Abstract
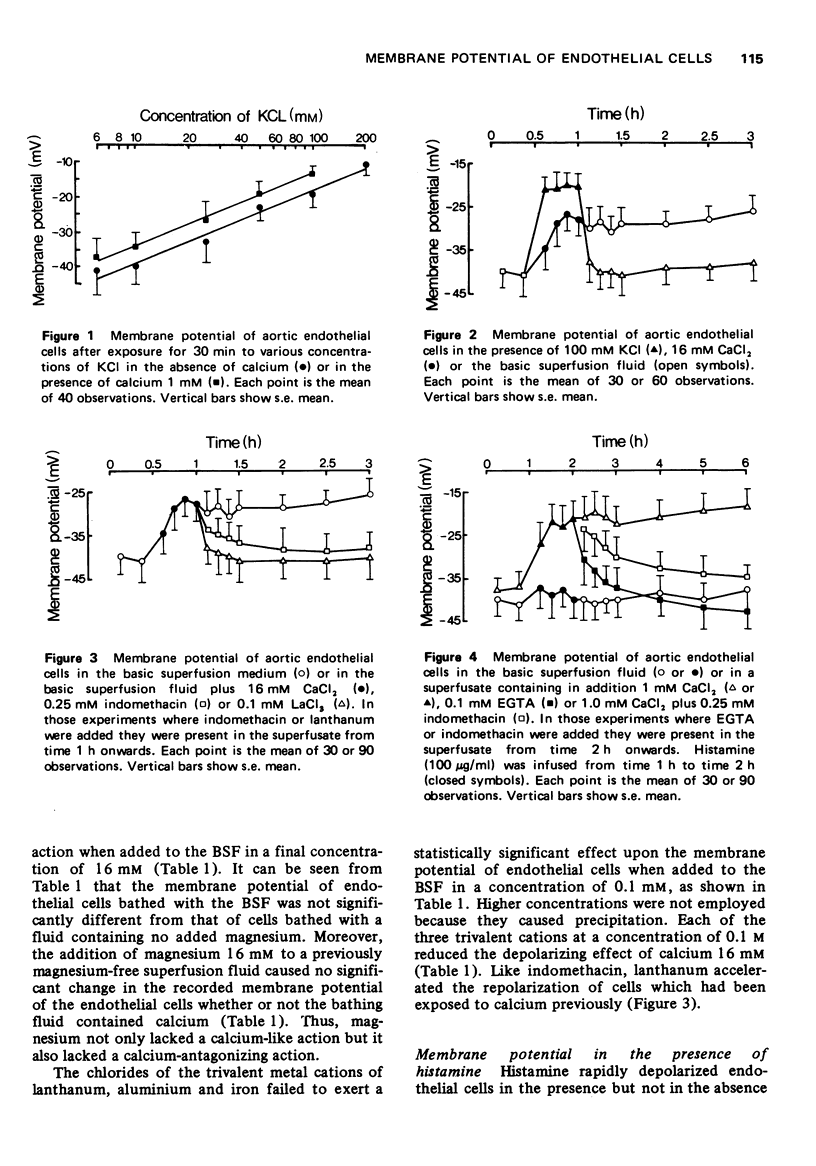
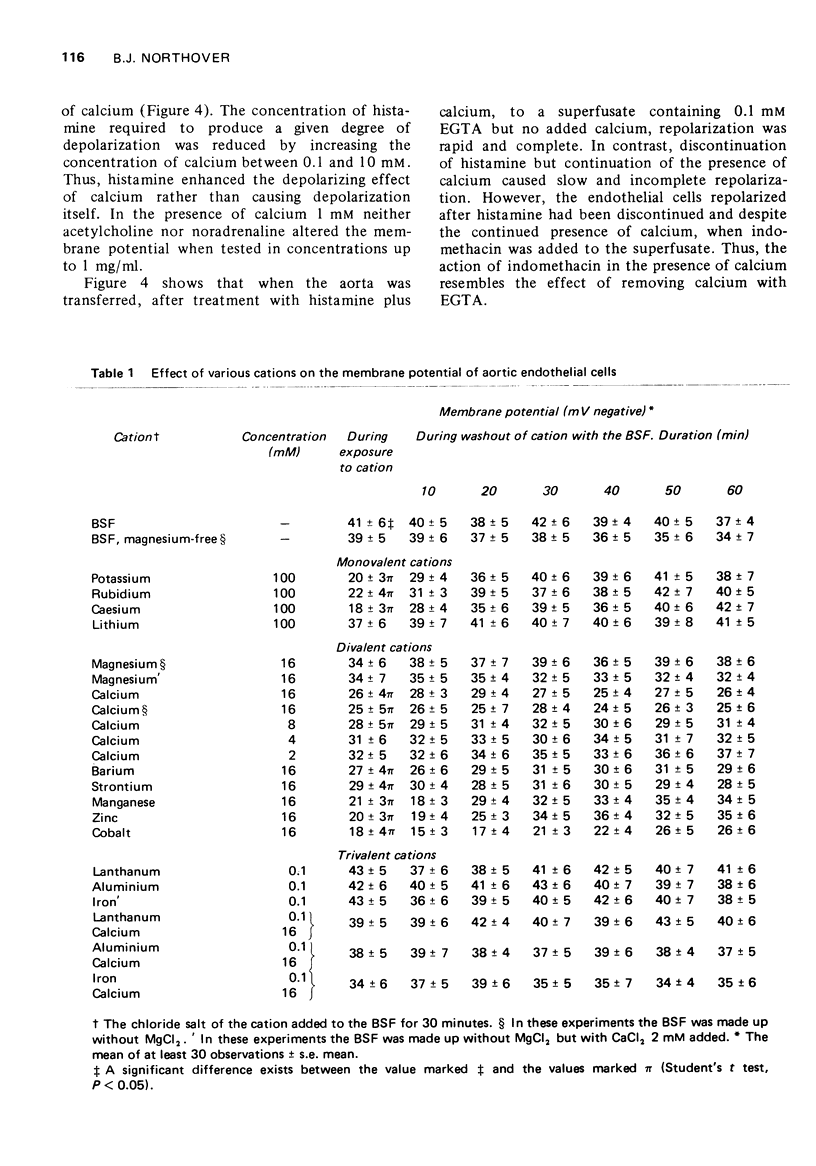
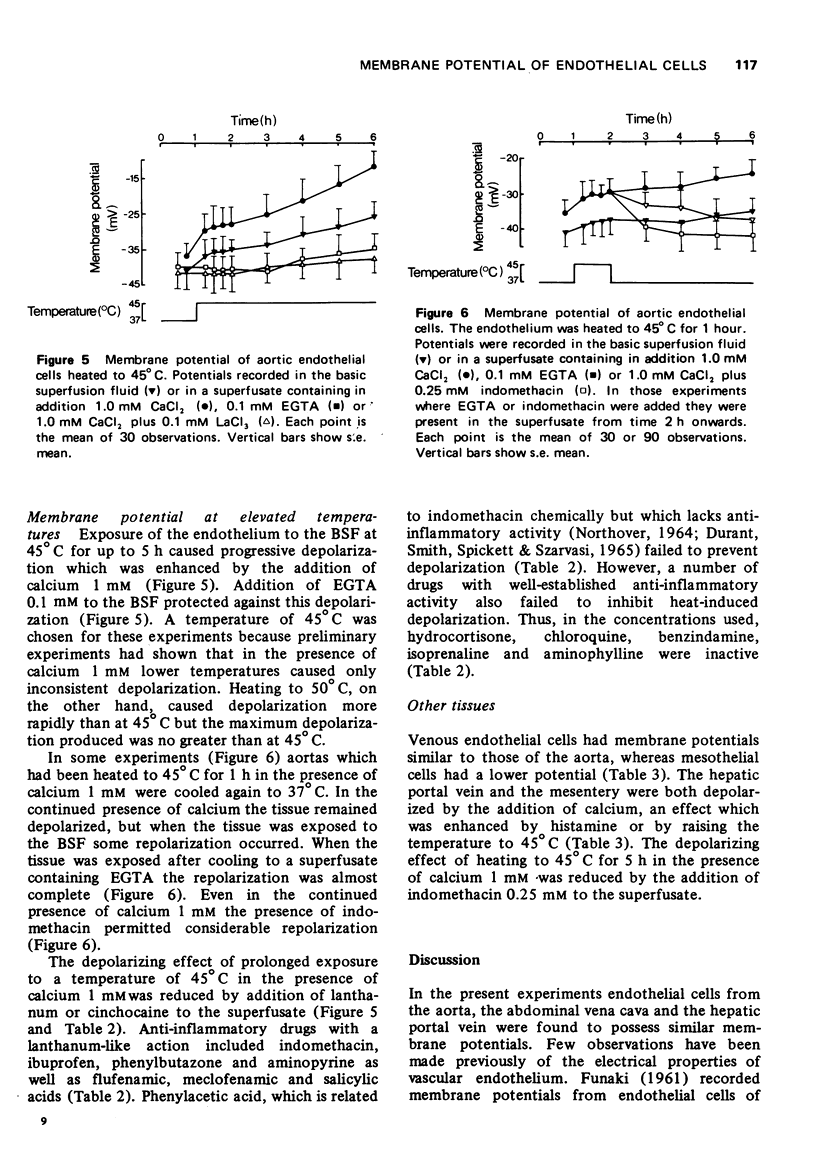
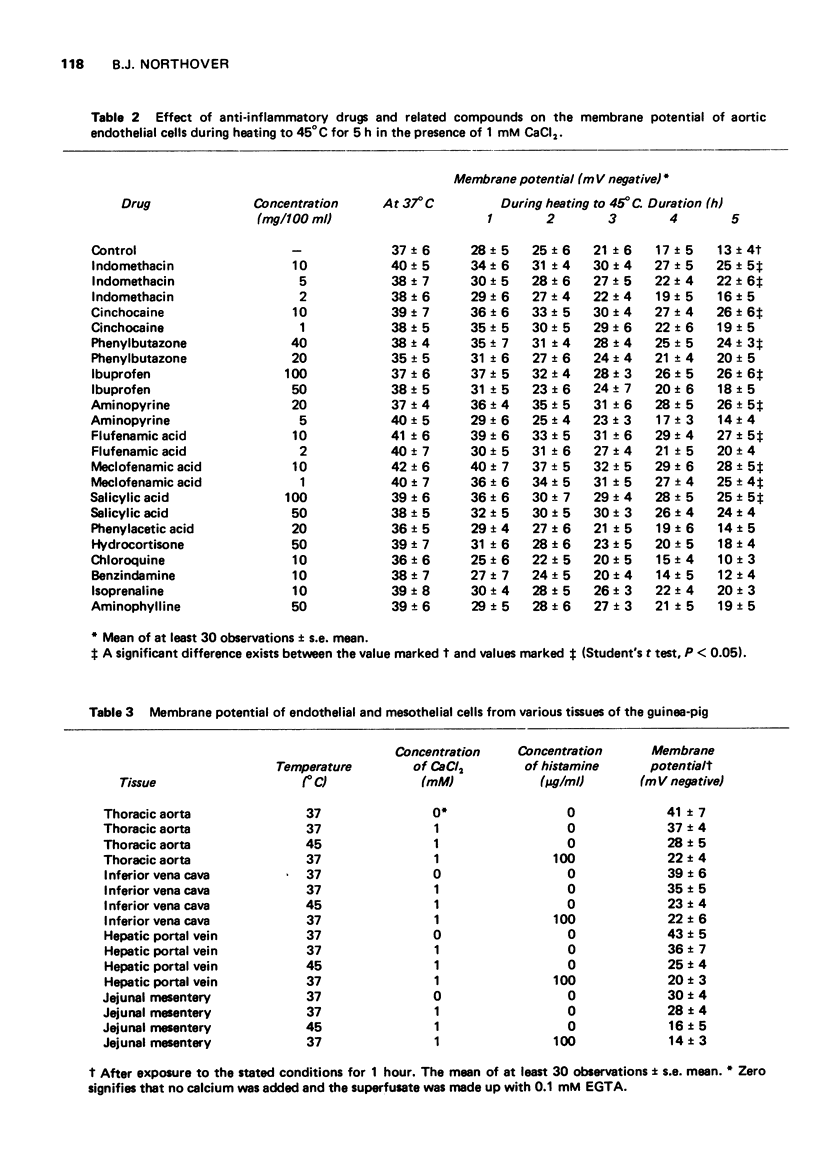
1 Cells of the aortic endothelium isolated from the guinea-pig and bathed at 37 degrees C with a calcium-free superfusion fluid had membrane potentials of minus 41 plus or minus 7 mV (mean plus or minus s.e mean). 2 Depolarization was produced by addition of potassium (50-200 mM) or certain other monovalent metal cations to the superfusion fluid. Depolarization was rapidly reversed on return to the original superfusate. 3 Several divalent metal cations, notably calcium (16 mM), caused depolarization which was only slowly and incompletely reversed on return to the original calcium-free superfusate. 4 Repolarization after exposure to calcium was accelerated and made more complete by addition of indomethacin (0.25 mM) to the superfusate, 5 The trivalent cations of lanthanum, aluminium or iron (0.1 mM) inhibited the depolarizing effect of calcium (16 mM). 6 Exposure to histamine (100 mug/ml) or heating to 45 degrees C for 1 h caused depolarization in the presence but not in the absence of calcium. Subsequent removal of histamine or cooling again to 37 degrees C in the continued presence of calcium permitted only slow and partial repolarization. However, repolarization was more rapid and complete in the presence of indomethacin (0.25 mM). 7 Heating to 45 degrees C for 5 h in the presence of calcium caused progressive and almost complete depolarization. Lanthanum, cinchocaine, indomethacin, flufenamic, meclofenamic and salicylic acids, phenylbutazone and aminopyrine each reduced the depolarization, but hydrocortisone, chloroquine, benzindamine, isoprenaline and aminophylline did not.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNAKI S. Spontaneous spike-discharges of vascular smooth muscle. Nature. 1961 Sep 9;191:1102–1103. doi: 10.1038/1911102a0. [DOI] [PubMed] [Google Scholar]
- Frank G. B., Inoue F. Effects of reducing the extracellular calcium concentration on the resting potential of frog's skeletal muscle fibers. Jpn J Physiol. 1973 Apr;23(2):183–197. doi: 10.2170/jjphysiol.23.183. [DOI] [PubMed] [Google Scholar]
- Hava M., Hurwitz A. The relaxing effect of aluminum and lanthanum on rat and human gastric smooth muscle in vitro. Eur J Pharmacol. 1973 May;22(2):156–161. doi: 10.1016/0014-2999(73)90006-x. [DOI] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHOVER B. J. THE ACTION OF ANTI-INFLAMMATORY COMPOUNDS IN MICE WITH PERITONITIS. J Pathol Bacteriol. 1964 Apr;87:395–404. doi: 10.1002/path.1700870219. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Effect of anti-inflammatory drugs on the binding of calcium to cellular membranes in various human and guinea-pig tissues. Br J Pharmacol. 1973 Jul;48(3):496–504. doi: 10.1111/j.1476-5381.1973.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northover B. J. The effects of indomethacin on calcium, sodium, potassium and magnesium fluxes in various tissues of the guinea-pig. Br J Pharmacol. 1972 Aug;45(4):651–659. doi: 10.1111/j.1476-5381.1972.tb08124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Ogawa M., Aoki N., Izutsu K. The effect of K + on the membrane potential in HeLa cells. Biochim Biophys Acta. 1973 Jan 2;291(1):116–126. doi: 10.1016/0005-2736(73)90066-7. [DOI] [PubMed] [Google Scholar]
- Starr M. S., West G. B. Bradykinin and oedema formation in heated paws of rats. Br J Pharmacol Chemother. 1967 Sep;31(1):178–187. doi: 10.1111/j.1476-5381.1967.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


