Abstract
In the present study we sought to find interneurones responsible for the group I-evoked disynaptic excitation of hindlimb extensor motoneurones that occurs during fictive locomotion. Locomotion was produced by stimulation of the mesencephalic locomotor region (MLR) in decerebrate paralysed cats in which activation of ankle extensor group I afferents evoked a disynaptic excitation of motoneurones during the extension phase of fictive locomotion. Extracellular recordings were used to locate interneurones fulfilling all, or five of the six following criteria: (i) weak or no response to stimulation of extensor group I afferents in the absence of locomotion; (ii) strong group I activation during locomotion; (iii) group I activation at monosynaptic latencies; (iv) strong group I activation during only the extensor phase of locomotion; and (v) antidromic activation from the extensor motor nuclei; but (vi) no antidromic activation from rostral spinal segments. Candidate excitatory interneurones were located in mid to caudal parts of the L7 segments in areas where monosynaptic field potentials were evoked by group I afferents, within 2 mm of the stimulation site in the ventral horn from which they were antidromically activated. All were activated during extension by stimulation of group I afferents in extensor nerves. In the absence of peripheral nerve stimulation, six of the seven candidate excitatory interneurones were rhythmically active with maximal activation during the extension phase of fictive locomotion. Rhythmic activity during extension was also seen in five additional interneurones located near candidate interneurones but not activated by group I strength stimulation of the tested nerves. We suggest that the lumbosacral interneurones located in the intermediate laminae that can be activated by extensor group I afferents during the extension phase are a previously unknown population of interneurones, and may mediate group I-evoked disynaptic excitation of extensor motoneurones. Their rhythmic activity suggests that they also provide central excitatory drive to extensor motoneurones during locomotion.
Under several experimental conditions (i.e. in anaesthetized and decerebrate cats with or without spinalization) the dominant effect of stimulation of ankle extensor group I afferents is inhibition of extensor motoneurones (Eccles et al. 1957; Jankowska et al. 1981b, c; for review see Jankowska, 1992). Since the inhibition is most potent in homonymous and synergist extensor motoneurones and is mediated by neurones co-excited by group Ia muscle spindle and group Ib tendon organ afferents, it has been termed ‘group I non-reciprocal inhibition’ (Jankowska et al. 1981b). Many of the interneurones responsible for non-reciprocal inhibition are located in the intermediate zone (laminae V–VI) of the lumbosacral cord (Jankowska et al. 1981a; Brink et al. 1983b) and have ascending axon collaterals in the dorsolateral funiculus (DLF) as well as descending collaterals projecting to motoneurones (Hongo et al. 1983; see also Fern et al. 1988). In fact all group I excited interneurones with such axonal projections were found to be inhibitory (Brink et al. 1983a; Hongo et al. 1983).
During locomotion the interneuronally mediated reflex actions of group I extensor afferents on extensor motoneurones reverse from inhibitory to excitatory. This has been demonstrated using a number of experimental approaches. Short trains of stimuli at group I strength during the extensor phase of fictive locomotion (Conway et al. 1987; Gossard et al. 1994: Guertin et al. 1995), as well as treadmill locomotion (Pearson & Collins, 1993; Whelan et al. 1995; Hiebert & Pearson, 1999), can enhance the activity of hindlimb extensor motoneurones. These actions have been attributed in part to the activation of the spinal locomotor neuronal networks by group I afferents (Gossard et al. 1994; Guertin et al. 1995; McCrea, 2001).
Intracellular recordings from extensor motoneurones in decerebrate cats show that the reversal of group I reflexes during fictive locomotion also involves a suppression of non-reciprocal group I inhibition (Gossard et al. 1994; McCrea et al. 1995; Angel et al. 1996) and the appearance of disynaptic excitation from extensor group I afferents to extensor motoneurones during fictive locomotion (Schomburg & Behrends, 1978; McCrea et al. 1995; Angel et al. 1996). Group I non-reciprocal inhibition is suppressed during both the flexion and extension phases of MLR-evoked fictive locomotion while the disynaptic excitation is only recorded in extensor motoneurones during the extension phase of fictive locomotion. The central latency of the group I-evoked EPSPs (mean, 1.5–1.6 ms) indicates a disynaptic pathway with a single interneurone interposed between extensor group I afferents and extensor motoneurones (Angel et al. 1996). Evidence has also been presented that both group Ia and Ib afferents from extensor nerves evoke disynaptic EPSPs during extension in homonymous (McCrea et al. 1995; Angel et al. 1996) and close synergist extensor motoneurones (McCrea et al. 1995; Angel et al. 1996), as well as in extensor motoneurones operating at different joints (Angel et al. 1996).
Based on the incidence and distribution of group I disynaptic EPSPs recorded in extensor motoneurones during fictive locomotion (McCrea et al. 1995; Angel et al. 1996) and on previous studies of inhibitory interneurones (see above), the following criteria were used to identify the responsible excitatory interneurones: (i) weak or no response to stimulation of extensor nerve group I afferents at rest; (ii) a strong response to stimulation of group I afferents during locomotion; (iii) central latencies compatible with monosynaptic coupling between afferents and interneurones; (iv) activation during the extension, but not flexion phase; and (v) antidromic activation by stimuli applied within extensor motor nuclei; but (vi) not to the DLF in more rostral segments.
The principal aims of this investigation were to locate candidate interneurones mediating group I disynaptic excitation of extensor motoneurones during locomotion on the basis of these criteria, and to examine their activity during fictive locomotion. Some of this work has been presented previously (McCrea, 1998, 2001).
Methods
Preparation
Data on interneurones were collected from six decerebrate and paralysed cats in which fictive locomotion was elicited by monopolar electrical stimulation of the MLR. All surgical and experimental protocols were in compliance with the guidelines set out by the Canadian Council on Animal Care and the University of Manitoba. Surgery was performed on cats anaesthetized with a 1–1.6% halothane delivered in a mixture of 70% nitrous oxide and 30% O2 mixture. A surgical plane of anaesthesia was confirmed by continuous monitoring of the arterial blood pressure via a carotid artery cannula and by repeatedly testing for the lack of pedal withdrawal and corneal reflexes as well as muscle tone. Data from an additional five cats used in other studies (Angel et al. 1996) were examined to determine whether MLR stimulation facilitated group I disynaptic EPSPs recorded in extensor motoneurones. Details of the preparation and brainstem stimulation are provided elsewhere (Guertin et al. 1995; Quevedo et al. 2000). Briefly, the sequence was to induce anaesthesia, dissect the peripheral nerves and make the laminectomy and then perform the decerebration removing the cortices and rostral brainstem structures before withdrawal of anaesthetic agents, induction of paralysis and mechanical ventilation.
The following peripheral nerves were cut, dissected and placed on bipolar electrodes for either stimulation or recording: medial gastrocnemius (MG), lateral gastrocnemious–soleus (LGS), or MG and LGS taken together as GS, plantaris (Pl), semimembranosus and anterior biceps taken together (SmAB), posterior biceps and semitendinosus taken together (PBSt), tibialis anterior (TA), flexor digitorum and hallucis longus (FDHL), the cutaneous branch of the superficial peroneal nerve, caudal and lateral cutaneous branches of the sural nerve, and the mixed cutaneous and muscular tibial nerve. Quadriceps (vasti and rectus femoris) and sartorius nerves (Sart, medial and lateral branches) were placed in subcutaneous bipolar cuff electrodes. Contralateral (Co) PBSt and SmAB nerves were cut and dissected and used for monitoring fictive locomotion. The remaining sciatic, femoral and obturator nerve branches were cut bilaterally and the tendons around both hips severed. Following laminectomy at T13 and L3–S1 levels a precollicular–postmammillary decerebration was performed by blunt transection. Both cortices and all tissue rostral to the transection were removed and anaesthesia stopped. The cats were paralysed with gallamine triethiodide (Flaxedil, 2–3 mg kg−1 h−1) and artificially ventilated. End tidal CO2 levels were monitored and kept between 3 and 5%. A lethal injection of barbiturate anaesthetic was administered at the end of the experiment.
Stimulation and recording
The search for candidate interneurones began with assessing the presence of disynaptic EPSPs evoked by group I afferents in extensor motoneurones during locomotion. The methods of recording disynaptic EPSPs during locomotion are detailed elsewhere (McCrea et al. 1995; Angel et al. 1996). Briefly, glass microelectrodes (tip diameter, 1.8 μm; resistance, 2–5 MΩ) filled with 2 m potassium citrate and 50 mm QX-314 (N-[2, 6-dimethylphenylcarbomonyl-methyl]triethylammonim bromide; Alamone Laboratories, Jerusalem, Israel) to block action potentials, were used to record intracellularly from antidromically identified extensor motoneurones. Averages of extensor nerve group I afferent-evoked disynaptic EPSPs were made during the extension and flexion phases of locomotion. The range of latencies for accepting EPSPs as disynaptic was 1.0–1.9 ms (Angel et al. 1996). Only after the presence of group I disynaptic EPSPs in motoneurones was established did the experiments continue. The intracellular recording microelectrode was replaced with a tungsten stimulating electrode (impedance, 1 MΩ) at the same location. Electrodes in the ventral horn were inserted at an angle of 15 deg from the vertical (tip pointing rostrally). Constant current pulses (0.2 ms) were then applied along this electrode track while recording from the MG, LGS, Pl and SmAB nerves in order to locate extensor motor nuclei at the border between the L7 and S1 or in the S1 segment. Intraspinal stimulation (10–200 μA) was applied at depths ranging from 1.5 to 2.8 mm to antidromically activate interneurones that project to the extensor motor nuclei. Intraspinal stimulation of 50 μA should excite axonal branches of interneurones within about a 0.5 mm radius (see Gustafsson & Jankowska, 1976).
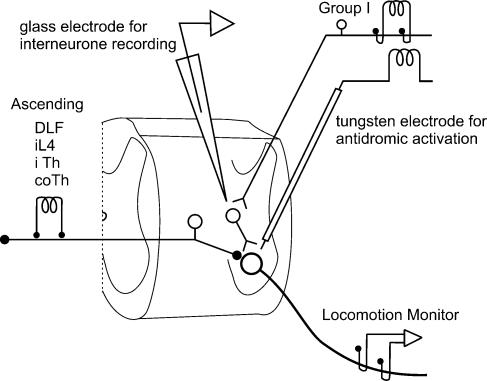
A second microelectrode (a standard glass capillary tubing, filled with 2 m sodium citrate solution, with a 2.0–2.2 μm tip diameter and ∼2 MΩ resistance) was used for extracellular recording from interneurones within a similar or more rostral region. This microelectrode was mounted on a second arc-styled manipulator and moved independently of the tungsten microelectrode used for antidromic activation of interneurones (see Fig. 1); it was inserted at an angle of 25 deg from the vertical (tip pointing caudally). The tips of the recording and stimulating electrodes were often about 1 mm from each other in the rostro-caudal plane at the surface of the cord. At a depth corresponding to the motor nucleus and intermediate nucleus, the distance separating the two was reduced substantially. The typical search strategy involved tracking for cells in the absence of locomotion which were antidromically activated from the extensor motor nucleus but not (or very weakly), orthodromically activated from group I afferents. Fictive locomotion was then initiated by MLR stimulation, and group I input onto these cells was then re-assessed.
Figure 1. Experimental arrangement used to locate premotor interneurones in pathways from group I afferents.
The glass recording microelectrode and tungsten stimulating electrodes were inserted through the dorsal columns and the lateral funiculus, respectively. Within the grey mater the stimulating and recording microelectrode tips were often separated by < 1 mm in the rostro-caudal plane. Pairs of stimulating electrodes were placed on the ipsilateral and contralateral DLF at L4 and on both ipsilateral and contralateral thoracic dorsal columns for antidromic activation of laminae V–VI inhibitory cells and ascending tract cells.
Bipolar AgCl ball electrodes were placed on the left (ipsilateral) DLF at L4 for antidromic activation of ascending axon collaterals of laminae V–VI interneurones which mediate non-reciprocal inhibition of motoneurones, and inhibition of cells of Clarke's column (Brink et al. 1983b; Hongo et al. 1983). Bipolar stimulating electrodes were also positioned bilaterally at the division between the DLF and dorsal columns in T13. Cells antidromically activated from either of these electrodes (ascending tract cells) were excluded from further analysis. Data were captured on-line and stored on computer for subsequent averaging and analysis using programs developed with the Spinal Cord Research Centre. The sampling rates of the microelectrode, cord dorsum and rectified-integrated electroneurogram (ENG) recordings were 5 or 10 kHz, 2500 Hz and 500 Hz, respectively. Both continuous records for the 1–2 min of the run and stimulus locked sweeps were captured. A second data system operating at a higher rate (20 kHz) was also used to store shorter duration microelectrode and cord dorsum records for subsequent display as superimposed sweeps and to determine latencies of interneurone activation.
Assessment of MLR-evoked facilitation of group I disynaptic EPSPs
Intracellular records from motoneurones from a previous study (Angel et al. 1996) were re-analysed to determine whether the amplitudes of group I evoked disynaptic EPSPs in extensor motoneurones were facilitated by stimulation of the MLR. In those experiments (and the present) the MLR was stimulated continuously at approximately 15 Hz and randomly with respect to peripheral extensor nerve stimulation (typically at 4 Hz). To assess whether a MLR shock delivered at short intervals from the peripheral nerve stimulus increased disynaptic EPSPs, intracellular records obtained during the extension phase were sorted into three groups: group 1, those in which a stimulus from the MLR preceded the peripheral nerve stimulation by 1–10 ms (potentially facilitating the disynaptic EPSP); group 2, where MLR stimulation was delivered up to 10 ms after nerve stimulation and was unable to affect the disynaptic EPSP (control disynaptic EPSP); and group 3, those in which MLR stimuli occurred more than 10 ms after the disynaptic EPSP to avoid effects of the disynaptic EPSP on the MLR-evoked EPSP (pure MLR-evoked EPSP). Averages of the disynaptic EPSPs obtained without an immediately preceding MLR stimulus (group 2) were compared to those preceded by MLR stimulation (group 1). Facilitation of the disynaptic EPSP was concluded when the peak amplitude of the disynaptic EPSP preceded by MLR stimuli was larger than the sum of the MLR EPSP and the disynaptic EPSP which was not preceded by an MLR stimulus.
Results
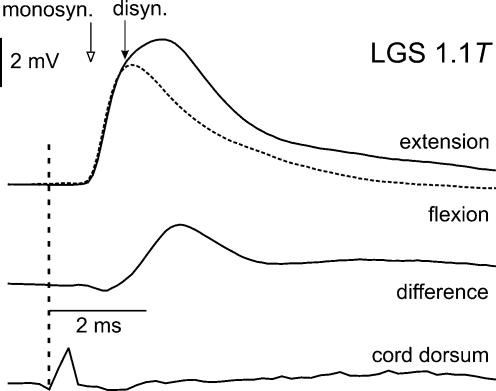
All experiments began with intracellular recording from hindlimb extensor motoneurones to confirm the presence of disynaptic group I EPSPs evoked by extensor nerve stimulation during the extension phase of fictive locomotion. Averages of EPSPs evoked during the flexion and extension phases were calculated immediately after collecting the intracellular records. The top traces in Fig. 2 show averaged intracellular records from an LGS motoneurone obtained while stimulating the homonymous nerve at 1.1 times the threshold for the most excitable fibres in the nerve (1.1T) during the extension (continuous line) and flexion (dashed line) phases of the fictive locomotor step cycle. Note the depolarization (filled arrow) that follows the monosynaptic EPSP (open arrow) during the extension phase. The arithmetic difference of the records obtained during extension and flexion is plotted underneath. The latency of the extensor-related depolarization is about 1.6 ms, as expected for disynaptic excitation. This preparation was thus deemed suitable for searching for the interneurones responsible for group I excitation during locomotion. Because of the possibility of changes in the preparation (see Discussion in Angel et al. 1996) the presence of disynaptic excitation of motoneurones was verified both at the beginning and at later stages of the experiments. Data obtained from interneurones without a subsequent demonstration of disynaptic EPSPs in extensors are not reported.
Figure 2. Example of disynaptic EPSPs in extensor motoneurones indicates the preparation is suitable for locating relevant interneurones during extension.
Top traces are superimposed averaged records of EPSPs evoked in a LGS motoneurone by near-threshold (1.1 T) stimulation of group I afferents in the LGS nerve during extension (continuous line) and flexion (dashed line) phases of the locomotor cycle. The difference between the two records is shown underneath. Open arrow indicates the onset of monosynaptic EPSPs. The filled arrow indicates the onset of disynaptic EPSPs which were evoked only during the extension phase. Bottom trace shows the afferent volley.
In the initial two experiments the search for interneurones was made in the L6 and the rostral part of the L7 segments of the spinal cord, where inhibitory interneurones with group Ia and Ib input are known to be located (Jankowska et al. 1981a, Brink et al. 1983a; Hongo et al. 1983). No interneurones were encountered at these locations which fulfilled the criteria for the excitatory interneurones in question. In the subsequent four experiments the search was carried out more caudally and closer to the tungsten stimulating electrode located in the motor nuclei. The caudal part of the L7 and the rostral part of the S1 segments proved to be the most reliable for locating candidate interneurones.
Locomotion-related inhibition of interneurones mediating non-reciprocal inhibition from group I afferents
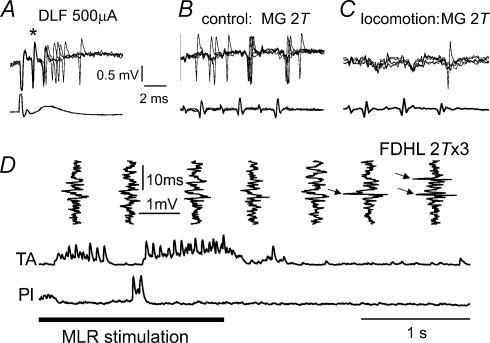
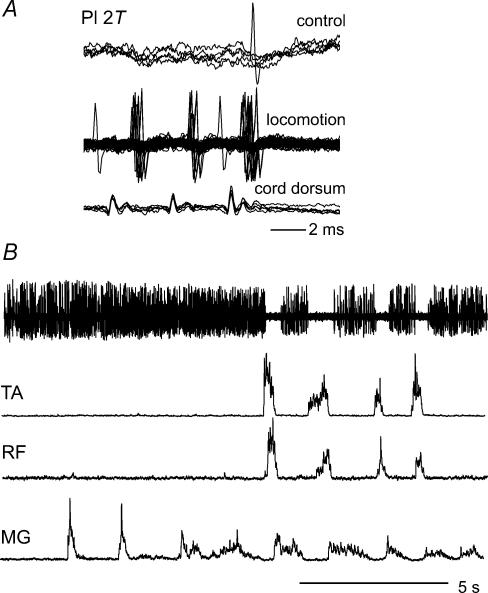
Although cells with strong group I input in the absence of fictive locomotion were usually rejected from further analysis (see criteria in Introduction), Fig. 3 illustrates changes in the responses to peripheral nerve stimulation during fictive locomotion of two such interneurones. Figure 3A–C are records from a lamina V–VI interneurone in L7 that was antidromically activated from the L4 DLF (Fig. 3A, asterisk) and activated at monosynaptic latencies by stimulation of the MG nerve in the absence of locomotion (Fig. 3B). This unit was also monosynaptically activated by Pl group I afferents and disynaptically activated by low threshold cutaneous afferents (data not shown). Records in Fig. 3C show that the effectiveness of activation of this neurone by group I afferents was markedly reduced during fictive locomotion. Only one of the 15 stimulus presentations (a three-shock train, five overlaid sweeps) evoked a spike. In view of the responses of this cell to stimulation of group I afferents in the absence of locomotion, and the collateral projection to the L4 segment, this interneurone is likely to mediate non-reciprocal inhibition. The vertical traces in Fig. 3D are extracellular records from another neurone antidromically activated from the gastrocnemius–soleus motor nucleus during fictive locomotion. Stimulation of the FDHL nerve evoked no spikes during locomotion but became effective (arrows) within 1 s of the end of fictive locomotion. The depression of group I activation is in keeping with the depression of non-reciprocal inhibition recorded in motoneurones during fictive locomotion (Gossard et al. 1994; McCrea et al. 1995; Angel et al. 1996).
Figure 3. Inhibition during locomotion of responses of two interneurones which probably mediate group I non-reciprocal inhibition of motoneurones.
A–C, records from an interneurone antidromically activated by stimulation of the L4 DLF (A, *), activated by MG group I afferents at monosynaptic latencies in the absence of locomotion (B) and only weakly activated by the same stimuli during MLR-evoked locomotion (C). D, another interneurone monosynaptically activated by group I afferents at rest, which failed to respond to FDHL stimulation during locomotion, but became responsive to the same stimuli (arrows) within 1 s after terminating MLR stimulation (horizontal bar).
Identification of candidate excitatory interneurones
Seven interneurones in the present study displayed five or all six of the criteria expected of interneurones mediating disynaptic excitation of extensors during fictive locomotion (outlined in Introduction). None were antidromically activated by stimulation of the ipsilateral DLF at L4 or by stimulation at ipsi- or contralateral thoracic levels. All were unresponsive or only weakly responsive to extensor group I afferent input in the absence of locomotion but were readily activated by these afferents during the extension phase of locomotion. The latencies of their activation during extension (< 1 ms) were consistent with a monosynaptic coupling between group I afferents and the interneurones. Four of these seven cells were, in addition, antidromically activated from an extensor motor nucleus and thus fulfilled all the requirements for being considered as mediating disynaptic excitation (Figs 4–6). Sural or superficial peroneal stimulation failed to activate all three of these seven interneurones tested in the absence of fictive locomotion. The effects of cutaneous stimulation were not examined during locomotion.
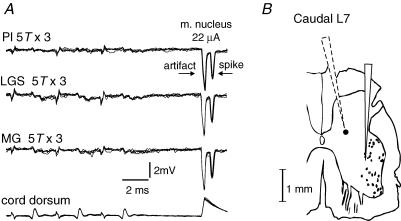
Figure 4. Examples of records from a candidate excitatory interneurone which is unresponsive to group I stimulation in the absence of locomotion.
A, overlayed extracellular recordings (n = 5) from an interneurone that was antidromically activated by stimuli applied in the ankle extensor motor nuclei (latency, 0.6 ms) but failed to respond to stimulation supramaximal for group I afferents (5T × 3, 300 Hz). Bottom trace is the cord dorsum recording of the afferent volleys. B, camera lucida reconstruction of the recording microelectrode track (dotted outline) indicating the estimated location (filled circle) of the interneurone illustrated in Figs 4A, 5, 6 and 8A. The solid outline to the right is the track of the tungsten stimulating electrode. The scale bar takes into account a 10% shrinkage due to mounting. This interneurone was located in the intermediate nucleus.
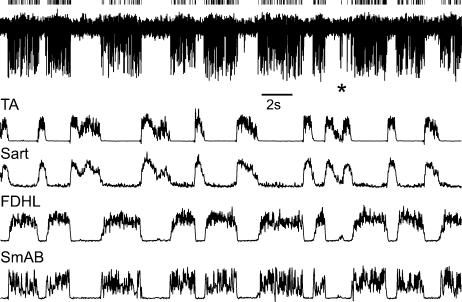
Figure 6. Spontaneous activity of a candidate excitatory interneurone during extension.
Top trace, activity of the interneurone illustrated in Figs 4 and 5 (largest spikes; represented by small vertical ticks) during MLR-evoked fictive locomotion in the absence of peripheral nerve stimulation. The responses are superimposed on responses of at least two other interneurones. The remaining four traces show discharges in two flexor (TA, Sart) and two extensor (FDL, SmAB) muscle nerves evoked simultaneously with responses of the interneurones.
Antidromic activation of candidate interneurones from extensor motor nucleus
Figure 4A illustrates two properties of interneurones considered as candidates for mediating group I-evoked disynaptic excitation; antidromic activation from the ventral horn and the lack of synaptic activation from extensor afferents in the absence of fictive locomotion. Figure 4A is a series of extracellular recordings from an interneurone obtained shortly after disynaptic EPSPs were recorded in the motoneurone illustrated in Fig. 2. This interneurone was activated by stimuli applied in the motor nuclei in the caudal part of the L7 segment, as shown in the right hand parts of the upper traces. The location of the tungsten stimulating electrode was judged to be near GS motoneurones because of the much larger amplitude of the efferent discharge recorded from GS than other peripheral nerves (data not shown). The threshold current for activation of this interneurone was 15 μA, indicating that the interneurone axon was stimulated within a short distance from the electrode tip. The latencies from stimulation within the motor nucleus to activation of the interneurones were 0.6 ms (for the interneurone illustrated) and 0.5, 0.65 and 0.8 ms for the other three antidromically activated interneurones. Since these latencies include the time for activation of the axon (0.2–0.3 ms) the conduction times are even shorter and indicate an antidromic activation of the interneurones. Such short latencies are too short to be compatible with synaptic activation from other interneurones or by afferents with collaterals terminating both within the motor nuclei and on interneurones. Antidromic identification was also indicated by constant latencies of the responses and by their capability to follow trains of stimuli at 400 Hz. The use of the collision test to ensure that the responses evoked from the motor nuclei were induced antidromically was precluded by the short latencies of these responses.
Failure to antidromically activate the remaining three interneurones may not indicate the lack of their projections to the extensor motor nucleus: once the tungsten electrode was placed within the motor nuclei it was only moved in the dorsal–ventral plane. Thus rostral or caudal axonal branches of interneurones may not have been activated. In addition, the close proximity of the stimulating electrode to the recording electrode produced stimulus artefacts that may have obscured very short latency antidromic spikes.
Group I activation of candidate interneurones during fictive locomotion
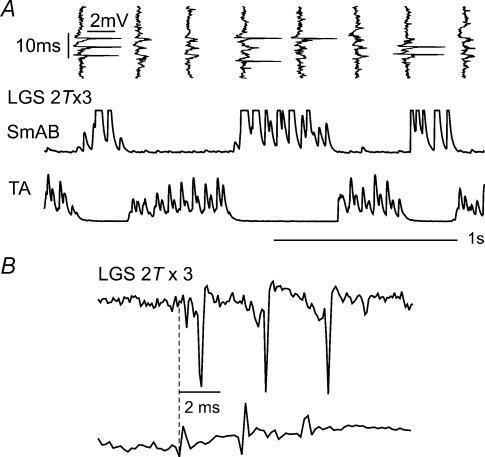
The records in Fig. 4 also show that this antidromically activated interneurone was neither spontaneously active nor able to be activated by peripheral nerve stimulation in the absence of fictive locomotion. Stimulation of ankle extensor nerves (three-shock trains, five sweeps overlaid) at 5T (i.e. supra-maximal for activation of group I afferents) failed to evoke any spikes. However, Fig. 5 shows that the same interneurone was activated by group I afferents during the extension phase of the locomotor step cycle, as expected of interneurones mediating disynaptic excitation of extensors (see Introduction). For example, a spike can be seen after each of the three 2T stimuli in the left-most vertically orientated records in Fig. 5A. These records were obtained as discharges were occurring in the hip extensor (SmAB) muscle nerve. Records in Fig. 5B show that the central latency of these spikes from the group I afferent volley was 0.9 ms and consistent with a monosynaptic linkage. MG and Pl group I afferents evoked responses with similar latencies (data not shown). The upper records in Fig. 7A show the locomotor-dependent group I excitation of another interneurone which was not antidromically activated from within the stimulated motor nucleus. They show that before locomotion only one of the 15 shocks delivered to the Pl nerve (three-shock trains, five presentations overlaid) evoked an action potential. During the extension phase of locomotion, the same stimuli activated the neurone most of the time (minimum latency of 0.8 ms for spikes evoked by the third shock in the train). Group I stimulation did not discharge this cell during flexion. This cell did not have an ascending axonal projection but we were unable to antidromically activate it from the motor nucleus. The latencies of activation of the remaining five interneurones were 0.8 ms from the incoming group I afferent volley and were as required for monosynaptically evoked responses. Figure 5A also illustrates the common finding that the interneurones were as unresponsive to peripheral nerve stimulation during the flexion phase of the fictive locomotor step cycle (i.e. during TA activity) as they were in the absence of locomotion.
Figure 5. Activation of a candidate excitatory interneurone by group I afferents during fictive locomotion.
A, top traces are vertically orientated extracellular records from the interneurone illustrated in Figs 4 and 6, showing its responses to stimulation of the LGS nerve during locomotion. The records were obtained at the same time as the horizontally displayed discharges in the extensor (SmAB) and flexor (TA) nerves during locomotion. B, records obtained during fictive locomotion at an expanded time base, showing the latency of activation of the interneurone (0.9 ms) with respect to the incoming volleys.
Figure 7. Tonic activity of a candidate interneurone in the absence of flexor nerve activity.
A, a comparison of responses of an interneurone to group I afferents before and during fictive locomotion (at a minimal latency of 0.8 ms from the third incoming volley). B, activity of the same interneurone (largest spikes) during MLR-evoked fictive locomotion in the absence of peripheral nerve stimulation superimposed on spike potentials of at least one other interneurone. Note that tonic activation of the interneurones occurred when MLR stimulation produced rhythmic activation of only extensors (MG). The interneurones discharge rhythmically only when activation of flexors started to alternate with activation of extensors.
Figure 4B shows the location of the interneurone illustrated in Figs 4A, and 5A and B in Rexed's Lamina VI. Its location was estimated from a camera lucida tracing of the recording electrode track and the known depth of the recording site, taking into consideration a 10% shrinkage during histological procedures. The tips of the recording electrode and of the tungsten stimulating electrode were less than 300 μm apart in the rostro-caudal plane. The remaining interneurones were located at similar depths from the dorsal surface of the spinal cord, at sites where distinct monosynaptic focal field potentials were evoked by group I afferents of ankle extensors (see the traces in Fig. 4A with LGS and Pl nerve stimulation and the top traces in Fig. 7A). Six of these interneurones were found in mid to caudal parts of the L7 segment; within 1–2 mm from the tip of the tungsten stimulating electrode in the motor nuclei in the rostro-caudal plane, and one was found in the caudal part of the L6 segment. Estimation of distances between the cell body and the axonal projections to the motor nuclei took into account the insertion angles of the stimulating and the recording electrodes as well as the depths from the surface.
Spontaneous locomotor activity of candidate excitatory interneurones during extension
The two interneurones illustrated in Figs 4A and 6 and the remaining five interneurones in the present sample were not spontaneously active in the absence of locomotion. However, spontaneous rhythmic activity appeared in six of the seven interneurones during fictive locomotion. The top trace of Fig. 6 shows the rhythmic activity during fictive locomotion when the peripheral nerve stimulation was discontinued. The activity of several (at least three) neurones during fictive locomotion is apparent. The records in Fig. 6 were obtained immediately after those illustrated in Fig. 5. The largest spikes are of the interneurone illustrated in Figs 4 and 5. They were isolated using a window discriminator and are indicated by the small vertical bars above the record. The activity of this interneurone was approximately 15 Hz during the extension phase. Rhythmic activity of an interneurone which we failed to activate from motor nuclei is illustrated in the right part of the top trace in Fig. 7B; its activity coincided with that of extensor motoneurones.
The spontaneous rhythmic activity during fictive locomotion of an additional five interneurones not activated by extensor group I afferents was recorded while monitoring the activity of interneurones with group I activation during locomotion (e.g. the neurones in Figs 6 and 7). These additional cells include the smaller spikes in the top record of Fig. 6. The tight coupling of the activity of all of the neurones in Fig. 6 to the extension phase of fictive locomotion is evident, and similar coupling was seen in the case of the other interneurones without group I input. However, because there was no other characterization of these neurones, they are not included among the seven candidate interneurones.
Recruitment of candidate excitatory interneurones during fictive locomotion appears to occur in parallel with even minimal activation of extensor motoneurones. The asterisk in Fig. 6 shows a fictive locomotor step where there was an incomplete but still visible extensor phase (see the weak activity in the FDHL ENG and the reduction in TA and Sart activity). Both the largest and another smaller unit fired single action potentials during this brief extension phase.
The records in Fig. 7B show another feature, that flexor motoneurone activity is associated with a silencing of interneurone activity. The left portion of the records in Fig. 7B shows a period in which MLR stimulation produced extensor bursts but no flexor motoneurone bursts. There is sustained interneurone activity without obvious variation despite the modulation of extensor motoneurone activity as seen in the MG ENG. These tonic discharges become rhythmic with the appearance of alternating flexor (TA, RF) and extensor motoneurone activity.
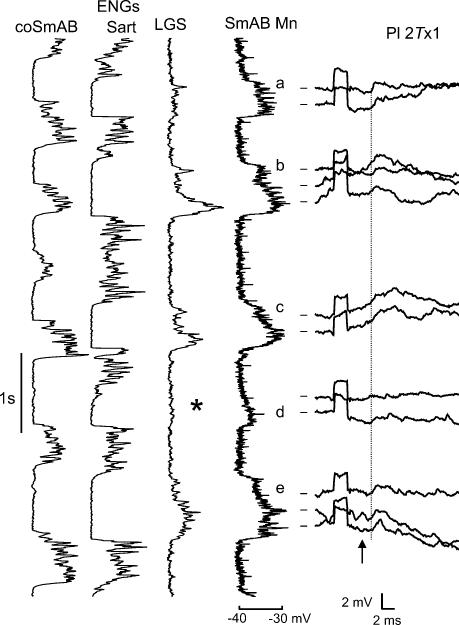
The close relationship between activation of extensor motoneurones and the appearance of disynaptic EPSPs in extensor motoneurones is illustrated in Fig. 8. This figure is from a bout of fictive locomotion in which there was an occasional failure in the alternating activation of flexors and extensors. In this figure, intracellular records from a SmAB motoneurone are shown at both low gain (vertical trace) and high gain (horizontal records). The Pl nerve was stimulated continuously at about 5 Hz. For clarity only those high gain records occurring during extension are plotted. The vertical intracellular records show five periods of depolarization (a–e) indicating the extension phases of the step cycle. The vertical dashed line (1.4 ms from the arrival of the Pl nerve volley at the cord dorsum) indicates the onset of distinct disynaptic EPSPs during each depolarizing period (except d). The failure to evoke disynaptic excitation (at d) coincides with weak depolarization of the SmAB motoneurone and the failure of activity in the ankle extensor (LGS) nerve. Thus it appears that the activity of candidate interneurones (e.g. Fig. 6) and the expression of disynaptic EPSPs in motoneurones (Fig. 8) are reduced when the activity of extensor motoneurones fails during fictive locomotion.
Figure 8. Relationship between disynaptic EPSPs and activity of the extensor rhythm generator.
Vertically orientated records from an ipsilateral flexor (Sart) and extensor (LGS) nerves are plotted alongside a low gain intracellular record from a SmAB motoneurone during MLR-evoked fictive locomotion. Horizontally plotted high gain intracellular records from this SmAB motoneurone show disynaptic EPSPs evoked by stimulation of Pl nerve during five extension phases (a–e). The arrow indicates arrival of group I afferent volleys. The dashed vertical line indicates onset of disynaptic EPSPs following these volleys (latency, 1.4 ms). Calibration pulse, 2 mV, 2 ms. Notice that the disynaptic EPSPs are largest when Pl stimulation occurs during large locomotor drive potentials (a, b, e). In contrast, when there is no activity in the extensor nerve (*) and the locomotor drive potential is small, the Pl-evoked disynaptic EPSPs are absent (d).
MLR activation of candidate excitatory interneurones
In these experiments fictive locomotion was evoked by continuous brainstem stimulation at 10–20 Hz. To investigate the possibility that the interneurones investigated can be activated by MLR stimulation, the continuous records of the cell illustrated in Figs 4, 5, 6 and the LGS ENG were divided into 60-ms segments triggered by the MLR stimulus delivery (Fig. 9, vertical dashed line). Fifty-one segments occurring in the extension phase are superimposed in Fig. 9A. Interneurone activity (top records) and corresponding LGS ENG activity are clearly related to MLR stimulation with the shortest latency between shock artefact and interneurone activity being about 6.8 ms. Two other interneurones of the sample of seven and two additional interneurones, rhythmically active during extension but not activated by the group I afferents tested, were activated following MLR stimulation at similar latencies. Two LGS ENG traces show large increases in LGS ENG activity but in most there is a small increase in activity 7–10 ms following the shock that becomes larger 40–50 ms after the MLR stimulation and at a time when interneurone activity is again prominent. The low digitization rate (500 Hz) of the ENG recording prevents an accurate estimation of the latencies, but the latencies of MLR-evoked responses in the interneurone and LGS nerve appear similar.
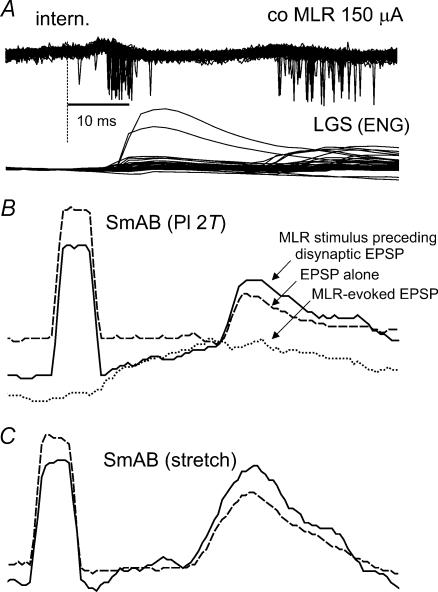
Figure 9. Evidence for spatial facilitation of synaptic actions of neurones activated by the MLR stimulation and of group I afferents onto interneurones mediating locomotion-related disynaptic EPSPs in motoneurones.
A (top traces), superimposed records (n = 51) of spikes of two or three interneurones locked to MLR stimuli during fictive locomotion; the traces were collected during the extension phase and aligned to the MLR stimulation indicated by the dashed vertical line. A (bottom traces), discharges recorded in the LGS peripheral nerve. B, three averaged intracellular records of potentials evoked in a SmAB motoneurone: disynaptic EPSPs evoked by Pl group I afferents (n = 25, continuous line) preceded by MLR stimuli by 1–10 ms (see text); disynaptic EPSPs evoked by the same stimuli (n = 187, dashed line) which were not preceded by MLR stimulation; EPSPs evoked by MLR stimulation alone without peripheral nerve stimulation (n = 17, dotted line). C, as in B but for EPSPs evoked by selective activation of group Ia triceps surae and Pl afferents by muscle stretch. Calibration pulse, 2 mV, 2 ms.
Evidence for MLR facilitation of the disynaptic component of the group I-evoked disynaptic EPSPs was found in two of eight motoneurones recorded during a previous study (Angel et al. 1996). Using records from these motoneurones the temporal relationship between MLR stimuli and amplitudes of the EPSPs evoked during the extension phase of MLR-evoked fictive locomotion was analysed as described in Methods. Figure 9B shows a comparison of an averaged Pl-evoked disynaptic EPSP recorded in a SmAB motoneurone when group I stimuli were preceded within 10 ms by MLR stimulation (continuous line) or occurred without MLR stimulation (dashed line). The dotted traces show the effect of MLR stimulation alone. Such MLR-evoked effects are usually excitatory during the locomotor phase in which the motoneurone is depolarized (Shefchyk & Jordan, 1984; Degtyarenko et al. 1998b; Noga et al. 2003). The facilitation of disynaptic EPSP amplitude when peripheral nerve stimulation was preceded by MLR stimulation is consistent with the finding (Fig. 9B) of an MLR-evoked spatial facilitation of the candidate interneurones. Figure 9C illustrates a similar facilitation of disynaptic EPSPs in another SmAB motoneurone. The EPSPs were evoked in this case, by selective activation of Ia afferents from triceps surae and plantaris by single brief stretches of the Achilles tendon (see Methods in Angel et al. 1996).
Discussion
In anaesthetized preparations and in decerebrate preparations in the absence of locomotion, activation of extensor group I afferents evokes a general inhibition of extensor motoneurones, the non-reciprocal inhibition. This inhibition is suppressed with the onset of fictive locomotion and during both the flexor and extensor phases (Gossard et al. 1994; McCrea et al. 1995; Angel et al. 1996). Figure 3A–C shows the suppression during locomotion of group I activation of an interneurone likely to mediate non-reciprocal inhibition. As argued elsewhere (Angel et al. 1996) it is unlikely that a presynaptic inhibition of group I afferent terminals ending on these interneurones is responsible for the suppression of non-reciprocal inhibition during locomotion. Instead a tonic inhibition (from unknown sources) is more likely to account for the reduction in non-reciprocal inhibition of motoneurones during locomotion. The primary objective of the present study was to locate candidate interneurones that could mediate the group I disynaptic excitation of extensor motoneurones that emerges during fictive locomotion. It was thus important to search for these interneurones in preparations in which stimulation of group I ankle extensor afferents evoked EPSPs instead of IPSPs in extensor motoneurones during fictive locomotion. The presence of disynaptic EPSPs in extensor motoneurones during the extension phase of fictive locomotion (e.g. Figure 2) was confirmed either before or after obtaining the interneurone records for all interneurones described here.
Our main finding is the description of a group of interneurones that can be activated by extensor group I afferents during the extension, but rarely the flexion phase of fictive locomotion. Some of these cells are also rhythmically active during fictive locomotion in the absence of nerve stimulation, their activity coinciding with activation of extensor motoneurones. Direct evidence that these interneurones are excitatory is still missing. However, this study presents data on the characteristics and location of candidate interneurones that provide the foundation for future investigations of the functions of these interneurones.
Four interneurones fulfilled all criteria of interneurones mediating disynaptic excitation from group I afferents. Three were located in the intermediate zone in mid to caudal parts of the L7 segment of the spinal cord and the fourth in the L6 segment. These four interneurones were antidromically activated from an extensor motor nucleus. In common with three other interneurones not antidromically activated from the motor nucleus, they were unresponsive or only weakly responsive to group I strength stimulation of extensor nerves in the absence of locomotion. All seven neurones could be readily activated at monosynaptic latencies by group I afferents during the extension phase of locomotion. All seven were not antidromically activated from the L4 or thoracic spinal levels. Although three interneurones failed to be activated from the region of the ventral horn traversed by the stimulating electrode, the possibility that these interneurones projected to a motor nucleus some distance away from the stimulation site cannot be ruled out. Alternatively, these interneurones may be part of a subpopulation of cells recruited by extensor group I afferents during locomotion, with as yet unknown projections.
The latency of interneurone activation from group I afferents was about 0.8 ms. Adding the latencies of antidromic activation from the motor nucleus (0.5–0.8 ms) and an estimated 0.3 ms for the synaptic delay between the interneurone and motoneurones gives a total latency of 1.6–1.9 ms. This is consistent with the possibility that these interneurones mediate the group I-evoked EPSPs observed in extensors during extension (range, 1.1–1.9 ms; Angel et al. 1996). Such latencies have been considered to indicate disynaptically mediated reflexes evoked by group I afferents at rest (Eccles et al. 1957; Jankowska et al. 1981b, c) and during locomotion (McCrea et al. 1995; Angel et al. 1996). They are also similar to the latencies of locomotor-dependent excitation recorded on the falling phase of monosynaptic EPSPs during fictive locomotion reported in earlier studies (Schomburg & Behrends, 1978; Shefchyk et al. 1984). We postulate therefore that these candidate interneurones mediate the group I-evoked disynaptic excitation of extensor motoneurones during the extension phase of fictive locomotion.
The ability of group I afferents from different nerves to excite the candidate interneurones was not systematically investigated nor was the relative strength of activation from group I afferents from various nerves evaluated. Tests for interneurone activation by group Ia afferents were made primarily using stimulation of ankle extensor nerves because these afferents evoke a widespread distribution of disynaptic EPSPs in extensor motoneurones (Angel et al. 1996). However, the pattern of disynaptic EPSPs evoked in hindlimb motoneurones suggests that subpopulations of excitatory interneurones exist that differ in the sources of their group I input (Angel et al. 1996). For example hip extensor afferents evoke disynaptic EPSPs in hip extensor but not ankle motoneurones during extension whereas ankle extensor afferents evoke EPSPs in hip, knee and ankle extensor motoneurones (Angel et al. 1996). Further analysis, preferably based on intracellular recording, will be needed for an assessment of the pattern of group I (and other) afferent input to these interneurones.
Is this a new population of spinal interneurones?
It is unlikely that the interneurones investigated in this study are laminae V–VI interneurones mediating group I non-reciprocal inhibition. Lumbosacral laminae V–VI inhibitory interneurones have an ascending axon collateral in the DLF (Hongo et al. 1983; Brink et al. 1983) and the majority of those activated by group I afferents in non-locomoting preparations have been found to have such collaterals (Fern et al. 1988). In approximately 50% of such interneurones disynaptic cutaneous input has been found (Harrison & Jankowska, 1985). In contrast to the interneurone described in Fig. 3A–C, candidate excitatory interneurones neither projected to the level of Clarke's column nor were effectively activated by group I or (when tested) cutaneous afferents in the absence of locomotion.
Gossard et al. (1994) recorded from interneurones proposed to be interposed in a locomotion-related polysynaptic group I pathway. These interneurones were located in lamina VII in the L6 segment and were maximally activated from group I afferents during flexion, with a minimum central latency of 3 ms. This contrasts with the monosynaptic activation of the interneurones in the present study which were active during extension. Thus, the population of interneurones described by Gossard et al. (1994) clearly differs from that reported here.
During a search for first order interneurones in polysynaptic pathways activated by group I afferents (Fedirchuk et al. 1994) a few interneurones were encountered with properties similar to those of the neurones described here. The study by Fedirchuk et al. (1994) predated a full appreciation of the pattern of disynaptic excitation of motoneurones and the consequent development of the criteria for interneurone classification used in the present study. Therefore, it is possible that some of the interneurones they investigated belonged to the same class of cells described here.
It is unlikely that candidate excitatory interneurones are Ia inhibitory interneurones mediating reciprocal inhibition. Such interneurones are located in the ventral horn adjacent to lamina IX (Jankowska & Lindstrom, 1972) whereas the interneurones in the present study were located in the intermediate nucleus. Furthermore, Ia inhibitory interneurones can be readily activated by stimulation of group Ia afferents, both in non-locomoting preparations and during fictive locomotion (e.g. McCrea et al. 1980). It is even more unlikely that any interneurones investigated here were Renshaw cells even though stimulation of peripheral nerves at 5T (e.g. Fig. 4) would have recruited most of the motoneurone axons antidromically, and hence activated Renshaw cells. Renshaw cells are located more ventrally and have characteristic repetitive firing upon stimulation of muscle efferents both at rest and during MLR-evoked fictive locomotion (McCrea et al. 1980).
There are two additional interneuronal populations described in non-locomoting preparations from which the present interneurones should be differentiated. One is the population of interneurones co-excited by group I afferents (monosynaptically) and high threshold muscle, skin and joint afferents (polysynaptically) during fictive locomotion in spinal non-anaesthetized preparation treated with l-3,4-dihyhdroxyphenylalanine (l-DOPA) (Jankowska et al. 1967). However, as most of those interneurones are Ia inhibitory interneurones (Fu et al. 1975) it is unlikely that they are the same population as those reported here.
The second population is that of intermediate zone interneurones with strong excitation from group II afferents, weak excitation from group I afferents (Edgley & Jankowska, 1987; Riddell & Hadian, 2000) and including last order excitatory as well as inhibitory interneurones (Cavallari et al. 1987). Half of the interneurones of this type investigated during fictive locomotion are rhythmically active during flexion and the other half are tonically inhibited throughout locomotion (Shefchyk et al. 1990). It is thus unlikely that the present interneurones with strong group I activation and rhythmic activity during extension are interneurones in these group II reflex pathways.
To our knowledge, there have been no previous attempts to identify the interneurones investigated in the present study. Because most studies in non-locomoting preparations have used stimulation of peripheral afferents as a means to locate interneurones, previous surveys of interneurones with group I input would probably have missed those that only become activated by group I afferents during fictive locomotion. Figures 4 and 6 illustrate the difficulty in finding such interneurones. Rhythmic activity of other neurones in the immediate vicinity during fictive locomotion further complicates assessment of their locomotor activity. However, the change in the responsiveness of these neurones to group I input associated with the appearance of fictive locomotion provides a strong indication that these interneurones belong to a previously undescribed population of excitatory interneurones.
There is also a locomotor-related disynaptic group I excitation of flexor (Degtyarenko et al. 1998a; Quevedo et al. 2000) and bifunctional (Quevedo et al. 2000) motoneurones. Stimulation of group I afferents in flexor nerves evokes disynaptic excitation of flexor motoneurones and although larger during flexion, these EPSPs are present in both phases (Quevedo et al. 2000). Stimulation of group I afferents in bifunctional muscle nerves similarly produces disynaptic EPSPs in bifunctional motoneurones that are usually largest during the transitions between the flexion and extension phases (Quevedo et al. 2000). The dependence on extensor activity for the activity of interneurones reported here and their activation by extensor afferents makes it unlikely that any of the present interneurones also excite flexor motoneurones. The possibility that they might also project to and excite some bifunctional motoneurones is more difficult to assess. Angel et al. (1996) demonstrated extensor group I-evoked excitation of PBSt motoneurones during the extension phase that might be mediated by interneurones of the type described here. While it is unlikely that the present interneurones produce excitation of bifunctional motoneurones during the flexion phase, the possibility that afferents from bifunctional muscles can activate the interneurones was not tested.
Spontaneous activity during fictive locomotion
Spontaneous firing of the investigated interneurones during the extension phase of MLR-evoked fictive locomotion was an unexpected finding. If as predicted, these interneurones are excitatory they would serve two purposes. They would be responsible for evoking disynaptic reflex excitation and their spontaneous rhythmic activity would contribute to the excitation of extensor motoneurones during locomotion. The question arises as to whether these interneurones are an integral part of the spinal neuronal network of locomotion. The main line of evidence that suggests otherwise stems from the observation that some preparations with coordinated fictive locomotion may not display group I-evoked disynaptic EPSPs in extensor motoneurones (McCrea et al. 1995; Angel et al. 1996). Thus the basic locomotor rhythm in motoneurones does not require the contribution of the interneurones mediating group I-evoked disynaptic EPSPs. Nevertheless, the tight coupling of the activity of candidate interneurones to the extensor phase (Fig. 6), and the covariance of group I disynaptic EPSP amplitude and of extensor locomotor drive (Fig. 8) suggest that the activity of candidate interneurones is closely regulated by the same systems that produce excitation of extensor motoneurones. However in those preparations in which these interneurones are active, they would provide a portion of the excitatory drive to extensor motoneurones and hence should be considered as part of the central locomotor generating networks. It is possible that the present interneurones also evoke the disynaptic EPSPs in extensor motoneurones during fictive scratching (Perreault et al. 1999a). During the inspiration phase of respiration, longer latency EPSPs occur in intercostal motoneurones on the falling portion of muscle afferent-evoked monosynaptic EPSPs (Kirkwood & Sears, 1982). Interneurones with similar properties to those reported here maybe responsible for such excitation in the thoracic cord.
MLR-evoked facilitation of disynaptic EPSPs
By examining the presence of spatial facilitation in motoneurones, and by directly recording from interneurones, evidence has been obtained that the MLR can activate interneurones of the group I disynaptic pathway. The latencies of interneurone activation from the MLR reported here are thus consistent with the possibility that these interneurones also mediate excitation to motoneurones from the MLR via one of the descending and spinal neuronal systems. One complication in interpreting the present observations on the locomotor activity of the interneurones is separating their excitation from the MLR stimulation from that evoked by the locomotor circuitry. Thus it would be desirable to re-examine their activity in the locomotor period immediately after MLR stimulation when disynaptic, group I EPSPs can still be evoked in extensor motoneurones (Angel et al. 1996) or in high spinal cats during fictive locomotion without MLR stimulation (Schomburg & Behrends, 1978).
In addition to producing fictive locomotion, MLR stimulation evokes EPSPs in motoneurones during the same phase of the step cycle in which the motoneurones are depolarized (Shefchyk et al. 1985; Degtyarenko et al. 1998b; Noga et al. 2003). These EPSPs occur at disynaptic and trisynaptic latencies with some motoneurones displaying two EPSP peaks (Noga et al. 2003). In the case of extensor motoneurones, MLR stimulation both facilitates disynaptic group I EPSPs during extension (Fig. 9B and C) and evokes spikes in candidate group I excitatory interneurones (Fig. 9A). Thus it is possible that the MLR-evoked activation of extensor motoneurones during the extension phase (e.g. the LGS in the ENG trace in Fig. 9A) is mediated in part by candidate interneurones found in the present study. As the latency of the earliest EPSPs in motoneurones produced by MLR stimulation ranges from 4.4 to 8.2 ms (mean, 6.4 ms; Noga et al. 2003) and MLR-evoked firing in the three interneurones examined occurred at about 7 ms, it is more likely that the candidate interneurones contribute to the trisynaptic components of MLR-evoked excitation of extensor motoneurones. It remains to be determined whether MLR stimulation can also facilitate the locomotor-related disynaptic excitation of flexor motoneurones produced by flexor group I afferents (Degtyarenko et al. 1998a; Quevedo et al. 2000).
In summary, this study reports on a previously unknown population of interneurones that can be activated by stimulation of ankle extensor group I afferents during the extension phase of fictive locomotion. The hypothesis is that these are excitatory interneurones interposed in a disynaptic pathway from group I afferents to extensor motoneurones. Further work is needed to verify that such interneurones are excitatory and to examine the organization of descending and segmental muscle and cutaneous afferent input to these cells. Further investigation is also required to identify the mechanisms responsible for the tonic inhibition of these interneurones in the absence of locomotion and for their activation during extension. The locus of such control of interneurone excitability is likely to be postsynaptic because there is no evidence to suggest that an augmentation of presynaptic transmission from group I afferents occurs during fictive locomotion. On the contrary, there appears to a presynaptic reduction in transmission from group I afferents that would if anything decrease the group I excitation of interneurones mediating disynaptic excitation during extension (Perreault et al. 1999b; Gosgnach et al. 2000). The emergence of group I disynaptic excitation in non-locomoting decerebrate preparations following intravenous administration of strychnine (K. Stecina, J. Quevedo and D.A. McCrea, unpublished observations), suggests that these interneurones are subject to a tonic glycinergic inhibition that is removed during locomotion.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research to D.M. M.A. was supported by Rick Hansen Man in Motion Foundation. The authors thank Dr Peter Kirkwood for comments on the manuscript and are grateful for the expert technical support of Ms Sharon McCartney.
References
- Angel MJ, Guertin P, Jiménez I, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurons during fictive locomotion. J Physiol. 1996;494:551–561. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E, Harrison PJ, Jankowska E, McCrea DA, Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983a;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E, Jankowska E, McCrea DA, Skoog B. Inhibitory interactions between interneurones in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol. 1983b;343:361–373. doi: 10.1113/jphysiol.1983.sp014897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Postsynaptic actions of midlumbar interneurones on motoneurones of hindlimb muscles in the cat. J Physiol. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cord. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden-Krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol. 1998a;79:447–463. doi: 10.1152/jn.1998.79.1.447. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden-Krichmar T, Burke RE. Locomotor modulation of disynaptic EPSPs from the mescencephalic locomotor region in cat motoneurons. J Neurophysiol. 1998b;80:3284–3296. doi: 10.1152/jn.1998.80.6.3284. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurons caused by impulses in Golgi tendon organ afferents. J Physiol. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley JA, Jankowska E. An interneuronal relay for group I and II muscle afferents in middle lumbar segments of cat spinal cord. J Physiol. 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Noga B, Carr P, Jordan LM, Hultborn H. Candidate first order interneurones in a locomotor-related group Ib pathway from hindlimb extensors in the cat. Eur J Neurosci. 1994;166(7) Abstract 95.09. [Google Scholar]
- Fern R, Harrison PJ, Riddell JS. The ascending projection of interneurones activated by group I muscle afferent fibres of the cat hindlimb. J Physiol. 1988;405:275–288. doi: 10.1113/jphysiol.1988.sp017333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T-C, Jankowska E, Lundberg A. Reciprocal Ia inhibition during the late reflexes evoked from the flexor reflex afferents after DOPA. Brain Res. 1975;85:99–102. doi: 10.1016/0006-8993(75)91012-4. 10.1016/0006-8993(75)91012-4. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic Ia EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol. 2000;526:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR-evoked fictive locomotion in the cat. J Physiol. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Johannisson T, Lipski J. Common interneurones in reflex pathways from group Ia and Ib afferents of ankle extensors in the cat. J Physiol. 1981a;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lindstrom S. Morphology of interneurons mediating reciprocal inhibition of motoneurons in the spinal cord of the cat. J Physiol. 1972;226:805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, McCrea, Mackel R. Pattern of ‘non-reciprocal’ inhibition of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981b;316:393–409. doi: 10.1113/jphysiol.1981.sp013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, McCrea, Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981c;316:411–425. doi: 10.1113/jphysiol.1981.sp013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Neuronal basis of afferent-evoked enhancement of locomotor activity. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. New York: The New York Academy of Sciences; 1998. pp. 216–225. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Pratt CA, Jordan LM. Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. J Neurophysiol. 1980;44:475–488. doi: 10.1152/jn.1980.44.3.475. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Quevedo J, Stecina K, Gosgnach S. Glycinergic inhibition of locomotor-related group I disynaptic excitation in cat hindlimb motoneurons. Soc. Neurosci. 2001 Abstract. 402.8. [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464–1478. doi: 10.1152/jn.00034.2003. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Enríquez-Denton M, Hultborn H. Proprioceptive control of extensor activity during fictive scratching and weight support compared to fictive locomotion. J Neurosci. 1999a;19:10966–10976. doi: 10.1523/JNEUROSCI.19-24-10966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Shefchyk SJ, Jiménez I, McCrea DA. Depression of monosynaptic field potentials evoked by stimulation of group II and other afferents during fictive locomotion. J Physiol. 1999b;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Fedirchuk B, Gosgnach S, McCrea D. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol. 2000;525:549–564. doi: 10.1111/j.1469-7793.2000.t01-1-00549.x. 10.1111/j.1469-7793.2000.t01-1-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Interneurones in pathways from group II muscle afferents in the lower-lumbar segments of the feline spinal cord. J Physiol. 2000;522:109–123. doi: 10.1111/j.1469-7793.2000.t01-2-00109.xm. 10.1111/j.1469-7793.2000.t01-2-00109.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED, Behrends HB. The possibility of phase-dependent monosynaptic and polysynaptic Ia excitation to homonymous motoneurons during fictive locomotion. Brain Res. 1978;143:533–537. doi: 10.1016/0006-8993(78)90363-3. 10.1016/0006-8993(78)90363-3. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brain-stem evoked fictive locomotion. Exp Brain Res. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Stein RB, Jordan LM. Synaptic transmission from muscle afferents during fictive locomotion in the mesencephalic cat. J Neurophysiol. 1984;51:986–997. doi: 10.1152/jn.1984.51.5.986. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res. 1995;103:20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]