Abstract
TREK-1 is a member of the two-pore domain potassium (K2P) channel family that is mechano-, heat, pH, voltage and lipid sensitive. It is highly expressed in the central nervous system and probably encodes one of the previously described arachidonic acid-activated K+ channels. Polyunsaturated fatty acids and lysophospholipids protect the brain against global ischaemia. Since both lipids are openers of TREK-1, it has been suggested that this K2P channel is directly involved in neuroprotection. Recently, however, this view has been challenged by a report claiming that TREK-1 and its activation by arachidonic acid is inhibited by hypoxia. In the present study, we demonstrate that the bubbling of saline with gases results in the loss of arachidonic acid from solution. Using experimental conditions which obviate this experimental artefact we demonstrate that TREK-1 is resistant to hypoxia and is strongly activated by arachidonic acid even at low PO2 (< 4 Torr). Furthermore, hypoxia fails to affect basal as well as 2,4,6-trinitrophenol- and acid-stimulated TREK-1 currents. These data are supportive for a possible role of TREK-1 in ischaemic neuroprotection and in cell signalling via arachidonic acid.
Mammalian K2P channels are dimers of subunits comprising four transmembrane segments and two P domains in tandem (Lesage & Lazdunski, 2000; Goldstein et al. 2001; Patel & Honoré, 2001). This structural motif is associated with unusual functional properties. For instance TREK-1 is a mechano-gated K+ channel that is also activated by intracellular acidosis and heat (Patel et al. 1998; Maingret et al. 1999, 2000a; Honoréet al. 2002). Polyunsaturated fatty acids (PUFAs) such as arachidonic acid and lysophospholipids (LPs) are potent and reversible openers of TREK-1 (Patel et al. 1998; Maingret et al. 2000b; Han et al. 2003). It is highly expressed in the central nervous system and probably encodes one of the previously described arachidonic acid-activated K+ channels (KAA) channels (Kim et al. 1995).
Polyunsaturated fatty acids protect the rat brain against global ischaemia (Lauritzen et al. 2000), prevent neuronal cell death and paraplegia after spinal cord ischaemia and induce ischaemic and epileptic tolerance (Blondeau et al. 2002b; Lang-Lazdunski et al. 2003). Similarly LPs afford protection against global cerebral ischaemia and glutamate excitotoxicity (Blondeau et al. 2002a). Since both PUFAs and extracellular LPs are openers of TREK-1, it has been suggested that this K2P channel may play an important neuroprotective role (Lauritzen et al. 2000; Patel & Honoré, 2001). During brain ischaemia, endogenous arachidonic acid is released, intracellular pH falls and neurones swell. These pathological alterations will contribute to opening TREK-1 and the resulting hyperpolarization will lower intracellular calcium and limit excitotoxicity (Patel & Honoré, 2001; Honoréet al. 2002). Recently, the role of TREK-1 in PUFA and LP-induced neuroprotection was further indicated by the genetic inactivation of TREK-1. TREK-1(–/–) mice display an increased sensitivity to both ischaemia and epilepsy (Heurteaux et al. 2004). Remarkably, neuroprotection by PUFAs and LPs disappears in the KO mice. It is also of interest to note that the neuroprotective agents riluzole, nitrous oxide and xenon (gaseous anaesthetics) are additional openers of TREK-1 (Duprat et al. 2000; Gruss et al. 2004). These recent results strongly suggest that indeed TREK-1 may play an important role in neuroprotection during brain ischaemia (Lauritzen et al. 2000, 2002a; Blondeau et al. 2002b; Heurteaux et al. 2004). This view, however, has recently been challenged by a report claiming that acute hypoxia (PO2∼20 Torr) causes a rapid and reversible inhibition of hTREK-1 stimulation by arachidonic acid (Miller et al. 2003). The implication of this work was that the potential neuroprotective role of TREK-1 is unlikely to occur in the CNS as tissue PO2 (in the range of 20 Torr) is too low to allow the activation of TREK-1 by arachidonic acid (Miller et al. 2003). These findings are clearly in contradiction with the previously suggested role of TREK-1 in neuroprotection.
In order to resolve this apparent contradiction, we have re-examined the sensitivity of both human and mouse TREK-1 to hypoxia. In this study we report a problem encountered in bubbling (sparging) solutions containing arachidonic acid, wherein sparging leads to the rapid loss of arachidonic acid from the bulk phase of the solution. Using a strategy to control oxygen levels that avoids the direct sparging of arachidonic acid-containing solutions we demonstrate that, in contrast to the previous study of Miller et al. (2003), TREK-1 and its activation by arachidonic acid is resistant to hypoxia. This finding is supportive for a possible role of TREK-1 in ischaemic neuroprotection (Heurteaux et al. 2004).
Methods
Transient transfection of TREK mRNA
Cells from the human embryonic kidney cell line HEK 293 were grown in minimum essential medium (MEM) supplemented with 10% fetal calf serum, 1% essential amino acids, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin (all Gibco). Cells were cotransfected with two plasmids, one containing TREK-1 DNA and one containing DNA encoding for enhanced green fluorescent protein (pIRES-EGFP, Clontech) using lipofectamine 2000 (Invitrogen). Three TREK-1 plasmid constructs were used in this study; (1) a pCi IRES-CD8 variant of the pCi plasmid (Promega) containing DNA encoding mouse TREK-1 (Fink et al. 1996, 1998; Maingret et al. 2002) (GenBank Ref. NP 034737), referred to as mTREK-1; (2) a pCi IRES-CD8 plasmid containing a mouse splice variant of TREK-1 with an extended 15 amino acids in the amino terminal domain, referred to as mTREK-1long (GenBank submission); (3) a pCi IRES-CD8 containing human TREK-1 (GenBank Ref. NP 055032), referred to as hTREK-1. Twenty-four hours following transfection cells were re-plated onto poly d-lysine-coated glass coverslips and used within 24–48 h. Transfected cells were identified by their green fluorescence.
Solutions and cell perfusion
Hepes-buffered saline contained (mm): NaCl 140, KCl 4.5, CaCl2 2.5, MgCl2 1, glucose 10, Hepes 20 (pH adjusted to 7.4 at room temp with NaOH). The bicarbonate-buffered saline contained NaCl 117, NaHCO3 23, KCl 4.5, MgCl2 1, CaCl2 2.5, glucose 10; pH 7.4 at 35°C when equilibrated with 5% CO2.
Since the gas bubbling of solutions can result in evaporative heat loss, all solutions were kept in glass bottles placed in a water bath. Solutions from these glass bottles were delivered to a two-way tap through medical grade stainless steel tubing. The last 25 cm of this tubing was held against a copper tube fed with water from the water bath so as to form a heat exchanger; this ensured that solutions were delivered at the desired temperature (room temp or 35°C). The tap was connected to the recording chamber via a 5 cm length of PharMed Tubing (Norton Performance Plastics). The recording chamber had a volume of ∼100 μl; solution flow rate was ∼2 ml min−1. Oxygen levels in the chamber were monitored using a commercially available 50 μm fibre-optic probe coated with an oxygen-sensitive luminophore and a Microx TX2 fibre-optic oxygen meter (PreSens, GmbH). The fibre-optic sensor was calibrated daily using air-saturated saline and a saline equilibrated with nitrogen containing 0.5 mm Na2S2O4 (0 Torr).
Stock solutions (100 mm) of arachidonic acid sodium salt (Calbiochem) in water were prepared freshly each day (using trituration and vortexing to dissolve the arachidonic acid) and were used at a final concentration of 5–10 μm by diluting into saline and swirling to mix. All arachidonic acid-containing solutions were clear. 2,4,6-Trinitrophenol (TNP) at a concentration of 1 mm was dissolved directly into saline and pH readjusted. Gas mixtures were supplied by BOC. 3H-labelled arachidonic acid was from Sigma.
Electrophysiology
Patch pipettes were fabricated from Corning 7052 glass (WPI) and had resistances of 5–7 MΩ. Recordings were performed using an Axopatch-1D with 80% series resistance compensation. For recordings conducted in Hepes-buffered saline the patch pipette filling solution comprised (mm): KCl 111, MgCl2 6 (1.2 mm as free ion), CaCl2 1.6 (100 nm as free ion), EGTA 5, Hepes 20, K2ATP 5, KOH ∼40 (pH 7.2 at 22°C). The pipette filling solutions used in HCO3−-buffered media comprised (mm): KCl 95, MgCl 6 (1 mm free ion), EGTA 5, CaCl2 2.1 (100 nm free ion), Hepes 20, NaCl 10, K2ATP 5, KOH ∼31.5 (pH 7.2 at 35°C). Inside-out patch recordings were performed at room temperature using a pipette solution containing KCl 140, MgCl2 2, EGTA 1, Hepes 10 (pH adjusted to 7.4 with KOH) and a bath solution containing KCl 140, MgCl2 1.5, EGTA 5, Pipes 10 (pH adjusted to 7.2, 6.5 or 6.0 with KOH).
Statistics
Measurements of both [3H]arachidonic acid levels and membrane currents recorded at 0 mV are reported as values relative to control unless otherwise indicated. Channel activity is reported as the open probability times the number of channels in a patch (NPopen). Data are presented as mean values ± s.e.m. Significance of difference was assessed by Student's paired t test or ANOVA.
Results
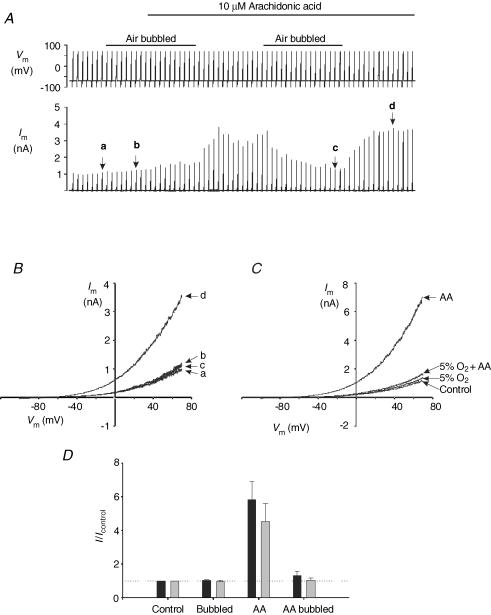
In initial experiments we sought to examine the effects of varying oxygen levels upon mTREK-1 currents and their regulation by arachidonic acid. Hepes-buffered solutions at room temperature were either bubbled with gas (sparged) for > 20 min prior to use or were used as is (i.e. without any bubbling; referred to as non-aerated). During the course of these studies it became apparent that sparging arachidonic acid-containing solutions caused a complete loss of their ability to activate mTREK-1 currents. Figure 1A shows an example of one such experiment in which a cell was voltage clamped at a holding potential of −70 mV and subject to a series of 1 s voltage ramps from −100 to +70 mV at 0.1 Hz. In non-aerated solutions 10 μm arachidonic acid caused a marked stimulation of mTREK-1 currents of, on average, 5- to 6-fold (measured at 0 mV; P < 0.02 relative to control n = 5; see Fig. 1A, B and D). In contrast in solutions sparged with compressed air arachidonic acid had no effect. The effects of arachidonic acid were therefore inhibited simply by bubbling arachidonic acid-containing solutions with gas even whilst maintaining PO2 at levels comparable to non-aerated solutions. A similar inhibition of the effects of arachidonic acid were also noted when solutions were sparged with 5% O2–95% N2; arachidonic acid in non-aerated solutions induced an average 4.5-fold rise in current at 0 mV (P < 0.05, n = 6) but had no effect in solutions bubbled with 5% O2 (Fig. 1C and D).
Figure 1. Effects of bubbling on arachidonic activation of mTREK-1.
mTREK-1 currents recorded at room temperature in Hepes buffer. A, effects of 10 μm arachidonic acid on membrane currents in non-aerated and air-sparged solutions. Upper panel, voltage clamp protocol; lower panel, membrane current. B, ramp currents from the experiment in A; a, in control, non-aerated, media; b, media sparged with air; c, media containing arachidonic acid sparged with air; d, in a non-aerated medium containing arachidonic acid. C, mTREK-1 currents recorded in non-aerated Hepes buffer or in buffer sparged with 5% O2–95% N2. AA indicates currents recorded in the presence of 10 μm arachidonic acid. D, summary of effects of sparging and arachidonic acid on membrane current at 0 mV (expressed relative to currents recorded in control non-aerated solutions). Black columns are data obtained using air to sparge solutions (n = 5); grey columns are data obtained using 5% O2–95% N2(n = 6).
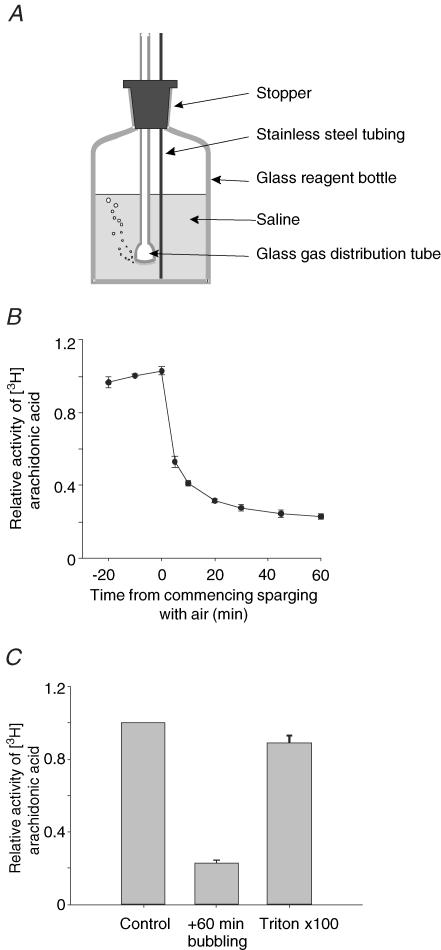
The forgoing preliminary experiments indicated a serious problem with the method used to equilibrate solutions containing arachidonic acid with gases. To investigate the nature of this problem we examined the effects of sparging solutions of arachidonic acid with air upon the concentration of arachidonic acid in free solution. One hundred millilitres of a standard Hepes-buffered solution containing 10 μm arachidonic acid together with a tracer amount of [3H]arachidonic acid was placed in a glass reagent bottle fitted with a plastic stopper. A glass gas distribution tube and a stainless steel tube were inserted through holes in the plastic stopper into the solution (see Fig. 2A). Samples of the solution were withdrawn from the bulk phase of the solution via the stainless steel tubing and the amount of [3H]arachidonic acid present determined by liquid scintillation counting. Three samples were withdrawn at 10 min intervals prior to the commencement of sparging. The solution was then sparged with air (∼150 ml min−1) and further samples taken over a 60 min period. This experiment (Fig. 2B) shows clearly that upon sparging there is a rapid decline in the amount of arachidonic acid in solution with only 20% of 3H activity remaining after 1 h. Following bubbling, much of the arachidonic acid could be returned to solution by inclusion of 100 μl of Triton X-100 and shaking (Fig. 2B). The interpretation of these observations is reserved for the Discussion. The practical significance, however, is clear: in order to study the effects of oxygen on TREK-1 responses to arachidonic acid an alternative method for equilibrating solutions with oxygen other than continuous bubbling is needed.
Figure 2. Effects of bubbling on arachidonic acid solutions.
A, method used for sparging solutions with air (see Results). B, effects of bubbling on arachidonic acid levels in solution. Data presented are means ± s.e.m.(n = 4) of the activity of [3H]arachidonic acid in samples withdrawn at the indicated time points expressed relative to the mean activity in the three samples withdrawn prior to sparging with air. C, relative activity of [3H]arachidonic acid in solution under control conditions (prior to sparging), after 60 min sparging, and after 60 min sparging followed by addition of 100 μl Triton X-100 and remixing of the solution.
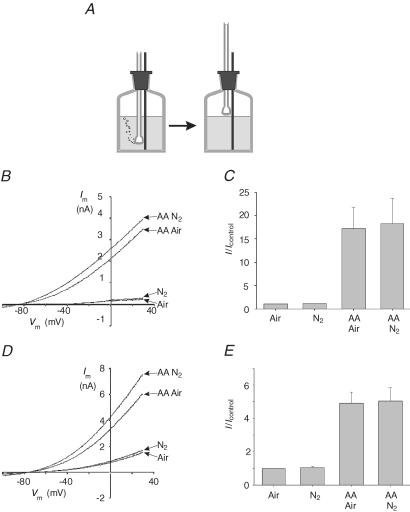
In order to circumvent the problems associated with the sparging of arachidonic acid-containing solutions we adopted the following protocol. Solutions were placed in bottles (as above) and were sparged with gas for ∼20 min. The gas distribution tube was then removed from the solution and suspended in the bottle just above the surface of the solution and the flow of gas continued to prevent the ingress of atmospheric air (Fig. 3A). Arachidonic acid, dissolved in a small volume of Hepes-buffered saline, was introduced into solution after the cessation of sparging. When using solutions equilibrated with nitrogen in this manner PO2 in the experimental chamber was found to be ≤ 4 Torr.
Figure 3. Effects of hypoxia and arachidonic acid on mTREK-1.
A, method used for equilibrating solutions with gases (see Results). B, effects of 10 μm arachidonic acid (AA) on mTREK-1 currents in air-equilibrated (Air) and nitrogen-equilibrated (N2) Hepes buffer at room temperature. The cell was voltage clamped at a holding potential of −70 mV and subject to repetitive 0.5 s voltage ramps from −100 mV to +30 mV at 0.1 Hz. C, mean (± s.e.m.) of mTREK-1 currents at 0 mV expressed relative to control (air equilibrated) in Hepes buffer at room temperature (as in B; n = 6). D, effects of 5 μm arachidonic acid (AA) on mTREK-1 currents in 5% CO2–95% air-equilibrated (Air) and 5% CO2–95% N2-equilibrated (N2) HCO3− buffer at 35°C. Voltage clamp protocol as in B. E, mean (± s.e.m.) of membrane currents measured at 0 mV expressed relative to control (5% CO2–95% air equilibrated) in HCO3−-buffered medium at 35°C (as in D; n = 5).
Figure 3B shows current traces from HEK 293 cells expressing mTREK-1 obtained in solutions equilibrated with air (PO2= 160 Torr), and with nitrogen (PO2≤ 4 Torr) both in the presence and absence of 10 μm arachidonic acid (using the method described above). It is apparent from these recordings (Fig. 3B and C) that arachidonic acid substantially increases mTREK-1 currents in both air-equilibrated and N2-equilibrated solutions. The mean current measured at 0 mV in the presence of arachidonic acid in air was 4.6 ± 1.2 nA and that measured in nitrogen was 4.7 ± 1.0 nA; both values were significantly different from their respective control levels (0.37 ± 0.1 nA in air and 0.4 ± 0.1 nA in N2, P < 0.02, n = 6). Hypoxia (N2 equilibrated) had no significant effect upon either basal mTREK-1 currents or current recorded in the presence of arachidonic acid.
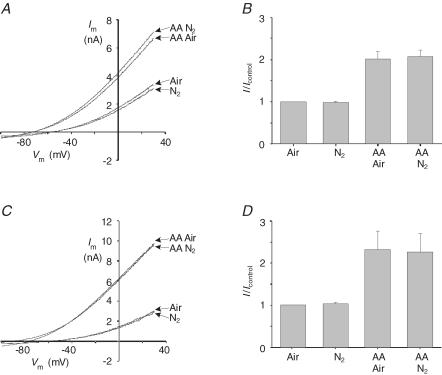
Having established a method for equilibrating arachidonic acid solutions with gases and confirming that mTREK-1 responds robustly to arachidonic acid at room temperature in Hepes-buffered solution at both high and very low oxygen levels we next sought to confirm these observations in a more physiological environment, i.e. at 35°C in a bicarbonate-buffered medium equilibrated with 5% CO2. Figures 3 and 4 show results obtained from a series of experiments in which we re-evaluated the effects of hypoxia and arachidonic acid on mTREK-1 (Fig. 3D and E), the splice variant mTREK-1long (Fig. 4A and B) and the human orthologue hTREK-1 (Fig. 4C and D) expressed in HEK 293 cells. In all of these experiments TREK-1 channel activity was significantly (P < 0.05) stimulated upon application of 5 μm arachidonic acid both in solutions equilibrated with 5% CO2 in air (PO2= 150 Torr) and in solutions equilibrated with 5% CO2 in nitrogen (PO2≤ 4 Torr). Indeed we were unable to detect any significant difference between the responses to arachidonic acid evoked at PO2= 150 Torr and those at PO2≤ 4 Torr. We were also unable to detect any significant difference between basal TREK-1 currents recorded at high PO2 (150 Torr) from those recorded at very low PO2 (see Figs 3E, 4B and Fig. 4D).
Figure 4. Effects of hypoxia and arachidonic acid on mTREK-1long and hTREK-1.
Effects of 5 μm arachidonic acid (AA) onTREK-1 currents in bicarbonate buffer at 35°C. Solutions were equilibrated with 5% CO2–95% air (Air) or 5% CO2–95% N2 (N2). Cells were voltage clamped at −70 mV and subject to repetitive 0.5 s voltage ramps from −100 mV to +30 mV at 0.1 Hz. A, representative recording of mTREK-1long currents. B, summary of the effects of hypoxia and arachidonic acid on mTREK-1long. Data are mean (± s.e.m.) of currents at 0 mV expressed relative to control (5% CO2–95% air equilibrated); n = 5. C, representative recording of hTREK-1 currents. D, summary of the effects of hypoxia and arachidonic acid on hTREK-1. Data are mean (± s.e.m.) of currents at 0 mV expressed relative to control (5% CO2–95% air equilibrated); n = 6.
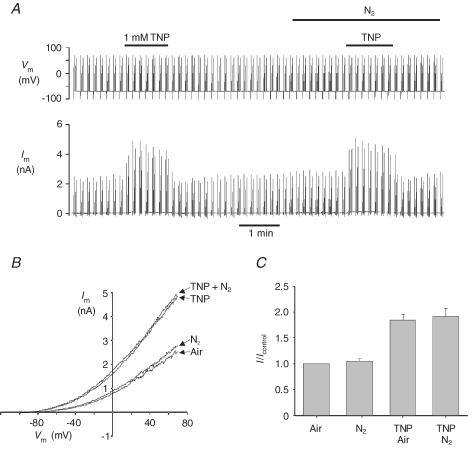
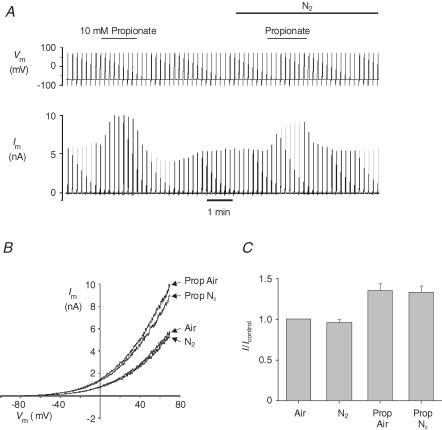
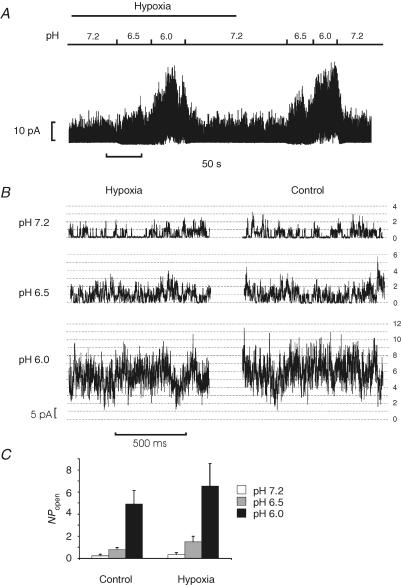
Hypoxia has also been reported to abolish the stimulation of TREK-1 by both the membrane crenator TNP and intracellular acidosis (induced by application of the weak acid propionate) (Miller et al. 2003, 2004). In Hepes-buffered media we observed that 1 mm TNP increased mTREK-1 currents in both air- (mean current at 0 mV = 1.4 ± 0.42 nA, control; 2.5 ± 0.71 nA, TNP; P < 0.05, n = 6) and nitrogen-equilibrated solutions (mean current at 0 mV = 1.44 ± 0.42 nA, N2; 2.6 ± 0.73 nA, TNP + N2; P < 0.05). There was no significant difference between the effects of TNP on mTREK-1 currents in air versus nitrogen-equilibrated solutions (Fig. 5). Mild intracellular acidosis, induced by perfusion with a weak acid (10 mm sodium propionate), also increased mTREK-1 currents in both air- (current at 0 mV = 0.78 ± 0.21 nA, air; 1.0 ± 0.29 nA, propionate; P < 0.05, n = 5) and N2- (current at 0 mV = 0.73 ± 0.18 nA, N2; 0.99 ± 0.24 nA, propionate + N2; P < 0.05, n = 5) equilibrated Hepes-buffered saline to a similar extent (Fig. 6). To further investigate any possible effects of hypoxia upon acid activation of mTREK-1 we used excised inside-out patches. Reduction of bath (intracellular) pH from 7.2 to 6.5 or 6.0 induced a robust activation of mTREK-1 channel activity (Fig. 7) by up to approximately 20-fold under both control (air equilibrated) and hypoxic (N2 equilibrated) conditions (NPopen control, pH 7.2 = 0.22 ± 0.06, pH 6.0 = 4.9 ± 1.2; NPopen hypoxia, pH 7.2 = 0.36 ± 0.16, pH 6.0 = 6.5 ± 2.0; n = 6; measured at a membrane potential of −70 mV). Two-way ANOVA revealed no significant effect of hypoxia on channel activity but a highly significant effect of acidosis (P < 0.0001); analysis of the effects of acidosis alone by one-way ANOVA confirmed that acidosis induced a significant increase in channel activity under both control (P < 0.001) and hypoxic conditions (P < 0.01).
Figure 5. Effects of hypoxia and TNP on mTREK-1.
mTREK-1 currents recorded at room temperature in Hepes buffer. A, effects of 1 mm TNP on membrane currents in air-equilibrated and nitrogen-equilibrated solutions. Upper panel, voltage clamp protocol; cells were held at −70 mV and subject to a 500 ms voltage from −100 mV to +70 mV followed by four 25 ms depolarizing pulses to −30, 0, 30 and +60 mV at 0.1 Hz. Lower panel, membrane current. B, ramp currents from the experiment in A in air-equilibrated (Air) and nitrogen-equilibrated (N2) saline in the presence and absence of 1 mm TNP. C, mean (± s.e.m.) of mTREK-1 currents at 0 mV expressed relative to control (air equilibrated) in Hepes buffer at room temperature (n = 6).
Figure 6. Effects of hypoxia and intracellular acidosis on mTREK-1.
mTREK-1 currents recorded at room temperature in Hepes buffer. A, effects of 10 mm sodium propionate on membrane currents in air-equilibrated and nitrogen-equilibrated solutions. Upper panel, voltage clamp protocol; cells were held at −70 mV and subject to 500 ms voltage ramps from −100 mV to +70 mV at 0.1 Hz. Lower panel, membrane current. B, ramp currents from the experiment in A in air-equilibrated (Air) and nitrogen-equilibrated (N2) saline in the presence and absence of 10 mm propionate (Prop). C, mean (± s.e.m.) of mTREK-1 currents at 0 mV expressed relative to control (air equilibrated) in Hepes buffer at room temperature (n = 5).
Figure 7. Effects of hypoxia and acidosis on mTREK-1 channel activity.
Inside-out patch recordings of mTREK-1 channel activity at a membrane potential of −70 mV (+70 mV pipette potential). A, continuous recording of mTREK-1 activity in an inside-out patch showing the effects of changing perfusate (intracellular) pH and of hypoxia (N2-equilibrated PO2 < 4 Torr). B, 1 s excerpts from above recording shown at higher temporal resolution. Number of open channels is indicated by scale on right of figure. Single channel amplitude was 3.5 pA. C, summary of effects of acidosis and hypoxia upon single channel activity (reported as NPopen calculated for a single channel amplitude of 3.5 pA using a 50% threshold); n = 6 patches.
Discussion
Sparging of arachidonic acid-containing solutions
In the course of this study we have noted that sparging of arachidonic acid solutions can result in an insidious artefact in which arachidonic acid is lost from the bulk phase of the solution. We are not aware of this problem having been previously reported. One possible explanation for our observations lies in the amphipathic nature of fatty acids which are known to partition to the air–water interface. It is possible therefore that as bubbles pass through the solution they trap arachidonic acid molecules at their air–water interface and then deposit them at the surface. In addition when gas bubbles burst at the surface a fine droplet of solution can be ejected. Such droplets could be coated with a high level of arachidonic acid as they are formed at the surface of the solution. Some of these droplets will inevitably be deposited on the vessel walls, and if no lid is present some may be ejected from the vessel completely. In support of this tentative hypothesis we have noted in preliminary experiments that, following sparging, much higher levels of [3H]arachidonic acid are found in solution coating the sides of the vessel than in the bulk phase. This hypothesis is also supported by the observation that most of the [3H]arachidonic acid lost from solution during sparging could be recovered by the addition of Triton X-100 and shaking the contents of the bottle. If this is indeed the correct explanation for our observations then it would suggest that similar problems may also be encountered when working with other amphipathic compounds.
Effects of arachidonic acid on TREK-1 currents
Previous studies have shown that in Hepes-buffered air-equilibrated (non-aerated) solutions arachidonic acid greatly stimulates mTREK-1 currents (Patel et al. 1998). Similar effects have also been reported for other unsaturated fatty acids and for lysophospholipids (Patel et al. 1998; Maingret et al. 2000b). The effects of arachidonic acid on TREK-1 are retained in excised patches, in the presence of cyclo-oxygenase and lipoxygenase inhibitors, and in the presence of agents which disrupt the cytoskeleton (Patel et al. 1998, 2001). It is therefore thought that arachidonic acid activates TREK-1 either by direct interaction with the channel and or by deformation of the membrane lipid bilayer (Patel et al. 1998, 2001; Maingret et al. 2000b).
The properties and distribution of TREK-1 channels have led to the suggestion that these, and other closely related channels such as TREK-2 and TRAAK, are the molecular correlates of endogenous fatty acid-activated channels found in the brain and in other tissues where they are thought to play an important role in cell signalling by arachidonic acid (Kim & Clapham, 1989; Kim et al. 1995). It has also been suggested that activation of endogenous TREK channels by polyunsaturated fatty acids could play an important role in neuroprotection during ischaemia (Lauritzen et al. 2000; Blondeau et al. 2002a, b; Heurteaux et al. 2004).
The putative role of TREK-1 in mediating the protective effects of polyunsaturated fatty acids in ischaemia has recently received considerable support from a study showing that injection of linolenic acid decreased mortality in wild-type mice subject to bilateral carotid artery occlusion but had no effect in TREK-1 knockout mice (Heurteaux et al. 2004). The proposed role of TREK-1 in mediating neuroprotection has, however, been called into question by a recent report claiming that the effects of arachidonic acid on the human orthologue of TREK-1 are ablated by lowering the oxygen levels in solution to around 20 Torr (Miller et al. 2003). The data presented here, wherein the effects of arachidonic acid remain at a much lower PO2, contrast markedly with those of Miller et al. (2003). We note that in the study of Miller et al. 2003) nitrogen-equilibrated solutions appeared to have been routinely bubbled with gas, but that some of the control solutions were only equilibrated with room air (i.e. non-aerated). Whilst it is possible that some other reason may account for the difference between our results and those of Miller et al. (2003), the most likely explanation is that the failure of nitrogen-bubbled arachidonic acid solutions to stimulate TREK-1 currents in the study by Miller et al. (2003) is an artefact caused by the loss of arachidonic acid from solution and not a consequence of lowering oxygen levels.
Effects of TNP and acidosis on TREK-1 currents
In this study, we failed to observe any effect of hypoxia upon the response of mTREK-1 to the membrane crenator TNP or to intracellular acidosis induced either by extracellular perfusion with a weak acid or by direct application of acidic solutions to the internal aspect of an inside-out patch of membrane. It is notable that with respect to propionate, Miller et al. observed a transient stimulation of TREK-1 currents upon adding propionate under hypoxic conditions (Fig. 5B of Miller et al. 2004). However, they attributed this activation to a transient increase in temperature (although it is not clear why switching between two gassed solutions at room temperature should give rise to an increase in temperature). This, combined with the high temperature sensitivity of TREK-1 (Maingret et al. 2000a) and the fact that the intracellular acidosis induced by weak acids is likely to be only transient (due to cellular pH regulation), confounds comparison between our data on propionate-induced intracellular acidification and those of Miller et al. (2004). The data we have obtained using inside-out patches, however, clearly show that strong intracellular acidosis induces robust activation of mTREK-1 under both normoxic and hypoxic conditions. Acid modulation of TREK-1 channel activity is not therefore dependent upon oxygen.
Effects of hypoxia on TREK-1 currents
We failed to observe any effect of hypoxia on basal mTREK-1 currents in Hepes-buffered media at room temperature, or on mTREK-1, mTREKlong and hTREK-1 currents at 35°C in HCO3/CO2-buffered media. These results contrast markedly with reports claiming that reducing PO2 to ≤ 60 Torr inhibits hTREK-1 (Miller et al. 2003, 2004). This lack of reproducibility may reflect differences in the experimental preparation (e.g. transient versus stable expression in HEK 293 cells) or other methodological causes or artefacts. Whatever the reasons for the differences between our study and that of Miller et al. (2003) it is apparent that as TREK-1 currents are not inhibited by hypoxia under the conditions employed in this study, TREK-1 channels themselves cannot therefore be considered to exhibit intrinsic O2 sensitivity. Thus if TREK-1 channels display O2 sensitivity in other preparations it must presumably be conferred by factors either not universally present in all cells or which are not functionally integrated with the channels during transient transfection.
A lack of reproducibility has also been noted with other putative O2-sensitive K+ channels when expressed in different cell types or even between different batches of the same cell line (Lopez Barneo et al. 2001). We have similarly been unable to reproduce oxygen sensitivity in two other tandem-P-domain K+ channels, TASK-1 and TASK-3 (expressed in either COS-7 cells or HEK 293 cells; authors' unpublished observations) contrary to reports from other groups (Lewis et al. 2001; Kemp et al. 2003). Clearly there is a growing need to establish the causes of this lack of reproducibility in respect of the oxygen sensitivity of an increasing number of ion channels.
Conclusions
In summary we have shown TREK-1 to be strongly stimulated by arachidonic acid even at very low PO2 under physiological recording conditions (i.e. at 35°C and in the presence of a HCO3/CO2 buffer). These data are consistent with the hypothesized role of TREK-1 in mediating the neuroprotective effects of polyunsaturated fatty acids under ischaemic conditions. However, we were unable to demonstrate any direct inhibition of basal TREK-1 currents by hypoxia, contrary to other studies (Miller et al. 2003, 2004). The reasons for the lack of reproducibility of oxygen sensitivity in TREK-1 channels remain to be elucidated.
Acknowledgments
This work was supported by the CNRS and the British Heart Foundation.
References
- Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab. 2002a;22:821–834. doi: 10.1097/00004647-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience. 2002b;109:231–241. doi: 10.1016/s0306-4522(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, et al. A neuronal two P domain K+ channel activated by arachidonic acid and polyunsaturated fatty acid. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2003;546:625–639. doi: 10.1113/jphysiol.2002.032094. 10.1113/jphysiol.2002.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca L, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PJ, Searle GJ, Hartness ME, Lewis A, Miller P, Williams S, et al. Acute oxygen sensing in cellular models: relevance to the physiology of pulmonary neuroepithelial and carotid bodies. Anat Rec. 2003;270A:41–50. doi: 10.1002/ar.a.10008. 10.1002/ar.a.10008. [DOI] [PubMed] [Google Scholar]
- Kim D, Clapham DE. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989;244:1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sladek CD, Aguadovelasco C, Mathiasen JR. Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. J Physiol. 1995;484:643–660. doi: 10.1113/jphysiol.1995.sp020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg. 2003;38:564–575. doi: 10.1016/s0741-5214(03)00473-7. 10.1016/S0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Poly-unsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lewis A, Hartness ME, Chapman CG, Fearon IM, Meadows HJ, Peers C, et al. Recombinant hTASK1 is an O2-sensitive K+ channel. Biochem Biophys Res Commun. 2001;285:1290–1294. doi: 10.1006/bbrc.2001.5310. 10.1006/bbrc.2001.5310. [DOI] [PubMed] [Google Scholar]
- Lopez Barneo J, Pardal R, Ortega Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Maingret F, Honore E, Lazdunski M, Patel AJ. Molecular basis of the voltage-dependent gating of TREK-1, a mechano-sensitive K+ channel. Biochem Biophys Res Commun. 2002;292:339–346. doi: 10.1006/bbrc.2002.6674. 10.1006/bbrc.2002.6674. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000a;19:2483–2491. doi: 10.1093/emboj/19.11.2483. 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000b;275:10128–10133. doi: 10.1074/jbc.275.14.10128. 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Miller P, Kemp PJ, Lewis A, Chapman CG, Meadows H, Peers C. Acute hypoxia occludes hTREK-1 modulation: re-evaluation of the potential role of tandem P domain K+ channels in central neuroprotection. J Physiol. 2003;548:31–37. doi: 10.1113/jphysiol.2003.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Peers C, Kemp PJ. Polymodal regulation of hTREK1 by pH, arachidonic acid, and hypoxia: physiological impact in acidosis and alkalosis. Am J Physiol Cell Physiol. 2004;286:C272–C282. doi: 10.1152/ajpcell.00334.2003. 10.1152/ajpcell.00334.2003. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. 10.1016/S0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honoré E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. 10.1016/S0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]