Abstract
Electrical slow waves in gastrointestinal (GI) muscles are generated by interstitial cells of Cajal (ICC), and these events actively propagate through networks of ICC within the walls of GI organs. The mechanism by which spontaneously active pacemaker sites throughout ICC networks are entrained to produce orderly propagation of slow waves is unresolved. A three-chambered partition bath was used to test the effects of agents that affect metabolism, membrane potential and voltage-dependent Ca2+ entry on slow wave propagation in canine antral smooth muscle strips. Slow waves evoked by electrical field stimulation actively propagated from end to end of antral muscle strips with a constant latency between two points of recording. When the central chamber of the bath was perfused with low-temperature solutions, mitochondrial inhibitors, reduced extracellular Ca2+ or blockers of voltage-dependent Ca2+ channels, active propagation failed. Depolarization or hyperpolarization of the tissue within the central chamber also blocked propagation. Blockade of propagation by reduced extracellular Ca2+ and inhibitors of dihydropyridine-resistant Ca2+ channels suggests that voltage-dependent Ca2+ entry may be the ‘entrainment factor’ that facilitates active propagation of slow waves in the gastric antrum.
Electrical slow waves govern the timing of segmental and peristaltic contractions in gastrointestinal (GI) muscles (Szurszewski, 1987), and loss of these events leads to abnormal motility and slowed transit (e.g. Der-Silaphet et al. 1998). Slow waves are generated by interstitial cells of Cajal (ICC), and these events spread actively within ICC networks and conduct passively to neighbouring smooth muscle cells electrically coupled to ICC (Sanders, 1996; Dickens et al. 1999; Horowitz et al. 1999; Cousins et al. 2003). Loss of ICC or damage to the continuity of ICC networks compromises the generation and spread of slow waves (Ward et al. 1994; Huizinga et al. 1995; Torihashi et al. 1995; Ördög et al. 1999). In the stomach slow waves are generated throughout the myenteric ICC network (ICC-MY) from the orad corpus to the pylorus (see Szurszewski, 1987).
Progress has been made in determining the pacemaker mechanism that generates slow wave activity in ICC, but there is still controversy regarding the exact mechanism. There are currently two views suggesting that intracellular Ca2+ handling is responsible for activation of the plasma membrane conductance(s) responsible for the pacemaker current. In both concepts pacemaker events are triggered by release of Ca2+ from IP3 receptor-operated stores, and this idea has been supported by pharmacological studies (Suzuki & Hirst, 1999; Ward et al. 2000; van Helden et al. 2000; Malysz et al. 2001) and a genetic study employing IP3R1 knock-out mice (Suzuki et al. 2000). Some investigators have thought that the local increase in cytoplasmic [Ca2+] due to release from IP3 stores is the primary activator of the pacemaker conductance and suggested that a Ca2+-activated Cl− conductance provides pacemaker current (e.g. Nose et al. 2000; Hirst et al. 2002b). Our observations have suggested that mitochondrial Ca2+ uptake is interposed between IP3 receptor-operated Ca2+ release and pacemaker current activation, and it is the falling phase of Ca2+ transients that activates the pacemaker mechanism (Ward et al. 2000). In support of this idea we found that ICC express a prominent Ca2+-inhibited, non-selective cation conductance that is activated in phase with the pacemaker current (Koh et al. 2002). Involvement of this conductance in the pacemaker current of ICC would be consistent with a model involving mitochondrial Ca2+ uptake, and studies of intact GI muscles also show that inhibitors of mitochondrial Ca2+ uptake block slow waves (Ward et al. 2000; Kito et al. 2002).
Both concepts for the mechanism for slow wave generation suggest that non-voltage-dependent conductances are responsible for pacemaker currents. At the present time it is unclear how the pacemaker activity of one ICC synchronizes the activity of other ICC within a network to produce active slow wave propagation. We have proposed that membrane depolarization caused by the inward pacemaker current activates a voltage-dependent dihydropyridine-resistant Ca2+ conductance, and ICC express such a conductance (Lee & Sanders, 1993; Kim et al. 2002). Influx of Ca2+ may synchronize release of Ca2+ from IP3 receptors (e.g. via Ca2+-induced Ca2+ release) and entrain pacemaker activity in networks of ICC. Thus, while the pacemaker conductance (and pacemaker mechanism) is not voltage dependent, regenerative propagation may depend upon a voltage-dependent mechanism.
In the present study we have investigated the propagation mechanisms in muscles of the canine antrum. We have tested conditions or drugs that would be predicted to interfere with propagation based on the above hypothesis. We have used a three-chambered recording apparatus to study slow wave propagation across a region of muscle exposed to drugs that interfere with active propagation. Our data suggest that the slow wave propagation requires voltage-dependent Ca2+ influx to synchronize the spontaneous activity of ICC.
Methods
Tissue preparation
Mongrel dogs approximately 1 year of age and of both sexes were killed with sodium pentobarbitone (100 mg kg−1) followed by exsanguination. The abdomen was opened and the entire stomach including 5 cm of the oesophagus and duodenum were removed. The gastric antrum, approximately 10.0 cm from the pyloric sphincter, was isolated and opened along the lesser curvature. The mucosa was cleaned of gastric juices with Krebs–Ringer bicarbonate solution (KRB) prior to its removal from the underlying muscle layers by sharp dissection. Sheets of antral muscle from the greater to lesser curvature of one side of the stomach were pinned out onto a Sylgard elastomer (Dow Corning Inc.) lined dissecting dish and immersed in oxygenated KRB. Strips of tissue (1 × 80 mm) were cut parallel to the circular muscle fibres, turned onto their side and additional submucosa removed by sharp dissection. The use and treatment of animals was approved by the Animal Use and Care Committee at the University of Nevada.
Electrophysiological recordings
In this study we used a partitioned recording bath that was separated into three chambers by thin strips of Plexiglass with 5 mm holes drilled at the level of the Sylgard elastomer floor (Fig. 1). Sheets of latex rubber (Armkel LLC) were glued to one side of the Plexiglass partitions, and small holes were made in the latex. Antral muscle strips were pulled through the latex diaphragms and pinned to the Sylgard elastomer floor such that cells could be impaled in any of the chambers. The strips were pinned ‘on side’ such that cells at any point through the muscularis externa could be selectively impaled. The width of the centre chamber was designed to be at least five space constants for canine antral muscle (Bauer & Sanders, 1986). The three chambers could be perfused independently with KRB or test solutions. The latex partitions sealed around the muscle strips, so the solutions perfusing one chamber did not leak into the adjacent chamber(s). This was confirmed by adding Methylene Blue dye to individual chambers. The dye did not leak into the adjacent chambers and stained the muscle strips along a discrete line defined by the latex partition.
Figure 1. Schematic of recording chamber used to study entrainment of slow waves.
A recording chamber was divided by two latex partitions separated by a distance of 12 mm (5 space constants for antral muscles). Strips of muscle were pulled through the latex partitions and secured to the Sylgard elastomer base for intracellular recording. Chambers A and C were continuously perfused with KRB maintained at 37°C. Intracellular microelectrodes were used to impale cells in chambers A and C, 30 mm apart. Slow waves were evoked by two parallel platinum electrodes placed across the end of the muscle in chamber A (filled bars in A). After control recordings were made, test solutions containing agents or conditions to affect slow wave propagation were added to the solution perfusing chamber B. In A and B dual electrical recordings showed that slow waves occurred in chambers A and C at the same frequency and with approximately the same waveform. The site of the dominant slow wave pacemaker varied because the latency between slow waves recorded in chambers A and C changed from cycle to cycle as denoted by the dotted arrows (see summaries of slow waves in E and F). C and D show that slow waves could be driven by electrical field stimulation delivered in chamber A. Evoked events were first recorded at the electrode in chamber A (C) and then at the electrode in chamber C (D). A constant latency was noted in these recordings (dotted arrows). E shows changes in latencies between spontaneous slow waves recorded in chambers A and C in the experiment shown in A and B. Recordings were binned as the time (s) between slow waves (from chamber A to C or chamber C to A). F summarizes the same data from experiments on ten muscle strips.
Slow waves occurred spontaneously and propagated without decrement through all three chambers under control conditions (i.e. with KRB perfusing all chambers; Fig. 1A and B). Tips of the microelectrodes were typically positioned 30 mm apart to impale cells simultaneously in chambers A and C. Recordings from these impalements showed 1:1 coupling of spontaneous slow wave activity. When recording spontaneous activity, the latency between slow waves changed from event to event since the position of the dominant pacemaker site in a strip of muscle shifts with time (Publicover & Sanders, 1984). When events were evoked in chamber A with electrical field stimulation (EFS; single pulses, 1–5 ms, 0.05–0.08 Hz; Fig. 1C and D) propagation between the recordings sites in chambers A and C occurred with a constant latency. In all experiments chambers A and C were continuously perfused with KRB to allow normal generation and propagation of slow waves through these regions of the muscle. After control recordings to demonstrate the continuity of the muscle strips and the ability of slow waves to propagate actively from chamber A to C, the solution perfusing the middle chamber (B) was switched to test the effects of temperature, drugs and different ionic concentrations on the propagation of slow waves. Cells near the myenteric plexus side of the circular muscle layer were selectively impaled because these cells are close to the source of pacemaker activity (Bauer et al. 1985).
Impalements of individual cells were made with glass microelectrodes having resistances of 50–90 MΩ. Transmembrane potentials were recorded with a standard electrometer (Intra 767; World Precision Instruments, Sarasota, FL, USA). Data were recorded on digital tape (Vetter, Robersburg, PA, USA) and hard copies were made by replaying the tapes through a polygraph (Gould RS 3200, Cleveland, OH, USA).
Solutions and drugs
Tissues were constantly perfused with oxygenated KRB of the following composition (mm): NaCl, 118.5; KCl, 4.5; MgCl2, 1.2; NaHCO3, 23.8; KH2PO4, 1.2; dextrose, 11.0; and CaCl2, 2.4; pH 7.4 at 37 ± 0.5°C. The pH of the KRB was 7.4 when bubbled with 17% O2–3% CO2. In some experiments external K+ was increased in the KRB perfusing chamber B by equimolar replacement of Na+ with K+. In other experiments the temperature of the KRB perfusing chamber B was decreased from 37 ± 0.5 to 17.0 ± 0.5°C, and the pH was adjusted to maintain the solution at 7.4. Atropine sulphate, tetrodotoxin, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), antimycin A, nickel chloride and nifedipine were all obtained from Sigma (St Louis, MO, USA). Mibefradil was a gift from Hoffmann-La Roche. Atropine, tetrodotoxin, nickel, mibefradil and manganese were dissolved in distilled H2O. Antimycin A, FCCP, oligiomycin and nifidipine were all dissolved in absolute ethanol. CCCP was dissolved in DMSO. Further dilutions of each drug were performed with the superfusion buffer to the concentration stated in the text. Drugs were typically perfused into the middle chamber or directly for 20 min before recordings were made.
Analysis of intracellular microelectrode data
Data are expressed as means ± s.e.m. Differences in the data were evaluated by Student's unpaired t test. P values less than 0.05 were taken as a statistically significant difference. The ‘n values’ reported in the text refer to the number of tissue strips from which recordings were performed. Each muscle strip that was exposed to the same drug was taken from a different animal. Several slow wave parameters were analysed: (i) resting membrane potential (RMP); (ii) slow wave amplitude; (iii) duration (time from 10% maximum depolarization to 90% repolarization); and (iv) frequency. Conduction velocity (CV) between two electrodes was measured in some experiments. Slow waves were evoked at the end of the muscle strip in these studies, and the distance was measured between points of recording. Conduction velocity was calculated as the distance between recording sites divided by the latency between the times of 10% of maximal slow wave depolarization at each recording site. Figures displayed were made from digitized data using Adobe Photoshop 4.0.1 (Adobe Co., Mountain View, CA, USA) and Corel Draw 7.0 (Corel Corp. Ontario, Canada).
Results
Spontaneous electrical slow waves were recorded from smooth muscle cells along the myenteric border of the circular muscle layer in chambers A and C. Resting membrane potentials recorded from this region in chamber A averaged −71.3 ± 1.0 mV and slow waves possessed an average upstroke amplitude of 40.4 ± 1.5 mV and a plateau amplitude of 25.0 ± 1.8 mV. Slow waves were 6.3 ± 0.4 s in duration and occurred at a frequency 2.8 ± 0.2 cycles min−1 (n = 24). There was no statistical difference in any of these parameters when recordings were made from chamber A or chamber C (i.e. in chamber C: RMP was −72 ± 1.2 mV; upstroke amplitude, 40.4 ± 1.0 mV; plateau amplitude, 24.3 ± 1.7 mV; slow wave duration, 6.1 ± 0.3 mV; and frequency, 2.8 ± 0.1 cycles min−1; n = 24, P > 0.05 for all parameters). Activity was consistent in waveform and frequency to previously published recordings, and the data suggest, as previously documented, that slow wave propagation occurs actively, without significant decrement along strips of antral smooth muscle (Bauer et al. 1985).
Dual impalements of cells in chambers A and C revealed that slow wave activity was coupled in the two chambers. Thus, cells in both chambers were part of a continuous electrical syncytium through which slow waves actively propagated from the point of initiation through the muscle strips. Although there was 1:1 coupling between slow waves, the latencies between events in the two chambers varied dynamically (Fig. 1A, B, E and F). This is evidence, as previously shown, that the site of the dominant pacemaker shifts from cycle to cycle (Publicover & Sanders, 1984). Single pulses of electrical field stimulation (EFS; 1.0–5.0 ms in duration; 0.05–0.08 Hz), delivered via two parallel platinum wires to the end of the muscle strip in chamber A, evoked slow waves that occurred first in this chamber and propagated to chamber C. The latency between paced slow waves recorded in chambers A and C was constant and a function of the distance between the recording electrodes. The conduction velocity of slow waves averaged 21.9 ± 0.9 mm s−1 (n = 50; Fig. 1C and D). Resting membrane potentials and slow wave parameters were not significantly different in cells in chambers A and C for preparations paced with EFS (e.g. in chamber A: RMP was −71.4 ± 0.7 mV; upstroke amplitude, 40.5 ± 1.0 mV; plateau amplitude, 25.1 ± 1.1 mV; and slow wave duration, 5.8 ± 0.2 s. In chamber B: RMP was −71.4 ± 0.7 mV; upstroke amplitude, 41.8 ± 0.65 mV; plateau amplitude, 21.8 ± 1.1 mV; and slow wave duration, 5.3 ± 0.2 s (n= 55 for both chambers; P > 0.05 when comparing slow wave parameters from cells in chambers A and C). When muscles were paced at a frequency above the frequency of intrinsic pacemakers, EFS became the dominant pacemaker and maintained dominance over an extended period.
Propagation of slow waves
In the next series of experiments we altered the solution perfusing chamber B to determine mechanisms contributing to the propagation of slow waves from chamber A to C. In the first series of experiments we added atropine to examine the role of muscaranic receptors in the propagation of slow waves from chamber A to C. Atropine (1 μm; n = 8; not shown), added to the middle chamber, had no effect on the coupling of slow waves in chambers A and C or on propagation of slow waves evoked by EFS. To further evaluate the role of action potential-dependent neural activity on coupling of slow waves between the two chambers we added tetrodotoxin (TTX; 1 μm) to the middle chamber. TTX also did not have any effect on the 1:1 coupling of spontaneous slow waves or the propagation of slow waves evoked by EFS (n = 9; not shown). These data suggest that neural mechanisms are not involved in the active propagation of slow waves.
The role of metabolic processes in the propagation of slow waves was also investigated. First, the temperature of the solution perfusing chamber B was decreased gradually from 37°C to 20°C. Reduction of the temperature in chamber B by 10°C decreased the propagation rate of slow waves from chamber A to C by 63% (i.e. from 19.0 ± 2.5 mm s−1 at 37°C to 7.0 ± 1.2 mm s−1 at 27°C; n = 6; Fig. 2A). When the temperature in chamber B was reduced to 24°C, propagation from chamber A to C often failed. At temperatures below 21°C, slow waves never propagated from chamber A to C. Increasing the temperature of chamber B produced a return in the propagation of slow waves between chambers A and C (Fig. 2B). However, we noted that the temperature in chamber B needed to increase above 28°C for several seconds before propagation was restored. A plot of the average reduction in conduction velocity (CV) as a function of temperature resulted in a decay in CV that could be fitted with a liner regression of − 1.1 ± 0.05 mm s−1 reduction in CV per degree centigrade (r2= 0.9860; P < 0.0001; Fig. 2C). A problem with this calculation is that only the tissue in chamber B was exposed to reduced temperature (i.e. 12 mm or 40% of the total distance between imapalements in chambers A and C). Sixty per cent (or 18 mm) of the propagation pathway was located in chambers A and C and the temperature of solution perfusing these regions was maintained at 37°C. Thus, calculation of the CV over the entire length of the muscle strip, with only a portion of the strip exposed to reduced temperature, underestimates the effect of temperature on the rate of propagation. It is possible to correct this relationship by calculating the drop in CV along the 12 mm of muscle exposed to reduced temperatures using the equation:
Figure 2. Effects of temperature on slow wave propagation.
A shows the effects of reducing temperature of the KRB perfusing chamber B from 37 to 24°C on slow wave latency and propagation. Slow waves were evoked by EFS in chamber A. Temperature reduction increased the latency between slow waves recorded in chambers A and C, suggesting that there was a reduction in conduction velocity (CV) across the area of reduced temperature. In this experiment 24°C blocked slow wave propagation. Reheating the KRB in chamber B to 37°C caused recovery of slow wave propagation between chambers A and C (B). After beginning the warming of KRB in chamber B it took several slow wave cycles to restore propagation (note failure of slow wave propagation at 27°C after rewarming was initiated). The temperature that was required to produce a return in the propagation was often higher than that at which propagation failure occurred and had to be maintained for several seconds (dotted arrows between upper and lower traces). C shows a plot of the conduction velocity between chambers A and C as a function of temperature in chamber B (•). Linear regression analysis revealed a −1.1 ± 0.05 mm s−1 reduction in conduction velocity per degree centigrade (r2= 0.9860; P < 0.0001). These data were corrected for the length of muscle that was exposed to low temperature in chamber B (see text for details; ○). The latter data were fitted with an exponential function with Q10= 5.9 calculated between 27°C and 37°C. D shows the direct actions of temperature on slow waves in chamber B. There was a marked reduction in the rate of rise of the upstroke component, a reduction in slow wave frequency and an increase in the slow wave duration.
where t12(T) = 30/VTotal− 18/19 s at temperature T, V12(T) = 12/t12(T) and VTotal is the total velocity measured over the distance between the two microelectrodes. VTotal at 37°C had an average velocity of 19 mm s−1. The distance between the two recording electrodes was 30 mm. The distance between the latex partitions in the middle chamber (B) was 12 mm. It is assumed that the conduction velocity of slow waves remained constant over the length of tissue between the electrodes (18 mm) that was maintained at 37°C.
In this model CV dropped from 19 mm s−1 at 37°C to 3.6 mm s−1 at 27°C. A plot of the corrected effects of temperature on CV reveals an exponential decay in the CV as a function of temperature. When fitted with an exponential function, a Q10 of 5.9 was calculated between 27°C and 37°C (r2= 0.9976;Fig. 2C).
The direct effects of temperature on slow waves were also studied by reducing the temperature in chamber B and recording from cells in the same chamber. Reduction of the temperature in increments from 37°C to 24°C did not affect membrane potential significantly (from −71.0 ± 2.0 mV at 37°C to −75 ± 1.3 mV at 24°C (n = 9; P > 0.05 at all temperatures examined). The amplitude of slow waves was also not affected (41.5 ± 2.0 mV at 37°C versus 46.0 ± 1.2 mV at 24°C); however, the rate of rise of the upstroke component of slow waves (dV/dt) decreased from 720 ± 30 mV s−1 at 37°C to 522 ± 48 mV s−1 at 24°C (P < 0.01). There was also a significant decrease in slow wave frequency from 2.0 ± 0.24 to 0.96 ± 0.18 cycles min−1 (P < 0.05). Associated with a decrease in temperature was an increase in slow wave duration from 6.5 ± 0.3 s at 37°C to 18.2 ± 1.6 s at 24°C (P < 0.001;Fig. 2D).
Effect of mitochondrial inhibitors on slow wave propagation
We have previously reported that the mitochondrial uncouplers cyanide P-(trifluoromethoxy) phenylhydrazone (FCCP) and carbonyl cyanide m-chlorophenyl hydrazone (CCCP), or respiratory chain inhibitors such as antimycin A, block the propagation of slow waves in the canine colon (Ward et al. 2003). Here we performed studies to determine whether common mechanisms are involved in the propagation of slow waves in gastric antral muscles. These agents were added to chamber B while recording simultaneously from chambers A and C. FCCP (1 μm; n = 5) and CCCP (1 μm; n = 8) blocked the 1:1 coupling of spontaneous slow waves and propagation of slow waves evoked by EFS (Fig. 3). In one of the eight muscles in which CCCP was tested, propagation persisted in 1 μm CCCP but was blocked by increasing the concentration of CCCP to 10 μm. Antimycin A also blocked the coupling and propagation of slow waves in five tissue samples (Fig. 4). These data suggest that propagation of slow waves (like generation of slow waves, see Ward et al. 2000) is dependent upon Ca2+ handling by mitochondria.
Figure 3. Effects of a mitochondrial uncoupler on the entrainment and propagation of slow waves.
A shows the 1:1 coupling of evoked slow waves in chambers A and C (dotted arrows). B shows block of propagation between chambers A and C when CCCP (1 μm) was added to the KRB in chamber B. Note recovery of spontaneous activity in chamber C (single slow wave) when propagation was blocked. C shows recovery of 1:1 coupling between slow waves in chambers A and C after the washout of CCCP (dotted arrows).
Figure 4. Effect of a mitochondrial respiratory chain inhibitor on propagation of slow waves.
A shows 1:1 coupling of evoked slow waves in chambers A and C during control recordings (dotted arrows between upper and lower traces). B shows block of propagation of every other slow wave, which was often noted before total block of slow wave propagation, after the addition of antimycin A (10 μm). Dotted arrows between upper and lower traces in both panels denote EFS.
Effect of membrane potential on slow wave propagation
We have proposed that voltage-dependent Ca2+ entry is required for slow wave propagation (Kim et al. 2002). Thus we evaluated the effects of both depolarization and hyperpolarization on propagation of slow waves from chamber A to C. To depolarize the region of muscle in chamber B, the solution perfusing this chamber was switched to a solution containing elevated external K+ ([K+]o = 36 mm). This [K+]o has been shown to depolarize canine antral muscles by at least 40 mV (Bauer & Sanders, 1985) and it completely uncoupled spontaneous slow waves and the propagation of slow waves evoked by EFS (n = 4). To examine the graded effects of depolarization more carefully, we studied the effects of 9, 12, 18 and 24 mm[K+]o. An increase of [K+]o to 9 mm (8–10 mV depolarization of cells in chamber B) blocked coupling of spontaneous slow waves in four of six muscles. The coupling was blocked in the remaining two muscles by 12 mm[K+]o (Fig. 5A–D). This would have caused a 12–15 mV depolarization of cells in chamber B (Bauer & Sanders, 1985). Although uncoupling of spontaneous slow waves was noted with these modest depolarizations, slow waves evoked by EFS remained capable of propagation in 9 and 12 mm [K+]o in one muscle (Fig. 5E). When [K+]o was raised to 18 mm, however, the propagation of slow waves was blocked in all tissues (n = 6). Elevation of [K+]o to 18 mm produces a depolarization of approximately 20 mV (Bauer & Sanders, 1985). Restoration of [K+]o in chamber B to the normal concentration in KRB (i.e. 5.9 mm) resulted in recovery of coupling of spontaneous slow waves and propagation of slow waves evoked by EFS.
Figure 5. Membrane depolarization blocked slow wave propagation.
A shows 1:1 coupling of spontaneous slow waves in chambers A and C under control conditions ([K+]o= 5.9 mm). Elevation in [K+]o to 9 mm blocked the 1:1 coupling of slow waves in chambers A and C (B). The block in propagation was also observed when [K+]o was raised to 12 mm (C). D shows that restoration of normal [K+]o led to recovery of 1:1 coupling in chambers A and C. In E the left set of traces shows 1:1 coupling of evoked slow waves in chambers A and C (dotted arrows). Blockade of slow wave propagation from chamber A to C occurred when [K+]o in chamber B was raised to 12 mm (middle set of traces). Slow wave propagation was restored when [K+]o was returned to 5.9 mm (right set of traces).
Hyperpolarization was achieved by treating the muscle in chamber B with KATP channel openers (i.e. lemakalim, 1–10 μm, and panicidil, 1–10 μm). Experiments were first performed to test the effects of these compounds on membrane potential to determine the magnitude of hyperpolarization that could be achieved with these concentrations. Under control conditions resting potentials of antral circular muscle cells in these experiments averaged −70.2 ± 1.3 mV and slow waves 36.0 ± 1.6 mV amplitude, 4.0 ± 1.4 s duration, with plateau potentials 26.8 ± 2.4 mV in amplitude occured at a frequency of 1.8 ± 0.4 cycles min−1 (n = 5). Lemakalim (1 μm) caused hyperpolarization to −76.0 ± 2.1 mV. In three of the five muscles, spontaneous slow wave activity ceased (Fig. 6A). In the other two muscles slow wave and plateau amplitudes were reduced to 4.0 ± 2.5 and 2.4 ± 1.5 mV, respectively; slow wave frequency was also reduced to 0.4 ± 0.25 cycles min−1 and to a duration of 1.4 ± 0.87 s. Washout of lemakalim restored resting membrane potential and slow wave activity. In the presence of lemakalim, EFS (single pulses, 2 ms in duration at supramaximal voltage delivered at a frequency of 0.05 Hz) evoked slow waves, but the amplitude and duration of slow waves was reduced in comparison to events evoked under control conditions (i.e. 27.5 ± 2.5 mV and 5.5 ± 0.5 s in control conditions versus 9.0 ± 1.0 mV and 3.0 ± 0.0 s in the presence of lemakalim; P < 0.05 for both parameters) and were reversible upon washout of the drug (Fig. 6B).
Figure 6. Membrane hyperpolarization blocked entrainment and propagation of slow waves.
A shows the direct effects of lemakalim (1 μm) on slow wave activity and membrane potential in chamber B. Lemakalim induced membrane hyperpolarization (dotted lines between excerpts of continuous records) and either blocked slow wave activity (as in the experiment depicted by the upper trace) or reduced the amplitude and duration of slow waves (as in a separate experiment depicted by the lower trace). In the presence of lemakalim, EFS-evoked slow waves were of reduced amplitude (B). C and D show 1:1 coupling of spontaneous and evoked slow waves, respectively, in chambers A and C. When lemakalim (1 μm) was added to chamber B, propagation of slow waves was blocked (E and F). Note that after active propagation was blocked, the intrinsic frequency of pacemakers in chamber C paced this region at a slower rate than the events evoked in chamber A. Time scale applies to upper and lower panels.
In the partition chambered bath, addition of lemakalim (1 μm) or pinacidil (1 μm, which has similar, reversible effects on membrane potential to lemakalim, not shown) to chamber B caused failure of slow wave propagation between chambers A and C in all preparations (n = 5 each; Fig. 6C–F).
Effects of reducing external calcium and calcium channel blockers on slow wave propagation
We further tested the idea that voltage-dependent Ca2+ entry is required for active propagation by changing the concentration of external Ca+ ([Ca2+]o) in chamber B and by evaluating the effects of Ca2+ channel blocking agents, such as nickel, mibefradil and nifedipine. The direct effects of reduced [Ca2+]o (from 2.5 to 0.25 mm; n = 4) on antral slow waves were tested first. Reduction of [Ca2+]o from 2.5 to 1.0 mm did not affect membrane potential but reduced slow wave amplitude, duration, dV/dt of the upstroke component, and frequency (Fig. 7A–C). Further reduction in [Ca2+]o to 0.5 mm did not alter membrane potential (i.e. −71.8 mV ± 2.1 compared to −71.3 ± 4.8 mV in 2.5 mm [Ca2+]o) but abolished slow waves in two of four preparations (Fig. 7D). Slow waves in the other two preparations were significantly attenuated. Reduction of [Ca2+]o to 0.25 mm caused a small, but insignificant, depolarization in membrane potential to −69.0 ± 3.7 mV (P > 0.05) and abolished all spontaneous slow wave activity (Fig. 7E). Plots of the change in dV/dt of the slow wave upstroke as a function of [Ca2+]o showed that decreasing [Ca2+]o from 2.5 to 0.5 mm reduced the rate of rise by approximately 60% (from 639 ± 107 to 268 ± 74 mV s−1; P < 0.01; Fig. 7F). Although spontaneous activity was inhibited when [Ca2+]o was reduced, it was possible to evoke slow waves with EFS (inset in Fig. 7F). For example, reducing [Ca2+]o to 0.5 mm did not alter resting membrane potential, slow wave amplitude or the duration of evoked slow waves. However, the maximum rate of rise of the upstroke component was reduced from 700 ± 50 to 170 ± 80 mV s−1 (P < 0.05). Reduction of [Ca2+]o to 0.25 mm further reduced the maximum rate of rise of the upstroke depolarizations to 500 ± 10 mV s−1 (P < 0.05; n = 5).
Figure 7. Direct effects of reduced [Ca2+]o on slow waves.
Reduction of [Ca2+]o from 2.5 to 0.25 mm in chamber B caused reduction in upstroke velocity of spontaneous slow waves. A–E show representative slow waves at each concentration and the upstroke is shown at an increased sweep speed to the right of each slow wave. Note the drop in upstroke velocity as [Ca2+]o was reduced. F shows a plot of dV/dt of the slow wave upstroke as a function of [Ca2+]o. The [Ca2+]o that blocked slow wave propagation when added to chamber B (i.e. see Fig. 8) was associated with at least 50% reduction in dV/dt. In low-Ca2+ solution (0.5 mm) slow waves could be evoked locally by EFS (arrowheads indicate EFS in inset in F).
In the partition chamber apparatus, reduction of [Ca2+]o to 0.5 or 0.25 mm in the central chamber blocked coupling of spontaneous slow waves and propagation of slow waves evoked by EFS (Fig. 8; n = 7).
Figure 8. Effects of reduced [Ca2+]o on slow wave propagation.
A shows propagation of spontaneous and EFS-evoked slow waves (dotted arrows), respectively, between chambers A and C, under control conditions. After recording control activity, the solution in chamber B was switched to KRB with 0.5 mm[Ca2+]o. B, at 0.5 mm Ca2+ some events evoked by EFS still propagated from chamber A to C, but there were increased latencies between events recorded in the two chambers, indicating a reduction in conduction velocity. Reduced [Ca2+]o eventually blocked propagation of spontaneous and EFS-evoked slow waves (dotted lines in C, E and F, respectively). Blockade of spontaneous and EFS-evoked slow wave propagation was also observed at 0.25 mm [Ca2+]o.
The Ca2+ channel antagonist Ni2+ is known to inhibit slow waves and reduce the upstroke velocity of slow waves in a variety of GI muscles (Ward & Sanders, 1992; Kito et al. 2002; Kito & Suzuki, 2003). At low concentrations (50–200 μm), Ni2+ dramatically reduced the rate of rise of the slow wave upstroke and caused complete inhibition of slow waves at higher concentrations (e.g. 1 mm). We first tested the direct effects of Ni2+ on spontaneous slow waves and slow waves evoked by EFS. Nickel ions (50 μm) did not affect resting membrane potential, slow wave amplitude or duration (−76 ± 2.8 mV, 47 ± 1.0 mV and 7.0 ± 0.4 s under control conditions and −78 ± 1.9 mV, 46 ± 1.0 mV and 6.5 ± 0.6 s after 50 μm Ni2+, respectively). Nickel ions (50 μm) caused a reduction in the rate of rise of the upstroke from 710 to 400 mV s−1 (P < 0.05). Increasing the concentration of Ni2+ to 100 μm reduced the amplitude of the slow waves to 39.8 ± 3.5 mV and caused a further reduction in the maximum rate of rise of the upstroke component to 215 mV s−1 (Fig. 9A and B; P < 0.01; n= 4).
Figure 9. Effects of nickel on slow waves.
Slow waves could be evoked locally in the presence of Ni2+ (50 and 100 μm; latter shown; A and B). However, propagation from chamber A to chamber C (C shows propagation under control conditions; EFS at dotted arrows) was blocked by adding Ni2+ (50 μm) to the central chamber (D; EFS at dotted arrows). The intrinsic pacemaker frequency in chamber C paced spontaneous slow waves when propagation was blocked with Ni2+ in chamber B.
The effects of Ni2+ on the propagation of slow waves were examined by adding Ni2+ to the solution perfusing chamber B in the partitioned bath. Nickel ions at concentrations between 50 and 200 μm blocked the propagation of slow waves between chambers A and C when added to the central chamber of the partition bath (n = 8). These effects were time dependent, and the first indication of failure was the tendency for failure of every other evoked slow wave to propagate. In four of the eight tissues coupling of spontaneous slow waves and propagation of evoked slow waves was inhibited at 50 μm; in three of the remaining muscles slow wave propagation was blocked by increasing the concentration to 100 μm. One of the eight muscles required 200 μm Ni2+ to block propagation of slow waves from chamber A to chamber C (Fig. 9C and D).
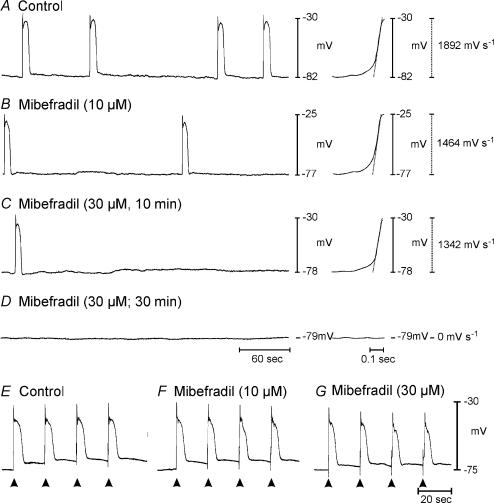
We have previously shown that ICC express both dihydropyridine-sensitive and dihydropyridine-resistant voltage-dependent Ca2+ conductances (Kim et al. 2002). These conductances are blocked by nifedipine and mibefradil, respectively; however, neither compound blocks the pacemaker conductance (Koh et al. 1998; Kim et al. 2002). Either of these Ca2+ conductances could contribute to the voltage-dependent Ca2+ entry that appears to be obligatory for slow wave propagation, but it is well known from a variety of studies that slow waves persist in dihydropyridines (e.g. see Ozaki et al. 1991). We examined the direct effects of nifedipine and mibefradil on the upstroke velocities of slow waves. Nifedipine (1 μm) caused a 20% reduction in the rate of rise of the upstroke component (i.e. from 1235 ± 164 to 991 ± 76 mV s−1; n = 4) and did not block slow wave activity. Mibefradil (10 μm) reduced the rate of rise of spontaneous slow waves by 42% (i.e. from 1205 ± 192 to 701 ± 299 mV s−1 and inhibited spontaneous activity in one of the four muscle strips (Fig. 10A and B). Mibefradil did not cause a significant change in resting potential (control, −75 ± 4.0 mV; 10 and 30 μm mibefradil, −72 ± 3.0 and −71 ± 3.0 mV, respectively; n = 4). Increasing the concentration of mibefradil to 30 μm further reduced the rate of rise of spontaneous slow waves to 228.8 mV s−1 (n = 4) and blocked spontaneous activity in two of the four muscles (Fig. 10C and D). Mibefradil also decreased the frequency of spontaneous slow waves from 0.83 ± 0.18 cycles min−1 in control conditions to 0.52 ± 0.22 and 0.44 ± 0.34 cycles min−1 with 10 and 30 μm mibefradil, respectively.
Figure 10. Direct effects of mibefradil on slow waves.
Mibefradil caused a decrease in dV/dt of the upstroke phase and frequency of slow waves. A shows a control recording of slow wave activity and the upstroke of one slow wave is shown at an increased sweep speed to the right of the recording. B and C show the effects of mibefradil at 10 and 30 μm, respectively, on slow wave frequency and dV/dt of the upstroke of an individual slow wave (right of each trace). Spontaneous slow waves were abolished by 30 μm mibefradil in this preparation (D). Slow waves could be evoked by EFS (arrowheads) in the presence of 10 and 30 μm mibefradil (E–G).
In the presence of 10 and 30 μm mibefradil, slow waves were evoked by EFS (Fig. 10E–G) in all preparations. Mibefradil (10 μm) had no effect on evoked slow wave amplitude or duration but decreased the maximum rate of rise of the upstroke phase of spontaneous slow waves from 440 ± 100 to 260 ± 50 mV s −1 (P < 0.05). Increasing the concentration of mibefradil to 30 μm reduced the amplitude of evoked slow waves from 37.5 ± 2.7 mV under control conditions to 28.2 ± 2.4 mV after mibefradil and further reduced the maximum rate of rise to 120 ± 30 mV s−1 (P < 0.05; n= 4).
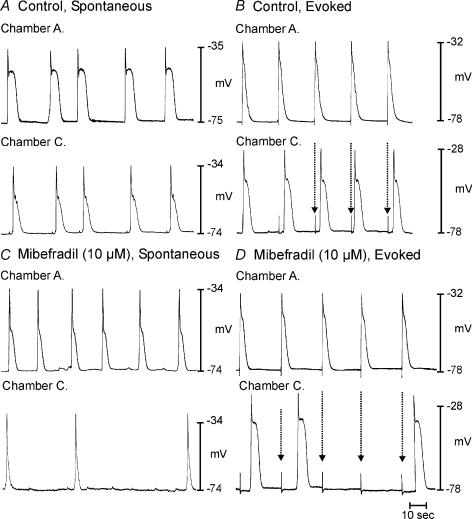
The role of Ca2+ conductances in slow wave propagation was also studied using the partitioned bath. Nifedipine (1 μm) did not affect coupling of spontaneous slow waves in chambers A and C or propagation of slow waves (n = 10; not shown). Addition of mibefradil (10 μm) to chamber B inhibited coupling of spontaneous slow waves in chambers A and C and propagation of slow waves (Fig. 11; n = 5).
Figure 11. Mibefradil blocks propagation of slow waves.
A and B show propagation of spontaneous and evoked slow waves (dotted arrows), respectively, from chamber A to C. Addition of mibefradil (10 μm) to chamber B blocked propagation of spontaneous and evoked slow waves (dotted arrows in D).
Discussion
Recent studies have shown that ICC in the myenteric region (ICC-MY) between the circular and longitudinal muscle layers are the pacemakers of gastric slow wave activity (Ördög et al. 1999; Dickens et al. 1999). Interstitial cells of Cajal generate and propagate slow waves, and pathophysiological conditions, where ICC networks are disrupted or lost, result in either incomplete spread of slow waves or total loss of slow wave activity (see Ördög et al. 1999, 2002). Our experiments have suggested that generation of pacemaker activity by ICC involves a mechanism that includes intracellular Ca2+ handling by IP3 receptor-operated stores and mitochondria (Ward et al. 2000). Mitochondrial uptake of Ca2+ regulates the open probability of a non-selective cation conductance in the plasma membrane (Ward et al. 2000; Koh et al. 2002). Slow waves actively propagate through ICC networks in GI smooth muscle tissues, but when the above mechanism was blocked in canine colonic muscles, slow waves were unable to regenerate and decayed in amplitude as a function of distance (Ward et al. 2003). Data from the present study also suggest that metabolic activity, most likely through mitochondrial Ca2+ handling, is also involved in the active propagation of gastric slow waves, because slow waves failed to propagate across a region of muscle treated with mitochondrial uncouplers or respiratory chain inhibitors, or cooled below 24°C. Our data also suggest that voltage-dependent Ca2+ entry is critical for cell to cell propagation. This conclusion is supported by the following: (i) either depolarization (which would tend to inactivate Ca2+ channels in ICC; see Kim et al. 2002) or hyperpolarization (which would tend to deactivate and stabilize the open probability of Ca2+ channels in ICC; see Kim et al. 2002) blocked propagation of slow waves; (ii) reduced extracellular Ca2+ blocked propagation; and (iii) Ca2+ channel blocking agents (Ni2+ and mibefradil) blocked propagation. These data are consistent with a mechanism in which depolarization of ICC from spontaneous activation of the ‘pacemaker’ current increases the open probability of voltage-dependent Ca2+ channels. Ca2+ entry causes a localized increase in Ca2+ near Ca2+ stores and enhances the open probability of IP3 receptors. This reinitiates the pacemaker mechanism and promotes active propagation of slow waves by coordinating IP3 receptor-operated Ca2+ release from cell to cell through the ICC networks.
Our concept of slow wave generation and propagation suggests that different conductances are responsible for the pacemaker current and the voltage-dependent current required for active propagation (Ward et al. 2000; Koh et al. 2002; Kim et al. 2002). Most studies of the slow wave mechanism in the literature have not been designed to distinguish between the conductance that generates pacemaker activity and the mechanism responsible for coordinating the firing of multitudes of spontaneously active ICC in networks. This is because these experiments have tested pharmacological agents in ‘open baths’, where inhibition of slow waves might result from blockade of either the generation or the propagation mechanism. Here we used a triple chambered apparatus where slow waves could be evoked reliably in one area of the muscle and propagation occurred across a region of muscle (of at least 5 space constants in breadth) that could be independently exposed to test substances. We showed that none of the test substances blocked local generation of slow waves evoked by EFS, but substances that would be expected to block voltage-dependent Ca2+ entry reduced the safety factor for regenerative spread of slow waves and blocked propagation from chambers A to C. Canine antral muscles were chosen for these studies because: (i) stomachs of these animals are of sufficient size to make the long strips necessary to extend through the three chambers; (ii) antral slow waves are generated spontaneously at 1–3 cycles min−1, allowing time for events to propagate through the entire muscle strip before another event is generated; (iii) it is easy to evoke slow waves with an external current source and drive activity above the intrinsic frequency from a fixed site of extrinsic pacing; and (iv) robust slow waves with fast upstroke potentials can be recorded from antral muscles, making it easy to precisely determine conduction velocities and when propagation was disrupted.
A potential drawback of studying slow wave propagation in strips of muscle containing both ICC and smooth muscle cells is that some of the treatments we used to affect pacemaker activity may also affect the input resistance of smooth muscle cells. Thus, along with changes in the availability of voltage-dependent Ca2+ channels in ICC, there may have been effects on propagation due to changes in the electrical load on the ICC network. For example, it is possible that the effects of depolarization (with high K+) and hyperpolarization (with KATP channel openers) on blocking propagation could have been over estimated. It should be noted that slow waves could be evoked in high K+ (Bauer & Sanders, 1985) or in the presence of KATP openers (this study), suggesting that the block of propagation by these manipulations was not due to an inability of the pacemaker apparatus to generate slow waves. It is unlikely that changes in input resistance of smooth muscle cells affected our interpretations of the effects of reduced external Ca2+ and block of voltage-dependent Ca2+ channels (with Ni2+ and mibefradil), since these agents did not significantly affect membrane potential. Ideally, it would be better to perform the kinds of experiments we have described on isolated networks of ICC, but this is not technically possible at present.
The precise pathway for active propagation in intact strips of smooth muscle, which contain several species of electrically coupled cells, is potentially complicated. Many studies have shown that smooth muscle cells lack the ionic apparatus necessary to generate or regenerate slow waves (e.g. Horowitz et al. 1999), so active propagation of slow waves is unlikely to occur in these cells. Both ICC-MY and intramuscular ICC (ICC-IM) (see Hirst & Edwards, 2001, Hirst et al. 2002a) possess intrinsic pacemaker capability. Thus, it is possible that either cell type could support active propagation. It appears, however, that the ICC-IM pathway is not obligatory, since slow wave activity persists in the stomachs of W/Wv mice that lack ICC-IM (Hirst et al. 2002b; Beckett et al. 2003) and that small isolated bundles of canine gastric antrum that contain ICC-IM do not produce slow wave activity (unpublished observation).
Strips of canine antral muscles are spontaneously active and generate slow waves with an intrinsic frequency of about 1–3 cycles min−1 (e.g. El-Sharkawy et al. 1978; Horiguchi et al. 2001). Equivalent activity has been recorded in vivo when the antrum is separated from the dominant pacemaker region in the orad corpus (Kelly & Code, 1971). In isolated antral muscles the site of initiation of spontaneous pacemaker activity is dynamic, and we have shown previously that the location of the primary pacemaker shifts from cycle to cycle (Publicover & Sanders, 1984). This makes it difficult to determine the site of slow wave origination or the conduction velocities of spontaneous slow waves from two recording sites because the latencies between slow waves arriving at the two recording sites constantly change (see Fig. 1). Therefore, we paced the muscles from a defined point in chamber A (which was always perfused with KRB) at 0.05–0.08 Hz, which is above the intrinsic frequency, to fix the site of the dominant pacemaker. Under these conditions constant latencies were recorded between sites of impalements in chambers A and C.
As shown in a variety of GI muscles (e.g. Ward et al. 2000; Kito et al. 2002), inhibitors of mitochondrial Ca2+ uptake blocked slow wave regeneration in the central chamber and thus blocked propagation from chamber A to chamber C. Lowering temperature in the central chamber (B) slowed propagation velocity and inhibited slow wave propagation below 24°C. These data further suggest that a metabolic mechanism is involved in slow wave propagation, but it is also possible that extension of the refractory period of slow waves could have contributed to the failure of propagation when the temperature was reduced in the central chamber, since slow waves were enhanced in duration by low temperature. It is also possible that the availability of voltage-dependent Ca2+ channels may have been reduced by lowering the temperature in chamber B, since it is known that voltage-dependent Ca2+ conductances are affected by temperature (Klockner et al. 1990).
Previously, we have suggested that Ca2+ entry into ICC via a voltage-dependent Ca2+ conductance may be the ‘entrainment factor’ essential for slow wave propagation, and data obtained in this study support this hypothesis. ICC express a smooth muscle-like, dihydropyridine-sensitive Ca2+ conductance (Kim et al. 2002), but it is well known that dihydropyridines do not block slow waves in most GI muscles (e.g. Ozaki et al. 1991). Thus, a conductance other than one encoded by CaV1.2 channels is likely, and we have characterized a dihydropyridine-resistant Ca2+-selective current in freshly dispersed and cultured ICC (Lee & Sanders, 1993; Kim et al. 2002). This current is blocked by Ni2+ and mibefradil. We have also shown that mibefradil does not block the non-selective cation conductance responsible for the pacemaker current (Kim et al. 2002), so this compound is an important tool in distinguishing the role of voltage-dependent Ca2+ channels in slow wave propagation. In the present study we found that lowering the driving force for Ca2+ influx and the Ca2+ channel blocking agents, Ni2+ and mibefradil, were effective in blocking slow wave propagation. It should be noted that while nifedipine failed to block slow wave propagation, it reduced dV/dt of the slow wave upstroke by about 20%. This is an indication that the depolarization induced by pacemaker activity in ICC activates the dihdropyridine-senstive channels that are present in ICC (Lee & Sanders, 1993; Kim et al. 2002). The dihydropyridine-sensitive channels in ICC contribute to the safety factor for propagation, but blocking these channels does not reduce Ca2+ entry sufficiently to block propagation.
Our data show a bell-shaped dependence of propagation on membrane potential. Either hyperpolarization (with KATP agonists) or depolarization (with elevated external K+) blocked propagation. This property of slow wave propagation is consistent with the voltage-dependent properties of Ca2+ channels: hyperpolarization tends to deactivate and stabilize the closed state of channels, and depolarization causes steady-state inactivation and reduces channel availability. These data suggest that Ca2+ entry could be the key factor that entrains pacemaker activity in ICC networks and facilitates regenerative slow wave propagation.
How Ca2+ entry entrains pacemaker activity is an important question. The pacemaker conductance we have identified in ICC is a voltage-independent conductance that is inhibited by Ca2+ (Koh et al. 2002). Thus, neither the charge associated with Ca2+ entry nor changes in submembrane [Ca2+] are likely to activate the pacemaker conductance directly. Release of Ca2+ from IP3 receptor-operated (IP3R1) stores is critical for the initiation of slow waves (Ward et al. 2000; Suzuki et al. 2000; Malysz et al. 2001). The open probabilities of IP3R1 receptors are regulated by IP3 and by Ca2+ (Iino, 1990; Bezprozvanny et al. 1991; Mak et al. 1998). Thus, we suggest that the open probability of IP3 receptors may be enhanced by small increases in Ca2+ concentration that result from Ca2+ entry via a voltage-dependent Ca2+ conductance, producing the phenomenon of ‘Ca2+-induced Ca2+ release’. Our concept is that all ICC in the network have intrinsic activity, and the primary pacemaker for each cycle is the first ICC to generate enough inward current to entrain the activity of its neighbours. The amount of Ca2+ current necessary to do this is determined by the relative excitability and degree of depolarization of surrounding ICC caused by the initial pacemaker current. Depolarization causes activation of voltage-dependent Ca2+ channels, Ca2+ entry, a rise in submembrane Ca2+ concentration, and phase-advancement of IP3-receptor-operated Ca2+ release. The latter regenerates the pacemaker mechanism in ICC surrounding the primary pacemaker and leads to active propagation of slow waves.
When microelectrode recordings are made from syncytia of ICC and smooth muscle cells, propagated activity is almost always recorded because the likelihood of recording from the cell that generates a slow wave is extremely low. Propagated events are indicated by the exponential ‘foot’ depolarization preceding the upstroke potential. Slow waves recorded from all areas of the GI tract are generally composed of two distinct components (a rapid upstroke depolarization and a sustained ‘plateau’ component; see Szurszewski, 1987). Data from the present study suggest that the first component of slow waves, the upstroke, is due to the Ca2+ current that entrains the spontaneous activity of a multitude of pacemakers within an ICC network. The plateau phase results from a summation current from activation of the pacemaker conductance in many cells and activation of dihydropyridine-sensitive Ca2+ channels in both ICC and smooth muscle cells. Similar conclusions have been reached about the nature of the upstroke component of the pacemaker potentials recorded from ICC in the small intestine (Kito & Suzuki, 2003).
Many GI motility disorders are associated with loss of ICC or defects in ICC networks (for reviews see Sanders et al. 1999; Vanderwinden & Rumessen, 1999). Few of these studies show complete loss of ICC. Thus, the question of slow wave propagation is important because it is possible that ICC remaining in tissues may function normally but be incapable of actively propagating slow waves through the muscularis. Thus, the real defect in motor pathologies associated with loss of ICC may be propagation disorders. Without active propagation through ICC networks the slow waves would be impotent as a means of organizing phasic contractions in GI muscles, and important physiological behaviours, such as segmentation and gastric peristalsis, would be compromised. Reduced expression or defects in the voltage-dependent Ca2+ conductance required for propagation could produce motor defects in the absence of ICC loss.
Acknowledgments
This project was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH DK57236 to S.M.W. and NIH DK40569 to K.M.S. The authors are grateful to Gareth Semple for excellent technical assistance. We are also grateful to Dr Terence K. Smith for advice with conduction velocity measurements.
References
- Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Physiol. 1985;249:G800–G806. doi: 10.1152/ajpgi.1985.249.6.G800. [DOI] [PubMed] [Google Scholar]
- Bauer AJ, Sanders KM. Gradient in excitation–contraction coupling in canine gastric antral circular muscle. J Physiol. 1985;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Sanders KM. Passive and active membrane properties of canine gastric antral circular muscles. Am J Physiol. 1986;251:C268–C273. doi: 10.1152/ajpcell.1986.251.2.C268. [DOI] [PubMed] [Google Scholar]
- Beckett EA, McGeough CA, Sanders KM, Ward SM. Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J Physiol. 2003;553:545–559. doi: 10.1113/jphysiol.2003.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5),P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–736. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy TY, Morgan KG, Szurszewski JH. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Beckett EA, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002a;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002b;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Semple GSA, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537:237–250. doi: 10.1111/j.1469-7793.2001.0237k.x. 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz BM, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Ann Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J General Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971;220:112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002;445:202–217. doi: 10.1007/s00424-002-0884-z. 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockner U, Schiefer A, Isenberg G. L-type Ca-channels: similar Q10 of Ca-, Ba- and Na-conductance points to the importance of ion–channel interaction. Pflugers Arch. 1990;415:638–641. doi: 10.1007/BF02583518. [DOI] [PubMed] [Google Scholar]
- Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in cultured interstitial cells of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Sanders KM. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP(3)-sensitive calcium release in the murine small intestine. Am J Physiol. 2001;280:G439–G348. doi: 10.1152/ajpgi.2001.280.3.G439. [DOI] [PubMed] [Google Scholar]
- Nose K, Suzuki H, Kannan H. Voltage dependency of the frequency of slow waves in antrum smooth muscle of the guinea-pig stomach. Jap J Physiol. 2000;50:625–633. doi: 10.2170/jjphysiol.50.625. [DOI] [PubMed] [Google Scholar]
- Ördög T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/WV mutant mice. Gastroenterology. 2002;123:2028–2040. doi: 10.1053/gast.2002.37056. 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- Ördög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991;260:C917–C925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Publicover NG, Sanders KM. A technique to locate the pacemaker in smooth muscles. J App Physiol. 1984;57:1586–1590. doi: 10.1152/jappl.1984.57.5.1586. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ördög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastro Mot. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hirst GD. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, et al. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- Torihashi S, Ward SM, Nishi K, Kobayashi S, Sanders KM. c-kit-Dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tiss Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–360. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Baker SA, de Faoite A, Sanders KM. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol. 2003;549:207–218. doi: 10.1113/jphysiol.2003.040097. 10.1113/jphysiol.2003.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ördög T, Koh SD, Abu Baker S, Jun JY, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Dependence of electrical slow waves of canine colonic smooth muscle on calcium gradient. J Physiol. 1992;455:307–319. doi: 10.1113/jphysiol.1992.sp019303. [DOI] [PMC free article] [PubMed] [Google Scholar]