Abstract
Abscisic acid (ABA) plays a key role in the control of stomatal aperture by regulating ion channel activities and water exchanges across the plasma membrane of guard cells. Changes in cytoplasmic calcium content and activation of anion and outward-rectifying K+ channels are among the earliest cellular responses to ABA in guard cells. In Arabidopsis suspension cells, we have demonstrated that outer plasmalemma perception of ABA triggered similar early events. Furthermore, a Ca2+ influx and the activation of anion channels are part of the ABA-signaling pathway leading to the specific expression of RAB18. Here, we determine whether phospholipases are involved in ABA-induced RAB18 expression. Phospholipase C is not implicated in this ABA pathway. Using a transphosphatidylation reaction, we show that ABA plasmalemma perception results in a transient stimulation of phospholipase D (PLD) activity, which is necessary for RAB18 expression. Further experiments showed that PLD activation was unlikely to be regulated by heterotrimeric G proteins. We also observed that ABA-dependent stimulation of PLD was necessary for the activation of plasma anion current. However, when ABA activation of plasma anion channels was inhibited, the ABA-dependent activation of PLD was unchanged. Thus, we conclude that in Arabidopsis suspension cells, ABA stimulation of PLD acts upstream from anion channels in the transduction pathway leading to RAB18 expression.
Abscisic acid (ABA) regulates seed maturation and germination, adaptation of plants to water shortage, cold, and high salinity (Leung and Giraudat, 1998). Several ABA transduction mutants have been isolated in Arabidopsis in which diverse loci affecting ABA response have been identified (for review, see Merlot and Giraudat, 1997; Leung and Giraudat, 1998). Among the best characterized are the ABA-insensitive abi-1 and abi-2 mutations, which affect protein phosphatases (Leung et al., 1997); the abi-3 (Giraudat et al., 1992; Parcy et al., 1994), abi-4 (Finkelstein et al., 1998), and abi-5 (Finkelstein and Lynch, 2000), which are mutated in transcription processes; and the ABA-hypersensitive era1 mutant, deleted for the β-subunit of a farnesyl transferase which acts as a negative regulator (Cutler et al., 1996). In guard cells, ABA transduction pathways have been extensively analyzed. Stomatal aperture is controlled by ABA through the activation of anion currents, which depolarizes the plasma membrane and promotes the activation of outward-rectifying K+ currents (Schroeder et al., 2001). In aleurone cells, ABA causes a decrease in cytoplasmic Ca2+ content, which is necessary for the inhibition of GA promotion of α-amylase activity (Ritchie and Gilroy, 1998). In Arabidopsis suspension cells, similar ion channel activation has been observed (Jeannette et al., 1999; Ghelis et al., 2000a, 2000b; Takahashi et al., 2001). However, the precise sequence of events triggered by ABA in these models remains unknown.
Recent research has provided new data about the role of phospholipases in plant physiological responses (Chapman, 1998), including the implication of phospholipase C (PLC) in ABA signaling. In Arabidopsis, ABA was shown to induce the expression of the Ca2+-dependent AtPLC1 (Hirayama et al., 1995). In the same species, it was demonstrated that an increase in PLC1 activity was necessary for the induction of the RD29a, KIN2, and RD22 ABA-responsive genes (Sanchez and Chua, 2001). In Commelina communis guard cells, Staxen et al. (1999) have shown that U73122, an inhibitor of phosphatidylinositol-PLC (PI-PLC) activity, abolished ABA-induced cytosolic [Ca2+] oscillations and stomatal closure.
The involvement of phospholipase D (PLD) in ABA responses is better understood (Wang, 1999; Munnik, 2001). PLD hydrolyzes constitutive phospholipids to phosphatidic acids (PAs) and free alcohols. A plasma membrane localization of PLD was observed in rice (Oryza sativa; Young et al., 1996), tobacco (Nicotiana tabacum; Gardiner et al., 2001), and Arabidopsis (Fan et al., 1997; Ritchie and Gilroy, 2000). Three groups of PLD are distinguished according to the Ca2+ concentration required in in vitro activity tests (Wang, 2001). PLDs of the first group, named PLDα, are active at millimolar levels of Ca2+. The second group is PI-dependent PLD activated by micromolar Ca2+ concentration. The third group is Ca2+-independent PLD. Because of their higher affinity for primary alcohols than for water, Ca2+-dependent PLDs are able to transfer the phosphatidyl group to the alcohols, thus, synthesizing phosphatidyl alcohols. This reaction, associated with radioactive phosphate labeling of phospholipids, allows easy measurement of PLD activity (de Vrije and Munnik, 1997; Munnik, 2001). This method makes it possible to show that PLD activity mediates ABA responses. For example, in broad bean (Vicia faba) guard cells, ABA induced a transient stimulation of PLD (Jacob et al., 1999). Moreover, PA application was able to mimic ABA action because, in broad bean, PA treatment inhibited inward K+ currents in guard cell protoplasts and regulated stomatal aperture (Jacob et al., 1999). In barley (Hordeum vulgare) aleurone protoplasts, PA treatment causes a reduction in the cytoplasmic [Ca2+] and inhibits the GA activation of α-amylase activity (Ritchie and Gilroy, 1998).
In animal models, heterotrimeric G proteins relay extracellular signals perceived from cell surface receptors to ion channels or phospholipases (Neer, 1995). Activation of a G protein-coupled receptor provokes the exchange of GDP for GTP in the guanine nucleotide-binding site of the α-subunit, which dissociates from the βγ-dimer. Subsequent dephosphorylation of the GTP α-subunit allows the reassociation of the three G protein subunits. In plants, many biochemical and molecular studies argue in favor of the involvement of G proteins in the transduction of plant hormone signals (Assmann, 1996; Munnik et al., 1998; Bischoff et al., 1999; Hooley, 1999). For instance, in aleurone protoplasts, the ABA stimulation of PLD is GTP dependent and is inhibited by pertussis toxin, thus, demonstrating an association with G proteins (Ritchie and Gilroy, 2000). Heterotrimeric (Wang, 2001) and small (Lemichez et al., 2001) G proteins were recently shown to be involved in ABA-triggered stomatal mechanism. However, in Arabidopsis, only one gene, GPA1, coding for an α-subunit of G protein, has been found (Ma, 2001).
In Arabidopsis suspension cells, we have previously shown that ABA perception, at the outer side of the plasma membrane, was accompanied by the activation of outward-rectifying K+ channels and led to the expression of RAB18 (Jeannette et al., 1999). We demonstrated also that ABA perception was followed by a Ca2+ influx (Ghelis et al., 2000b). Furthermore, the activation of anion channels and the inhibition of inward K+ channels occurred very quickly after ABA perception (Ghelis et al., 2000a). Ca2+ influx and anion channel activation belong to the signaling pathway leading to the expression of RAB18, whereas K+ exchanges are independent of this pathway. The activation of specific Ca2+ channels by impermeant ABA prompted us to investigate Ca2+-dependent elements in the cascade of events starting from the outer-plasmalemma perception of ABA and leading to RAB18 expression in Arabidopsis suspension cells. In this report, we analyze the possible role of PLC and PLD. PLC activity was not involved in this signaling pathway, but we demonstrate that ABA stimulates a specific PLD activity that seems unlikely to be connected with heterotrimeric G proteins. This PLD stimulation precedes the activation of anion channels and is required for the expression of RAB18.
RESULTS
PLD Activity Is Necessary for RAB18 Expression
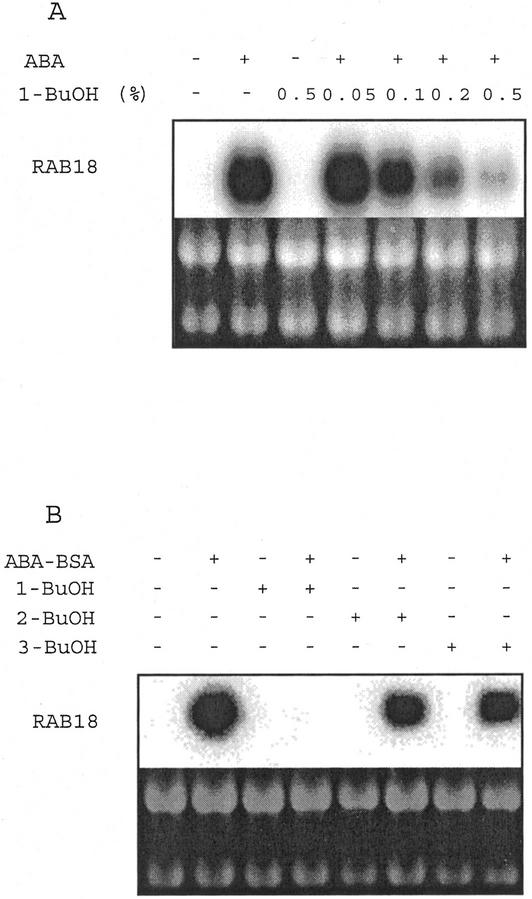
In Arabidopsis suspension cells, RAB18 expression was elicited by both free and protein-conjugated ABA. When 1-butanol (1-BuOH), known as a PLD substrate for transphosphatidylation, was co-incubated for 3 h with 10−5 m ABA, a dose-dependent inhibition of RAB18 expression was observed (Fig. 1A). With 0.5% (v/v) 1-BuOH, a total inhibition occurred, whereas 2-BuOH and 3-BuOH, which are not recognized by PLD, did not affect RAB18 expression (Fig. 1B).
Figure 1.
ABA-induced RAB18 gene expression is inhibited by 1-BuOH in Arabidopsis suspension cells. Northern-blot analysis of total RNA (10 μg) extracted from cells. A, Cells were incubated for 3 h with 10−5 m ABA and 0.05% to 0.5% (v/v) 1-BuOH. B, Cells were incubated for 3 h with ABA-BSA (10−5 m equivalent ABA) and 0.5% (v/v) 1-BuOH, 2-BuOH, or 3-BuOH. Ethidium bromide staining of rRNAs is shown as control.
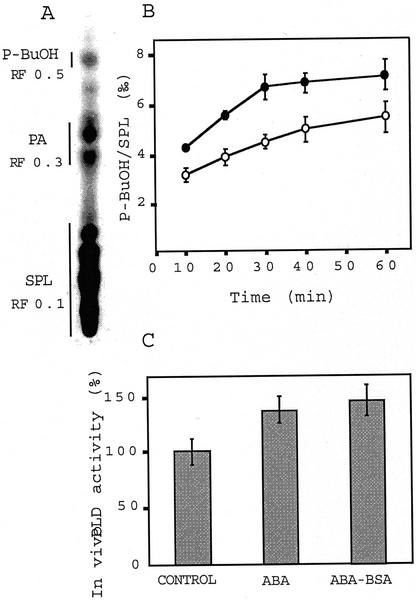
Because the PA produced by PLD activity was required for ABA-induced RAB18 expression, we measured how in vivo PLD activity was modulated by ABA. After an 18-h 33P pulse, 0.5% (v/v) 1-BuOH and 10−5 m ABA were simultaneously added to the suspension cells. Lipids were extracted and phosphatidyl-BuOH (P-BuOH) synthesized was separated by thin-layer chromatography (Fig. 2A). Direct measurement of in vivo PLD activity was expressed as the relative amount of P-BuOH compared with structural phospholipids. Figure 2B shows that accumulation of P-BuOH is almost linear for 1 h in control cells, whereas it presents a bimodal pattern in ABA-treated cells: 10 min after ABA treatment, PLD increased 20%, reached about 30% at 30 min, and then remained almost unchanged (Fig. 2B). Therefore, ABA induced a transient increase in PLD activity. Figure 2C illustrates relative in vivo PLD activity measured from cells treated for 30 min with free or conjugated ABA. ABA-bovine serum albumin (ABA-BSA; 10−5 m equivalent ABA) stimulated PLD activity to the same extent as free ABA.
Figure 2.
In vivo PLD activity is stimulated by ABA in Arabidopsis suspension cells. A, Thin-layer chromatography separation of phospholipids after treatment with ABA-BSA (10−5 m equivalent ABA) and 0.5% (v/v) 1-BuOH after an 18-h 33P pulse. B, Time course of in vivo ABA-stimulated PLD activity. ○, Control; ●, ABA (10−5 M). Bars, sd, n = 3. C, Free (10−5 m) or conjugated (10−5 m equivalent ABA) ABA-stimulated PLD activity. PLD activity was measured after 30 min of treatment. SPL, Structural phospholipids. Bars, sd, n = 7.
PLC Activity Is Not Involved in ABA-Induced RAB18 Expression
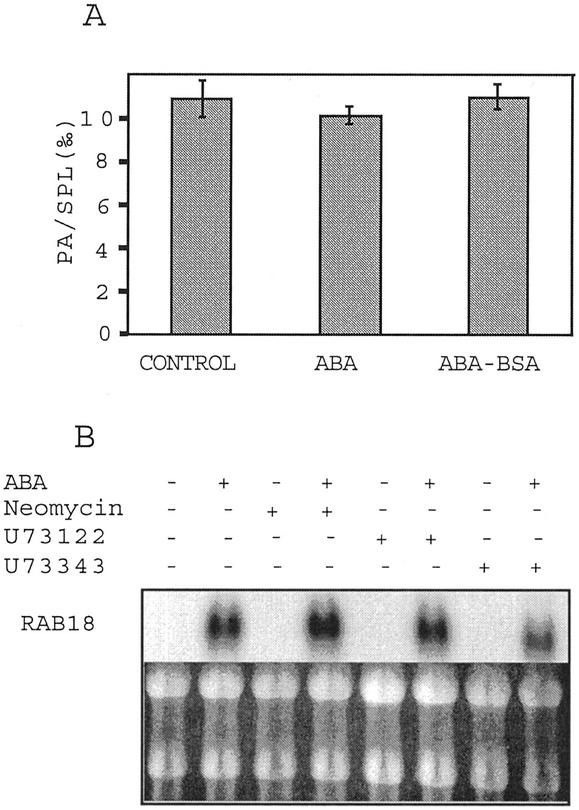
Activation of PLD is necessary for ABA-induced RAB18 expression, thus, indicating that PA synthesis is a step of this signaling pathway. Because PA can also be produced by PLC associated with diacylglycerol kinase (DAG kinase) activity, we investigated the involvement of PLC in ABA-specific RAB18 expression. Short incubation of cells with 33P was done to label the ATP pool, thus, making PA labeling only through DAG-kinase activity. Neither free nor conjugated ABA induced significant increase in the measured PA level (Fig. 3A). Figure 3B shows that RAB18 expression induced by ABA was not modified by neomycin (50 μm) or by the specific PI-PLC inhibitor U73122 (10 μm) and its biologically inactive analog U73343 (10 μm). Furthermore, measurement of inositol 1,4,5-triphosphate (IP3) produced by PI-PLC showed no variation between control and 30-min ABA-treated cells (4.6 pmol IP3 mL−1, sd = 0.14, n = 4; data not shown). These results show that PLC is not implicated in ABA-induced RAB18 expression in Arabidopsis suspension cells.
Figure 3.
PLC is not involved in ABA-induced RAB18 expression in Arabidopsis suspension cells. A, Relative amount of PA extracted from cells preincubated for 2 h with 33P and treated with ABA (10−5 m) or ABA-BSA (10−5 m equivalent ABA) for 30 min. Bars, sd, n = 3. B, Northern-blot analysis of total RNA (10 μg) extracted from cells treated for 3 h with ABA-BSA (10−5 m equivalent ABA) and neomycin (50 μm), U73122 (10 μm), or U73343 (10 μm). Ethidium bromide staining of rRNAs is shown as control.
G Proteins Do Not Seem to Be Involved in ABA-Triggered PLD Activation
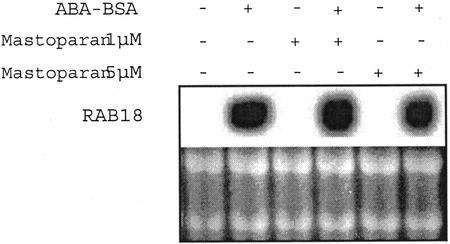
PLD has been shown to be regulated by heterotrimeric G proteins (Munnik et al., 1998). Thus, we tested mastoparan, a specific heterotrimeric G protein activator, on RAB18 expression.
Mastoparan did not mimic ABA, and when added to ABA, it did not potentialize ABA activation of RAB18 expression (Fig. 4). These data are in accordance with those displayed in Figure 1B. 2-BuOH, a secondary alcohol that enhances G protein activity (Munnik et al., 1995), did not modify ABA-specific expression of RAB18. From these data, we conclude that heterotrimeric G proteins do not seem to be involved in ABA stimulation of PLD activity required for RAB18 expression.
Figure 4.
Heterotrimeric G proteins do not seem to be involved in RAB18 expression in Arabidopsis suspension cells. Northern-blot analysis of total RNA (10 μg) from cells incubated simultaneously with ABA-BSA (10−5 m equivalent ABA) and mastoparan (1 and 5 μm) for 3 h. Ethidium bromide staining of rRNAs is shown as control.
PLD Precedes Anion Channel in ABA-Signaling Cascade
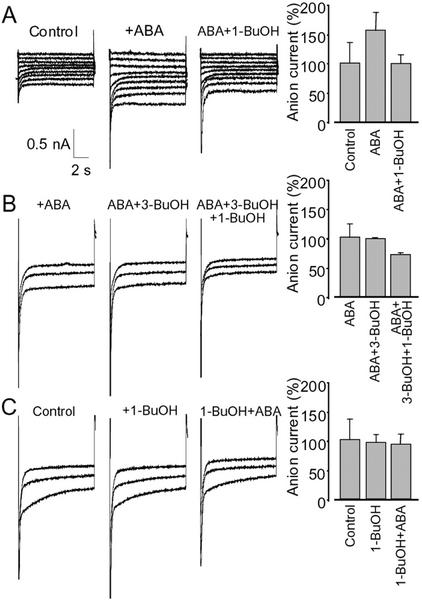
In a previous study, we showed that ABA outer-plasmalemma perception triggers early activation of plasmalemma anion currents, which is required for RAB18 expression (Ghelis et al., 2000a). We tested ABA anion current activation when PLD was inhibited and vice versa to better understand the link between PLD and anion current activation.
After pulses from −200 to −40 mV, most of the cells (70%; n = 91) exhibited recordings typical of S-type anion currents, which are enhanced by ABA (Fig. 5A). Successive application of 0.1% (v/v) 1-BuOH reduced ABA-enhanced currents by 57% ± 15% (mean value ± sd; n = 4) for −200 mV clamping (Fig. 5A). This reduction was observed in six of the nine cells exhibiting an anion current. By contrast, when cells were first treated with 1-BuOH, ABA could not activate anion currents (Fig. 5B). When ABA application was followed with 3-BuOH treatment, ABA activation of anion channels was not changed, but further addition of 1-BuOH resulted in a reduction (30% ± 4%) of anion currents (Fig. 5C). These results indicate that ABA activation of anion currents depends on PA formation by PLD activation.
Figure 5.
Shunt of PLD with 1-BuOH suppresses ABA activation of whole cell anion currents in Arabidopsis suspension cells. Whole cell currents were activated by a depolarizing prepulse (+100 mV for 4.5 s), not shown. Then, hyperpolarizing pulses ranging from −200 to −40 mV, in 20-mV steps (A) or from −200 to −120 mV, in 40-mV steps (B and C) were applied for 9.5 s. Representative traces are shown. A, Current recordings from intact cells before (control) and 15 min after application of 10 μm ABA and 6 min after addition of 0.1% (v/v) 1-BuOH. The diameter of the cell was 45 μm. Right, Histogram represents the mean anion current at −200 mV for at least four replicates (bars, sd). B, Currents were recorded 5 min after successive additions, at 5-min intervals, of ABA, 3-BuOH, and 1-BuOH (0.1%, v/v). The diameter of the cell was 42 μm. Right, Histogram represents the mean anion current at −200 mV for at least three replicates (bars, sd). C, Currents were recorded from intact cells (control) and after successive additions of 0.1% (v/v) 1-BuOH (2 min later) and then 10 μm ABA (4 min later). The diameter of the cell was 48 μm. Right, Histogram represents the mean anion current at −200 mV for at least four replicates (bars, sd).
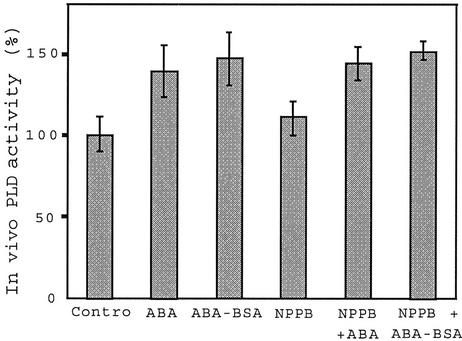
The anion channel blocker 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) inhibits ABA activation of anion currents and RAB18 expression in Arabidopsis suspension cells (Ghelis et al., 2000a). Addition of NPPB (25 μm) did not significantly affect PLD activity: Only a slight increase was observed in control (110% versus 100%) and, when ABA and NPPB were simultaneously added, the stimulation of PLD activity by free or conjugated ABA was still recorded (Fig. 6). Taken together, the data presented demonstrate that the ABA-induced stimulation of PLD activity is upstream the activation of plasmalemma anion currents (Figs. 5 and 6).
Figure 6.
Inhibition of plasma membrane anion currents does not alter ABA activation of in vivo PLD activity in Arabidopsis suspension cells. Cells were treated for 30 min with ABA (10−5 m) or ABA-BSA (10−5 m equivalent ABA) and 25 μm NPPB. Bars, sd, n = 4.
DISCUSSION
PLD Activity Is Involved in ABA-Triggered RAB18 Expression in Arabidopsis Suspension Cells
In the present study, we established that treatment of Arabidopsis suspension cells with free or protein-conjugated impermeant ABA triggered a stimulation of PLD activity. Therefore, PLD is an element of the signaling cascade associated with ABA perception at the plasmalemma level. The role of PLD and its PA product in the ABA-induced RAB18 expression was demonstrated because addition of 1-BuOH led to the accumulation of P-BuOH, at the expense of PA formation, and inhibited RAB18 expression (Figs. 1 and 2). Such an involvement of PLD has already been described in ABA transduction pathways. For example, in barley aleurone cells, PLD activity mediates ABA inhibition of GA-induced α-amylase production (Ritchie and Gilroy, 1998). Using transient gene expression in rice protoplasts, PLD was demonstrated to regulate expression of the ABA-inducible wheat (Triticum aestivum) gene Em (Gampala et al., 2001).
In Arabidopsis suspension cells treated with 10−5 m ABA, the range of in vivo PLD activation observed (i.e. 30%–40%; Fig. 1) is comparable with the 50% increase in PLD activity measured in vitro in the microsomal fraction of barley aleurone protoplasts treated by ABA at the same concentration (Ritchie and Gilroy, 2000). In broad bean guard cell protoplasts, a transient increase in PLD activity, within the same range, was obtained after a 30-min application of 10−5 m ABA (Jacob et al., 1999). The activation of PLD by ABA in Arabidopsis suspension cells follows a similar pattern (Fig. 2). Nevertheless, our data are in contrast with the observation reported in Arabidopsis T87 culture cells by Takahashi et al. (2001). They did not detect any PLD activation by exogenous ABA. The reason for this discrepancy remains unknown.
Specificity of the PLD Stimulated by ABA
PLDs represent a multiple gene family that is divided into three groups according to Ca2+ requirement (Wang, 1999). Ca2+-independent PLDs are unable to achieve the transphosphatidylation reaction and cannot belong to the transduction cascade analyzed here because 1-BuOH addition blocks RAB18 expression. PLDβ and PLDγ are Ca2+-dependent PLDs that are inhibited by neomycin (Qin et al., 1997). We have demonstrated that neomycin did not interrupt the signaling cascade triggered by ABA leading to RAB18 expression (Fig. 3A). Therefore, this result suggests that the ABA-activated PLD belongs to PLDα group. Our observation corroborates other reports in Arabidopsis. In fact, the importance of PLDα has been confirmed in Arabidopsis leaves (Pappan et al., 1997; Sang et al., 2001), and the antisense suppression of PLDα retarded ABA-promotion of senescence in detached Arabidopsis leaves (Fan et al., 1997).
ABA-treated cells incubated with 1-BuOH accumulated 30% to 40% more P-BuOH than untreated ABA cells (Fig. 2), which is the reflection of specific PAs over-produced by the ABA-stimulated PLD activity. Thus, these PAs are the putative second messengers in the ABA transduction pathway understudied. PAs are considered as second messengers in physiological responses to stresses and pathogen attacks or in symbiosis (for review, see Munnik, 2001). In broad bean guard cells, PA was shown to exert similar effects as ABA on the regulation of stomatal aperture and on the inhibition of the activity of K+ inward channels (Jacob et al., 1999). In barley aleurone protoplasts and layers, similar inhibition of GA-activated α-amylase activity could be obtained with the addition of either ABA or PAs (Ritchie and Gilroy, 1998). In the same study, the authors highlighted the importance of the PA molecular species for a given biological effect and demonstrated that arachidonoyl-stearoyl PA was the most efficient antagonizer of GA action. In castor bean (Ricinus communis), it was shown that wounding activated a PLD and resulted in accumulation of polyunsaturated fatty acids, DAG, and PAs (Ryu and Wang, 1998). The analysis of the acyl composition of PAs in mechanically wounded Arabidopsis leaves has shown that a specific isoform of PLD, using phosphatidylcholine (PC) as substrate, was activated (Zien et al., 2001). The importance of acyl groups of phospholipids acting as putative second messengers is well established (Millar et al., 2000). Therefore, isolation and characterization of endogenous specific PAs produced by ABA-treated Arabidopsis suspension cells should be undertaken to confirm the role of PAs as the second messenger.
PLC Activity Does Not Contribute to ABA-Triggered RAB18 Expression in Arabidopsis Suspension Cells
In Arabidopsis suspension cells, we previously demonstrated that an influx of extracellular Ca2+ through specific plasmalemma Ca2+ channels was required for ABA-induced RAB18 expression (Ghelis et al., 2000b). Nevertheless, changes in cytoplasmic [Ca2+] may also be due to the activation of IP3 or cADPR-dependent intracellular Ca2+ channels (Leckie et al., 1998; MacRobbie, 2000). Hence, we examined the involvement of PI-PLC, responsible for IP3 production, in ABA signaling in Arabidopsis suspension cells.
ABA-induced RAB18 expression was not modified by treatment of the cells with the PI-PLC inhibitors neomycin and U73122 (Fig. 3B). Furthermore, no changes in the phosphorylation rate of PA extracted from cells preincubated for 2 h with 33P and treated with ABA was observed (Fig. 3A). The system PLC-DAG kinase is, thus, not involved in the ABA-triggered RAB18 expression pathway. As already reported by Takahashi et al. (2001) in similar Arabidopsis suspension cells, we were unable to detect any IP3 formation after ABA treatment (data not shown), indicating that RAB18 expression is not dependent on inner IP3-dependent Ca2+ channels. Taken together, these results show that a common regulation, independent of PLC but dependent on PLD, can be envisaged for RD20 (Takahashi et al., 2001) and RAB18, two ABA-inducible genes. However, in Arabidopsis seedlings, it was demonstrated, with transgenic lines expressing an AtPLC1 antisense, that PLC activity was involved for the induction of the RD29a, KIN2, and RD22 ABA-inducible genes. In contrast, the expression of COR47 was unaffected in the same AtPLC1 antisense line (Sanchez and Chua, 2001). Therefore, the control of the expression of ABA-inducible genes may act through different pathways. It should be noted that in other ABA-dependent responses that involve Ca2+, PI-PLC is not always implicated. For example, in broad bean, a total inhibition of ABA-induced stomatal closure requires both U73122 and nicotinamide, an inhibitor of cADPR synthesis (Jacob et al., 1999) whereas, in Commelina communis, treatment with U73122 is sufficient to inhibit ABA-induced cytosolic [Ca2+] oscillations and stomatal closure (Staxen et al., 1999). Thus, it appears that different Ca2+ channels may act in parallel or cooperate in ABA-signaling pathways according to the cell or the organ considered.
There Is No Evidence That PLD Involved in ABA-Triggered RAB18 Expression Is Regulated by Heterotrimeric G Proteins
Some reports suggest that G proteins act upstream from PLD in plants (Bischoff et al., 1999). In Chlamydomonas eugametos, deflagellation is associated with PLD activation and can be obtained with G protein activators (Munnik et al., 1995). The in vitro activity of a PLD recombinant protein, obtained from a cDNA clone isolated from tobacco, is inhibited by a recombinant G protein α-subunit expressed in Escherichia coli (Lein and Saalbach, 2001). In barley aleurone microsomal fraction enriched in plasma membrane, the addition of GDPβS rendered PLD activity insensitive to ABA, whereas GTPγS stimulated PLD in the absence of ABA (Ritchie and Gilroy, 2000). Our data show that mastoparan, which mimics the motif of 7TMS receptors that activates G proteins (Munnik et al., 1995; de Vrije and Munnik, 1997), was unable to reproduce the effect of ABA on RAB18 expression (Fig. 4). Furthermore, we have shown that 2-BuOH did not enhance the effect of ABA on RAB18 expression (Fig. 1B) Therefore, it is highly improbable that G proteins are involved in the regulation of PLD activated by ABA in this transduction pathway.
PLD Activity Precedes Activation of Plasmalemma Anion Currents
The activation of anion currents by ABA was shown to be required for RAB18 expression (Ghelis et al., 2000a). Here, we observed that the inhibition of anion currents by NPPB did not reduce the in vivo enhancement of PLD activity by ABA (Fig. 5). In contrast, the electrophysiological data clearly showed that addition of 1-BuOH but not of 3-BuOH suppressed the activation of anion currents by ABA (Fig. 6). ABA-specific PLD activation must, therefore, occur upstream from anion currents in the signaling pathway leading to RAB18 expression.
In conclusion, we demonstrated the role of a specific PLD in the signaling cascade, which begins with the plasmalemma perception of ABA and results in the expression of the RAB18 gene. PLD activation is a very early event in the ABA transduction pathway because it precedes the stimulation of anion currents. The PLD isoform involved remains to be analyzed. Further investigations need to characterize the PAs produced as second messengers and the targets modulated by PA.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotype Columbia cells were obtained by Axelos et al. (1992). They were cultured at 24°C, under continuous white light (40 μmol m−2 s−1) with an orbital agitation at 130 rpm, in 500-mL Erlenmeyer flasks containing 200 mL of Jouanneau and Péaud-Lenoël (1967) culture medium. A 25-mL aliquot of cell suspension was transferred to a fresh medium every week. All the experiments were conducted on 4-d-old cells. The pH of the culture medium was 6.8. The viability of the cells during the experimental treatment was systematically checked with Trypan blue tests (not shown).
RAB18-Responsive Test and Northern-Blot Analysis
A 5-mL suspension was incubated for 3 h under the conditions of culture. ABA-BSA purified conjugate (10−5 m equivalent ABA) was added in 50 mm Na2SO4, 50 mm phosphate buffer, pH 6.8. Northern-blot analyses were performed according to the protocol previously described (Jeannette et al., 1999). The 684-bp RAB18 cDNA probe used (GenBank accession no. X68042) contained the coding sequence (with the exception of the first 100 bp of 5′ sequence after the ATG codon) and the 3′ noncoding sequence ending with the polyadenylation site of the gene (Lang and Palva, 1992). 18S RNA gene probe was used as control of constitutive gene expression (data not shown).
In Vivo PLD Activity
PLD activity was measured as described by de Vrije and Munnik (1997). Arabidopsis suspension cells were first incubated in [33P]H3PO4 (53 MBq L−1) for 18 h to label all phospholipids. 1-BuOH was then added to probe PLD activity by transphosphatidylation leading to P-BuOH production. Phospholipids were extracted in 2:1 (v/v) MeOH:CHCl3 solvent mixture (15 mL for 6 mL of suspension cells), and separated on silica gel 60 thin-layer chromatography plates (Merck, Rahway, NJ) with the organic upper phase of 12:2:3:10 (v/v) ethyl acetate:iso-octane:formic acid:water according to Liscovitch and Amsterdam (1989). Radioactivity incorporated in structural phospholipids, PA, and P-BuOH spots was measured on a PhosphorImager (Storm, Molecular Dynamics, Sunnyvale, CA).
In Vivo PI-PLC Activity
PI-PLC activity was assayed with the Biotrak assay kit (TRK1000, Amersham, Buckinghamshire, UK). Six milliliters of suspension cells was fixed with 1.2 mL of perchloric acid and then centrifuged at 15,000g for 15 min, at 4°C. The supernatant was adjusted to pH 7.5 with ice-cold 60 mm HEPES in 1.5 m KOH. The KClO4 precipitated was removed, and IP3 content was determined in the supernatant according to the manufacturer's specifications.
Electrophysiology
Cells were immobilized by means of a microfunnel (approximately 30–80 μm o.d.). Impalements of the cells were carried out with a piezoelectric micromanipulator (PCS-5000, Burleigh Institute, New York) in a continuous-flow chamber (500 μL) made of perpex. ABA and BuOH were diluted in the bathing medium introduced via a polyethylene catheter. The cells were balanced for 24 h before electrophysiological experiments in fresh culture medium (20 mm KNO3, 0.9 mm CaCl2, and 0.45 mm MgSO4, pH 6.8). Microelectrodes were made from borosilicate capillary glass (Clark GC 150F, Clark Electromedical, Pangbourne Reading, UK) pulled on a vertical puller (Narischige PEII, Japan). Their tips were of 0.5 μm diameter, they were filled with 600 mm KCl, and had electrical resistances from 50 to 80 MΩ with the buffer solution.
Voltage-clamp measurements of whole cell currents from intact cells were carried out at room temperature (20°C–22°C) using the technique of the discontinuous single voltage-clamp microelectrode (Bouteau et al., 1996; Jeannette et al., 1999). A specific software (pCLAMP 8) drives the voltage clamp amplifier (Axoclamp 2A, Axon Instruments, Foster City, CA). Voltage and current were digitalized with a PC computer fitted with an acquisition board (Labmaster TL 1, Scientific Solution, Solon, OH). In whole cell current measurements, the membrane potential was held at −40 mV. Anion currents were activated by a depolarizing prepulse (+100 mV for 4.5 s), and then hyperpolarizing pulses ranging from −200 to −120 or to −40 mV for 9.5 s (in 40- and 20-mV steps, respectively) were applied. We systematically checked that cells were correctly clamped by comparing the protocol voltage values with those really imposed.
ACKNOWLEDGMENTS
We thank Drs. Jenny Zeitlin and Alain Zachowski for critical reading of the manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004168.
LITERATURE CITED
- Assmann SM. Guard cell G proteins. Trends Plant Sci. 1996;1:73–74. [Google Scholar]
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128. [Google Scholar]
- Bischoff F, Molendijk A, Rajendrakumar CSV, Palme K. GTP-binding proteins in plants. Cell Mol Life Sci. 1999;55:233–256. doi: 10.1007/s000180050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouteau F, Bousquet U, Pennarun AM, Convert M, Dellis O, Cornel D, Rona JP. Time dependent K+ currents through plasmalemma of laticiferous vessel protoplasts from Hevea brasiliensis. Physiol Plant. 1996;98:97–104. [Google Scholar]
- Chapman K. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998;11:419–426. [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- de Vrije T, Munnik T. Activation of phospholipase D by calmodulin antagonists and mastoparan in carnation petals. J Exp Bot. 1997;48:1631–1637. [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase D retards abscisic acid- and ethylene senescence of postharvest Arabidopsis leaves. Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell. 1998;10:1048–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala S, Hagenbeek D, Rock C. Functional interactions of lanthanum and phospholipase D with the abscisic acid signaling effectors VIVIPARIOUS1 and ABA-INSENSITIVE1–1 in rice protoplasts. J Biol Chem. 2001;276:9855–9860. doi: 10.1074/jbc.M009168200. [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J. A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell. 2001;13:2143–2158. doi: 10.1105/TPC.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelis T, Dellis O, Jeannette E, Bardat F, Cornel D, Miginiac E, Rona JP, Sotta B. Abscisic acid specific expression of RAB18 involves activation of anion channels in Arabidopsis thaliana suspension cells. FEBS Lett. 2000a;474:43–47. doi: 10.1016/s0014-5793(00)01574-x. [DOI] [PubMed] [Google Scholar]
- Ghelis T, Dellis O, Jeannette E, Bardat F, Miginiac E, Sotta B. Abscisic acid plasmalemma perception triggers a calcium influx essential for RAB18 gene expression in Arabidopsis thaliana suspension cells. FEBS Lett. 2000b;483:67–70. doi: 10.1016/s0014-5793(00)02088-3. [DOI] [PubMed] [Google Scholar]
- Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. A gene coding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley R. A role for G proteins in plant hormone signalling? Plant Physiol Biochem. 1999;37:393–402. [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S. Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannette E, Rona JP, Bardat F, Cornel D, Sotta B, Miginiac E. Induction of RAB18 gene expression and activation of K+ outward-rectifying channels depend on an extracellular perception of ABA in Arabidopsis thaliana suspension cells. Plant J. 1999;18:13–22. doi: 10.1046/j.1365-313x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Jouanneau JP, Péaud-Lenoël C. Growth and synthesis of proteins in cell suspensions of kinetin dependent tobacco. Physiol Plant. 1967;20:834–850. [Google Scholar]
- Lang V, Palva ET. The expression of rab-related gene RAB18 is induced by abscisic acid during cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1992;20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- Leckie CP, MacAinsh MR, Montgomery L, Priestley AJ, Staxen I, Webb AAR, Hetherington AM. Second messengers in guard cells. J Exp Bot. 1998;49:339–349. [Google Scholar]
- Lein W, Saalbach G. Cloning and direct G protein regulation of phospholipase D from tobacco. Biochim Biophys Acta. 2001;1530:172–183. doi: 10.1016/s1388-1981(00)00182-7. [DOI] [PubMed] [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001;15:1808–1846. doi: 10.1101/gad.900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE 2 (ABI2) and ABI1 genes encode homologous protein phosphatase 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M, Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. J Biol Chem. 1989;264:11762–11767. [PubMed] [Google Scholar]
- Ma H. Plant G proteins: the different faces of GPA1. Curr Biol. 2001;11:R869–R871. doi: 10.1016/s0960-9822(01)00519-x. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc Natl Acad Sci USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Giraudat J. Genetic analysis of abscisic acid signal transduction. Plant Physiol. 1997;114:751–757. doi: 10.1104/pp.114.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Smith MA, Kunst L. All fatty acids are not equal: discrimination in plant membrane lipids. Trends Plant Sci. 2000;5:95–101. doi: 10.1016/s1360-1385(00)01566-1. [DOI] [PubMed] [Google Scholar]
- Munnik T. Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci. 2001;6:227–233. doi: 10.1016/s1360-1385(01)01918-5. [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, de Vrije T, Musgrave A. G protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signalling in plant. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;73:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Pappan K, Zheng S, Wang X. Identification and characterization of a novel plant phospholipase D that requires polyphosphoinositides and submicromolar calcium for activity in Arabidopsis. J Biol Chem. 1997;272:7048–7054. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Pappan K, Wang X. Molecular heterogeneity of phospholipase D (PLD): cloning of PLDgamma and regulation of plant PLDgamma, -beta, and -alpha by polyphosphoinositides and calcium. J Biol Chem. 1997;272:28267–28273. doi: 10.1074/jbc.272.45.28267. [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1998;95:2697–2702. doi: 10.1073/pnas.95.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid stimulation of phospholipase D in the barley aleurone is G protein-mediated and localized to the plasma membrane. Plant Physiol. 2000;124:693–702. doi: 10.1104/pp.124.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Wang X. Increase in free linoleic acids associated with phospholipase D-mediated hydrolysis of phospholipids in wounded castor bean leaves. Biochim Biophys Acta. 1998;1393:193–202. doi: 10.1016/s0005-2760(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Sanchez JP, Chua NH. Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell. 2001;13:1143–1154. doi: 10.1105/tpc.13.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Zheng S, Li W, Huang B, Wang X. Regulation of plant water loss by manipulating the expression of phospholipase Dα. Plant J. 2001;28:135–144. doi: 10.1046/j.1365-313x.2001.01138.x. [DOI] [PubMed] [Google Scholar]
- Schroeder J, Allen G, Hugouvieux V, Kwak J, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- Staxen I, Pical C, Montgomery L, Gray J, Hetherington A, McAinsh M. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Katagiri T, Hirayama T, Yamaguchi-Shinozaki K, Shinozaki K. Hyperosmotic stress induces a rapid and transient increase in inositol 1,4,5-triphosphate independent of abscisic acid in Arabidopsis cell culture. Plant Cell Physiol. 2001;42:214–222. doi: 10.1093/pcp/pce028. [DOI] [PubMed] [Google Scholar]
- Wang X. The role of phospholipase D in signaling cascade. Plant Physiol. 1999;120:645–651. doi: 10.1104/pp.120.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Plant phospholipases. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:211–213. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- Young SA, Wang X, Leach JE. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell. 1996;8:1079–1090. doi: 10.1105/tpc.8.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zien CA, Wang C, Wang X, Welti R. In vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim Biophys Acta. 2001;1530:236–248. doi: 10.1016/s1388-1981(01)00091-9. [DOI] [PubMed] [Google Scholar]