Abstract
The signaling events generated by a cold exposure are poorly known in plants. We were interested in checking the possible activation of enzymes of the phosphoinositide signaling pathway in response to a temperature drop. In Arabidopsis suspension cells labeled with 33PO43−, a cold treatment induces a rapid increase of phosphatidic acid (PtdOH) content. This production was due to the simultaneous activation of phospholipase C (through diacylglycerol kinase activity) and phospholipase D, as monitored by the production of inositol triphosphate and of transphosphatidylation product, respectively. Moreover, inhibitors of the phosphoinositide pathway and of diacylglycerol kinase reduced PtdOH production. Enzyme activation occurred immediately after cells were transferred to low temperature. The respective contribution of both kind of phospholipases in cold-induced production of PtdOH could be estimated. We created conditions where phospholipids were labeled with 33PO43−, but with ATP being nonradioactive. In such conditions, the apparition of radioactive PtdOH reflected PLD activity. Thus, we demonstrated that during a cold stress, phospholipase D activity accounted for 20% of PtdOH production. The analysis of composition in fatty acids of cold-produced PtdOH compared with that of different phospholipids confirmed that cold-induced PtdOH more likely derived mainly from phosphoinositides. The addition of chemical reagents modifying calcium availability inhibited the formation of PtdOH, showing that the cold-induced activation of phospholipase pathways is dependent on a calcium entry.
During their development, plants are submitted to various stresses, either abiotic ones like changes in light intensities, in temperature conditions, or in soil water potential, or biotic ones like interactions with microorganisms. Among these stresses, changes in temperature are very important for plants cultivated in temperate climates. They are submitted to temperature variations between day and night, but also over the different seasons. Contrary to mammals, no system to maintain temperature homeostasis exists in plants that are going to adapt to a temperature change by modifying their metabolism. It has been observed for instance that in response to a cold treatment, specific genes are expressed such as those leading to the accumulation of sugar and Pro. Most of these metabolic changes explain why some plants submitted for a while to nonchilling, cold temperatures can resist to subzero temperature thanks to the so called cold-acclimation phenomenon (Thomashow, 1998, 2001).
Although many genes have been shown to be induced by a cold treatment (Seki et al., 2001), the signaling pathway implicated in the perception and in the transduction of cold signal into the cells is poorly known. In Synechocystis sp., Vigh and his collaborators (Vigh et al., 1993) proposed that rigidification of the plasma membrane might be the event that initiates all the downstream signaling cascade. Suzuki and collaborators (Suzuki et al., 2000) identified a His kinase that may serve as a cold sensor (Suzuki et al., 2001). In higher plants, a cold treatment is known to elicit an immediate rise in cytosolic free calcium concentration (Knight et al., 1996; Plieth et al., 1999) or to stimulate protein phosphorylation. For instance, a cold treatment activates some mitogen-activated protein (MAP) kinases by posttranslational modification in Arabidopsis (Ichimura et al., 2000) and in alfalfa (Medicago sativa; Jonak et al., 1996). Cold stress simultaneously induces genes encoding a MAP kinase kinase kinase and a MAP kinase in Arabidopsis (Mizoguchi et al., 1996). In rice (Oryza sativa), it induces a gene encoding a calcium-dependent protein kinase, and the overexpression of this gene confers cold tolerance (Saijo et al., 2000). Cold treatment also activates expression of transcription factors, such as those of the DREB1 family (Stockinger et al., 1997; Shinwari et al., 1998), leading to the induction of target genes (Liu et al., 1998).
De Nisi and Zocchi (1996) showed that in roots of maize (Zea mays) plantlets, a cold exposure induced a decrease in the level of membrane polyphosphoinositide. Knight and collaborators (1996) showed that in Arabidopsis seedlings, the cold-induced cytoplasmic calcium rise could be disturbed by altering inositol triphosphate (InsP3) metabolism. These findings lead to the question of whether some membrane enzymes of phospholipid metabolism, such as phospholipases C (PLC) and D (PLD), could be implicated in the cold signal transduction pathway.
Here, we provide the first direct evidence that in plant cells, a drop in temperature induces the activation of PLC and PLD, leading to an increase in phosphatidic acid (PtdOH) content. This activation was inhibited by inhibitors of the phosphoinositide signaling pathway and was calcium dependent. The fatty acid composition of the cold-generated PtdOH was determined.
RESULTS
PtdOH Formation Is Induced by a Cold Treatment
As a preliminary experiment, we studied the kinetic of labeling of the different phospholipids by [33P] orthophosphate. Lipids were extracted at different times after addition of the radioactive phosphate in the medium, and were separated by thin-layer chromatography (TLC) using alkaline and acidic solvent systems. The radiolabeled phospholipids were identified by comigration with standards and comparison with published data (Munnik et al., 1994, 2000; Gawer et al., 1999; Pical et al., 1999). After 90 min, all of the phospholipid classes were labeled and the labeling appeared to reach a plateau, indicating that the isotopic equilibrium was practically attained (data not shown).
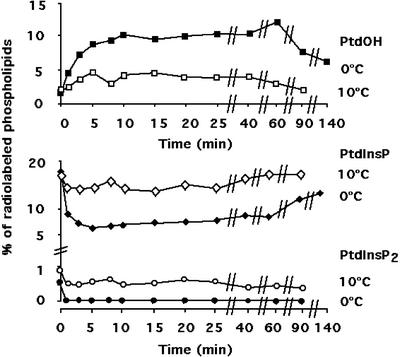
To study the turn-over of phospholipids during a cold treatment, after 2 h of labeling, the cell flasks were transferred at 0°C or 10°C, and the lipids were extracted at different times. The temperatures reached inside the flasks were measured in parallel. It took 1 or 1.7 min to equilibrate the suspension at 10°C or 0°C, respectively. It clearly appears that a cold treatment had an impact on phospholipid metabolism (Figs. 1 and 2). When the temperature was set at 0°C, the quantity of PtdOH, a minor phospholipid in nontreated cells (less than 1% of the total lipids), rose up to reach about 9% (8.8% ± 3.2%, mean and sd calculated from three independent experiments) of total phospholipids after 10 min, and then the level of PtdOH decreased slowly and still represented about 6% (w/v) of total phospholipids after 140 min (Fig. 2). Phosphatidylinositol bis-phosphate (PtdInsP2) became undetectable within 1 min (Fig. 2). Phosphatidylinositol monophosphate (PtdInsP) level dramatically decreased shortly after the temperature drop and remained low. If the temperature treatment was performed at 10°C, PtdOH also accumulated but reached only about 5% of total phospholipids (Fig. 2). PtdInsP level decreased, but less than in the experiment performed at 0°C, and then slowly recovered. The PtdInsP2 level decreased, but remained detectable (Fig. 2). No diacylglycerol pyrophosphate (DGPP) could be detected in either condition.
Figure 1.
Autoradiograph of a plate showing the turnover of phospholipids during an exposure at 0°C. Phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 2 h at 22°C. The cold treatment was then performed by transferring the culture flasks into a water bath at 0°C. Lipids were extracted at different times after the temperature treatment and were separated by TLC using an alkaline solvent. The TLC plate was analyzed with a Storm system (Molecular Dynamics, Sunnyvale, CA). An autoradiograph of a plate representative from a typical experiment is shown. Unlabeled phospholipid standards were run in parallel to identify the spots and were revealed by iodine vapor.
Figure 2.
Turnover of radiolabeled phospholipids during a cold treatment at 0°C (black symbols) or 10°C (white symbols). The turnover of PtdOH (square), PtdInsP (diamonds) and PtdInsP2 (circles) are displayed. Values were obtained after analysis of chromatography plates with a Storm system (Molecular Dynamics) and quantification of the labeled spots. PtdOH level was quantified from an autoradiograph of a plate submitted to migration in the acidic TLC system, whereas PtdInsP and PtdInsP2 levels were quantified from an autoradiograph of a plate used in the alkaline TLC system. Results from a typical experiment for each condition are displayed.
PtdOH could be synthesized by two pathways: directly by the action of a PLD or by the combined action of a PLC, which produces diacylglycerol, followed by the action of a diacylglycerol kinase (DAGK). Therefore, we investigated which one of these pathways was activated by a cold treatment.
PLC Activity Is Stimulated during Cold Treatment
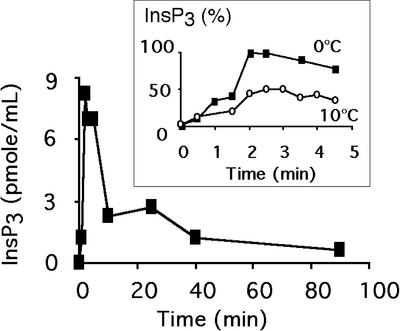
PLC activity was assayed by measuring the apparition of InsP3. Cell flasks were submitted to a cold exposure at 0°C or 10°C. Although no InsP3 could be detected in nontreated cells, it was rapidly produced during a 0°C treatment (Fig. 3). Maximum of InsP3 accumulation was attained after 2 min of exposure, and then the level decreased. When InsP3 accumulation was compared between a 0°C treatment and a 10°C treatment, the InsP3 production was approximately two times lower at 10°C (Fig. 3, inset).
Figure 3.
InsP3 production of cells submitted to an exposure at 0°C. At different times after the temperature shift, InsP3 was extracted and quantified with an InsP3 radioreceptor assay kit according to the manufacturer's recommendations. Data from a typical experiment are displayed. Inset, Comparison of InsP3 production in cells shifted to 0°C (black symbols) or 10°C (white symbols). At different times after the temperature shift, InsP3 was extracted and quantified with an InsP3 radioreceptor assay kit according to the manufacturer's recommendations. Data from a typical experiment are displayed. Data were normalized by the maximum InsP3 amount detected during the treatment at 0°C.
An Increase in PLD Activity Is Detected during Cold Treatment
PLD activity can be measured in vivo by the transphosphatidylation reaction, leading to the formation of phosphatidylbutanol (PtdBut) in presence of butanol added in the culture medium. [33P]orthophosphate was added to the cell medium, and after 105 min, 0.7% (v/v) butanol was introduced in the suspension medium. Fifteen minutes later, cells were transferred at 0°C or at 10°C. After 10 min, lipids were extracted and separated by TLC using an ethyl acetate-isooctane solvent system. In nontreated cells, PtdBut represented 0.36 (±0.01) ‰ of total phospholipids. After 10 min at 0°C, its levels increased to 1.17 (±0.07) ‰, whereas after 10 min at 10°C, its levels was 0.80 (±0.05) ‰, showing that PLD was activated at both temperatures.
PLC Activity Accounts for 80% in the Formation of Cold-Induced PtdOH
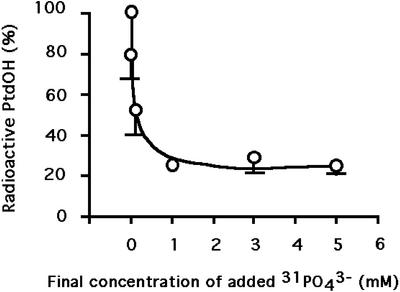
To estimate the relative contribution of PLC and PLD pathways in PtdOH formation, cells were first labeled by [33P]orthophosphate for 105 min, and then millimolar concentrations of [31P]orthophosphate were added to the medium. Fifteen minutes later, cells were transferred at 0°C for 10 min before stopping the reaction. In these conditions, structural phospholipids that contain a diesterified phosphate, such as phosphatidyl ethanolamine (PdtEtn) or phosphatidylcholine (PtdCho) remained labeled. On the contrary, ATP, which has a very short half-life time, had incorporated a lot of nonradioactive phosphate. PtdOH produced by PLD from PtdCho (or PtdEtn) should be radioactive as the substrate is radioactive. On the contrary, PtdOH produced by phosphorylation of diacylglycerol (resulting from PLC action) should not be radioactive, as ATP was not. To test this, an experiment was performed with increasing concentrations of [31P]orthophosphate (Fig. 4). As expected, the relative abundance of radiolabeled PtdOH among radiolabeled phospholipids decreased with increasing concentrations of [31P]orthophosphate, and then reached a plateau. An approximate 20% of the PtdOH formed was still radioactive in presence of high concentrations (3 mm and higher) of cold phosphate. This should represent the fraction of PtdOH produced by PLD after 10 min of treatment at 0°C, the remaining 80% being produced via the PLC pathway.
Figure 4.
Radioactive PtdOH detected after 10 min of exposure at 0°C in cells supplemented with increasing concentrations of nonradioactive PO43− prior to the temperature shift. Phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 105 min at 22°C. Cells were then supplemented with increasing concentrations of nonradioactive PO43−. After 15 min, cells were transferred to 0°C. Lipids were extracted after 10 min of exposure, and were separated by TLC using an acidic solvent system. PtdOH content was quantified as a fraction of total radiolabeled phospholipids. PtdOH produced in presence of nonradioactive PO43− is expressed as a percentage of the level of PtdOH attained in cells with no added PO43−. Data are the mean of three independent experiments (±sd).
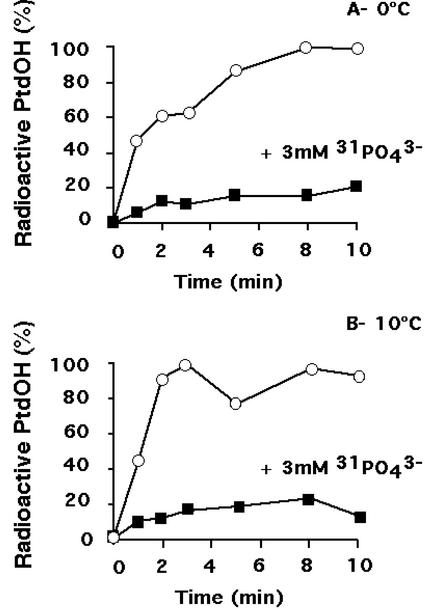
To follow the kinetics of PLD activation, radioactive PtdOH formed was measured in presence or absence of excess 31 PO43− (3 mm) for different times at 0°C (Fig. 5A) or at 10°C (Fig. 5B). It appears that PLD activity contributed to approximately 20% of the accumulation of PtdOH during a cold treatment at both temperatures. Moreover, PLD activation was always a fast phenomenon, taking place immediately after the temperature drop.
Figure 5.
Relative level of radioactive PtdOH formed in absence (white symbols) or presence (black symbols) of 3 mm nonradioactive PO43− during an exposure at 0°C (A) or 10°C (B). Phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 105 min at 22°C. Nonradioactive PO43−, if required, was then added. Fifteen minutes later, cells were transferred at 0°C or 10°C. Lipids were extracted at different times after the beginning of the cold treatment, and were separated by TLC using an acidic solvent system. PtdOH content was quantified as a fraction of total radiolabeled phospholipids. PtdOH formed was normalized to the maximum amount detected in the absence of nonradioactive phosphate. A typical experiment for each condition is shown.
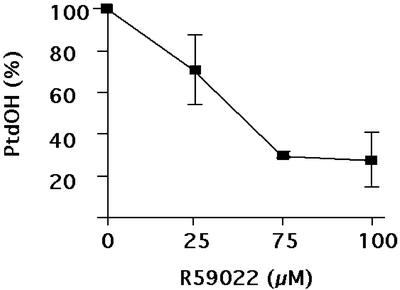
To further assess that some of the PtdOH originated from the DAGK action, cells were preincubated with R59022, a DAGK inhibitor (Lundberg and Sommarin, 1992), prior to the temperature exposure. The decrease in PtdOH formed was dependent on the concentration of the inhibitor (Fig. 6): PtdOH resistant to R59022 appeared to represent 30% of the total PtdOH. This is another indication that the PLC/DAGK pathway was responsible for the major part of the PtdOH formed.
Figure 6.
Effects of R59022, an inhibitor of DAGK, on the cold-induced production of PtdOH during an exposure at 0°C. R59022 was added 15 min prior to the cold treatment. Phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 2 h at 22°C. Lipids were extracted 10 min after the beginning of the cold treatment, and were separated by TLC using the acidic solvent system. PtdOH content was quantified as a fraction of total radiolabeled phospholipids. Values are expressed as the percentage of values obtained in non treated cells (mean of three independent determinations ± sd).
Determination of Fatty Acid Composition of Cold-Produced PtdOH
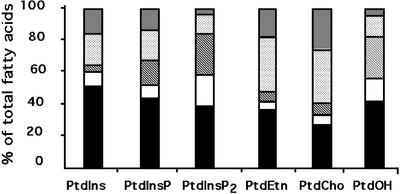
It is known that each phospholipid class exhibits an unique pattern of molecular species, thus a peculiar distribution of fatty acids (Olsson and Salem, 1997). Therefore, the origin of the PtdOH formed after a cold exposure could be ascertained by comparing its fatty acid composition with that of PtdCho (or PtdEtn) and PtdInsP2. PtdCho, PtdEtn, PtdIns, PtdInsP, and PtdInsP2 were isolated from nontreated suspension cells, and PtdOH was isolated from cells treated for 10 min at 0°C. The polyphosphoinositides (PtdInsP and PtdInsP2) were separated by TLC using an alkaline solvent system (Munnik et al., 1994). The other phospholipids were separated by two-dimensional TLC (Guillot-Salomon et al., 1987). The phospholipids were submitted to methanolysis, and the methyl esters of fatty acids were analyzed by gas chromatography. The relative composition in 16:0, 18:0, 18:1, 18:2, and 18:3 of cold-produced PtdOH was compared with the relative composition in these fatty acids of PtdEtn, PtdCho, PtdIns, PtdInsP, and PtdInsP2 (Fig. 7). PtdEtn and PtdCho were characterized by a high quantity of 18:2 and 18:3 fatty acids, which represented more than 50% of the total fatty acids. On the contrary, PtdIns, PtdInsP, and PtdInsP2 were characterized by a high amount of saturated acids, with 16:0 and 18:0 representing 50% to 60% of total fatty acids. Cold-induced PtdOH composition appeared to be more of the phosphoinositide kind, with nearly 60% of saturated acids. Moreover, the important quantity of 18:1 and the relatively low amount of 18:3 in cold-induced PtdOH made it very similar to PtdInsP2.
Figure 7.
Fatty acid composition of PtdIns, PtdInsP, PtdInsP2, PtdEtn, PtdCho, and PtdOH. PtdOH was extracted after an exposure at 0°C for 10 min, whereas the others phospholipids were extracted from nontreated cells. Lipids were separated by TLC. PtdInsP and PtdInsP2 spots were scrapped off plates developed in the alkaline solvent, and the other phospholipid spots were scrapped off plates developed by two-dimensional TLC. Fatty acids were transformed in methylesters before being separated by gas chromatography (see “Materials and Methods”). Data are the mean of five independent determinations. Fatty acids are 16:0 (black), 18:0 (white), 18:1 (hatched), 18:2 (light gray), and 18:3 (dark gray) molecular species.
Effects of Phosphoinositide Pathway Inhibitors
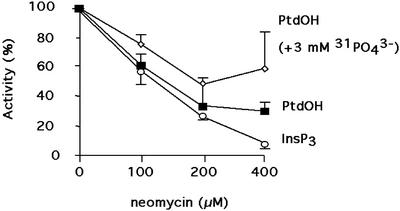
To further assess that the cold-produced PtdOH was due to the simultaneous action of a PLD and a PLC/DAGK system, the treatment at 0°C was performed in presence of different known inhibitors of the phosphoinositide pathway. The effects of the inhibitors were investigated on InsP3 produced after a 3-min cold treatment (as a probe of PLC activity), and on PtdOH generated after a 10-min cold treatment in absence (PtdOH originating from the two phospholipases) or in presence (PtdOH originating from PLD alone) of an excess of nonradioactive PO43−. Neomycin, known to inhibit the phosphoinositide pathway by encaging PtdInsP2 (Schacht, 1978), inhibited the production of PtdOH and InsP3 (Fig. 8). PLC activity could be almost totally inhibited, as expected. However, the production of PtdOH by PLD seemed to be less affected than the total PtdOH production.
Figure 8.
Effects of neomycin on the cold-induced production of PtdOH (in absence or presence of added excess nonradioactive PO43) and InsP3 during an exposure at 0°C. InsP3 (circle), PtdOH in absence of nonradioactive PO43− (square), and PtdOH in the presence of 3 mm of nonradioactive PO43− (diamonds). Values are expressed as a percentage of values obtained in nontreated cells (mean of three determinations ± sd). Neomycin was added 15 min prior to the cold treatment. When necessary, 3 mm PO43− was also added. For PtdOH measurement, phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 2 h at 22°C. Lipids were extracted 10 min after the beginning of the cold treatment and were separated by a TLC using the acidic solvent system. For InsP3 measurement, biological reactions were stopped by the addition a 0.2-volume of ice-cold 20% (w/v) perchloric acid 3 min after the beginning of the cold treatment. PtdOH and InsP3 were quantified as detailed in “Materials and Methods.”
Edelfosine, a PLC inhibitor (Arthur and Bittman, 1998), inhibited PtdOH and InsP3 productions: At concentrations of 150 μm or higher, PtdOH content was decreased by 51% ± 1%, and InsP3 production was inhibited by 52% ± 12% when compared with the control in absence of inhibitor.
Effects of Ca2+ Channel Inhibitors or Chelators
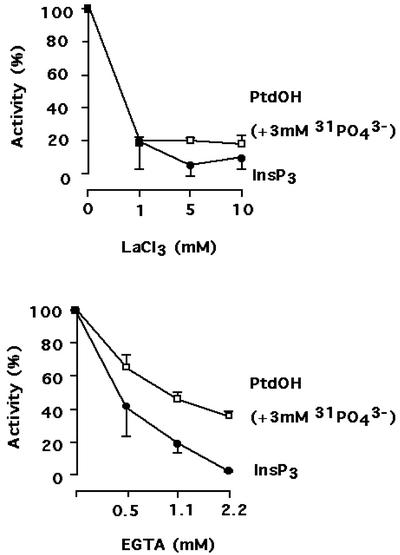
PLC and PLD are known to be dependent on Ca2+ for their activity. During a cold treatment, the concentration of cytosolic calcium was reported to dramatically increase, mostly due to the entry of extracellular calcium (Knight et al., 1996). To investigate if this Ca2+ entry was necessary for the activation of PLC and PLD during a cold treatment, lanthanum, an inhibitor of calcium channels, was added at different final concentrations 15 min prior to the cold treatment. PLC activation (as reported by InsP3 formation) was very sensitive to the presence of La3+ (Fig. 9). In contrast, PLD stimulation (as reported by PtdOH production in presence of cold phosphate) was slightly less affected. The origin of calcium necessary for InsP3 and PtdOH production was confirmed by adding EGTA in the cell medium. Chelating extracellular calcium ions inhibited PLC and PLD activation: Again, PLC seemed to be more sensitive to Ca2+ deprivation than PLD (Fig. 9). As a control, we verified that at 22°C, 2.2 mm EGTA and 10 mm LaCl3 had no effects on PtdOH production, even for incubation longer than 15 min (preincubation time) and reaching 30 min.
Figure 9.
Effects of LaCl3 and EGTA on the cold-induced production of PtdOH in presence of added cold PO43− and InsP3 during an exposure at 0°C. Values are expressed as a percentage of values obtained in nontreated cells (mean of three determinations ± sd). Chemicals were added 15 min prior to the cold treatment. When necessary, 3 mm PO43− was also added. For PtdOH measurement, phospholipids were labeled in presence of 53 MBq L−1 [33P]-PO43− for 2 h at 22°C. Lipids were extracted 10 min after the beginning of the cold treatment and were separated by a TLC using the acidic solvent system. For InsP3 measurements, biological reactions were stopped by the addition a 0.2-volume of ice-cold 20% (w/v) perchloric acid 3 min after the beginning of the cold treatment.
DISCUSSION
Our data show that a cold exposure of Arabidopsis suspension cells triggered PLC and PLD signaling pathways. Assaying InsP3 produced in cells submitted to 0°C revealed that the maximum InsP3 level was quickly attained in less than 2 min, and then diminished progressively. In parallel, PtdInsP2 (the PLC substrate) disappeared and could not be significantly detected after 1 min of cold exposure, whereas a 50% decrease in PtdInsP level was observed at this time. Most probably, PtdInsP was phosphorylated in PtdInsP2 and was immediately hydrolyzed by PLC; however, a direct use of PtdInsP as a substrate by PLC cannot be excluded. The activation of PLC appeared to be dependent on the amplitude of the temperature drop. When cells were transferred at 10°C, PLC activity, as determined by the rate of InsP3 production, was less important than in cells treated at 0°C. Such a PLC activation by cold exposure had been observed in oilseed rape (Brassica napus) leaves exposed at −5°C. However, the kinetics of InsP3 production was somehow different, with a maximum of InsP3 content observed after 30 min of stress (Smolenska-Sym and Kacperska, 1996). This difference could be due to the difference in the biological material used, isolated suspension cells being more likely able to rapidly perceive differences in temperature than leaf cells in plant. It could also be attributed to the difference in the stress temperature. A temperature of −5°C might be low enough to diminish all enzymatic activities by a simple thermodynamic effect. The PLC activation by cold treatment could not be correlated to any induction of AtPLC1 expression by cold temperature (Hirayama et al., 1995), as cold exposure had a much too rapid effect on PLC activation. Therefore, a posttranslational activation had to be envisaged. In our experiments, we were unable to detect a second PLC activation that could reflect an induced gene expression, most probably because it would require more than 140 min to occur at low temperature.
Cold exposure also activated a PLD, as revealed by the formation of PtdBut when cold treatment was performed in presence of 0.7% (v/v) butanol present in the medium, and by chase experiments. PLD activation was also detected when the temperature of the treatment was set at 0°C or 10°C. As for PLC, this rapid, posttranslational activation should certainly be followed by a transcriptional activation because Wang et al. (2000) showed that PLD expression was induced upon cold stress in Arabidopsis leaves. The relative insensitivity of PLD to the presence of neomycin indicated that it was not requiring PtdInsP2 as an activator. Moreover, formation of PtdBut was almost unaffected by the presence of neomycin (data not shown). This is in favor of the activated PLD being the α isoform because it is the only isoform that may be neomycin insensitive (Qin et al., 1997).
The contribution of each phospholipase in the cold-induced PtdOH production was estimated by controlling the availability of radioactive ATP, and the PLD contribution should be close to 20%. This means that a DAGK was also activated, even in low temperature conditions. This was verified in experiments where a kinase inhibitor was added in the medium. Under these conditions, a severe reduction in PtdOH production was evidenced and it confirmed that PtdOH originating from PLD activation accounted for 20% to 30% of the total PtdOH formed.
PtdOH is a general name for all glycerolipids with a phosphate at the sn-3 position of the glycerol backbone. Depending on the nature of the fatty acids that are linked by an ester bond to the first and second hydroxyls of the glycerol, the molecules are going to be quite different and might exhibit different biological properties (Hodgkin et al., 1998). Here, it was obvious that the major part of the PtdOH formed was low in polyunsaturated fatty acids, as it originated for the major part from PtdInsP2 (through PLC activity) rather than PtdCho or PtdEtn (through PLD activity). However, this analysis did not allow us to determine whether PLC preferred some precise PtdInsP2 molecular species.
PLC and PLD activation resulted in accumulation of PtdOH, an emerging plant lipid messenger (Munnik, 2001). The level of PtdOH remained high, with no phase of relaxation occurring at 0°C, contrary to what was observed with InsP3. PtdOH accumulation had to be correlated to the fact that no production of DGPP was detected. DGPP is a product of PtdOH phosphorylation, and has recently been detected in response to many stresses such as hyperosmotic stress (Pical et al., 1999; Munnik et al., 2000), nodulation (Den Hartog et al., 2001), or elicitation (Van der Luit et al., 2000). Thus, it appears that cold treatment did not lead to PtdOH kinase activation at 0°C or at 10°C. Changes in temperature were accompanied by a marked decrease in PtdInsP2 level (which rapidly became undetectable at 0°C) or in PtdInsP level. Most probably, the last phenomenon had to be related to a PtdInsP2 synthesis from PtdInsP. However, this synthesis was counterbalanced by PLC activity, and no increase in PtdInsP2 level was observed during a cold stress, contrary to hyperosmotic stress (Pical et al., 1999).
Our results show that calcium was necessary for InsP3 and PtdOH production during a cold exposure. The rise in Ca2+ was a very early event, as phospholipase activation was apparent as early as 15 s after the cold treatment. The calcium necessary for these production appeared to be primarily from extracellular origin because exogenously added EGTA or La3+ dramatically diminished the activation of PLC and PLD. This is in good accordance with results from other groups, showing that the rise in cytosolic calcium was mostly due to extracellular origin (Knight et al., 1996). In alfalfa cells and oilseed rape seedlings, respectively, Orvar et al. (2000) and Sangwan et al. (2001) proposed a sequence of signaling events where the decrease in membrane fluidity activates calcium channels on plasma membrane may be through an effect on cytoskeleton. It remains to be elucidated whether calcium entry is sufficient to activate phospholipases. Calcium could act through other activation mechanisms, such as protein G: Water deficit elicits PLD activity in Craterostigma plantagineum, and this activation involves trimeric protein G (Frank et al., 2000); in barley (Hordeum vulgare) aleurone, abscisic acid stimulation of PLD is mediated by a G-protein (Ritchie and Gilroy, 2000).
Because it appears that phosphoinositide signaling pathway and PLD pathway are implicated in cold response, it would be interesting to find which ones of the genes known to be induced by low temperature, such as LTI78, COR 47, or DREB1B, are activated via these pathways. The inhibitors of some steps of these signaling pathways are molecular tools for exploring this aspect of the cold response research field.
MATERIALS AND METHODS
Materials
Silica 60 TLC plates were obtained from Merck (Darmstadt, Germany). R59022 {6-(2-[4-{(p-fluorophenyl) phenylmethylene}-1-piperidinyl]ethyl)-7-methyl-5H-thiazolo(3, 2-a) pyrimidine-5-one} and edelfosine (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine) were from Calbiochem (San Diego). LaCl3, EGTA, and neomycin were purchased from Sigma (St. Louis). [33P]orthophosphate was purchased from Amersham Biosciences (Saclay, France). The culture medium for Arabidopsis suspension cells (Gamborg B5) was bought from (Duchefa, Haarlem, The Netherlands). R59022 (10 mm in ethanol), edelfosine (15 mm in ethanol), EGTA (660 mm in water), neomycin (10 mm in culture medium), and LaCl3 (1 m in water) were used as stock solutions.
Arabidopsis Cell Culture
Cell suspension culture of wild-type Arabidopsis ecotype Columbia was provided by Dr. Michèle Axelos (Centre National de la Recherche Scientifique, Toulouse, France). Every 7th day, 20-mL aliquots of cell suspension were transferred into 500-mL Erlenmeyer flasks containing 200 mL of fresh medium. Cells were grown under continuous light (100 μmol m−2 s−1) at 22°C, with orbital agitation (140 rpm).
Metabolic Radiolabeling and Cold Treatment
Phospholipids were metabolically labeled by incubating 6-d-old cells for 2 h (unless otherwise indicated) in presence of 53 MBq L−1 [33P]orthophosphate.
The cold treatment was initiated by transferring the flasks in a water bath at 0°C or 10°C, while maintaining the orbital agitation. Light conditions were constant throughout the whole experiment.
Extraction and Analysis of Lipids
Lipid extraction was performed by adding into the flasks 2.14 volumes of ice-cold chloroform:methanol:37% (v/v) HCl (50:100:1.5, v/v). The mixture was transferred into tubes, and a two-phase system was induced by the addition of 0.7 volume of chloroform and 0.7 volume of 9% (w/v) NaCl in water. The tubes were vigorously shaken and phases were allowed to form at 4°C. The upper phase was discarded and the organic phase was evaporated under nitrogen stream. When indicated, neutral lipids were extracted with ice-cold acetone and the remaining lipids were dissolved in chloroform.
Lipids were separated by TLC using an acidic solvent system (chloroform:methanol:acetone:acetic acid:water, 50:10:20:10:5, v/v; Lepage, 1967) or an alkaline solvent system (chloroform:methanol:25% [w/v] ammonia/water, 90:70:4:16, v/v; Munnik et al., 1994). When the alkaline solvent system was used, TLC plates were presoaked with 1.2% (w/v) potassium oxalate and 2 mm EDTA in methanol:water (2:3, v/v) and were heat activated. The presence of PtdBut was visualized by separating this phospholipid from the rest of phospholipids by a modified ethyl acetate TLC system (organic upper phase of a mixture of ethyl acetate:isooctane:acetic acid:water, 12:2:3:10, v/v; de Vrije and Munnik et al., 1997).
When necessary, phospholipids were separated using two-dimensional chromatography. The first migration was realized in chloroform:methanol:water (130:50:8, v/v), and the second migration was performed in the acidic solvent system (chloroform:methanol:acetone:acetic acid:water, 50:10:20:10:5, v/v; Guillot-Salomon et al., 1987).
Radiolabeled phospholipids were detected by autoradiography, and radioactivity was determined using a Storm (Molecular Dynamics). Unlabeled phospholipids standards (approximately 10 μg) were visualized by exposure to iodine vapor.
Extraction and Quantification of Ins(1, 4, 5) P3
Seven-milliliter cell suspension samples were mixed with 1.4 mL of ice-cold 20% (w/v) perchloric acid on ice, and then insoluble material was removed by centrifugation at 15,000g for 15 min at 4°C. The supernatant was recovered and adjusted to pH 7.5 with ice-cold 1.5 m KOH containing 60 mm HEPES. After removal of the sedimented KClO4, the neutralized supernatant was used for the measurement of Ins(1, 4, 5) P3 content with an Inositol 1,4,5-triphosphate [3H] Radioreceptor Assay kit (Biotrak; Amersham Biosciences). Assays were carried out according to the manufacturer's protocol with 100 μL of sample per assay.
Methanolysis of Phospholipids and Determination of Methyl Esters of Fatty Acid
After two-dimensional TLC or alkaline solvent system TLC, lipids were revealed with iodine vapor. The spots containing the phospholipids of interest were scrapped off the plate and were deposited in a tube. Methyl esters were formed by adding 3 mL of 2.5% (v/v) sulfuric acid in methanol to the silica. The reaction was run at 70°C for 1 h, and was stopped by transferring the tubes on ice. A two-phase system was formed by adding 3 mL of pentane and 3 mL of water. After at least 2 h, the upper (apolar) phase was recuperated and the pentane was evaporated under nitrogen stream. Methyl esters of fatty acids were resuspended in heptane, and 1 μL was injected in a gas chromatograph (Varian, Palo Alto, CA).
Footnotes
This work was supported by the Ministère de la Recherche, by the Centre National de la Recherche Scientifique, and by the Université Pierre et Marie Curie.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006080.
LITERATURE CITED
- Arthur G, Bittman R. The inhibition of cell signaling pathways by antitumor ether lipids. Biochim Biophys Acta. 1998;1390:85–102. doi: 10.1016/s0005-2760(97)00163-x. [DOI] [PubMed] [Google Scholar]
- Den Hartog M, Musgrave A, Munnik T. Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: a role for phospholipase C and D in root hair deformation. Plant J. 2001;25:55–65. doi: 10.1046/j.1365-313x.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- De Nisi P, Zocchi G. The role of calcium in the cold shock responses. Plant Sci. 1996;121:161–166. [Google Scholar]
- De Vrije T, Munnik T. Activation of phospholipase D by calmodulin antagonists and mastoparan in carnation petals. J Exp Bot. 1997;48:1631–1637. [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D. Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell. 2000;12:111–124. doi: 10.1105/tpc.12.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawer M, Norberg P, Chervin D, Guern N, Yaniv Z, Mazliak P, Kader JC. Phosphoinositides and stress-induced changes in lipid metabolism of tobacco cells. Plant Sci. 1999;141:117–127. [Google Scholar]
- Guillot-Salomon T, Farineau N, Cantrel C, Oursel A, Tuquet C. Isolation and characterization of developing chloroplasts from light-grown barley leaves. Physiol Plant. 1987;69:113–122. [Google Scholar]
- Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. AtPLC1, a gene encoding a phosphoinositol-specific phospholipase C is induced by dehydration salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AL, Wakelam MJ. Diacylglycerols and phosphatidates: Which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200–204. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Jonak C, Kiergerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H. Stress signaling in plants: A mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight M. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M. Identification and composition of turnip root lipids. Lipids. 1967;2:44–250. doi: 10.1007/BF02532563. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg GA, Sommarin M. Diacylglycerol kinase in plasma membranes from wheat. Biochim Biophys Acta. 1992;1123:177–183. doi: 10.1016/0005-2760(92)90109-9. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–789. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T. Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci. 2001;6:227–233. doi: 10.1016/s1360-1385(01)01918-5. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Rapid turnover of phosphatidylinositol 3-phosphate in the green alga Chlamydomonas eugametos: signs of a PI 3-kinase signaling pathway in lower plants? Biochem J. 1994;298:269–273. doi: 10.1042/bj2980269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Meijer HJG, ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A. Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J. 2000;22:147–154. doi: 10.1046/j.1365-313x.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- Olsson NU, Salem N., Jr Molecular species analysis of phospholipids. J Chromatogr B. 1997;692:245–256. doi: 10.1016/s0378-4347(96)00507-5. [DOI] [PubMed] [Google Scholar]
- Orvar BL, Sangwan V, Omann F, Dhindsa RS. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- Pical C, Westergen T, Dove SK, Larsson C, Sommarin M. Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4, 5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J Biol Chem. 1999;274:38232–38240. doi: 10.1074/jbc.274.53.38232. [DOI] [PubMed] [Google Scholar]
- Plieth C, Hansen UP, Knight H, Knight M. Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J. 1999;18:491–497. doi: 10.1046/j.1365-313x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Qin W, Pappan K, Wang X. Molecular heterogeneity of phospholipase D (PLD): cloning of PLDγ and regulation of plant PLDγ, -β, and -α by polyphosphoinositides and calcium. J Biol Chem. 1997;272:28267–28273. doi: 10.1074/jbc.272.45.28267. [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol. 2000;12:693–702. doi: 10.1104/pp.124.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- Sangwan V, Foulds I, Singh J, Dhindsa RS. Cold-activation of Brassica napus BN115 promoter is mediated by structural changes in membranes and cytoskeleton, and requires Ca2+ influx. Plant J. 2001;27:1–12. doi: 10.1046/j.1365-313x.2001.01052.x. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978;19:1063–1067. [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13:61–72. doi: 10.1105/tpc.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwari ZK, Nakashima K, Miura S, Kasuga M, Seki M, Yamaguchi-Shinozaki K, Shinozaki K. An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low-temperature-responsive gene expression. Biochem Biophys Res Commun. 1998;250:161–170. doi: 10.1006/bbrc.1998.9267. [DOI] [PubMed] [Google Scholar]
- Smolenska-Sym G, Kacperska A. Inositol 1, 4,5-triphosphate formation in leaves of winter oilseed rape plants in response to freezing, tissue water potential and abscisic acid. Physiol Plant. 1996;96:692–698. [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Protein Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Kanesaki Y, Mikami K, Kanehisa M, Murata N. Cold-regulated genes under control of the cold sensor Hik33 in Synechocystis. Mol Microbiol. 2001;401:235–244. doi: 10.1046/j.1365-2958.2001.02379.x. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Los DA, Kanesaki Y, Mikami K, Murata N. The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J. 2000;19:1327–1334. doi: 10.1093/emboj/19.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998;118:1–7. doi: 10.1104/pp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. So what's new in the field of plant cold acclimation? Lots! Plant Physiol. 2001;125:89–93. doi: 10.1104/pp.125.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T. Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol. 2000;123:1507–1515. doi: 10.1104/pp.123.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L, Los DA, Horvath I, Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci USA. 1993;90:369–374. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang C, Sang Y, Zheng L, Qin C. Determining functions of multiple phospholipase Ds in stress response of Arabidopsis. Biochem Soc Trans. 2000;28:813–816. [PubMed] [Google Scholar]