieved at strongly depolarized potentials (Fig. 3D; open triangles in Fig. 3E and F). In contrast, the fast potentiation of ITRPC6 at sub- to low millimolar Ca2+o exhibited little sign of voltage dependence, as evidenced by an almost symmetrically increased I–V relationship (dotted versus thick continuous curves in Fig. 3C) and unaltered unitary amplitude or NPo values at different potentials (filled circles in Fig. 3E and F). These results suggest that the mechanism underlying the potentiation may be different from that for the inhibition, but in the present study, we could not unequivocally figure out what changes in single channel properties contribute to the former.
Ca2o+ inhibits TRPC7 channels
TRPC7 is a closest homologue of TRPC6 and has been shown to exhibit much higher spontaneous activity than TRPC6 and undergo inhibitory actions of Ca2+o (Okada et al. 1999). As demonstrated in Fig. 4, over a broad range of [Ca2+]o, Ca2+o merely caused a concentration-dependent inhibition of currents due to TRPC7 expression (spontaneous plus CCh-induced currents; ITRPC7), irrespective of the species of activators used (i.e. CCh, GTPγS, OAG; Fig. 4A and B). Part of this inhibition was, however, eliminated by increasing the buffering capacity for [Ca2+]i (Fig. 4AversusB; open versus filled circles: * in Fig. 4B), suggesting the involvement of elevated [Ca2+]i in Ca2+o-induced inhibition.
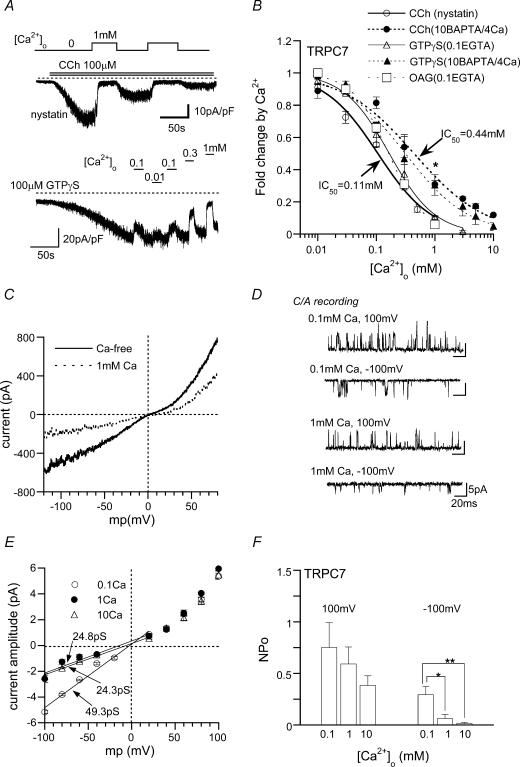
Figure 4. Inhibitory effects of Ca2o+ on ITRPC7 and single TRPC7 channels.
A, typical examples of Ca2+o-induced inhibition of ITRPC7 recorded at −60 mV, with nystatin-perforated (upper panel) or conventional whole-cell (10 BAPTA/4 Ca internal solution: lower panel) recordings. In the latter, GTPγS (100 μm) was included in a patch pipette. B; relationships between [Ca2+]o and relative ITRPC7 amplitude (fold change induced by Ca2+o) under various conditions. Different activators (100 μm CCh, OAG and GTPγS) and different modes of voltage-clamp (nystatin-perforated and conventional whole-cell recording with either 10 BAPTA/4 Ca or 0.1 EGTA-internal solution) were tested. *P < 0.05 with unpaired t test for open versus filled circles. Curves are drawn according to the results of single Hill fitting (see Methods). n = 5–10. C, typical I–V relationships for ITRPC7 obtained just before (Ca2+-free) and after the addition of 1 mm Ca2+o. D–F, C/A recording. Typical examples of single CCh-activated TRPC7 channels at [Ca2+]o of 0.1, 1 or 10 mm and membrane potentials of −100 or 100 mV (D), and I–V relationships (E) and [Ca2+]o dependence of NPo (F) of CCh-activated TRPC7 channels. n = 5. Continuous lines and the meaning of numerals in E are the same as in Fig. 3E. *P < 0.05, **P < 0.01 with ANOVA and pooled variance t test.
The fast inhibition of ITRPC7 remaining after strong [Ca2+]i buffering occurred in about a 10-fold lower concentration range of Ca2+o (apparent IC50: 0.44 mm) as compared with ITRPC6, but showed a very similar voltage dependency to ITRPC6, being characterized by more prominent inhibition of the macroscopic I–V relationship and reductions of unitary conductance and NPo at negative potentials (Fig. 4C–F). Furthermore, exchanging the transmembrane (TM) region of TRPC7 with that of TRPC6 converted the fast inhibition caused by 1 mm Ca2+ to potentiation (Supplementary Fig. 1). Taken together, these results are consistent with the idea that the site(s) of fast Ca2+o actions are located on the extracellular side of the TM region, of which only an inhibitory site may commonly exist in TRPC6 and 7 channels.
Mechanisms involved in intracellular Ca2+'s actions on TRPC6 and TRPC7 channels
The abolition of slow potentiating and inactivating effects of Ca2+o on ITRPC6 by vigorous [Ca2+]i buffering implies that the level of [Ca2+]i may both positively and negatively regulate TRPC6 channel activity. Consistent with this speculation, when cells were intracellularly perfused with various values of [Ca2+]i, the density of CCh-induced ITRPC6 showed a biphasic dependence on [Ca2+]i consisting of incremental (< ∼200 nm) and decremental (> ∼200 nm) phases (Fig. 5A). A similar [Ca2+]i dependence was also observed for ITRPC7, but in this case, the range for positive regulation is shifted to much lower concentrations as compared with ITRPC6 (Fig. 6A). The [Ca2+]i dependence of TRPC6 and TRPC7 channels did not change appreciably, even when they were activated by OAG (data not shown). It seems thus likely that the site(s) of the actions of intracellular Ca2+ (Ca2+i) are also located on the channel proteins themselves.
Calmodulin (CaM) is a ubiquitous intracellular calcium-binding protein involved in Ca2+-mediated regulation of many ionic channels (Levitan, 1999; Saimi & Kung, 2002). In addition to direct binding to channel proteins, the Ca2+-bound CaM (Ca2+−CaM) activates several physiologically important kinases and phosphatases including CaM-kinase II, myosin light chain kinase (MLCK) and calcineurin, thereby altering the phosphorylated state of channel proteins to modulate their functions (Braun & Schulman, 1995a; Rusnak & Mertz, 2000; Kamm & Stull, 2001). To assess the potential importance of these mechanisms in Ca2+i-mediated regulation of TRPC6 and TRPC7 described above, we next tested how inhibitors for CaM and Ca2+−CaM-dependent enzymes affect these channel functions.
Pretreatment of TRPC6-expressing cells with calmidazolium (CMZ; 3 μm), a potent CaM antagonist (IC50: ∼10 nm), or coexpression of a Ca2+-insensitive mutant of CaM (mutCaM), strongly attenuated the activation of ITRPC6 by CCh, GTPγS or OAG. (Fig. 5B and C). Such a marked attenuation also occurred by decreasing [Ca2+]i to an extremely low level (< 10 nm; Fig. 5A), by pretreatment with an organic CaM kinase II inhibitor KN-62 (2 μm), or with intracellular perfusion of CaM-kinase II inhibitory peptide (CAMK-IP(281–309); Fig. 5D). Furthermore, substitution of ATP with its non-hydrolysable analogue AMP-PNP in the patch pipette solution dramatically reduced the ITRPC6 density (Fig. 5D). These results collectively suggest that phosphorylation by CaM kinase II is a prerequisite for activation of the TRPC6 channel. In contrast, inhibitory peptides for MLCK (MLCK-IP(11–19), MLCK-IP(480–501)), a non-specific protein phosphatase inhibitor, okadaic acid (10 μm), or a potent calcineurin inhibitor, FK506 (1 μm), did not significantly affect the ITRPC6 density (Fig. 5D).
In sharp contrast with the critical importance of CaM kinase II for TRPC6 activation, activation of ITRPC7 by CCh was not impaired by pretreatment with CMZ (3 μm) or coexpression of mutCaM, but rather enhanced (Fig. 6B and C; however, spontaneous ITRPC7 did not exhibit a significant increase; not shown). Inhibitors for CaM kinase II, MLCK and calcineurin also failed to affect ITRPC7 (Fig. 6E). These results exclude the requirement of Ca2+−CaM and phosphorylation for TRPC7 channel activation. However, the extent of Ca2+o (1 mm)-induced inhibition of ITRPC7 was significantly reduced by pretreatment with CMZ or coexpression of mutCaM (Fig. 6D), being comparable to that observed for strongly [Ca2+]i-buffering conditions (Fig. 4B). This observation suggests the presence of some inhibitory actions of Ca2+−CaM on TRPC7 channels. To elucidate these actions more directly, we next employed the inside-out (I/O) configuration of single channel recording.
Ca2+−CaM actions on TRPC6 and 7 channels
Single TRPC6 or TRPC7 channels recorded in I/O mode with patch pipettes of ordinary size (5–10 MΩ) showed a rapid rundown, which presumably reflects a gradual loss of intracellular constituents maintaining the channel activity (i.e. washout phenomenon). To circumvent this problem, we needed to use patch pipettes having a very low input resistance (1.5–2 MΩ), by which, in more than 30% of patch membranes tested, the decline of channel activity could be minimized over a period of 5–10 min.
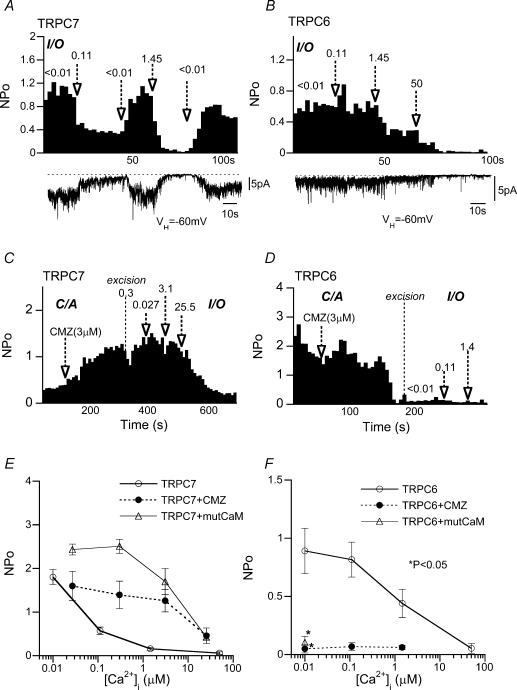
Figure 7 demonstrates typical examples of CCh (100 μm)-activated TRPC6 and TRPC7 channel activity at various [Ca2+]i levels (I/O mode). The activity of the TRPC7 channel was decreased by elevation of [Ca2+]i in the nanomolar range (< 10–110 nm) and completely suppressed by further increase in [Ca2+]i (Fig. 7A and open circles in E). The TRPC6 channel was also subject to inhibition by Ca2+i but required more than 10-fold higher [Ca2+]i (1.45 μm) than TRPC7 to be significantly inhibited (Fig. 7B and F). Ca2+i-mediated inhibition of TRPC7 channel activity was strongly attenuated by pretreatment with CMZ (3 μm) or coexpression of mutCaM. As demonstrated in Fig. 7C, application of CMZ in the C/A configuration gradually enhanced TRPC7 channel activity, which showed, after excision of patch membrane (i.e. I/O mode), a much decreased sensitivity to Ca2+i; the effective range of [Ca2+]i to inhibit TRPC7 channels was shifted from nano- to micromolar concentrations (filled circles in Fig. 7E). A similar shift was also observed with coexpression of mutCaM (Fig. 7E). These results strongly suggest that TRPC7 channel activity is negatively regulated by Ca2+−CaM.
Figure 7. Differential dependence of single TRPC6 and TRPC7 channels on Ca2i+ and CaM.
I/O recording at −60 mV. A and B, typical traces (lower panels) and corresponding NPoversus time plots (upper panels) for CCh-activated TRPC7 (A) and TRPC6 (B) channels at different [Ca2+]i. Numerals and arrows indicate the value of [Ca2+]i (micromolar) and the timing of solution change, respectively. C and D, effects of CMZ on CCh-activated TRPC7 (C) and TRPC6 (D) channels. NPoversus time plots. CMZ (3 μm) was applied in C/A mode, and then patch membranes were excised (I/O mode). E and F, NPo−[Ca2+]i relationships (I/O mode) for CCh-activated TRPC7 and TRPC6 channels, without (open circles) or with CMZ (3 mm) treatment (filled circles) or mutCaM coexpression (open triangles). n = 5–9. *P < 0.05 with pooled variance t test for filled circle or open triangle versus open circle at the same [Ca2+]i (control).
The effects of CMZ and mutCaM on single TRPC6 channels were entirely different from those on TRPC7 channels. Consistent with their effects observed for macroscopic ITRPC6, application of CMZ greatly diminished the CCh-induced TRPC6 channel activity in C/A mode, and subsequent exposure of excised membrane to various [Ca2+]i levels (I/O mode) could not restore the channel activity (Fig. 7D and F). Coexpression of mutCaM produced essentially the same results (Fig. 7F). These results confirm that the Ca2+−CaM-mediated process is obligatory for TRPC6 channel activation. However, we cannot exclude the possibility that inhibition by Ca2+−CaM observed at micromolar [Ca2+]i, which was not experimentally seen after abolition of the activation process, may also exert some modulatory role on TRPC6 channels.
The mechanism for the negative regulation of TRPC7 channels by Ca2+−CaM seems compatible with a recently proposed hypothesis of competitive regulation by CaM and IP3 of TRPC3 for its common binding domain (e.g. CIRB; see Introduction). Addition of IP3 (10 μm) increased single TRPC7 activity, and this was significantly counteracted by elevated [Ca2+]i or exogenously applied CaM (1 μm) (Fig. 8A and B). Macroscopic ITRPC7 evoked by CCh or OAG was also greatly enhanced by intracellular perfusion of IP3 (Fig. 8D; there was, however, no dramatic increase in spontaneous ITRPC7; 5.5 ± 1.4 versus 7.4 ± 1.3 pA pF−1 without and with 10 μm IP3, respectively; n = 7). In contrast, IP3 exerted little effect on TRPC6 at either the whole-cell or the single channel level (Fig. 8B and D). Instead, as reported recently (Estacion et al. 2004), marked enhancement of OAG-induced ITRPC6 occurred by pretreatment with a subthreshold activating concentration of CCh (0.5 μm; Fig. 8C and D). At least under our experimental conditions, the major part of this enhancement seems ascribable to synergistic effects of Ca2+i, as it was observed only with weak [Ca2+]i buffering; OAG (100 μm)-induced ITRPC6 recorded with 10 BAPTA/4 Ca internal solution was not significantly affected by pretreatment with 0.5 μm CCh (at −60 mV; 27.0 ± 6.7 pA pF−1(n = 5) versus 26.3 ± 4.8 pA pF−1(n = 6) for control and 0.5 μm CCh pretreatment, respectively).
Differential regulation by Ca2+−CaM and IP3 observed between TRPC7 and 6 channels probably reflects their C-terminal differences. Their chimeras with exchanged C-termini T667 and T776 showed essentially the same dependence on Ca2+−CaM and IP3 as TRPC7 and TRPC6, respectively (Supplementary Fig. 2).
Involvement of PKC in TRPC6 inactivation
According to previous studies (Trebak et al. 2003; Estacion et al. 2004), protein kinase C (PKC) negatively regulates some TRPC members including TRPC6. This raises the possibility that activation of PKC may be involved in the rapid inactivation process of ITRPC6 in the presence of Ca2+o (Fig. 1A and B) and its decreased current density at micromolar [Ca2+]i (Fig. 5). Consistent with this expectation, inclusion of a PKC inhibitor, calphostin C (500 nm) or a PKC inhibitory peptide (PKC-IP(19–36): 5 μm) in the patch pipette significantly decelerated the time course of inactivation (or decreased τ10–90 value; Fig. 9A and B) and enhanced the ITRPC6 density at micromolar [Ca2+]i (Fig. 9C). Conversely, activation of PKC by a phorbol ester, PDBu (1 μm; pretreated 10 min), caused almost complete suppression of ITRPC6 (Fig. 9C). Similar enhancement of current density by PKC inhibitors and inhibition by PDBu were also observed for ITRPC7 (Fig. 9C).
Discussion
TRPC6 and 7 share more than 70% identity in the overall amino acid sequence (N-terminus: 80%; transmembrane region: 77%; C-terminus: 73%) and both code for Ca2+-permeable cation channels activated by diacylglycerol (Hofmann et al. 1999; Okada et al. 1999). Despite these molecular and functional similarities, the results of the present study have provided the evidence that heterologously expressed TRPC6 and 7 channels undergo contrasting regulation by Ca2+ from both extracellular and intracellular sides, via CaM-dependent and -independent mechanisms.
Whole-cell (with 10 mm BAPTA) and single channel data (Fig. 4) together suggest that the major part of fast voltage-dependent inhibitory actions by Ca2+o on ITRPC7 is ascribable to its interaction with an ‘extracellular’ site(s) which can sense the transmembrane potential. A similar inhibitory site having a weaker efficacy is probably present in TRPC6 channels (Figs 2 and 3). In contrast, the fast potentiating effect of Ca2+o is unique to TRPC6, did not show voltage dependence and was not associated with changes in unitary conductance (compare 0.1 and 1 mm in Fig. 3E), but also probably occurred through the extracellular actions of Ca2+, as unequivocally demonstrated by the O/O recording (Fig. 2A and B). The finding that the type of TM region critically determines the pattern of response to Ca2+o (i.e. ‘inhibitory’ or ‘facilitatory’; Supplementary Fig. 1) further reinforces this speculation. These extracellular Ca2+ effects may most simplistically be accounted for by the permeation blockade and/or stabilization of gating in ‘non-conductive’ states by Ca2+o, which are often found in many cation-selective channels (e.g. Hille, 2001).
Complex extracellular actions of Ca2+ and of its mimetic lanthanides (La3+ and Gd3+), which are strongly reminiscent of Ca2+o actions on TRPC6 and 7, have recently been reported for heterologously expressed mouse TRPC5 channel (Jung et al. 2003). In this channel, the effect of lanthanides is biphasic with concentration-dependent potentiation in the low micromolar range and inhibition in a higher concentration range, whereas that of Ca2+ is merely facilitatory only at high millimolar concentrations. Site-directed mutagenesis has revealed that three glutamate residues adjacent to the putative pore-forming loop (Glu543, Glu595, Glu598) are crucial for the observed La3+- as well as Ca2+-induced potentiation (Jung et al. 2003). However, alignments of the TM5–TM6 linker region of TRPC5, 6 and 7 (Supplementary Fig. 3) indicate little similarity present for these glutamates between the three TRPC channels (see also Jung et al. 2003). Except for Glu598, negatively charged residues corresponding to Glu543 or Glu595 are absent in TRPC6 and 7, which instead possess four additional glutamates or aspartate in the more central part. Furthermore, the overall amino acid sequence of TRPC6 and 7 in this linker region, especially the number and position of negatively charged residues, is highly homologous (Supplementary Fig. 3), thus suggesting that these residues might not suffice to elucidate the differential effects of Ca2+o on TRPC6 and 7 channels.
The observed [Ca2+]o sensitivity of recombinant TRPC6 channel is substantially different from that reported for a number of native ROC channels in which this protein is probably involved (Inoue et al. 2001; Jung et al. 2002). For instance, the α1-adrenonceptor-activated cationic channel (α1-AD-NSCC) in portal vein smooth muscle displays much higher sensitivity to Ca2+o potentiation (EC50 = 3 μm) showing a rapid declining feature (Helliwell & Large, 1998), and is also potentiated by submillimolar concentrations of Ba2+ and Sr2+ (Aromolaran & Large, 1999). However, in the present study, Ba2+ was found to be ineffective in causing either potentiation or inhibition of expressed TRPC6 channels, and their potentiation by Ca2+ and Sr2+ was rather long-lasting (Figs 1D and 2D). A more strikingly different [Ca2+]o sensitivity is found for TRPC6-like currents in A7r5 cells, where millimolar Ca2+o exerts only inhibitory actions, although reduction of this current by changing [Ca2+]o from 200 μm to nominally zero suggests a stimulatory role of Ca2+o (Ba2+ and Sr2+ are, however, ineffective; Jung et al. 2002). A possible molecular background for such discrepancies is that these native TRPC6-like channels may be heteromultimerically assembled with other endogenously expressed TRP isoforms, thereby acquiring diverse divalent cation sensitivities. In support of this possibility, TRPC6 has been shown to coimmunoprecipitate with TRPC3 and 7 in adult tissues (Hofmann et al. 2002; Goel et al. 2002) and in embryonic brain interact with other TRPC isoforms in complex combinations (Strübing et al. 2003), and the presence of some of these isoforms has been confirmed by RT-PCR technique in portal vein myocytes and A7r5 cells (Inoue et al. 2001; Jung et al. 2002). However, it should be noted that the expression system used in this study (HEK293 cells) has been reported to endogenously express several TRPC isoforms including TRPC1, 3, 4 and 6 (Garcia & Schilling, 1997). Considering this fact, we cannot completely exclude the possibility that overexpressed TRPC6 or 7 channels could also be affected by these endogenously TRPC proteins thus bearing somewhat altered properties as compared with genuine homomeric channels.
Single channel data in large I/O membranes have indicated that, once receptor-activated, TRPC7 channels are concentration-dependently inhibited by Ca2+i in the nanomolar range. These effects are probably mediated via CaM-dependent actions on its C-terminus, as indicated by mutCaM coexpression and TRPC6/7 chimera experiments. Previous biochemical studies have shown that all TRPC members possess a common binding region for CaM and IP3 receptor (IP3R) on the C-terminus (CIRB domain), to which CaM binds in a strictly Ca2+-dependent manner (Tang et al. 2001). It has been hypothesized that under resting conditions, CaM bound to this region tonically inhibits TRPC3 channels, and its displacement by activated IP3R (by receptor stimulation) or its inactivation by the CaM antagonist CMZ leads to channel activation (Zhang et al. 2001). Overall, this picture seems to apply to TRPC7; pretreatment with CMZ or coexpression of mutCaM enhanced CCh-activated TRPC7 channel activity at both single channel (C/A mode; Fig. 7) and whole-cell (Fig. 6) levels; in I/O recording, inhibitory effects of Ca2+i on the TRPC7 channel, which were strongly attenuated by CMZ or mutCaM (Fig. 7), were antagonized and enhanced by IP3 and CaM, respectively (Fig. 8). However, the observed [Ca2+]i range for CaM-dependent TRPC7 channel inhibition (apparent IC50 ≈ 100 nm; Fig. 7) is far lower when compared to the Ca2+-dependence of CaM binding domain (i.e. CIRB) evaluated in vitro (Tang et al. 2001). The effect of Ca2+i still persisted even after exposure to a very low [Ca2+]i level (< 10 nm) which was expected to unbind CaM (Fig. 7A). Coexpression of mutCaM enhanced the magnitude of ITRPC7, but did not appreciably induce a current by itself unless the receptor was stimulated (e.g. Fig. 6B). All these findings are not simply compatible with the antagonism between CaM and IP3R for channel activation (Zhang et al. 2001). The most straightforward interpretation of the above results is that CaM is constitutively bound to TRPC7 channel C-terminus and negatively regulates the channel activity in a Ca2+-dependent fashion regardless of receptor activation process. Thus, the role of CaM would not be obligatory for TRPC7 channel activation but rather modulatory as a negative feedback operating during receptor stimulation. However, despite the observed [Ca2+]i range for negative regulation of single TRPC7 channels (Fig. 7E) roughly matching that for ITRPC7 (Fig. 6A), at an extremely low [Ca2+]i (< 10 nm), receptor activation of ITRPC7 was severely impaired. This is unexpected from single channel recordings, and unlikely to involve the actions of CaM, IP3R or phosphorylation, e.g. by CAMKII (see above). It seems thus likely that an additional, as-yet-unidentified, Ca2+-dependent mechanism participates in the activation process of TRPC7 channels.
Despite the presence of almost identical CaM binding domain (CIRB; Tang et al. 2001), the inhibitory effect of Ca2+i on single TRPC6 channels is much weaker than TRPC7 (it occurred only at micromolar [Ca2+]i), and intracellular application of IP3 failed to activate or enhance the channel activity at whole-cell and single channel levels (Fig. 8). This excludes the involvement of competitive regulation by Ca2+−CaM and IP3R in the TRPC6 channel activation process. Instead, we have found that activation of macroscopic TRPC6 current is positively regulated by nanomolar Ca2+i and highly susceptible to procedures that eliminate intracellular ATP and CaM or directly inhibit CAMKII, regardless of the activator (i.e. CCh or OAG) (Figs 5 and 7). These results are consistent with the idea that phosphorylation by CaMKII is an obligatory step for TRPC6 channel activation and occurs independently of the process of generating DAG. Similar CaMKII phosphorylation-mediated activation has also been reported for epithelial Ca2+-activated non-selective cation channels, the properties of which are strongly reminiscent of some TRP members (Braun & Schulman, 1995b).
Somewhat puzzlingly, however, activation of the native counterpart of TRPC6, α1-AD-NSCC in vascular smooth muscle, has been reported to be mediated by MLCK rather than CAMKII (Aromolaran et al. 2000). In this native channel, MLCK-specific peptides, one of which (MLCK(11–19) amide) was found to be ineffective for expressed TRPC6 channel in this study, strongly prevented the activation of α1-AD-NSCC. One plausible explanation for this discrepancy is that a rather muscle-specific distribution of MLCK may allow its preferential interaction with the TRPC6 channel in muscle tissues (Kamm & Stull, 2001) whereas in other tissues, the more ubiquitous CaMKII may play a similar role. However, it should be pointed out that while CaMKII can target a multitude of substrates including ion channels (Braun & Schulman, 1995a), the only known physiological target of MLCK is myosin, which is implicated in the regulation of muscle contraction and cytoskeletal activity but not directly in channel activation (Kamm & Stull, 2001). These discrepancies, together with the fact that synergism between IP3 and OAG observed for α1-AD-NSCC activation (Albert & Large, 2003) is deficient in expressed TRPC6 channels (Fig. 8; Estacion et al. 2004), again suggest that essential differences may be present in subunit compositions or accessory regulatory mechanisms between these two channels. Obviously, more studies will be needed to elucidate the exact mechanism underlying phosphorylation-mediated activation of the TRPC6 channel.
In summary, the activity of the TRPC6 channel is probably regulated by Ca2+ in a multifold fashion. In physiological ionic milieu, in addition to direct tonic enhancement by Ca2+o, Ca2+i-dependent mechanisms seem to dynamically regulate TRPC6 channels during PLC-linked receptor stimulation. In the early phase, Ca2+ released from internal stores via activated IP3R and Ca2+ influx through activated TRPC6 channels by DAG generation elevate [Ca2+]i thereby facilitating the channel activation process via CaMKII-mediated phosphorylation (Fig. 5). When the elevation of [Ca2+]i is sustained, however, this turns to inactivation via PKC activation (Fig. 9). Such sequential activation of kinases can be seen as accelerated activation and inactivation processes by Ca2+o (Fig. 1A and C). In contrast, Ca2+ regulates TRPC7 channels almost negatively via direct extracellular, and Ca2+−CaM- (but phosphorylation-independent) and PKC-dependent mechanisms (Figs 4, 7 and 9). In this channel, generation of IP3 seems to synergistically enhance the channel activity with its primary activator DAG (Fig. 8).
Multifold regulation by Ca2+ reminiscent of TRPC6 has been reported for some native ROC channels. For instance, muscarinic cation channels in gastrointestinal smooth muscle has been shown to be strongly potentiated by [Ca2+]i elevation (Inoue & Isenberg, 1990; Pacaud & Bolton, 1991), part of which may involve a Ca2+−CaM–MLCK-dependent pathway (Kim et al. 1995; Kim et al. 1997). This mechanism seems to operate as an effective positive feedback synchronized with action potentials and IP3-mediated intracellular Ca2+ release, contributing to enhanced excitatory actions of visceral cholinergic nerves on gut motility (Kuriyama et al. 1998). In contrast, prolonged [Ca2+]i elevation inactivates these channels and terminates the muscarinic response rapidly (Kim et al. 1998; Zholos et al. 2003). A similar situation may hold for the α1-AD-NSCC in portal vein smooth muscle, in which the molecular contribution of TRPC6 has been more unequivocally demonstrated (Inoue et al. 2001; Large, 2002). In this respect, the results of this study would provide an important molecular basis for elucidating such multifold effects of Ca2+ in native tissues.
Supplementary Material
Acknowledgments
We thank Miss Yoko Takashiro for expert technical assistance. This study is supported in part by a grant-in-aid from the Ministry of Education, Culture and Science to R.I. J.S. is a fellow of the Tokyo Biochemical Research Foundation and supported by the National Natural Science Foundation of China (grant No. 30400154).
Supplementary material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2004.075051/http://jp.physoc.org/cgi/content/full/jphysiol.2004.075051/DC1 and contains supplementary material consisting of three figures:
Supplementary Figure 1. Effects of Ca2+o on TRPC6/7 chimeras Supplementary Figure 2. C-terminus determines the pattern of Ca2+i response
Supplementary Figure 3. CLUSTALW amino acid alignments of putative TM5–6 linker region between TRPC7, 6 and 5 channels.
This material can also be found at:
http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp575/tjp575sm.htm
References
- Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol. 2003;552:789–795. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Albert AP, Large WA. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. J Physiol. 2000;524:853–863. doi: 10.1111/j.1469-7793.2000.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Large WA. Comparison of the effects of divalent cations on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J Physiol. 1999;520:771–782. doi: 10.1111/j.1469-7793.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G. Ca2+-calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium. 2002;32:201–207. doi: 10.1016/s0143416002001550. [DOI] [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995a;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. A non-selective cation current activated via the multifunctional Ca2+-calmodulin-dependent protein kinase in human epithelial cells. J Physiol. 1995b;488:37–55. doi: 10.1113/jphysiol.1995.sp020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;14:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Estacion M, Li S, Sinkins WG, Gosling M, Bahra P, Poll C, Westwick J, Schilling WP. Activation of human TRPC6 channels by receptor stimulation. J Biol Chem. 2004;279:22047–22056. doi: 10.1074/jbc.M402320200. [DOI] [PubMed] [Google Scholar]
- Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239:279–283. doi: 10.1006/bbrc.1997.7458. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Facilitatory effect of Ca2+ on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J Physiol. 1998;512:731–741. doi: 10.1111/j.1469-7793.1998.731bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA, USA: Sunauer Associates, Inc.; 2001. [Google Scholar]
- Hoffmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J General Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R. Effect of external Cd2+ and other divalent cations on carbachol-activated non-selective cation channels in guinea-pig ileum. J Physiol. 1991;442:447–463. doi: 10.1113/jphysiol.1991.sp018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Ito Y. Intracellular ATP slows time-dependent decline of muscarinic cation current in guinea pig ileal smooth muscle. Am J Physiol Cell Physiol. 2000;279:C1307–C1318. doi: 10.1152/ajpcell.2000.279.5.C1307. [DOI] [PubMed] [Google Scholar]
- Inoue R, Morita H, Ito Y. Newly emerging Ca2+ entry channel molecules that regulate the vascular tone. Expert Opin Ther Targets. 2004;8:321–334. doi: 10.1517/14728222.8.4.321. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenocepotor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Ahn SC, So I, Kim KW. Role of calmodulin in the activation of carbachol-activated cationic current in guinea-pig gastric antral myocytes. Pflugers Arch. 1995;430:757–762. doi: 10.1007/BF00386173. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Kang TM, Suh SH, So I, Kim KW. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:346–353. doi: 10.1007/s004240050407. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Sim JH, Jun JY, Kang TM, Suh SH, So I, Kim KW. Protein kinase C mediates the desensitization of CCh-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch. 1998;436:1–8. doi: 10.1007/s004240050597. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- Large WA. Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- Levitan IB. It is calmodulin after all! Mediator of the calcium modulation of multiple ion channels. Neuron. 1999;22:645–648. doi: 10.1016/s0896-6273(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001:1–17. doi: 10.1126/stke.2001.90.re1. http://www.stke.org/cgi/content/full/OC_sigtrans2001/90/re1. [DOI] [PubMed] [Google Scholar]
- Mori Y, Takada N, Okada T, Wakamori M, Imoto K, Wanifuchi H, Oka H, Oba A, Ikenaka K, Kurosaki T. Differential distribution of TRP Ca2+ channel isoforms in mouse brain. Neuroreport. 1998;9:507–515. [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient recepotr potential protein homologue TRP7. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Bolton TB. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: Form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annu Rev Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Shi J, Inoue R, Mori E, Mori Y, Ito Y. Contrasting nature of calcium regulation via calmodulin (CaM) for two murine transient receptor potential protein homologues TRPC6 and TRPC7. J Pharmacol Sci. 2003;91(sup I):95P. [Google Scholar]
- Siemen D. Nonselective cation channels. In: Siemen D, Hescheler J, Birkhaeuser Basel, editors. Nonselective Cation Channels. Pharmacology, Physiology and Biophysics. Boston Berlin: 1993. [Google Scholar]
- Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9:739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of Trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, St Bird G, J, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- Vaca L, Sampieri A. Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. J Biol Chem. 2002;277:42178–42187. doi: 10.1074/jbc.M204531200. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Yamada H, Wakamori M, Hara Y, Takahashi Y, Konishi K, Imoto K, Mori Y. Spontaneous single-channel activity of neuronal TRP5 channel recombinantly expressed in HEK293 cells. Neurosci Lett. 2000;285:111–114. doi: 10.1016/s0304-3940(00)01033-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Tsvilovskyy VV, Bolton TB. Muscarinic cholinergic excitation of smooth muscle: signal transduction and single cationic channel properties. Neurophysiol. 2003;35:311–329. [Google Scholar]
- Zitt C, Obukhov AG, Strübing C, Zobel A, Kalkbrenner F, Lückhoff A, Schultz G. Expression of TRPC3 in chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.