Abstract
Developmental changes in the coupling between Ca2+ entry and exocytosis were studied in mouse inner hair cells (IHCs) which, together with the afferent endings, form the primary synapse of the mammalian auditory system. Ca2+ currents (ICa) and changes in membrane capacitance (ΔCm) were recorded using whole-cell voltage clamp from cells maintained at body temperature, using physiological (1.3 mm) extracellular Ca2+. The magnitudes of both ICa and ΔCm increased with maturation from embryonic stages until postnatal day 6 (P6). Subsequently, ICa gradually declined to a steady level of about −100 pA from P13 while the Ca2+-induced ΔCm remained relatively constant, indicating a developmental increase in the Ca2+ efficiency of exocytosis. Although the size of ICa changed during development, its activation properties did not, suggesting the presence of a homogeneous population of Ca2+ channels in IHCs throughout development. The Ca2+ dependence of exocytosis changed with maturation from a fourth power relation in immature cells to an approximately linear relation in mature cells. This change applies to the release of both a readily releasable pool (RRP) and a slower secondary pool of vesicles, implying a common release mechanism for these two kinetically distinct pools that becomes modified during development. The increased Ca2+ efficiency and linear Ca2+ dependence of mature IHC exocytosis, especially over the physiological range of intracellular Ca2+, could improve the high-fidelity transmission of both brief and long-lasting stimulation. These properties make the mature cell ideally suited for fine intensity discrimination over a wide dynamic range.
Inner hair cells (IHCs) are the primary sensory cells of the mature mammalian cochlea, responsible for translating sounds into neuronal signals. Sound-induced bundle deflection depolarizes the IHC and leads to the activation of Cav1.3 L-type voltage-gated Ca2+ channels situated in the cell's basolateral membrane (Platzer et al. 2000; Brandt et al. 2003; Hafidi & Dulon, 2004). Ca2+ entry triggers the fusion of synaptic vesicles to the basolateral membrane and glutamate is released onto afferent terminals (Glowatzki & Fuchs, 2002). Mature IHCs respond to sound stimulation with graded receptor potentials that increase with sound intensity (Russell & Sellick, 1978) from around 12 days after birth (P12). Although immature IHCs do not respond to sound, they fire spontaneous and depolarization-induced Ca2+ action potentials (Kros et al. 1998; Marcotti et al. 2003a, b), thought to affect the remodelling of synaptic connections that occurs during development (Pujol et al. 1998). Synaptic machinery is present in mouse IHCs from birth (Sobkowicz et al. 1982) and appears already to be functional at this time (Beutner & Moser, 2001; Marcotti et al. 2003b). This suggests that action potentials could drive neurotransmitter release onto afferent fibres, which has been indirectly demonstrated from ‘bursts’ of afferent activity that match the duration and frequency of Ca2+ action potentials in P7 rat IHCs (Glowatzki & Fuchs, 2002).
At around the onset of hearing, the synaptic machinery changes from multiple spherical bodies, typical of immature cells, to single flat plate-like ribbons at each active zone (Sobkowicz et al. 1982). The change in synaptic architecture and response properties during development may have evolved to ensure that IHCs are optimally configured to respond to different membrane voltage excursions before and after the onset of hearing. In addition to these morphological findings, the onset of functional maturation in the cochlea is also characterized by an increase in the Ca2+ efficiency of readily releasable pool (RRP) exocytosis in IHCs (Beutner & Moser, 2001), although these results were obtained using unphysiological recording conditions (room temperature and 10 mm extracellular Ca2+).
In this study we compare directly whole-cell current recordings and membrane capacitance measurements from apical IHCs during development to investigate changes in the properties of their Ca2+ currents (ICa) and synaptic vesicle exocytosis, at body temperature and using a physiological concentration of extracellular Ca2+ (1.3 mm; Wangemann & Schacht, 1996). We show that in IHCs the Ca2+ efficiency of exocytosis of mainly an identified RRP of vesicles increases during development whereas, in contrast to previous investigations, the Ca2+ dependence of both RRP and secondary vesicle pools reduces, which could be consistent with a transition in the synaptic machinery to optimize the mature IHC for auditory transduction. Moreover, the kinetics and size of the RRP and the secondary pool differ from those obtained using high extracellular Ca2+.
Methods
Tissue preparation
Apical-coil IHCs were studied in acutely dissected organs of Corti from Swiss CD-1 mice (Charles Rivers, Margate, UK) ranging from embryonic-day 16.5 (E16.5) up to P20 where the day of birth (P0) corresponds to E19.5. For embryonic experiments only, mice were paired overnight and checked for vaginal plugs the following morning. Assuming ovulation occurs midway through the dark cycle, the midpoint of the light cycle of the day following mating is considered to be E0.5. Mature and neonatal mice were killed by rapid cervical dislocation and embryos by decapitation, in accordance with UK Home Office guidelines. Cochleae were transferred to a microscope chamber and immobilized under nylon mesh. The chamber was continuously perfused with extracellular solution containing (mm): 135 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 d-glucose, 10 Hepes-NaOH, 2 sodium pyruvate. Amino acids and vitamins (Invitrogen, Paisley, UK) for Eagle's MEM were added from concentrates. The pH was 7.5 and the osmolality 308 mmol kg−1. The chamber was mounted on an upright microscope (Olympus, Tokyo, Japan) and cells were observed with Nomarski DIC optics. The position of the cells was recorded as fractional distance along the cochlea starting from the extreme apex. In the immature cochlea, cells were positioned in the apical coil at a fractional distance of between 0.12 and 0.26. Mature cells were positioned between 0.06 and 0.19, corresponding to an approximate frequency range of 0.8–3.0 kHz (using eqn (13) in Ehret, 1975). Access to IHCs was gained using a suction pipette (tip diameter approximately 3–4 μm) filled with extracellular solution to create a small tear in the epithelium around the cells and expose their basolateral membranes. Only cells of a healthy appearance and with well-preserved hair bundles were investigated.
Electrical recording
Current recordings from apical IHCs (n = 226), maintained near body temperature (34–37°C) and in 1.3 mm extracellular Ca2+, were obtained using whole-cell patch clamp with an Optopatch amplifier (Cairn Research Ltd, Faversham, UK). Patch electrodes were pulled from soda glass capillaries (Harvard Apparatus Ltd, Edenbridge, UK) and coated with surf wax (Mr Zogs SexWax, Carpinteria, CA, USA) to minimize the fast electrode capacitative transients. The pipette filling solution contained (mm): 140 Cs-glutamate, 3 MgCl2, 5 Na2ATP, 0.3 Na2GTP, 1 EGTA-CsOH, 5 Hepes-CsOH (pH 7.3, 290 mmol kg−1). Data were acquired using pClamp software and a Digidata 1320 A (Axon Instruments, CA, USA), filtered at 2.5 kHz or 10 kHz (8-pole Bessel), sampled at 5 kHz or 50 kHz, respectively, and stored on computer. Offline data analysis was performed using Clampfit (Axon Instruments) and Origin software (OriginLab, Northampton, MA, USA). Current recordings were corrected for linear leak conductance (gleak) measured near −81 mV (1.6 ± 0.1 nS, n = 204). Since the leak conductance was not measured for some of the postnatal cells (P0–P6) in Fig. 1, averaged values from age-matched cells were used instead in these cases. Membrane potentials were corrected for residual series resistance (Rs, 6.0 ± 0.1 MΩ, n = 226) and a liquid junction potential of −11 mV, measured between electrode and bath solutions. The voltage-clamp time constant was 48.3 ± 0.2 μs (n = 226).
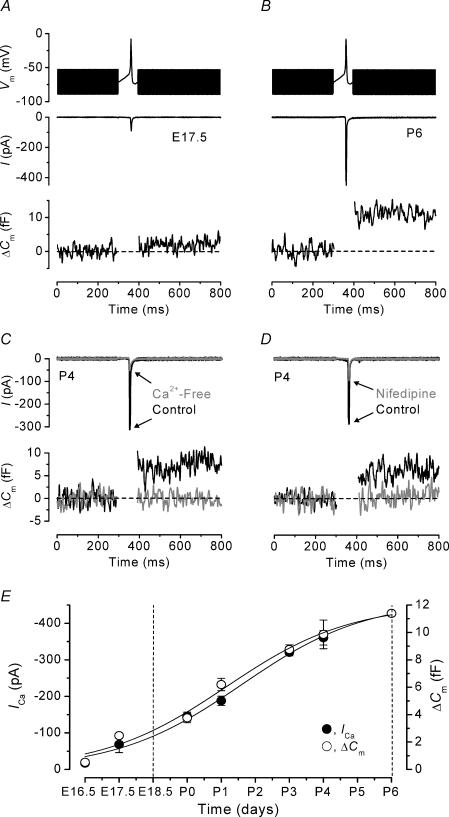
Figure 1. ICa and ΔΔCm responses to a voltage-clamp spike protocol.
A and B, ICa and ΔCm from an E17.5 and a P6 IHC, respectively. Top panels show the command protocol consisting of a sine wave (2.5 kHz) that appears as thick solid lines, which is interrupted for the duration of the spike and applied from a holding potential of −71 mV. Middle panels show the inward current elicited by the spike and lower panels show the corresponding ΔCm responses. Note that the region during the spike in the lower panels is blanked as the track-in circuitry is not operational. The recordings in A and B are averages of two and four protocol repetitions, respectively, and the broken horizontal lines represent the zero ΔCm level (as in subsequent figures). E17.5, Cm 5.3 pF; Rs 7.0 MΩ; gleak 0.8 nS. P6, Cm 8.0 pF; Rs 5.3 MΩ; gleak 2.0 nS. C, ICa (top panel) and ΔCm (bottom panel) responses to the spike protocol from a P4 IHC in control conditions (black traces) and during the superfusion of a Ca2+-free solution (grey traces). The recordings in control and Ca2+-free conditions are averages of three and two repetitions, respectively. Cm 7.6 pF; Rs 5.1 MΩ; gleak 1.7 nS. D, ICa and ΔCm elicited by the spike protocol from a P4 IHC in control conditions (black traces) and in the presence of 30 μm nifedipine (grey traces). The recordings in control conditions and during the application of nifedipine are averages of four and nine repetitions, respectively. Cm 7.7 pF; Rs 5.2 MΩ; gleak 1.8 nS. E, developmental changes (E16.5–P6) in the amplitudes of peak ICa (•) and ΔCm (◊) in response to the spike protocol. Solid lines are fits to the ICa and ΔCm data points using eqn (1). Numbers of cells are (E16.5–P6) 3, 2, 8, 9, 5, 4, 1. The broken vertical lines at E18.5 and P6 delineate the period during which apical-coil IHCs are capable of firing spontaneous action potentials.
Membrane capacitance measurement
Real-time change in membrane capacitance (ΔCm) was measured using the track-in circuitry of the Optopatch as previously described (Johnson et al. 2002). To enable the track-in circuitry to operate, a 2.5 kHz sine wave (amplitude 18.5 mV) was applied to IHCs from a holding potential of either −71 mV (Fig. 1) or −81 mV (Figs 2, 4, 5 and 6) using the internal oscillator of the Optopatch. The sine wave was small enough not to activate any significant membrane current since accurate membrane capacitance calculation requires a high and constant membrane resistance (Rm). The command sine wave was interrupted for the duration of the voltage step, or action potential waveform, so inward currents could be recorded. The capacitance signal from the Optopatch was amplified (×50), 2-pole filtered at 150 Hz with additional 8-pole Bessel filtering at 250 Hz, and sampled at 5 kHz. We allowed approximately 5–10 s per stimulus for vesicle pool replenishment. When multiple step protocols were used (Fig. 2A and Fig. 4A) the prestimulus Cm baseline for consecutive steps was set to zero during offline analysis. Changes in membrane capacitance were measured by averaging the Cm trace over a 300 ms period following the voltage step when the prestimulus baseline had been set to zero. Due to the apparent partial Cm recovery immediately after the voltage step, seen in some recordings (Fig. 2A), ΔCm was measured 50 ms after the end of the voltage step. This small decline is likely to be caused by the ICa tail currents, which significantly reduce Rm. Post-stimulus Cm recovery (endocytosis) was not observed in recordings of up to 2.5 s using 1.3 mm extracellular Ca2+ or up to 0.6 s using higher Ca2+ concentrations (the longest durations used). Although we allowed 5–10 s in between consecutive steps, no clear evidence for endocytosis was found. This could be explained by the loss of important elements of the endocytotic machinery due to the whole-cell configuration (Parsons et al. 1994). This does, however, give us a more accurate representation of the vesicle pool size and Ca2+ dependence of exocytosis since the ΔCm responses are not reduced by endocytosis.
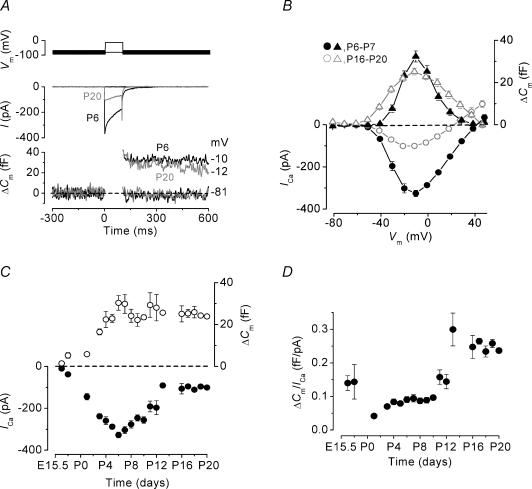
Figure 2. Comparison of ICa and ΔΔCm responses during IHC development.
A, inward current (middle panel) and ΔCm (lower panel) responses from a P6 (black traces) and a P20 (grey traces) IHC. Recordings (unaveraged single traces) were obtained in response to 100 ms voltage steps, in 10 mV increments, from the holding potential of −81 mV. For clarity, only responses to two voltage steps (top panel) are shown and the membrane potentials reached are indicated to the right of the ΔCm traces. P6, Cm 7.0 pF; Rs 6.9 MΩ; gleak 1.3 nS. P20, Cm 8.7 pF; Rs 5.3 MΩ; gleak 3.1 nS. B, average I–V (lower panel, circles) and ΔCm−V (upper panel, triangles) curves for peak ICa and ΔCm measured at each voltage step potential in immature (P6–P7, n = 8; black closed symbols) and mature (P16-P20, n = 8; grey open symbols) IHCs. C, average ΔCm (upper panel) and peak ICa (lower panel) responses from IHCs at each stage of development following a 100 ms voltage step to around −11 mV. D, changes in the Ca2+ efficiency of exocytosis during development where all ΔCm values have been normalized to ICa, in response to a voltage step to around −11 mV. Numbers of cells in C and D are (E16.5–P20): 3, 2, 3, 7, 11, 15, 21, 14, 7, 10, 15, 9, 5, 4, 3, 4, 5, 8, 3.
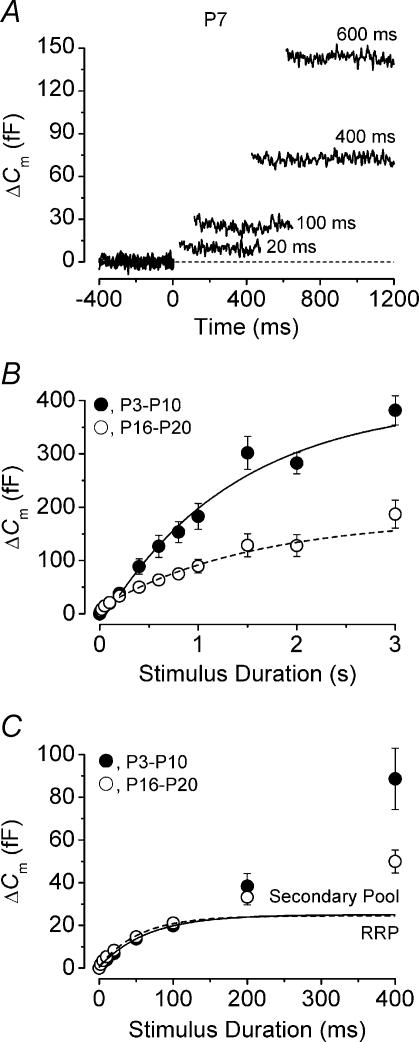
Figure 4. Developmental changes in the kinetics of neurotransmitter release.
A, ΔCm recordings (single traces) from a P7 IHC in response to voltage steps of different duration, shown next to the traces, to around −11 mV. Cm 8.4 pF; Rs 5.7 MΩ; gleak 0.7 nS. B, average ΔCm values obtained for each voltage step duration (2 ms to 3 s) from immature (•, P3–P10, n = 28) and mature (◊, P16–P20, n = 8) IHCs. P3–P10 and P16–P20 were chosen since the ΔCm/ICa ratios shown in Fig. 2D were very similar within each range. The lines are exponential fits to immature (solid line) and mature (broken line) ΔCm values from 200 ms to 3 s. C, average ΔCm values from B for stimulus durations up to 400 ms on an expanded time scale. ΔCm responses from immature and mature cells showed an initial foot region over the first 100 ms that could be approximated with an exponential function (immature: solid line; mature: broken line). The RRP (below the exponential fits) and secondary pool (above the exponential fits) are indicated.
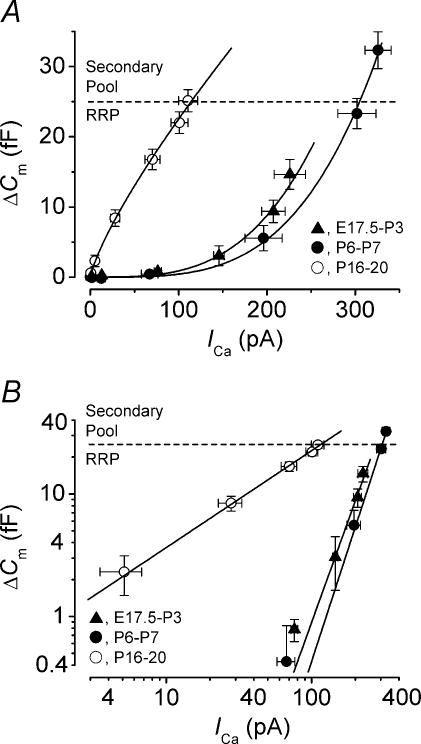
Figure 5. Synaptic transfer functions relating ICa and ΔΔCm at different membrane potentials.
A, average ΔCm responses from E17.5–P3 (▴, n = 12), P6–P7 (•, n = 8) and P16–P20 (◊, n = 8) IHCs plotted against the corresponding absolute ICa magnitude resulting from 100 ms voltage steps from the holding potential of −81 mV to different voltages up to around −11 mV in nominal 10 mV increments. For immature (P6–P7) and mature (P16–P20) IHCs, the data are from the I−V and ΔCm–V curves shown in Fig. 2B. Solid lines are fits to data points according to eqn (5) with powers N of: E17.5–P3, 3.3; P6–P7, 3.3; P16–P20, 0.7. The broken horizontal lines (also in B) represent the maximum size of the predicted RRP (from Fig. 4C). B, ICa and ΔCm responses as A but plotted using double-logarithmic coordinates. Straight lines are the same functions used in A with the same parameters.
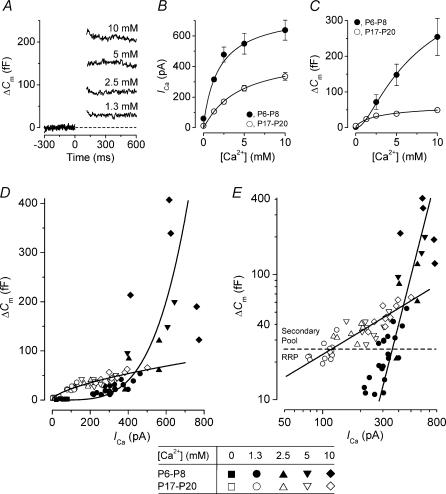
Figure 6. Dependence of ICa and ΔΔCm on extracellular Ca2+.
A, ΔCm (single traces) from four immature IHCs in different extracellular Ca2+ concentrations (indicated above each trace) in response to a 100 ms voltage step to around −11 mV. 1.3 mm (P8), Cm 9.9 pF; Rs 4.3 MΩ; gleak 1.4 nS. 2.5 mm (P6), Cm 8.0 pF; Rs 5.0 MΩ; gleak 0.7 nS. 5 mm (P7), Cm 8.1 pF; Rs 6.6 MΩ; gleak 1.1 nS. 10 mm (P6), Cm 8.0 pF; Rs 5.6 MΩ; gleak 1.4 nS. B and C, peak ICa magnitudes and corresponding ΔCm, respectively, as a function of extracellular Ca2+ for immature (•, P6–P8) and mature (◊, P17–P20) IHCs. Numbers of cells at each concentration are: immature, 8 (0 mm), 20 (1.3 mm), 4 (2.5 mm), 3 (5 mm) and 5 (10 mm); mature, 5 (0 mm), 18 (1.3 mm), 6 (2.5 mm), 11 (5 mm) and 10 (10 mm). Solid lines are fits according to eqn (6) (see Results). D, synaptic transfer functions relating ICa and ΔCm where ΔCm values for each cell at different Ca2+ concentrations are plotted against their corresponding ICa magnitude for immature (filled symbols) and mature (open symbols) IHCs. Immature and mature data points are fit (solid lines) using eqn (5) with powers N of: 3.97 (immature) and 0.62 (mature). E, as D but plotted using double-logarithmic coordinates. Straight lines are the same functions used in D with the same parameters. The Ca2+-free data points shown in D have been omitted. The broken horizontal line represents the maximum size of the predicted RRP (from Fig. 4C).
An action potential waveform recorded from a spontaneously active apical-coil P3 IHC under current clamp conditions at 37°C was used as a protocol in Fig. 1. During the spike protocol the changing membrane potential would introduce a capacitative current component that may affect the peak of the recorded currents. However, capacitance compensation was applied at the start of every recording and the track-in circuitry of the Optopatch keeps the membrane capacitance accurately compensated while the sine wave is active. When the sine wave is interrupted, during the spike or voltage step, the compensation remains at the prestimulus value. Therefore, any contamination of the ionic currents by the capacitative current would be minimal. In order to express ΔCm values as a number of vesicles we used a conversion factor of 37 aF per vesicle, which has previously been calculated from vesicle diameters measured using electron tomography on frog saccular hair cells (Lenzi et al. 1999).
Extracellular superfusion
ΔCm and ICa were recorded during the superfusion of 30 mm TEA (Fluka, Gillingham, UK), which was a constant in all experiments in order to block most IHC K+ currents. When ICa was studied in isolation (Fig. 3), immature IHCs were additionally superfused with 300 nm tetrodotoxin (TTX, Sigma, Gillingham, UK) to block the Na+ current (Marcotti et al. 2003b) and mature IHCs with 60 nm iberiotoxin (IbTx, Tocris, Bristol, UK), 10 mm 4-AP (Fluka) and 200 μm linopirdine (RBI, Natick, MA, USA) to block IK,f, IK,s and IK,n, respectively (Kros et al. 1998; Marcotti et al. 2003a). To test the Ca2+ dependence of ΔCm (Fig. 6), IHCs were superfused with a Ca2+-free solution (containing 0.5 mm EGTA) or solutions containing different Ca2+ concentrations (2.5, 5 and 10 mm, in addition to 1.3 mm). One set of experiments was designed to determine whether the inward current induced by the spike protocol was mainly carried by L-type Ca2+ channels containing the α1D (Cav1.3) subunit (Platzer et al. 2000). This was achieved by superfusing immature IHCs with an extracellular solution containing 30 μm nifedipine (Sigma). When chemicals or Ca2+ that were added to, or removed from, the solution had a concentration >1 mm, NaCl was adjusted to keep the osmolality constant. Solutions were applied via a multibarrelled pipette positioned close to the IHCs.
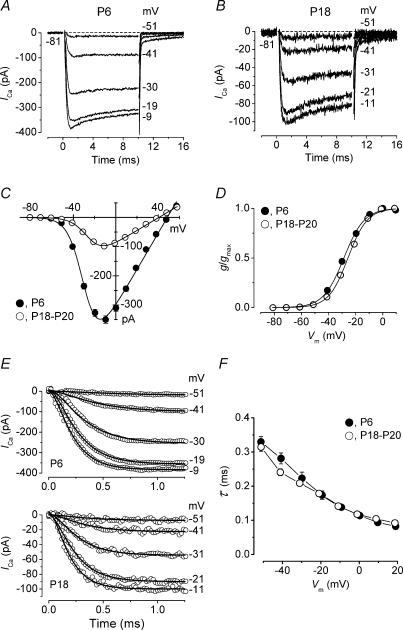
Figure 3. Properties of ICa in immature and mature IHCs.
A and B, ICa recorded from a P6 and a P18 IHC in response to 10 ms voltage steps from −81 mV in nominal 10 mV increments; for clarity only five traces are shown. Actual test potentials reached are shown next to the traces. Recordings are averaged from seven (•, P6) and eight (◊, P18) protocol repetitions. Residual capacitative transients have been blanked. P6, Cm 8.6 pF; Rs 4.0 MΩ; gleak 0.9 nS. P18, Cm 10.9 pF; Rs 5.1 MΩ; gleak 1.3 nS. C, average peak I–V curves for ICa at the different test potentials from immature (P6, n = 14) and mature (P18–P20, n = 21) IHCs. Continuous lines are fits obtained using eqn (2) for immature (gmax 7.1 nS, Vrev+48 mV, V1/2−29.3 mV, S 7.2 mV) and mature (gmax 3.8 nS, Vrev+41 mV, V1/2−25.2 mV, S 7.5 mV) cells. D, activation of ICa obtained by plotting the normalized chord conductance against the test potential. Same cells as for panel C. Continuous lines are fits obtained using eqn (3) for immature (gmax 7.1 nS, V1/2−28.7 mV, S 7.5 mV) and mature (gmax 3.8 nS, V1/2−25.6 mV, S 7.1 mV) cells. V1/2 was slightly but significantly (P < 0.0001) different between immature and mature IHCs. E, upper and lower panels show the ICa traces of A and B on an expanded time scale. Continuous lines are fits to the current traces obtained using eqn (4). F, average time constants of activation obtained from the fits to the current traces at different membrane potentials for immature (•, n = 9) and mature (◊, n = 14) IHCs.
Statistical analysis
Statistical comparisons of means were made using Student's two-tailed t test or, for comparisons of multiple data sets, one-way ANOVA (followed by the Tukey post test). Two-way ANOVA (followed by the Bonferroni post test) was used for Fig. 3F. For all statistical tests P < 0.05 was used as the criterion for statistical significance and mean values are quoted ±s.e.m. in text and figures.
Results
Neurotransmitter release in response to an action potential
Immature IHCs fire spontaneous Ca2+ action potentials (Marcotti et al. 2003b) from just before birth (E18.5 in apical IHCs), and this activity lasts until the end of the first postnatal week (Marcotti et al. 2003a). This spontaneous activity could be important for the remodelling of synaptic connections within the organ of Corti before the onset of sound-evoked responses (Shnerson et al. 1982; Echteler, 1992; Pujol et al. 1998). To influence the development of afferent fibres, Ca2+ influx into immature IHCs that is induced by an action potential should be sufficient to trigger exocytosis. The fusion of synaptic vesicles to the cell membrane can be measured as an increase in cell membrane capacitance (ΔCm) that is generally interpreted as a sign of neurotransmitter release from presynaptic cells (Neher & Marty, 1982; Parsons et al. 1994; von Gersdorff et al. 1998; Moser & Beutner, 2000).
To test this hypothesis, a spike protocol (see Methods) was applied to IHCs and the resulting current and ΔCm were recorded. Typical examples of the inward current and ΔCm from an E17.5 and a P6 IHC are shown in Fig. 1A and B, respectively. The inward current was mostly ICa since it was substantially reduced during the superfusion of a Ca2+-free solution (Fig. 1C and Marcotti et al. 2003b) and nifedipine (Fig. 1D), a blocker of L-type Ca2+ channels containing the α1D (Cav1.3) subunit (Engel et al. 2002). The amplitude of ICa was reduced by 82 ± 1% (P3–P4, n = 7) in a Ca2+-free solution and by 63 ± 6% (P4, n = 5) using 30 μm nifedipine. Moreover the Na+ current, known to be expressed in about 70% of immature IHCs, becomes largely inactivated during the slow depolarization leading up to the spike threshold potential of about −60 mV (Marcotti et al. 2003b). For this reason, the inward current elicited by an action potential was referred to as ICa. Consistent with the decrease in the size of ICa the ΔCm was reduced (Fig. 1C and D) by 89 ± 1% (P3–P4, n = 7) in a Ca2+-free solution and by 78 ± 2% (P4, n = 5) using nifedipine.
The peak ICa corresponds to the peak of the action potential, and the membrane capacitance increase was apparent immediately after the stimulating waveform. Figure 1E shows that the amplitudes of the peak inward current and ΔCm, in response to the spike protocol, increase as a function of development. Both ICa and ΔCm data sets in Fig. 1E could be approximated with a sigmoidal logistic growth curve:
| (1) |
where A is the amplitude of ICa or ΔCm, k a slope factor, and t1/2 the age where A is halfway between maximal (Amax) and minimal (Amin) values. The fits to ICa and ΔCm data points in Fig. 1E gave values for t1/2 and k of P1.6, 1.8 day−1 and P1.2, 1.8 day−1, respectively. These results show that the number of synaptic vesicles released is closely related to the development of ICa. Although small ICa and ΔCm responses were observed at E16.5, the small amplitude of ICa appears not to be sufficient to allow spontaneous action potentials before E18.5, indicating that the synaptic machinery is likely to be functional about two days before the onset of spontaneous activity.
Developmental changes in ICa and ΔΔCm
We used voltage steps to investigate the development of ICa and ΔCm from embryonic to mature stages (E16.5–P20). The use of action potential waveforms is not appropriate for mature cells, which respond to sound stimulation with graded receptor potentials, and the steps allowed a more detailed investigation of ΔCm dynamics by manipulating the stimulus amplitude and duration. Figure 2A shows the inward current and ΔCm recorded from an immature (P6) and a mature (P20) IHC using a series of 100 ms voltage steps from −81 mV to near −11 mV (peak responses). The current recordings are labelled ‘I’ rather than ‘ICa’ since they are likely to be contaminated by the presence of a residual unblocked SK current in immature IHCs and a delayed rectifier K+ current (IK,s) in mature cells (Marcotti et al. 2003a, b). However, the size of the peak inward current is referred to as ICa since these slowly activating K+ currents are relatively small at this time point but would become more evident towards the end of the voltage steps and cause the apparent large current inactivation (Fig. 2A). When a Na+ current was present, the inward current was measured at about 1.5 ms from the onset of the voltage step, by which time the Na+ current is inactivated (see Fig. 6A in Marcotti et al. 2003b). Although the mature IHC responded to the same voltage step with a significantly smaller peak ICa, the induced ΔCm reached a value comparable to that seen in the immature cell. The ΔCm–voltage (ΔCm–V) and peak ICa–voltage (I−V) curves for immature (n = 8) and mature (n = 8) cells were obtained from responses to 100 ms depolarizing voltage steps, in nominal 10 mV increments, from the holding potential of −81 mV to +49 mV (Fig. 2B). At both stages, ICa and ΔCm follow a bell-shaped pattern, but note the narrower voltage range for ΔCm for immature cells.
Figure 2C shows the developmental changes in ΔCm and peak ICa following a 100 ms depolarizing voltage step to around −11 mV. ICa (Fig. 2C, lower panel) increased in size (P < 0.0001) from E16.5 until P6 and then gradually declined (P < 0.0001), reaching a steady-state level of −100 ± 4 pA (P13–P20, n = 27) from P13. The average membrane potential that elicited the peak ICa (i.e. the peak of the I−V curves) was −12.4 ± 0.9 mV (n = 149) over the entire age range tested. The size of ΔCm (Fig. 2C, upper panel) did not decline during development as would be expected if ΔCm were directly linked to the size of ICa (Fig. 2C, lower panel). Early in development, the changes in ΔCm reflect that of ICa as ΔCm also increased significantly (P < 0.0005, E16.5-P6). However, ΔCm did not change significantly after P6 (26.6 ± 1.1 fF, n = 108, P6-P20). The average membrane potential that elicited the peak ΔCm response (peak of the ΔCm−V curves) was −11.5 ± 0.7 mV (n = 149).
The discrepancy between the development of ICa and ΔCm from about P6 onwards suggests that capacitance change, and therefore neurotransmitter release, becomes more efficient in mature cells, in the sense that less Ca2+ entry is required to elicit the same ΔCm response. A similar finding has previously been shown in IHCs but only for small ICa that elicited ΔCm of up to 5 fF (Beutner & Moser, 2001). The Ca2+ efficiency of exocytosis (Fig. 2D) was estimated by normalizing ΔCm to peak ICa (fF pA−1) and from P11 onwards the ΔCm/ICa ratio significantly increased (P < 0.05), showing that the release of neurotransmitter does indeed become more efficient in mature IHCs. Although embryonic IHCs show an apparently larger Ca2+ efficiency of exocytosis compared to that of early postnatal cells, the s.e.m. for these small responses was large and the mean values were not significantly different.
ICa in immature and mature IHCs
The reduction in ICa amplitude from the beginning of the second postnatal week (Fig. 2C) could be caused by a variety of factors such as a reduction in the number of Ca2+ channels per IHC, the expression of channel regulatory subunits or the insertion of another type of Ca2+ channel into the basolateral membrane in place of that expressed during earlier stages of development. A reduction in the number of Ca2+ channels should only affect the current magnitude and not the current kinetics, whereas the appearance of regulatory subunits (Singer et al. 1991) or the expression of a new channel type should have a significant effect on the kinetics. Therefore, we investigated whether the biophysical properties of ICa in immature (P5–P6) and mature (P18–P20) IHCs are different. To isolate ICa, IHCs were superfused with specific blockers for the other voltage-gated channels characteristic of the two stages of development (see Methods). Figure 3A and B show typical examples of ICa recordings from a P6 and a P18 IHC, respectively. Although the time course of ICa appears similar in immature and mature cells, ICa has a smaller amplitude in mature cells consistent with the findings of Fig. 2. The peak ICa measured at different membrane potentials from immature (P6, n = 14) and mature (P18–P20, n = 21) IHCs is shown in Fig. 3C. Both I−V curves are fits using the following equation:
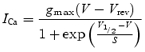 |
(2) |
where V is the membrane potential, Vrev the reversal potential, gmax the maximum chord conductance, V1/2 the membrane potential at which the conductance is half activated and the slope factor (S) describes the voltage sensitivity of activation. The peak ICa, measured near −11 mV, in immature IHCs (−350 ± 13 pA, n = 14) was significantly larger (P < 0.0001) than that of mature cells (−98 ± 5 pA, n = 21). The reversal potential of the isolated ICa in mature IHCs (Fig. 3C) was 19 mV more positive than that obtained when ICa was recorded simultaneously with ΔCm (Fig. 2B), most likely due to a more complete block of the outward K+ currents, which would be especially important for large depolarizing voltage steps. Such a discrepancy was not apparent for the immature IHCs. This more effective block of the K+ currents of mature IHCs was achieved by superfusing a solution containing IbTx, linopirdine and 4-AP, in addition to TEA (see Methods). The activation curves of Fig. 3D were obtained from the normalized chord conductance (Zidanic & Fuchs, 1995; Marcotti et al. 2003b), using reversal potentials from the fits in Fig. 3C, and approximated by a first-order Boltzmann equation:
| (3) |
where g is the chord conductance and the other parameters are as in eqn (2). In immature IHCs ICa activated positive to −63 mV (defined as 1% of gmax), 4 mV more hyperpolarized than that of mature cells (−59 mV). An example of the rapid activation of ICa in immature and mature cells is shown in detail in Fig. 3E. The time constants of ICa activation were obtained by fitting the traces with the following equation:
| (4) |
where ICa(t) is ICa at time t, Imax the peak ICa, τ the time constant of activation and α is 2, which gives a better fit than a power of 3, consistent with a Hodgkin–Huxley model with two opening gating particles (Hodgkin & Huxley, 1952). The time constants of ICa were not significantly different between immature and mature IHCs (Fig. 3F). We conclude from these experiments that the kinetic properties of ICa do not appreciably change during IHC development.
Developmental changes in the dynamics of vesicle pool recruitment in IHCs
Differences in the size, kinetics and Ca2+ dependence of distinct vesicle pools have previously been studied in mouse IHCs during development (Moser & Beutner, 2000; Beutner & Moser, 2001) using unphysiological recording conditions (10 mm Ca2+ and at room temperature). Therefore, as the results may not be representative of exocytosis in the physiological condition we have reinvestigated these properties using 1.3 mm extracellular Ca2+ and at body temperature.
Changes in the kinetics of neurotransmitter release were investigated using voltage steps, from 2 ms to 3 s in duration, to around −11 mV from the holding potential of −81 mV. By using stimuli of increasing duration, the emptying of different synaptic vesicle pools could be followed. Previous studies (Moser & Beutner, 2000; Beutner & Moser, 2001) have shown, using a similar voltage protocol, that in response to short (∼10 ms) stimuli there is an initial small component of ΔCm increase, which is likely to represent the exocytosis of the RRP of vesicles docked at the active zones. The same studies have also shown that ΔCm continues to increase at a slower rate for stimulus durations of up to 1 s. This slower component of exocytosis is likely to represent the release of vesicles from a secondary pool that is located further from the Ca2+ channels (von Gersdorff et al. 1996; von Gersdorff & Matthews, 1999; Voets et al. 1999).
Figure 4A shows ΔCm from a P7 IHC in response to depolarizing voltage steps of varying duration. The capacitance responses to voltage steps of different duration from immature (P3–P10, n = 28) and mature (P16–P20, n = 8) IHCs are shown in Fig. 4B. Figure 4C shows that when 1.3 mm Ca2+ is used instead of 10 mm Ca2+, voltage steps of up to about 100 ms are likely to recruit mainly the RRP in both immature and mature IHCs, since the ΔCm increase (up to 100 ms) could be well fitted using a single exponential (P3–P10: τ= 64.3 ms, maximal ΔCm = 25.0 fF; P16–P20: τ= 53.4 ms, maximal ΔCm = 24.5 fF). The exponential fits of Fig. 4C give an indication of an RRP in immature and mature IHCs consisting of 680 and 660 synaptic vesicles and initial release rates of 389 fF s−1 (10 500 vesicles s−1) and 459 fF s−1 (12 400 vesicles s−1), respectively. A slower additional component of ΔCm increase, most likely associated with the secondary releasable pool, became evident for voltage steps from around 200 ms. A previous study showed that a relatively high concentration of 5 mm intracellular EGTA was required to isolate the RRP from the slower secondary vesicle pool when 10 mm extracellular Ca2+ was used (Moser & Beutner, 2000). Here we show that in 1.3 mm Ca2+ the RRP could be isolated using 1 mm intracellular EGTA for stimulus durations of up to 100 ms. The ΔCm responses over the entire range of stimulus durations (2 ms to 3 s) could not be accurately approximated with a double exponential, possibly due to the delayed induction of the secondary vesicle pool (Figs 4B and C). The single exponential fits (Fig. 4B) to the slower component of ΔCm from 200 ms to 3 s (P3–P10 τ= 1.3 s, maximal ΔCm = 360 fF; P16–P20 τ= 1.5 s, maximal ΔCm = 147 fF) gave an indication of maximal release rates of the secondary pool of around 267 fF s−1 (7200 vesicles s−1) in immature cells and a slower rate of 96 fF s−1 (2600 vesicles s−1) in mature cells.
The calcium dependence of distinct vesicle pools
The relation between Ca2+ entry and exocytosis (Fig. 5A) for immature (E17.5–P3 and P6–P7) and mature (P16–P20) IHCs, estimated using a synaptic transfer function (Augustine et al. 1985), was obtained by plotting ΔCm against the peak ICa for 100 ms voltage steps (in order to elicit mainly the RRP of vesicles) from the holding potential to a range of potentials between –61 mV and −11 mV. The peak ICa was preferred rather than its time integral (Beutner & Moser, 2001) because the recordings were contaminated by the residual unblocked slower activating SK Ca2+-activated K+ current in immature IHCs (Marcotti et al. 2003b), and IK,s in mature IHCs (Marcotti et al. 2003a, 2004), which are likely to be responsible for the apparent large inactivation of the inward current. Since the slower activation of these currents does not affect the peak of the much faster ICa we decided that this was the most accurate representation of Ca2+ entry into immature and mature IHCs. Data were approximated using a power function:
| (5) |
where N is the power. The three transfer functions obtained using eqn (5) equate to straight lines when plotted on double-logarithmic coordinates (Fig. 5B). The average power obtained from the transfer functions of immature IHCs (E17.5–P3: 3.30 ± 0.11, n = 12; P6–P7: 3.34 ± 0.11, n = 8) indicates that each release event requires a minimum of four Ca2+ binding steps to occur (Dodge & Rahamimoff, 1967; Cohen & Van der Kloot, 1985). Somewhat surprisingly, the transfer function obtained from mature IHCs was best approximated using a power of 0.70 ± 0.02 (n = 8, P16-P20), significantly (P < 0.0001) smaller than that of immature cells, suggesting a near linear relation between Ca2+ entry and exocytosis (Llinas et al. 1976, 1981). The broken line in Fig. 5A and B represents the maximum size of the predicted RRP (from the exponential fits in Fig. 4C), and this indicates that 100 ms voltage steps in 1.3 mm Ca2+ elicit the release of vesicles mainly within the RRP. Therefore, the Ca2+ dependence indicated by the synaptic transfer functions (Fig. 5A and B) reflects mainly that of the RRP at all stages shown.
The developmental change in Ca2+ dependence of the RRP and secondary vesicle pools was investigated further by stimulating immature and mature IHCs with a 100 ms voltage step to a potential that elicited maximal responses (near −11 mV) from −81 mV while varying the extracellular Ca2+ concentration (Fig. 6). With the use of high extracellular Ca2+ concentrations, the resulting larger ICa allowed us to determine the Ca2+ dependence of the secondary vesicle pool to a greater extent. These experiments also allow a direct comparison between this and previous studies on hair cells where ΔCm responses have been recorded using different extracellular Ca2+ concentrations (4 mm Ca2+: Parsons et al. 1994; 5 mm Ca2+: Spassova et al. 2001; 10 mm Ca2+: Moser & Beutner, 2000; Beutner & Moser, 2001). Figure 6A shows the ΔCm responses near −11 mV, from four immature IHCs in different external Ca2+ concentrations. When 10 mm extracellular Ca2+ was used, the IHCs rapidly deteriorated, therefore no more than one viable cell was recorded for each dissected organ of Corti. Changing the Ca2+ concentration over the range from zero to 10 mm significantly (P < 0.0001 in all cases) increased both ICa (Fig. 6B) and ΔCm (Fig. 6C) in immature and mature IHCs. The individual data points were fitted using the following equation, which assumes the independent action of n Ca2+ binding sites (Augustine & Charlton, 1986):
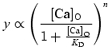 |
(6) |
where y is ICa (Fig. 6B) or ΔCm (Fig. 6C), [Ca]O the extracellular Ca2+ concentration, KD the dissociation constant for Ca2+ and n the order of the Ca2+-dependent reaction. The values for n and KD obtained from the fits were: immature ICa, 0.85 and 2.4 mm; immature ΔCm, 2.47 and 3.1 mm; mature ICa, 1.11 and 3.8 mm; mature ΔCm, 0.64 and 5.3 mm. The estimates for KD were independently verified using linear fits in double reciprocal (Lineweaver–Burk) plots (not shown) of the nth root of ICa or ΔCm against the extracellular Ca2+ concentration (Dodge & Rahamimoff, 1967). Using this plotting method the values for KD obtained from the x-axis intercepts were in all cases within 0.3 mm of those quoted above. The similar order of the Ca2+ dependence of ICa (n≈ 1) in both immature and mature IHCs (Fig. 6B) suggests that Ca2+ entry through the Ca2+ channels does not significantly contribute to the different power relations of exocytosis observed in these cells (Fig. 5). Instead, this developmental reduction in the power relation between ICa and exocytosis is likely to be caused by a reduced Ca2+ dependence of a step beyond Ca2+ entry as indicated by the different n-values for ΔCm in Fig. 6C. The direct relation between ICa and exocytosis (Fig. 6D and E) can be obtained by plotting synaptic transfer functions like those of Fig. 5, but now using maximum responses obtained near −11 mV in different Ca2+ concentrations from individual cells that have been used for the averages shown in Figs 6B and C. The immature and mature synaptic transfer functions were approximated using powers of 3.97 and 0.62, respectively, close to those obtained in Fig. 5. This result indicates that the relation between ΔCm and ICa at both immature and mature stages is not affected by the different methods (changing either membrane potential or extracellular Ca2+) used to vary Ca2+ entry. Studies investigating the Ca2+ dependence of exocytosis at the squid giant synapse have shown that a power of around 4 was obtained when ICa was varied either by using voltage steps to different membrane potentials (Augustine et al. 1985) or by changing extracellular Ca2+ over a range of concentrations between 1 mm and 50 mm (Augustine & Charlton 1986). For extracellular Ca2+ concentrations lower than the KD (i.e. if [Ca]O/KD < 1 in eqn (6)) eqn (6) approximates to a power function for both ICa and ΔCm:
| (7) |
By combining eqn (7) for both ICa and ΔCm the [Ca]O term is eliminated and the following power function obtained:
| (8) |
where nΔCm and nICa are the n-values for ΔCm and ICa, respectively, from eqn (7). Note that eqn (8) is formally equivalent to eqn (5). This approach could be applied reasonably well to IHCs since the average KD from Figs 6B and C was around 4 mm, a value larger than the physiological extracellular Ca2+ concentration (1.3 mm). In immature and mature IHCs the values for nΔCm/nICa (Figs 6B and C) were 2.91 and 0.58, respectively, which are similar to the powers N (eqn 5) of 3.97 and 0.62 obtained in Figs 6D and E. The method of simplifying eqn (6) is only used here to give an indication of the relationship between extracellular Ca2+ and transmitter release. We found that the most consistent way of measuring the Ca2+ dependency of exocytosis was to plot the actual ICa against ΔCm (Figs 5 and 6D or E), which is indicated by the similarity of the N-values obtained even though different methods were used to vary ICa. Even so, the dependence of ICa and ΔCm on extracellular Ca2+ seems to be sufficient to account for the different synaptic transfer curves of immature and mature IHCs, especially around the physiological range of Ca2+ concentrations.
The maximum size of the predicted RRP (indicated in Fig. 6E) suggests that 100 ms stimulation to around −11 mV, in extracellular Ca2+ concentrations >1.3 mm, generally elicits the release of the entire RRP and, to a varying extent, the secondary vesicle pool. Therefore, the synaptic transfer functions (Fig. 6E) reflect the Ca2+ dependence of both the RRP and secondary pools in immature and mature IHCs, and imply that there is a developmental modification of the release mechanism that controls the exocytosis of vesicles from both the RRP and secondary pools. The lower Ca2+ dependence of both vesicle pools in mature IHCs (Figs 5B and 6E) could perform a dual role by allowing more efficient release of the RRP at the lower range of ICa (Fig. 5B), while providing a more gradual recruitment of the secondary pool at higher ICa (Fig. 6E) or during prolonged stimulation (>100 ms, Fig. 4B).
Discussion
Much information regarding neurotransmitter release from mouse IHCs onto afferent terminals has been provided in recent years (Moser & Beutner, 2000; Beutner & Moser, 2001; Beutner et al. 2001), although the recording conditions used in these studies were largely unphysiological (room temperature and 10 mm extracellular Ca2+). Since exocytosis is a Ca2+-dependent process we investigated this phenomenon in 1.3 mm Ca2+ and at body temperature to provide further insights into exocytosis in mammalian IHCs.
The Ca2+ efficiency of IHC exocytosis
The amplitudes of ICa and ΔCm increased during development up until P6, when either voltage steps or spike protocols were used. At E16.5 a single spike elicited a small ICa and a ΔCm response that corresponded to the fusion of around 15 vesicles (Fig. 1). At P6 the number of vesicles per spike increased to about 310. The similar developmental trend between ICa and ΔCm (Fig. 1E) suggests that the synaptic machinery is fully functional from embryonic stages and the degree of release is governed by the development of ICa. Although the amplitude of ICa changed during development (Fig. 2C), its activation kinetics did not appreciably differ between immature and mature IHCs (Fig. 3). These results, together with the observation that the α1D (Cav1.3) Ca2+ channel subunit is responsible for >90% of ICa at both stages of development (Platzer et al. 2000; Brandt et al. 2003), indicate that the decline in ICa from P6 is due to a reduction in the number of Ca2+ channels rather than a change in channel composition.
After P6, while ICa declined to reach a steady value from about P13, ΔCm did not change significantly (Fig. 2C). The relatively stable ΔCm responses from P6, together with the decline in ICa obtained in 1.3 mm extracellular Ca2+, increased the Ca2+ efficiency of exocytosis, reaching statistical significance from P11 (Fig. 2D). Theoretically, if only Ca2+ channels that are not coupled to exocytosis would become eliminated with IHC maturation, this would give a false impression of an increase in Ca2+ efficiency. However, this is unlikely since extrasynaptic Ca2+ channels remain in mature IHCs (Hafidi & Dulon, 2004). A higher Ca2+ efficiency in mature IHCs compared to that of immature cells has previously been shown, using 10 mm Ca2+, only when ICa was elicited using short voltage steps (up to about 10 ms, Beutner & Moser, 2001) most likely due to the earlier recruitment of the secondary pool of vesicles (see below) in high extracellular Ca2+. Indeed, when unphysiologically high Ca2+ concentrations (>1.3 mm) were used, the smaller ICa in mature cells (Fig. 6B) was now accompanied by a simultaneous reduction in ΔCm (Fig. 6C) consistent with previous observations (Beutner & Moser, 2001). The increased Ca2+ efficiency of exocytosis with IHC maturation (Fig. 2D) could be due to an altered spatial relationship between Ca2+ entry and the release machinery, a manifestation of which may be the change in ribbon synapse morphology from spherical to plate-like (Sobkowicz et al. 1982; Liberman, 1980). Alternatively or additionally, the expression of molecules such as the Ca2+-binding protein synaptotagmin (Geppert et al. 1994) or the cysteine-string protein (Eybalin et al. 2002), thought to improve stimulus–secretion coupling (Chamberlain & Burgoyne, 2000), could be responsible for the increased Ca2+ efficiency. An enhancement of exocytotic Ca2+ efficiency has also been correlated with developmental changes in calyx of Held ultrastructure (Taschenberger et al. 2002). Whatever the developmental alteration turns out to be, it is also likely to be responsible for the changes in Ca2+ dependence as discussed in the next section.
The Ca2+ dependence of vesicle pool exocytosis
Vesicle exocytosis in excitable cells typically has a high-order dependence on presynaptic Ca2+ entry (Dodge & Rahamimoff, 1967; Augustine & Charlton, 1986). However, in the mouse cochlea this applies only to immature IHCs in which the relation between ICa and ΔCm was approximated by a power of about four, compared to 0.7 in mature cells (Figs 5 and 6), indicating that each release event requires the binding of around four and one Ca2+ ions, respectively. The change in Ca2+ dependence of exocytosis during development was evident for both the RRP and secondary pools (Fig. 6E), implying that a common release mechanism exists for both pools. An approximately linear relation between presynaptic ICa and transmitter release has been reported in previous studies (Llinas et al. 1976, 1981) although it has been suggested that the high extracellular Ca2+ concentrations of between 3 mm and 100 mm that were used in these studies could be responsible for the linearity (Augustine & Charlton, 1986). However, this possibility seems unlikely to apply to mature IHCs since a nearly linear Ca2+ dependence of exocytosis was also observed in physiological 1.3 mm Ca2+ (Fig. 5A). An alternative explanation for the reduced Ca2+ dependence in mature cells could be an increased resting intracellular Ca2+ caused by local buffer saturation (Cohen & Van der Kloot, 1985). Such a situation might potentially arise from damage to mature IHCs, which are considered to be more fragile than at immature stages, during tissue preparation, which would increase their leak conductance and cause them to become permanently depolarized. This might, in turn, activate a proportion of voltage-gated Ca2+ channels and result in an increased resting intracellular Ca2+ concentration. However, using the same preparation (Marcotti et al. 2003a), we found that the resting membrane potential of IHCs older than P8 (−74.4 ± 0.7 mV, n = 34, P8–P30) was significantly (P < 0.0001) more negative than that at earlier stages (−54.9 ± 0.6 mV, n = 70, P0–P7), suggesting that the cells are in good condition and that the resting intracellular Ca2+ level is not higher than at immature stages. One possibility that remains is that the local concentration of fixed buffers might be reduced during maturation.
A previous study on mature mouse IHCs (Beutner et al. 2001) estimated, using flash photolysis of caged Ca2+, five co-operative Ca2+ binding steps for each release event. However, flash photolysis recruits a pool of vesicles that is two orders of magnitude larger (Beutner et al. 2001) than the RRP activated by Ca2+ influx through Ca2+ channels and likely to be on average further removed from the active zones. This larger pool may not be coupled to synaptic proteins (Wiser et al. 1999), may experience a lower resting Ca2+ concentration than the RRP, and is probably not involved in IHC exocytosis over the physiological range of intracellular Ca2+ (Beutner et al. 2001). A linear relation between ICa and exocytosis in physiological extracellular Ca2+ has also recently been demonstrated at the photoreceptor synapse (Thoreson et al. 2004). The same study has also shown a near-linear relation between the rate of exocytosis and intracellular Ca2+ using flash photolysis when Ca2+ elevations were kept within the expected physiological range at the active zones (0.7 μm to 3 μm). However, a third-order Ca2+ dependency better described the data obtained over the entire range of intracellular Ca2+ concentrations investigated (0.2 μm to 5 μm, Thoreson et al. 2004). Similar considerations might explain the discrepancy between the linear Ca2+ dependence of mature IHCs in the present study and the fifth-order relation described by Beutner et al. (2001) that was measured over a range of very high intracellular Ca2+ concentrations (7 μm to 110 μm).
The power relation between ΔCm and ICa could reflect a number of steps along the excitation–exocytosis cascade and therefore one or more of these steps could contribute to the linearization in the Ca2+ dependence of exocytosis with IHC development. Changes in the proportion of Ca2+ channels contributing to neurotransmitter release or the coupling between Ca2+ channels and release sites could be responsible for this linearization. The former is unlikely because even if only a proportion of the total number of Ca2+ channels in immature cells were able to influence release (Rodriguez-Contreras & Yamoah, 2001), the similar voltage dependence of ICa in both immature and mature cells (Fig. 3C), that is mainly (>90%) determined by L-type Ca2+ channels at both stages (Platzer et al. 2000; Brandt et al. 2003), would generate the same overall Ca2+ dependence of exocytosis, even if ICa was reduced by any fraction. In mature IHCs, a very efficient coupling between Ca2+ channels and release sites could ensure that high Ca2+ concentrations are reached around the active zones, thus forcing the exocytotic mechanisms to operate at a range where the Ca2+ dependence becomes more linear (Dodge & Rahamimoff, 1967; Cohen & Van der Kloot, 1985). This more efficient coupling could arise from a closer colocalization between Ca2+ channels and release sites, or a change in the Ca2+ sensing molecules of exocytosis during IHC maturation. The Ca2+ sensor of exocytosis is generally believed to be synaptotagmin (Geppert et al. 1994; Koh & Bellen, 2003). This protein is integrated into the membrane of synaptic vesicles, is found in multiple isoforms with different Ca2+-binding properties (Südhof & Rizo, 1996; Südhof, 2002), and has been shown to form heteromultimers (Chapman et al. 1998). A reduced Ca2+ dependence of exocytosis was observed in the presence of synaptotagmin IV (Littleton et al. 1999) or mutations in a Ca2+-binding domain of synaptotagmin I (Littleton et al. 1994), both of which reduced the total number of Ca2+-binding sites. Although the expression of synaptotagmin IV has been demonstrated in mature guinea-pig hair cells (Safieddine & Wenthold, 1999), its presence is yet to be determined in the immature cochlea.
The kinetic properties of IHC exocytosis
Two kinetically distinct synaptic vesicle pools have been observed in sensory cells such as hair cells and photoreceptors (von Gersdorff & Matthews, 1999; Lenzi & von Gersdorff, 2001): an RRP of vesicles that are docked at the active zones and represent a small and rapid initial component of exocytosis, and a much slower but larger secondary pool representing the release of vesicles that are probably attached to the synaptic ribbon but located further away from the active zone. The synaptic ribbon architecture of sensory receptors appears to be a determinant of vesicle pool size and release kinetics (Lenzi & von Gersdorff, 2001; von Gersdorff, 2001; Parsons & Sterling, 2003; Fuchs et al. 2003).
The size and kinetics of the RRP remained relatively constant during postnatal development (Fig. 4C), consistent with a previous study (Beutner & Moser, 2001). While the kinetics of RRP release were slower than those obtained in high Ca2+ (Beutner & Moser, 2001), the estimated size of the RRP (670 vesicles) in immature and mature cells in the present study was 2.3 times larger than that reported by Beutner & Moser (2001). The possibility that the larger estimated RRP had been partially affected by the much slower secondary pool appears unlikely because this slower component was only recruited for steps longer than 100 ms in both immature and mature cells. Moreover, the rapidly releasable component (up to 100 ms, Fig. 4C) has been shown to be resistant to high concentrations of the slow Ca2+ buffer EGTA that affect the much slower secondary pool (Moser & Beutner, 2000). Finally, if the secondary pool was contaminating the RRP measurement (i.e. >100 ms in Fig. 4B), the amplitude of ΔCm would decline with IHC development due to the three-fold slower secondary pool release rate in mature cells. Therefore, the constant ΔCm responses in Fig. 2C, from P6 onwards, provide further support for the release of only the RRP following 100 ms voltage steps in physiological extracellular Ca2+.
Physiological implications of developmental changes in Ca2+ efficiency and Ca2+ dependence of exocytosis
Immature IHCs (E18.5–P6) generate spontaneous action potentials whereas mature cells respond to sound with graded receptor potentials. The properties of synaptic transmission change during maturation such that immature IHCs only release neurotransmitter in response to an action potential, while mature IHCs are capable of continuous vesicle release in vivo, both spontaneously and in response to small changes in membrane potential (Sewell, 1996). The high Ca2+ efficiency of transmitter release by mature IHCs would promote spontaneous neurotransmitter release whereas the low, near-linear, Ca2+ dependence would lead to a broadening of their dynamic range by allowing the cells to respond efficiently to both small and large stimuli, thus providing finely graded intensity discrimination over a wide dynamic range of sound stimuli. Interestingly, a linear relation between Ca2+ entry and exocytosis is also responsible for the high-fidelity transmission of small changes in receptor potential at sensory synapses of the photoreceptor (Thoreson et al. 2004) and olfactory receptor neurones (Murphy et al. 2004). On the other hand, the large ICa, lower Ca2+ efficiency and steeper Ca2+ dependence of exocytosis by immature IHCs may be specializations to ensure that the cells can fire action potentials and release neurotransmitter only during these spikes but not in the intervals between spikes. This would be important if spontaneous action potential activity in IHCs contributes to the maturation and refinement of synaptic connections in the developing auditory system. This possibility has been suggested on the basis of indirect evidence (Kros et al. 1998; Marcotti et al. 2003a). A similar fourth-power Ca2+ dependence to that of exocytosis by immature IHCs has generally been observed at synapses activated by action potentials (del Castillo & Katz, 1954; Dodge & Rahamimoff, 1967; Augustine et al. 1985; Nachshen & Sanchez-Armass, 1987; Borst & Sakmann, 1996).
Acknowledgments
This work was supported by the MRC. We thank Professor W. Van der Kloot for his comments on an earlier version of the manuscript. W.M. is a Royal Society University Research Fellow.
References
- Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD. Cysteine-string protein: the chaperone at the synapse. J Neurochem. 2000;74:1781–1789. doi: 10.1046/j.1471-4159.2000.0741781.x. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- Cohen I, Van der Kloot W. Calcium and transmitter release. Int Rev Neurobiol. 1985;27:299–336. doi: 10.1016/s0074-7742(08)60560-7. [DOI] [PubMed] [Google Scholar]
- del Castillo J, Katz B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954;124:553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the muscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci U S A. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Masked auditory thresholds, critical ratios, and scales of the basilar membrane of the housemouse (Mus musculus) J Comp Physiol. 1975;103:329–341. 10.1007/BF00612025. [Google Scholar]
- Engel J, Michna M, Platzer J, Striessnig J. Calcium channels in mouse hair cells: function, properties and pharmacology. Adv Otorhinolaryngol. 2002;59:35–41. doi: 10.1159/000059243. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Renard N, Aure F, Safieddine S. Cysteine- string protein in inner hair cells of the organ of Corti: synaptic expression and upregulation at the onset of hearing. Eur J Neurosci. 2002;15:1409–1420. doi: 10.1046/j.1460-9568.2002.01978.x. 10.1046/j.1460-9568.2002.01978.x. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. 10.1016/S0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Dulon D. Developmental expression of Cav1.3 (α1D) calcium channels in the mouse inner ear. Brain Res Dev Brain Res. 2004;150:167–175. doi: 10.1016/j.devbrainres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Thomas MV, Kros CJ. Membrane capacitance measurement using patch clamp with integrated self-balancing lock-in amplifier. Pflugers Arch. 2002;443:653–663. doi: 10.1007/s00424-001-0763-z. 10.1007/s00424-001-0763-z. [DOI] [PubMed] [Google Scholar]
- Koh TW, Bellen HJ. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MK, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, von Gersdorff H. Structure suggests function: the case for synaptic ribbons as exocytotic nanomachines. Bioessays. 2001;23:831–840. doi: 10.1002/bies.1118. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Serano TL, Rubin GM, Ganetzky B, Chapman ER. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 1999;400:757–760. doi: 10.1038/23462. 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- Littleton TJ, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci U S A. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Steinberg IZ, Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976;73:2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Steinberg IZ, Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981;33:323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003a;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca2+ on Ca2+-activated K+ currents in mature mouse inner hair cells. J Physiol. 2004;557:613–633. doi: 10.1113/jphysiol.2003.060137. 10.1113/jphysiol.2003.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rüsch A, Kros CJ. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J Physiol. 2003b;552:743–761. doi: 10.1113/jphysiol.2003.043612. 10.1113/jphysiol.2003.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci. 2004;24:3023–3030. doi: 10.1523/JNEUROSCI.5745-03.2004. 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen DA, Sanchez-Armass S. Co-operative action of calcium ions in dopamine release from rat brain synaptosomes. J Physiol. 1987;387:415–423. doi: 10.1113/jphysiol.1987.sp016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt? Neuron. 2003;37:379–382. doi: 10.1016/s0896-6273(03)00062-x. 10.1016/S0896-6273(03)00062-X. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lavigne-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer; 1998. pp. 146–192. [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca2+ currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol. 2001;534:669–689. doi: 10.1111/j.1469-7793.2001.00669.x. 10.1111/j.1469-7793.2001.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. Eur J Neurosci. 1999;11:803–812. doi: 10.1046/j.1460-9568.1999.00487.x. 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Sewell WF. Neurotransmitters and synaptic transmission. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 503–533. [Google Scholar]
- Shnerson A, Devigne C, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. II. Ultrastructural findings. Dev Brain Res. 1982;2:77–88. doi: 10.1016/0165-3806(81)90060-2. 10.1016/0165-3806(81)90060-2. [DOI] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M, Eisen MD, Saunders JC, Parsons TD. Chick cochlear hair cell exocytosis mediated by dihydropyridine-sensitive calcium channels. J Physiol. 2001;535:689–696. doi: 10.1111/j.1469-7793.2001.00689.x. 10.1111/j.1469-7793.2001.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. 10.1016/S0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. 10.1016/S0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron. 2004;42:595–605. doi: 10.1016/s0896-6273(04)00254-5. 10.1016/S0896-6273(04)00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. 10.1016/S0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H. Synaptic ribbons: versatile signal transducers. Neuron. 2001;29:7–10. doi: 10.1016/s0896-6273(01)00175-1. 10.1016/S0896-6273(01)00175-1. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Electrophysiology of synaptic vesicle cycling. Annu Rev Physiol. 1999;61:725–752. doi: 10.1146/annurev.physiol.61.1.725. 10.1146/annurev.physiol.61.1.725. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–1188. doi: 10.1016/s0896-6273(00)80634-0. 10.1016/S0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. 10.1016/S0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 130–185. [Google Scholar]
- Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidanic M, Fuchs PA. Kinetic analysis of barium currents in chick cochlear hair cells. Biophys J. 1995;68:1323–1336. doi: 10.1016/S0006-3495(95)80305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]