Abstract
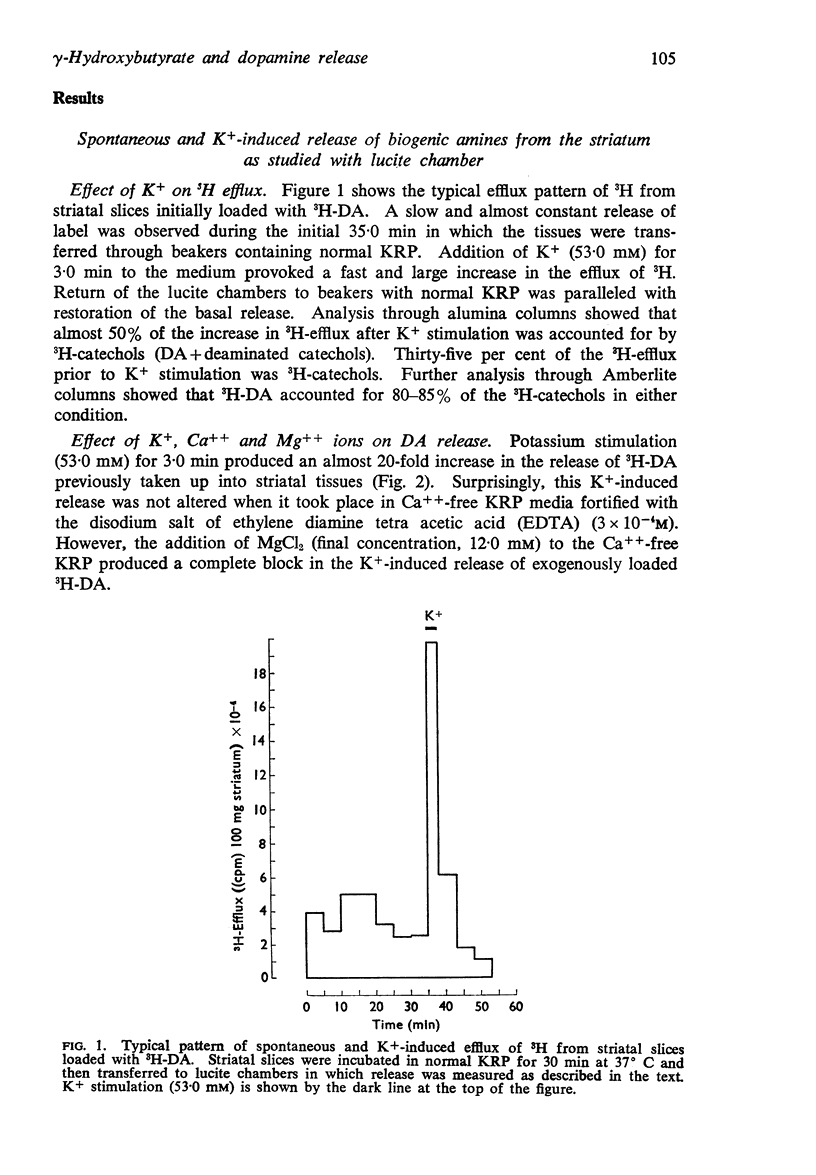
1. A simple in vitro system was developed to study the effect of γ-hydroxybutyrate on nerve cell depolarization-induced release of labelled dopamine, noradrenaline and 5-hydroxytryptamine from brain slices.
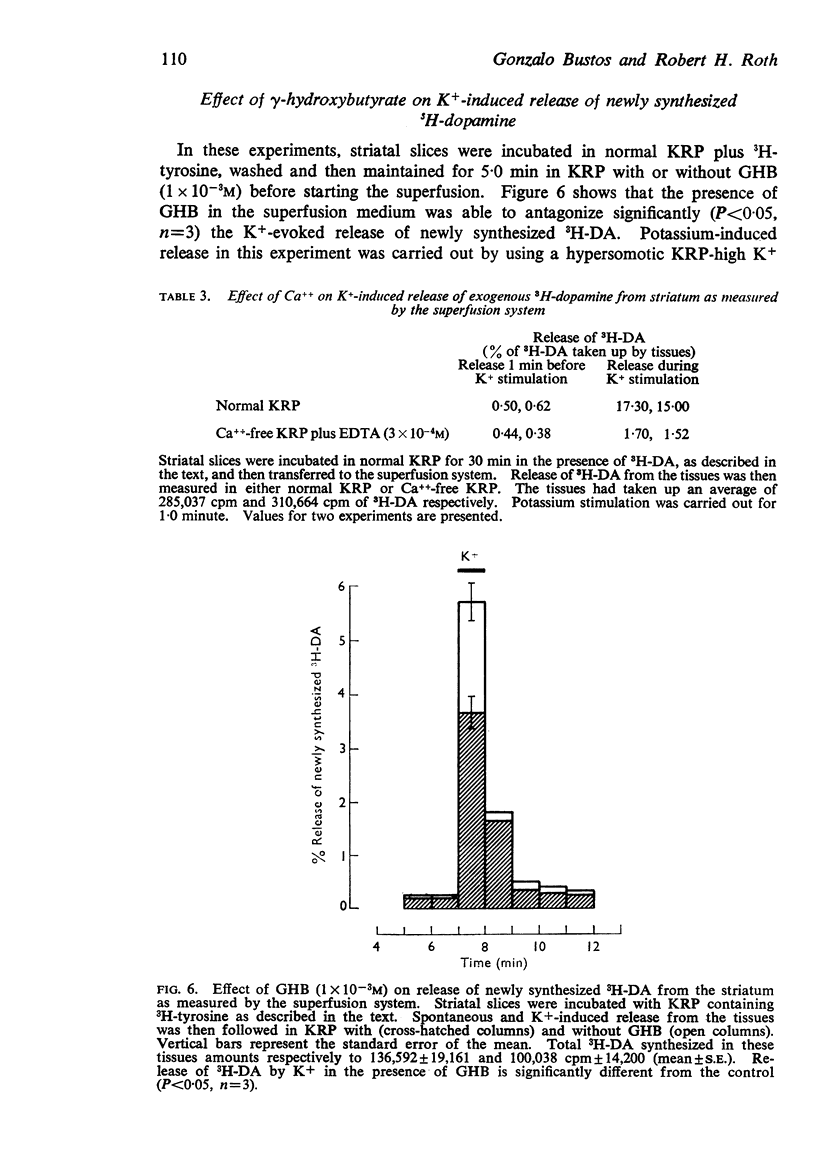
2. The release of 3H-dopamine formed in rat striatal slices incubated with 3H-tyrosine was followed either by transferring the slices through successive media or by using a superfusion system. A one to three minute exposure to K+ (53 mM) caused up to a thirty fold increase in the release of newly synthesized 3H-dopamine. This K+-induced release was antagonized when γ-hydroxybutyrate (1 × 10-3M) was present in the medium.
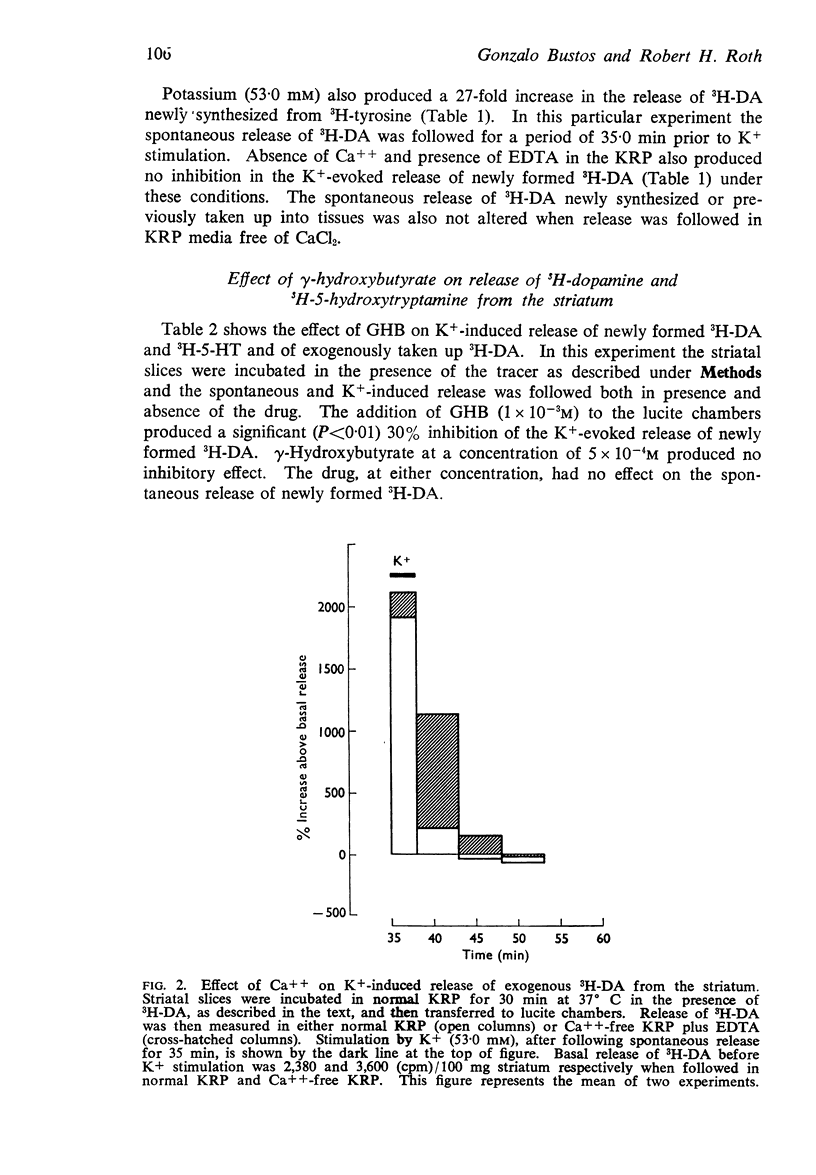
3. Potassium (53 mM) increased (eighteen to thirty fold) the release of 3H-dopamine from striatal slices initially loaded by preincubation with 3H-dopamine. However, the K+-induced release of this pool of dopamine was not antagonized by γ-hydroxybutyrate.
4. Potassium (53 mM) also increased the release from striatal slices of 3H-5-hydroxytryptamine newly synthesized from 3H-tryptophan. This K+-induced release of 5-hydroxytryptamine was also not inhibited by γ-hydroxybutyrate.
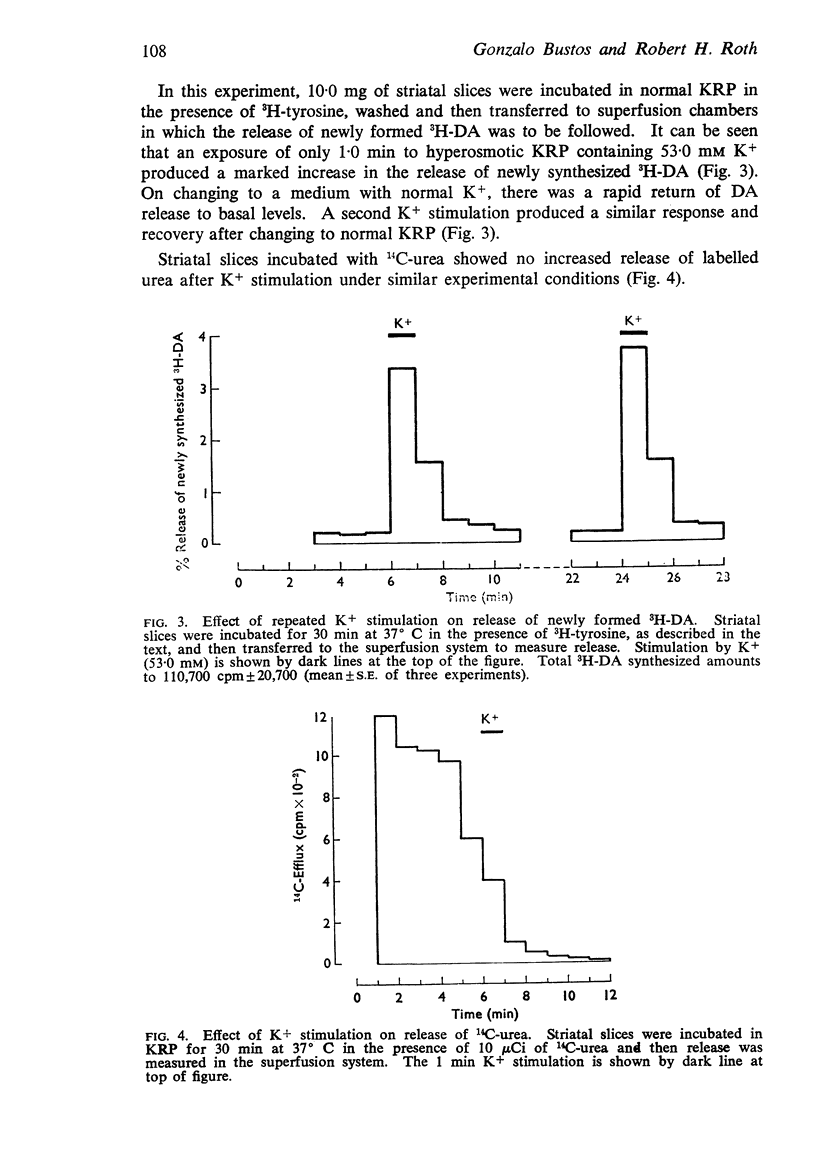
5. The release of newly synthesized 3H-noradrenaline from hypothalamic slices was also increased by K+. This K+-induced release, however, unlike that of 5-hydroxytryptamine, was antagonized when γ-hydroxybutyrate was present in the superfusion medium.
6. Removal of Ca++ had no effect on K+-induced release of 3H-dopamine when followed by transferring the slices through successive media. This K+-induced release was abolished, however, when Mg++ (12 mM) was present in the medium.
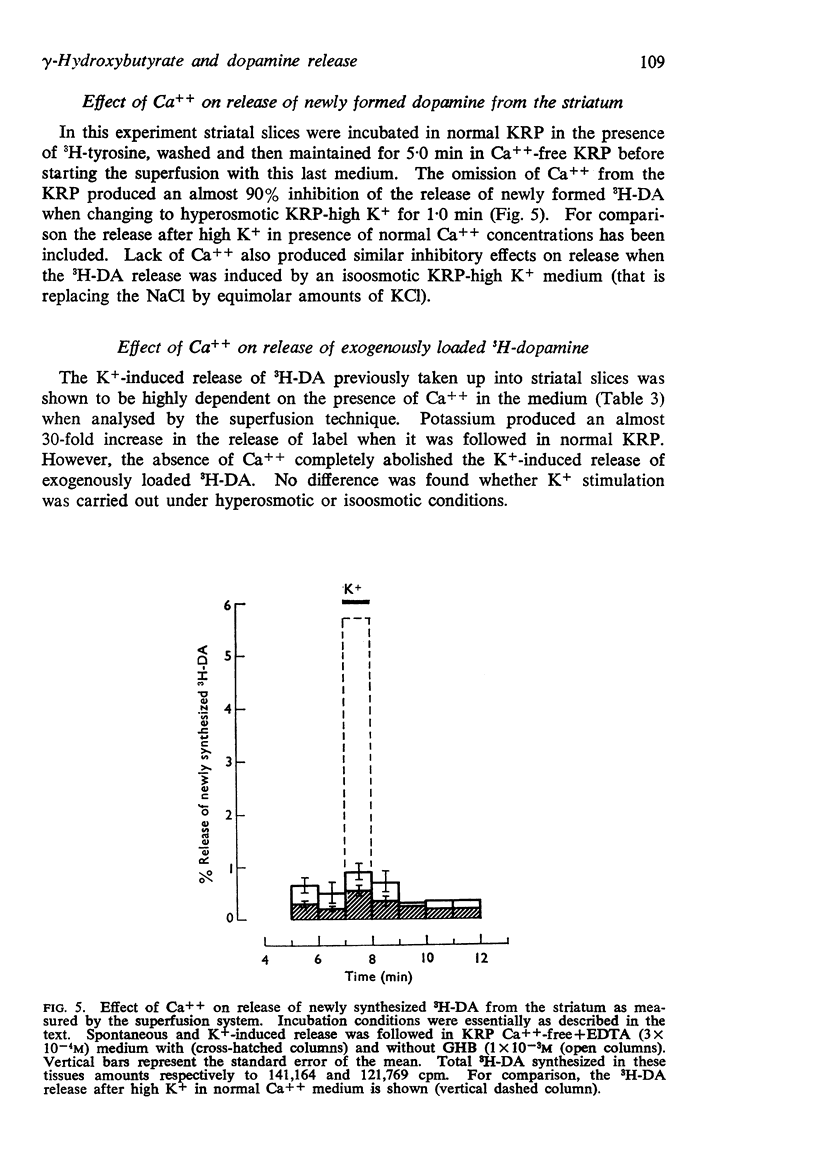
7. The removal of Ca++ from the superfusion medium abolished almost completely the K+-induced release from striatal slices of either newly synthesized 3H-dopamine or preloaded 3H-dopamine. This is presumably due to a more effective washout of tissue Ca++ by the superfusion technique.
8. The ability of γ-hydroxybutyrate to antagonize the K+-induced release of monoamines from brain slices does not appear to be unique to the release of newly synthesized dopamine from the striatum.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Roth R. H. Gamma-hydroxybutyrate-induced increase in brain dopamine: localization by fluorescence microscopy. J Pharmacol Exp Ther. 1970 Oct;175(1):131–138. [PubMed] [Google Scholar]
- BURN J. H., GIBBONS W. R. THE PART PLAYED BY CALCIUM IN DETERMINING THE RESPONSE TO STIMULATION OF SYMPATHETIC POSTGANGLIONIC FIBRES. Br J Pharmacol Chemother. 1964 Jun;22:540–548. doi: 10.1111/j.1476-5381.1964.tb01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini R. J., Kopin I. J. The effect of drugs on the release of norepinephrine-H from central nervous system tissued by electrical stimulation in vitro. J Pharmacol Exp Ther. 1967 Apr;156(1):31–38. [PubMed] [Google Scholar]
- Baldessarini R. J., Kopin I. J. Tritiated norepinephrine: release from brain slices by electrical stimulation. Science. 1966 Jun 17;152(3729):1630–1631. doi: 10.1126/science.152.3729.1630. [DOI] [PubMed] [Google Scholar]
- Boadle-Biber M. C., Hughes J., Roth R. H. Acceleration of noradrenaline biosynthesis in the guinea-pig vas deferens by potassium. Br J Pharmacol. 1970 Dec;40(4):702–720. doi: 10.1111/j.1476-5381.1970.tb10648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. The effects of inorganic ions on the storage and uptake of H3-norepinephrine by rat heart slices. J Pharmacol Exp Ther. 1969 Feb;165(2):181–189. [PubMed] [Google Scholar]
- Bogdanski D. F., Tissari A., Brodie B. B. Role of sodium, potassium, ouabain and reserpine in uptake, storage and metabolism of biogenic amines in synaptosomes. Life Sci. 1968 Apr 1;7(7):419–428. doi: 10.1016/0024-3205(68)90013-1. [DOI] [PubMed] [Google Scholar]
- Boullin D. J. The action of extracellular cations on the release of the sympathetic transmitter from peripheral nerves. J Physiol. 1967 Mar;189(1):85–99. doi: 10.1113/jphysiol.1967.sp008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos G., Roth R. H. Effect of -hydroxybutyrate on the release of monoamines from the rat striatum. Br J Pharmacol. 1972 Apr;44(4):817–820. doi: 10.1111/j.1476-5381.1972.tb07322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J. P., Wurtman R. J. The fate of intracisternally administered norepinephrine-3H in the brain and spinal cord of the rabbit. J Pharmacol Exp Ther. 1971 Jul;178(1):8–19. [PubMed] [Google Scholar]
- Chase T. N., Katz R. I., Kopin I. J. Release of [3H]serotonin from brain slices. J Neurochem. 1969 Apr;16(4):607–615. doi: 10.1111/j.1471-4159.1969.tb06860.x. [DOI] [PubMed] [Google Scholar]
- Cooke W. J., Robinson J. D. Factors influencing calcium movements in rat brain slices. Am J Physiol. 1971 Jul;221(1):218–225. doi: 10.1152/ajplegacy.1971.221.1.218. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. The mechanism of release of catecholamines from the adrenal medulla. Pharmacol Rev. 1966 Mar;18(1):471–480. [PubMed] [Google Scholar]
- GIARMAN N. J., ROTH R. H. DIFFERENTIAL ESTIMATION OF GAMMA-BUTYROLACTONE AND GAMMA-HYDROXYBUTYRIC ACID IN RAT BLOOD AND BRAIN. Science. 1964 Aug 7;145(3632):583–584. doi: 10.1126/science.145.3632.583. [DOI] [PubMed] [Google Scholar]
- GIARMAN N. J., SCHMIDT K. F. Some neurochemical aspects of the depressant action of gamma-butyrolactone on the central nervous system. Br J Pharmacol Chemother. 1963 Jun;20:563–568. doi: 10.1111/j.1476-5381.1963.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- HAEGGENDAL J. AN IMPROVED METHOD FOR FLUORIMETRIC DETERMINATION OF SMALL AMOUNTS OF ADRENALINE AND NORADRENALINE IN PLASMA AND TISSUES. Acta Physiol Scand. 1963 Nov;59:242–254. doi: 10.1111/j.1748-1716.1963.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Glowinski J. Regional studies of catecholamines in the rat brain. II. Rate of turnover of catecholamines in various brain regions. J Neurochem. 1966 Aug;13(8):671–682. doi: 10.1111/j.1471-4159.1966.tb09874.x. [DOI] [PubMed] [Google Scholar]
- Katz R. I., Kopin I. J. Electrical field-stimulated release of norepinephrine-H3 from rat atrium: effects of ions and drugs. J Pharmacol Exp Ther. 1969 Oct;169(2):229–236. [PubMed] [Google Scholar]
- Ng K. Y., Chase T. N., Colburn R. W., Kopin I. J. Dopamine: stimulation-induced release from central neurons. Science. 1971 Apr 30;172(3982):487–489. doi: 10.1126/science.172.3982.487. [DOI] [PubMed] [Google Scholar]
- Rizzoli A. A., Galzigna L. Molecular mechanism of unconscious state induced by butyrate. Biochem Pharmacol. 1970 Oct;19(10):2727–2736. doi: 10.1016/0006-2952(70)90099-7. [DOI] [PubMed] [Google Scholar]
- Roth R. H. Effect of anesthetic doses of hydroxybutyrate on subcortical concentrations of homovanillic acid. Eur J Pharmacol. 1971 Jun;15(1):52–59. doi: 10.1016/0014-2999(71)90078-1. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stone E. A. The action of reserpine on noradrenaline biosynthesis in sympathetic nerve tissue. Biochem Pharmacol. 1968 Aug;17(8):1581–1590. doi: 10.1016/0006-2952(68)90218-9. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Suhr Y. Mechanism of the gamma-hydroxybutyrate-induced increase in brain dopamine and its relationship to "sleep". Biochem Pharmacol. 1970 Dec;19(12):3001–3012. doi: 10.1016/0006-2952(70)90086-9. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Lishajko F. Drug-induced inhibition of noradrenaline synthesis in vitro in bovine splenic nerve tissue. Br J Pharmacol Chemother. 1966 Aug;27(2):398–404. doi: 10.1111/j.1476-5381.1966.tb01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A. M., Blanc G., Glowinski J. Effect of stress on the disposition of catecholamines localized in various intraneuronal storage forms in the brain stem of the rat. J Neurochem. 1971 Mar;18(3):449–461. doi: 10.1111/j.1471-4159.1971.tb11972.x. [DOI] [PubMed] [Google Scholar]


