Abstract
The regulation of intracellular Ca2+ levels is achieved in part by high-capacity vacuolar Ca2+/H+ antiporters. An N-terminal regulatory region (NRR) on the Arabidopsis Ca2+/H+ antiporter CAX1 (cation exchanger 1) has been shown previously to regulate Ca2+ transport by a mechanism of N-terminal auto-inhibition. Here, we examine the regulation of other CAX transporters, both within Arabidopsis and from another plant, mung bean (Vigna radiata), to ascertain if this mechanism is commonly used among Ca2+/H+ antiporters. Biochemical analysis of mung bean VCAX1 expressed in yeast (Saccharomyces cerevisiae) showed that N-terminal truncated VCAX1 had approximately 70% greater antiport activity compared with full-length VCAX1. A synthetic peptide corresponding to the NRR of CAX1, which can strongly inhibit Ca2+ transport by CAX1, could not dramatically inhibit Ca2+ transport by truncated VCAX1. The N terminus of Arabidopsis CAX3 was also shown to contain an NRR. Additions of either the CAX3 or VCAX1 regulatory regions to the N terminus of an N-terminal truncated CAX1 failed to inhibit CAX1 activity. When fused to N-terminal truncated CAX1, both the CAX3 and VCAX1 regulatory regions could only auto-inhibit CAX1 after mutagenesis of specific amino acids within this NRR region. These findings demonstrate that N-terminal regulation is present in other plant CAX transporters, and suggest distinct regulatory features among these transporters.
The level of intracellular Ca2+ is regulated in part by active efflux transporters that remove Ca2+ from the cytosol. As well as the P-type Ca2+-ATPase, an additional efflux mechanism is Ca2+/H+ antiport. Active efflux transporters perform a variety of roles, which include the restoration of cytosolic Ca2+ concentration to its resting level after a signal transduction event, to replenish internal Ca2+ stores, and to provide tolerance from toxic concentrations of Ca2+ (Sanders et al., 1999; Sze et al., 2000). The vacuole is the predominant Ca2+ store within the cell (Marty, 1999), and both a Ca2+-ATPase and a Ca2+/H+ antiporter exist at this organelle (Sze et al., 2000). The Ca2+/H+ antiporters are high-capacity, low-affinity transporters that efficiently sequester large amounts of Ca2+ when cytosolic Ca2+ concentrations are elevated during a signaling event (Hirschi, 2001). Recent studies suggest plants contain multiple Ca2+/H+ antiporters (Mäser et al., 2001). Given the importance of resetting cytosolic Ca2+ levels post-signal transduction, it is important to understand the regulation of the ensemble of Ca2+/H+ antiporters.
Yeast (Saccharomyces cerevisiae) has a single vacuolar Ca2+/H+ antiporter, VCX1, whereas Arabidopsis appears to have up to 11 putative Ca2+/H+ antiporters (Mäser et al., 2001), termed cation exchangers (CAXs), several of which appear to localize to the vacuole (Hirschi et al., 2000; Cheng et al., 2002). Vacuolar Ca2+/H+ antiporters have also been biochemically characterized from a variety of other plant species (Schumaker and Sze, 1985; Blumwald and Poole, 1986; Blackford et al., 1990; Ueoka-Nakanishi et al., 1999). Initially, plant Ca2+/H+ antiporter genes were identified by their ability to suppress the Ca2+-hypersensitive phenotype of a yeast mutant lacking the vacuolar Ca2+-ATPase PMC1 and VCX1 (Hirschi et al., 1996; Ueoka-Nakanishi et al., 2000). CAX1 from Arabidopsis and VCAX1 from mung bean (Vigna radiata) are high-capacity Ca2+ transporters, whereas CAX2 from Arabidopsis has a lower capacity for Ca2+ transport (Hirschi et al., 1996) and can also transport other metals (Hirschi et al., 2000). Understanding the diversity of function and regulation among the family of CAX transporters is a central component in understanding the details of Ca2+ signal transduction.
We have only begun recently to understand some of the mechanisms of posttranslational regulation of a single Ca2+/H+ antiporter, CAX1 (Pittman and Hirschi, 2001; Pittman et al., 2002). The full-length open reading frame of CAX1 contains an extended N-terminal tail of 36 amino acids, termed the N-terminal regulatory region (NRR), which prevents Ca2+ transport activity (Pittman and Hirschi, 2001). The CAX1 originally identified by the yeast suppression screen appears to be N-terminally truncated and, thus, constitutively activated; we refer to this as short-CAX1 (sCAX1). Subsequently, we have shown that the NRR regulates CAX1 by a mechanism of N-terminal auto-inhibition (Pittman et al., 2002). A synthetic peptide corresponding to all 36 amino acids of the CAX1 NRR can strongly inhibit Ca2+ transport mediated by sCAX1 expressed in a yeast expression system and this peptide can also inhibit Ca2+-induced Ca2+/H+ antiport activity from Arabidopsis root vacuolar-enriched membranes. These findings hint that CAX transporters are regulated by N-terminal domains.
To further address the question of diversity of function among the CAX transporters, we have characterized other antiporters, including CAX3 and CAX4 (Shigaki and Hirschi, 2000; Cheng et al., 2002). A CAX4 N-terminal truncation gives weak suppression of the yeast vacuolar Ca2+ transport deficiency, whereas CAX3 N-terminal truncations do not (Shigaki and Hirschi, 2000; Cheng et al., 2002). However, both CAX3 and CAX4 are able to strongly suppress the mutant phenotype if a region of nine amino acids of CAX1, called the Ca2+ domain, is inserted into N-terminal truncated versions of these transporters (Shigaki et al., 2001; Cheng et al., 2002). Furthermore, the addition of polypeptides, such as the epitope tag hemagglutinin, to the N termini of CAX1, CAX3, and CAX4 allows these transporters to suppress the yeast mutant phenotype (Cheng et al., 2002). These findings suggest that CAX3 and CAX4 are Ca2+ transporters and that the Ca2+ transport activity of these CAX transporters may also be dependent on N-terminal regulation. However, we are left with the question of whether there are common or distinct regulatory mechanisms among the CAX transporters.
Because many of the plant CAX transporters have significant sequence identity (Mäser et al., 2001; Cheng et al., 2002), we were interested to determine whether the CAX transporters do in fact differ in the regulation of their activity. By analogy to CAX1, we have proposed previously that all CAX transporters are regulated by the N terminus (Pittman and Hirschi, 2001; Cheng et al., 2002). Therefore, we investigated whether other CAX transporters also contain N-terminal regulatory domains and compare the function of these domains. In this study, we show that regulation of Ca2+/H+ antiporters by an N-terminal domain is not restricted to CAX1 but is a common mechanism in various CAX transporters both within Arabidopsis and in another plant species. We demonstrate for the first time, to our knowledge, that both Arabidopsis CAX3 and mung bean VCAX1 possess an NRR that has significant sequence similarity to that of CAX1, but that the precise mechanism of regulation varies between these CAX transporters. These findings suggest differential regulation of Ca2+/H+ antiporters and support the hypothesis that different interacting proteins may regulate each isoform.
RESULTS
VCAX1 Contains a Putative NRR
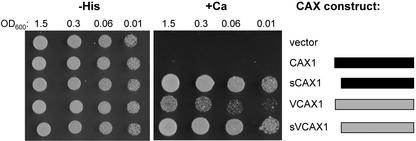
Sequence analysis of VCAX1 shows that like the Arabidopsis CAX genes CAX1 and CAX3, the mung bean Ca2+/H+ antiporter sequence also encodes a long hydrophilic N terminus (Fig. 1). However, unlike CAX1, full-length VCAX1 is able to suppress the Ca2+ sensitivity of a yeast vacuolar Ca2+ mutant K665, which lacks both vacuolar transporters, PMC1 and VCX1 (Ueoka-Nakanishi et al., 2000). Ca2+ transport into yeast microsomal vesicles by VCAX1 was also determined (Ueoka-Nakanishi et al., 2000). This initially suggested to us that VCAX1 might be regulated in a different manner to CAX1 (Pittman and Hirschi, 2001). To test this possibility, we directly compared the ability of VCAX1, CAX1, and the N-terminal truncated form sCAX1 to suppress the Ca2+-sensitive phenotype of a yeast mutant. We made this comparison using the K667 yeast triple mutant that lacks both vacuolar transporters as well as the Ca2+-dependent protein phosphatase calcineurin, and tested the ability of each CAX cDNA to suppress the Ca2+-sensitive phenotype. Although VCAX1 was able to suppress the Ca2+ sensitivity of K667 unlike vector alone or full-length CAX1, suppression was significantly stronger for yeast expressing sCAX1 (Fig. 2). To determine whether the N terminus of VCAX1 had any effect on Ca2+ transport activity, an N-terminally truncated mutant of VCAX1 (sVCAX1) lacking the first 31 residues was created by PCR. In this mutant, the residue that was Lys-32 of VCAX1 was mutated to Met so that translation would initiate from this Met. VCAX1 was truncated to this residue rather than to the second Met at Met-23, so as to allow direct comparison with sCAX1 (Fig. 1). sVCAX1 suppressed the Ca2+ sensitivity of K667 as efficiently as sCAX1 and significantly better than VCAX1 (Fig. 2). Both VCAX1 and sVCAX1 mRNA transcripts were present at approximately equal levels as determined by reverse transcriptase-PCR (data not shown), indicating that the difference in growth of K667 expressing VCAX1 or sVCAX1 was not due to altered expression.
Figure 1.
Partial amino acid alignment of the N-terminal tail region of CAX1 and CAX3 of Arabidopsis and VCAX1 of mung bean. The NRR of CAX1 is highlighted. The arrow indicates Met-37, which is the initiation codon for the truncated variants sCAX1 and sCAX3. The amino acid substitutions that were created in the CAX3 and VCAX1 NRR domains are highlighted. The alignment was performed using ClustalW version 1.8. Identical residues are in black, similar residues are in gray. Gaps introduced to maximize the alignment are denoted by hyphens. The accession numbers for CAX1, CAX3, and VCAX1 are AF461691, AF256229, and AB012932, respectively.
Figure 2.
Suppression of Ca2+ sensitivity of the pmc1 vcx1 cnb1 yeast mutant (K667) by full-length and truncated CAX1 and VCAX1 constructs. The CAX constructs are depicted as bars: A black bar represents portions of the CAX1 open reading frame and a gray bar represents portions of the VCAX1 open reading frame (not to scale). Saturated liquid cultures of K667 expressing various plasmids were diluted to the cell densities as indicated, then spotted onto selection medium lacking His (−His) and yeast-extract peptone dextrose (YPD) medium containing 200 mm CaCl2 (+Ca). Yeast growth at 30°C is shown after 3 d. A representative experiment is shown.
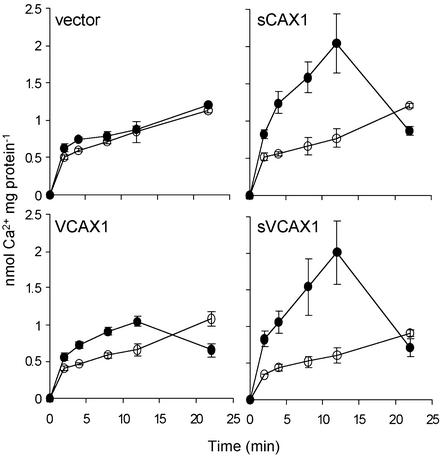
The assay of K667 yeast growth on the high-Ca2+ medium does not allow us to make precise comparisons of Ca2+/H+ antiport activity. Therefore, to obtain quantitative measurements of VCAX1, sVCAX1, and sCAX1 activity and to confirm that the difference in growth of K667 expressing both full-length and truncated antiporters was due to variation in their Ca2+ transport activity, vacuolar-enriched vesicles were prepared from yeast and 10 μm ΔpH-dependent 45Ca2+ transport was measured. The vector control yeast vesicles showed no Ca2+/H+ antiport activity, whereas all of the CAX-expressing vesicles showed significant antiport activity (Fig. 3). Although Ca2+/H+ antiport activity mediated by sCAX1 and sVCAX1 was essentially identical, VCAX1 vesicles always had less than 30% of the Ca2+ uptake capacity compared with sCAX1 and sVCAX1 (Fig. 3).
Figure 3.
Time courses of ΔpH-dependent 10 μm 45Ca2+ transport into vacuolar membrane-enriched vesicles prepared from pmc1 vcx1 cnb1 yeast (K667) expressing either vector alone, sCAX1, VCAX1, or sVCAX1 as indicated. Ca2+ transport was measured in the absence (black circle) or presence (white circle) of 5 μm protonophore gramicidin. All time course experiments were performed in the presence of 100 μm NaN3, 200 μm Na orthovanadate, and 1 mm Mg2+ATP. The Ca2+ ionophore A23187 (5 μm) was added after 12 min. Results are the mean (±se) of three independent experiments, each with two replicates.
CAX3 Chimeric Constructs Are Regulated by an N-Terminal Tail
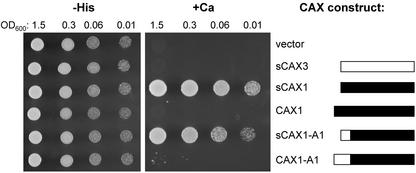
CAX3 is the most closely related gene to CAX1 (Mäser et al., 2001), yet it can only suppress the Ca2+-sensitive phenotype of K667 if modifications are made to its sequence. We have found previously that when particular regions of the sCAX1 open reading frame were swapped into CAX3, CAX3 could then transport Ca2+ (Shigaki et al., 2001). All of the CAX3 constructs that were used in this previous study were truncated at the N terminus to remove the first 36 amino acids. This allowed direct comparison of the CAX3 and CAX1 sequences because the CAX1 cDNA that was initially isolated by its function in yeast did not contain these 36 amino acids. To determine whether the CAX3 N terminus may have a regulatory role, some of the truncated CAX1/CAX3 chimeras were compared with versions that contain the N-terminal 36 amino acids of CAX3. Even without its first 36 amino acids, a truncated CAX3 (sCAX3) cannot suppress the Ca2+-sensitive phenotype of K667, whereas the sCAX1-expressing strain grows very efficiently (Fig. 4; Shigaki and Hirschi, 2000). We analyzed a CAX1/CAX3 chimera called CAX1-A1, which is the CAX3 N terminus (residues 1–73) fused in frame to CAX1 from residues 74 to 463. Suppression of the Ca2+-sensitive phenotype was only observed with the truncated chimera (sCAX1-A1), which lacked the first 36 amino acids of CAX3; almost no suppression was observed with the longer version containing the full-length CAX3 N terminus, indicating that this full-length construct was auto-inhibited (Fig. 4). Similarly, we found that other CAX1/CAX3 chimeric constructs no longer suppressed the mutant phenotype when the CAX3 N terminus was present (data not shown).
Figure 4.
Suppression of Ca2+ sensitivity of the pmc1 vcx1 cnb1 yeast mutant (K667) by full-length and truncated CAX constructs. The CAX constructs are depicted as bars: A black bar represents portions of the CAX1 open reading frame and a white bar represents portions of the CAX3 open reading frame (not to scale). The same assay conditions were used as described in Figure 2 except growth is shown after 2 d. A representative experiment is shown.
The Regulatory Function of the CAX3 NRR Is Specific to CAX3
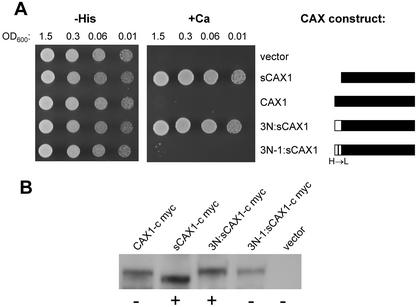
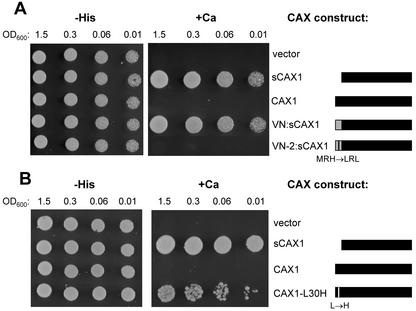
CAX1 is 77% identical to CAX3 and the NRR of CAX1 shares significant sequence identity (24 of 36 identical amino acids) with the NRR of CAX3 (Fig. 1). Despite such similarity, a CAX1-NRR peptide that can inhibit Ca2+ transport by CAX1 cannot inhibit transport by the “activated” CAX3 mutant, sCAX3-9 (Pittman et al., 2002). When the NRR of CAX3 was fused in frame to the N terminus of sCAX1, to give the chimera 3N:sCAX1, this construct was still able to suppress the Ca2+-sensitive phenotype of K667 (Fig. 5A). Site-directed mutagenesis was performed on the NRR of 3N:sCAX1 to change some of the amino acids that are unique to CAX3 to those present in the equivalent position of CAX1 (Fig. 1). When amino acids ANV of CAX3 (residues 17–19) were changed to PSI (to give construct 3N-3:sCAX1), suppression of the Ca2+-sensitive phenotype still occurred, indicating no auto-inhibition (data not shown). When amino acid His-30 was changed to Leu (to give construct 3N-1:sCAX1), this construct was now unable to suppress the Ca2+ phenotype, indicating auto-inhibition (Fig. 5A).
Figure 5.
A, Suppression of Ca2+ sensitivity of the pmc1 vcx1 cnb1 yeast mutant (K667) by sCAX1 with N-terminal fusions of either the wild-type NRR of CAX3 (3N:sCAX1) or the NRR of CAX3 containing a His to Leu single amino acid change (3N-1:sCAX1). The CAX constructs are depicted as bars: A black bar represents portions of the CAX1 open reading frame and a white bar represents portions of the CAX3 open reading frame (not to scale). The same assay conditions were used as described in Figure 2, except yeast growth is shown after 2 d. A representative experiment is shown. B, Western blot showing relative levels of the various CAX constructs used in the suppression assay. Equal amounts of total protein isolated from yeast strains expressing each c-myc-tagged construct as indicated were separated by SDS-PAGE, blotted, then subjected to western-blot analysis using an anti-c-myc monoclonal antibody. The ± signs designate whether the CAX construct was able to suppress the yeast vacuolar transport mutant, as shown in A.
To confirm that protein stability of sCAX1 was unaffected by the chimeric fusion of the CAX3 NRR, and that the expression of 3N:sCAX1 was not disrupted by the amino acid changes, epitope-tagged versions of each construct were created. It was important to introduce the epitope tags at the C terminus given that the N terminus of CAX1 is clearly important for regulation, and we have shown previously that addition of epitopes to the N terminus of CAX proteins affects protein function (Cheng et al., 2002). Therefore, a c-myc epitope tag was fused to the C terminus of each of the CAX1 variants. The addition of the five-copy c-myc epitope did not change the Ca2+ suppression phenotype of each CAX variant. For example, sCAX1:c-myc still suppressed the yeast vacuolar Ca2+ deficiency, whereas CAX1:c-myc did not (data not shown). Western analysis performed on total protein isolated from the various yeast strains showed that the expression of c-myc-tagged 3N:sCAX1 and 3N-1:sCAX1 was equivalent to CAX1 and sCAX1 (Fig. 5B).
The Regulatory Function of the VCAX1 NRR Is Specific to VCAX1
We have shown that the NRR of CAX3 was able to auto-inhibit sCAX1 activity only after specific modifications had been made to the NRR (Fig. 5A). To determine the degree of conservation between different plant CAX NRRs, we performed a similar experiment utilizing the VCAX1 NRR. A chimeric fusion was created in which the first 31 residues of VCAX1 were fused in frame to the N terminus of sCAX1 (VN:sCAX1). VN:sCAX1 strongly suppressed the Ca2+-sensitive phenotype of K667, suggesting that this N terminus is not capable of inhibiting sCAX1 (Fig. 6A). VCAX1 shares fewer residues in the NRR region with CAX1 compared with CAX3; 18 of the first 31 residues in the VCAX1 N terminus are identical to CAX1 (Fig. 1). Three significant regions of heterogeneity were identified in the C-terminal end of the VCAX1 NRR compared with CAX1: the absence of amino acids SITA following Pro-16, amino acids VLT (residues 18–20) instead of GSS, and amino acids MRH (residues 23–25) instead of LRL (Fig. 1). To determine if any of these regions within the VCAX1 NRR were actually responsible for perturbing auto-inhibition, three sets of VN:sCAX1 mutants were generated to add or change those amino acids in the VCAX1 NRR to those present in the equivalent position of CAX1 (Fig. 1). When amino acids SITA were inserted into VCAX1 after Pro-16 (VN-4:sCAX1), and when amino acids VLT were changed to GSS (VN-3:sCAX1), suppression of the Ca2+-sensitive phenotype still occurred (data not shown), indicating that auto-inhibition was not conferred. When amino acids MRH were changed to LRL (VN-2:sCAX1), this construct was unable to suppress the Ca2+ phenotype of K667, indicating that auto-inhibition had occurred (Fig. 6A).
Figure 6.
A, Suppression of Ca2+ sensitivity of the pmc1 vcx1 cnb1 yeast mutant (K667) by sCAX1 with N-terminal fusions of either the wild-type NRR of VCAX1 (VN:sCAX1) or the NRR of VCAX1 containing an MRH to LRL double amino acid change (VN-2:sCAX1). The CAX constructs are depicted as bars: A black bar represents portions of the CAX1 open reading frame and a gray bar represents portions of the VCAX1 open reading frame (not to scale). The assay conditions were the same as in Figure 2, except yeast growth is shown after 2 d. B, Suppression of Ca2+ sensitivity of the pmc1 vcx1 cnb1 yeast mutant (K667) by full-length and truncated CAX1 compared with a mutant CAX1 with a single Leu-30 to His amino acid change (CAX1-L30H). The CAX constructs are depicted as bars: A black bar represents portions of the CAX1 open reading frame and a white bar represents portions of the CAX3 open reading frame (not to scale). The assay conditions were the same as in Figure 2. Representative experiments are shown in A and B.
The mutagenesis experiments performed on 3N:sCAX1 and VN:sCAX1 both infer that the Leu residue at position 30 in the CAX1 NRR is important for auto-inhibition of this Ca2+/H+ antiporter (Figs. 5A and 6A). To test this directly, Leu-30 of CAX1 was mutated to His and the ability of the mutant to suppress the Ca2+ sensitivity of K667 was examined. As shown in Figure 6B, although no growth occurred for the strain expressing CAX1, yeast expressing CAX1-L30H grew almost as well as sCAX1 on high-Ca2+ medium, indicating that this single amino acid change abolished auto-inhibition.
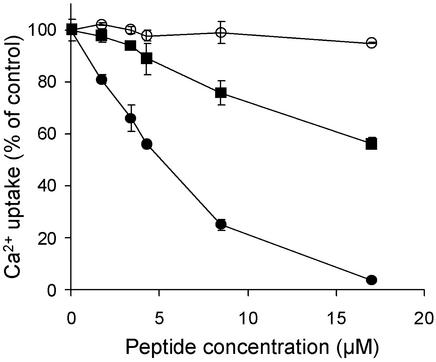
We have demonstrated previously that a synthetic peptide that corresponds to all 36 amino acids of the CAX1 NRR (CAX1-NRR peptide) strongly inhibits Ca2+ transport mediated by sCAX1 in a concentration-dependent manner but is unable to inhibit Ca2+ transport by sCAX2, VCX1, or sCAX3-9, even at high peptide concentrations (Pittman et al., 2002). The effect of the CAX1-NRR peptide was tested on Ca2+ transport by sVCAX1. The peptide slightly inhibited Ca2+ transport by sVCAX1 but was much less effective at inhibiting sVCAX1 compared with sCAX1 (Fig. 7). However, this inhibition of sVCAX1 was slightly more effective than the negligible inhibition of sCAX1 by a control peptide of similar charge and size to the CAX1-NRR peptide (Fig. 7). A CAX1-NRR peptide concentration of approximately 25 μm was required to inhibit 50% of sVCAX1 Ca2+/H+ antiport activity compared with 5 μm of peptide that could inhibit 50% of sCAX1 activity.
Figure 7.
The inhibitory effect of CAX1-NRR peptide concentration on ΔpH-dependent 10 μm Ca2+ transport by sCAX1 (black circle) or sVCAX1 (black square) into yeast endomembrane vesicles at a 10-min time point. The inhibitory effect of a control peptide of similar size and charge to CAX1-NRR peptide on ΔpH-dependent 10 μm Ca2+ transport by sCAX1 is also shown (white circle). Results are displayed as a percentage of the Ca2+ uptake measured in the absence of peptide. Results are the mean (±se) of two independent experiments.
DISCUSSION
Many genes encoding putative Ca2+/H+ antiporters have been identified in Arabidopsis, whereas yeast contains a single Ca2+/H+ antiporter (Mäser et al., 2001). To address the potential differences among these Arabidopsis transporters, we have set out to investigate the diversity of function and regulation between these transporters. Furthermore, we were interested to determine whether a Ca2+/H+ antiporter of another plant species, in this case VCAX1 of mung bean, has similar mechanisms of regulation to those from Arabidopsis. The Arabidopsis Ca2+/H+ antiporter CAX1 is regulated at the posttranslational level by a mechanism of N-terminal auto-inhibition (Pittman and Hirschi, 2001; Pittman et al., 2002). Apart from CAX1, very little is known regarding the posttranslational regulation mechanisms of Ca2+/H+ antiporters from any species. In this study, we suggest that the posttranslational mechanism of N-terminal regulation is not restricted to CAX1, but also exists in other plant antiporters, CAX3 and VCAX1.
The N terminus appears to be an important component in the regulation of Ca2+ transport by CAX3 as well as CAX1. So far, we have been unable to measure transport activity of any substrate by CAX3 unless minor modification are made to its sequence (Shigaki et al., 2001, 2002; Cheng et al., 2002). When sCAX3 contained portions of CAX1 that included the nine-amino acid Ca2+ domain (sCAX1-A1) or only the Ca2+ domain (sCAX3-9), the modified sCAX3 was able to transport Ca2+ as inferred by its ability to suppress the Ca2+ sensitivity of the yeast mutant K667 (Shigaki et al., 2001; Fig. 4). This was only observed when the first 36 amino acids of CAX3 were lacking, demonstrating that CAX3 contains an NRR. Similar experiments have suggested that CAX4 may also contain an NRR (Cheng et al., 2002). Unlike the Arabidopsis antiporters, full-length VCAX1 transports Ca2+. However, the activity of VCAX1 was greatly enhanced by a truncation of the N terminus as shown by increased suppression of the K667 phenotype (Fig. 2). Previously, VCAX1 was shown to efficiently suppress the Ca2+-sensitive phenotype of the yeast mutant K665 that lacks PMC1 and VCX1 (Ueoka-Nakanishi et al., 2000). In this study, we have used a yeast strain that besides lacking the vacuolar Ca2+ transporters, also lacks the calcium-/calmodulin-dependent phosphatase calcineurin, a positive regulator of various other Ca2+ transporters, such as the yeast Golgi Ca2+-ATPase PMR1 (Kingsbury and Cunningham, 2000). This triple mutant is substantially more sensitive to high levels of external Ca2+ than the double mutant, possibly due to the lack of activation of PMR1 (Cunningham and Fink, 1996). Therefore, with this Ca2+ hypersensitivity, we were able to detect more subtle differences in suppression of the mutant phenotype and observed that VCAX1-expressing cells grew less efficiently on 200 mm Ca2+ media than those expressing sCAX1 or sVCAX1 (Fig. 2). Furthermore, by direct Ca2+ transport measurements, VCAX1 was shown to transport more Ca2+ into yeast vacuolar-enriched membrane vesicles when the N terminus was removed (Fig. 3).
The reason why VCAX1 has slight transport activity in yeast but CAX1 has none may be due to differences in binding affinity of each putative N-terminal auto-inhibitor, and, thus, differences in efficiency of auto-inhibition. However, in planta, we believe that both full-length VCAX1 and CAX1 can efficiently transport Ca2+. Immunological detection of full-length VCAX1 in mung bean using an antipeptide antibody against the N terminus confirms that it is translated from Met-1 rather than Met-23 and demonstrates that it is not proteolytically cleaved in the plant cell (Ueoka-Nakanishi et al., 1999). Ca2+/H+ antiport activity measured from mung bean hypocotyl vacuolar-enriched membranes (Ueoka-Nakanishi et al., 1999) was much higher than the activity measured from vacuolar-enriched membranes of VCAX1-expressing yeast (Fig. 3). Therefore, in the plant, VCAX1 activity can be up-regulated without N-terminal truncations. By analogy to CAX1, we predict that the N terminus of VCAX1 regulates antiport activity, possibly by a mechanism of auto-inhibition, although this remains to be determined. Similarly, we believe that CAX1 exists only as a full-length version in Arabidopsis; thus, CAX1-mediated Ca2+ transport is also likely to be due to activation of the full-length antiporter.
Comparisons of various CAX transporters show that the amino acids that comprise the putative regulatory domains are very conserved (Fig. 1). However, the regulatory regions were not interchangeable between CAX1, CAX3, and VCAX1 (Figs. 5 and 6). Similarly, the NRR of CAX1 cannot inhibit sCAX3-9 activity (Pittman et al., 2002), and we have found that although the CAX1-NRR peptide could weakly inhibit sVCAX1, this was much less efficient than the inhibition of sCAX1 (Fig. 7). Coupled with our previous findings that the NRR of CAX1 is also unable to inhibit Ca2+/H+ antiport activity of CAX2 and yeast VCX1 (Pittman et al., 2002), it appears that the regulatory domains of each antiporter act specifically and that the subtle differences between these domains are enough to effect inhibition. These results contrast with studies that analyzed the effects of synthetic peptides derived from auto-inhibitory domains of various Ca2+ transporters and found that these domains cross-react. For example, a peptide against the calmodulin-binding auto-inhibitory domain of the cardiac Ca2+/Na+ exchanger can inhibit activity of both the plasma membrane and sarcoplasmic reticulum Ca2+-ATPases, whereas a peptide of the rabbit plasma membrane Ca2+ pump can inhibit activity of the Ca2+/Na+ exchanger and both Ca2+-ATPases (Li et al., 1991; Enyedi and Penniston, 1993). Similarly, a synthetic peptide that corresponds to the auto-inhibitory domain of the Arabidopsis Ca2+-ATPase ACA2 is almost as effective at inhibiting the activity of another Arabidopsis Ca2+-ATPase ECA1 as it is at inhibiting ACA2 (Hwang et al., 2000).
The NRR of CAX1 has been shown previously to directly bind to another part of CAX1, at a region immediately C-terminal to the NRR and just before the first predicted transmembrane span (Pittman et al., 2002). We suggest that the CAX3 NRR may also interact with the N terminus of CAX3 at a region within residues 37 to 73. A construct containing only this domain of CAX3 fused in frame to CAX1 (CAX1-A1) was not active when the CAX3 NRR was also present, indicating auto-inhibition (Fig. 4). The construct made up of the CAX3 NRR fused in frame to sCAX1 (3N:sCAX1) was active, indicating no auto-inhibition (Fig. 5A). This indicates that either the CAX3 NRR does not interact with sCAX1 or that it can interact but is unable to inhibit sCAX1 activity. The CAX3 NRR could inhibit sCAX1 when a single His residue at position 30 was substituted to Leu (Fig. 5A). The only mutation that allowed the VCAX1 NRR to inhibit sCAX1 was the MRH to LRL change (Fig. 6A). Although we cannot rule out the importance of the Met to Leu change, it is interesting that a His to Leu change was also identified. From these results, we decided to mutate Leu-30 to His in CAX1 and we found that with this mutation, auto-inhibition of CAX1 could be blocked (Fig. 6B). Site-directed mutagenesis of the CAX1 NRR has identified residues, such as Ser-25, Arg-29, and Thr-33, at the C-terminal end of this 36-amino acid domain, that when mutated, prevent auto-inhibition of CAX1; hence, these residues are important for auto-inhibition, maybe for the binding of the NRR to CAX1 (Pittman et al., 2002). The mutagenesis in this study clearly implies that Leu-30 is also required for CAX1 auto-inhibition.
The results presented here support the idea that one of the main differences between the CAX transporters may be in their regulation. In mammals, three Na+/Ca2+ exchanger genes exist, NCX1, NCX2, and NCX3, which appear very similar in function but the expression of these isoforms appears to be regulated by a variety of independent mechanisms (Blaustein and Lederer, 1999). For example, expression of NCX2 transcripts is switched off by calcineurin, whereas NCX1 and NCX3 are calcineurin independent (Li et al., 2000). Many Ca2+ transporters are posttranslationally regulated by the interaction of Ca2+ modulator proteins; for example, VCX1 is inactivated by calcineurin (Cunningham and Fink, 1996), and ACA2 is activated or repressed by calmodulin and Ca2+-dependent protein kinase, respectively (Sze et al., 2000). The CAX transporters may also be regulated by interaction with a modulator protein. In our model for CAX1 regulation, we propose that activity is inhibited by the binding of the N terminus to another region of CAX1, which we term the regulatory-dependent region, and possibly to other regions of the CAX transporter, and that inhibition is released upon the binding of a separate activator protein to the N terminus (Pittman et al., 2002). The differences in the N termini of the Arabidopsis CAX proteins may reflect their different roles within the plant and within signal transduction pathways. The N termini of the CAX transporters may determine which activators can interact and, therefore, which CAX isoform is activated during a particular signaling event. Alternatively, the CAX transporters might all be regulated by the same proteins, but the level of activation may vary with each isoform. Ca2+/H+ antiporters are partly responsible for restoring the cytosolic Ca2+ levels after a signaling event; therefore, they may, along with other Ca2+ efflux transporters, have a role in determining the characteristics of a Ca2+ transient (Harper, 2001). Any differences in activation between the Ca2+/H+ antiporter isoforms may explain the difference in characteristic of Ca2+ signal that could be produced. Depending on which isoforms are activated, different Ca2+ signals with varying dynamics may be produced. Before we can address these questions, we need to determine the relative expression patterns of the CAX transporters. Although northern analysis has provided some information (Hirschi, 1999; Shigaki and Hirschi, 2000; Cheng et al., 2002), we do not yet know whether the spatial expression patterns overlap and we may find that each CAX is present in a specific cell type.
High-level expression of deregulated and truncated Ca2+/H+ antiporters can lead to very severe consequences to the plant, as demonstrated by ectopic expression of sCAX1 and CAX3-9 in tobacco (Nicotiana tabacum) producing Ca2+ deficiency-like stress symptoms (Hirschi, 1999; Shigaki et al., 2002). VCAX1 is also a very active transporter. A high level of Ca2+/H+ antiport activity was measured in mung bean despite only low levels of VCAX1 protein expressed in the plant (Ueoka-Nakanishi and Maeshima, 2000). These results indicate the importance of regulation for these transporters. In this study, we have demonstrated that various plant Ca2+/H+ antiporters contain N-terminal domains that have the potential to be differentially regulated, indicating that the CAX isoforms may be regulated by different interacting proteins in planta.
MATERIALS AND METHODS
DNA Manipulations
VCAX1 in pBluescript SK(−) (Stratagene, La Jolla, CA) was subcloned into the EcoRI and KpnI sites of pGEM 7zf(−) (Promega, Madison, WI), then subcloned into the XbaI and SacI sites of the yeast (Saccharomyces cerevisiae) expression vector piHGpd (Nathan et al., 1999). Five tandem copies of the c-myc epitope (EQKLISEEDL) were used to produce in-frame fusions of c-myc to the 3′ ends of CAX constructs. The c-myc fusion was amplified by PCR from plasmid pT7-5Xmyc using the primers: forward, 5′-CAG GAT GAG GAG TTT TCT CAT CTA TGG AGC AAA AGC TCA TTT CTG-3′; and reverse, 5′-GAA TTC GAG CTC TTA ATT CAA GTC CTC TTC AGA AAT G-3′. A BseRI site (underlined) was included in the forward primer. This restriction site was generated into the 5′ end of the c-myc tag to allow ligation into the BseRI site present at the 3′ end of CAX1 immediately before the termination codon. All PCR amplifications were performed using the high-fidelity Expand polymerase kit (Roche, Indianapolis).
Construction of CAX Chimeric Clones
Chimeric clone sCAX1-A1 was constructed in a previous study (Pittman et al., 2002). CAX1-A1was constructed by utilizing unique internal restriction sites within the coding sequences of CAX1 and CAX3. The CAX3 NRR fused in frame to the N terminus sCAX1 (3N:sCAX1) was constructed using the primers 5′-GGG AGA ACA GCA CAC AAC ATG TCT TCT TCT TCT TTG-3′ (forward) and 5′-CCA AGA AGA AGA AGA CAT GTT GTG TGC TGT TCT CCC-3′ (reverse) to create the fusion, then the entire construct was amplified using a forward primer against the CAX3 5′ end (5′-GAA TTC GCG GCC GCT AGA TCT ATG GGA AGT ATC GTG GAG-3′) and a reverse primer against the CAX1 3′ end (Pittman and Hirschi, 2001). VCAX1 was truncated by removing the first 31 residues and mutating Lys-32 to Met to produce sVCAX1 by using a forward sVCAX1 primer 5′-GAA TTC GGA TCC ATG TCT TCC AAC TCA CTT CGC AC-3′ and a reverse primer against the VCAX1 3′ end (5′-GAA TTC GGT ACC CTA AGC ACT TAA AAC TCC-3′). The VCAX1 NRR fused in frame to the N terminus of sCAX1 (VN:sCAX1) was constructed using the primers 5′-GGT CGC ACT GCG CAC AGC ATG TCT TCT TCT TCT TTG-3′ (forward) and 5′-CAA AGA AGA AGA AGA CAT GCT GTG CGC AGT GCG ACC-3′ (reverse) to create the fusion, then the entire construct was amplified using a forward primer against the VCAX1 5′ end (5′-ACG CAA TCT AGA ATG GGT TCT CAC CAA CAC G-3′) and a reverse primer against the CAX1 3′ end. All chimeric constructs were subcloned into pGEM-T Easy (Promega) and were completely sequenced before they were subcloned into piHGpd.
Site-Directed Mutagenesis
All site-directed mutagenesis was performed using a PCR and type IIS restriction enzyme-based method (Shigaki and Hirschi, 2001). A type IIS BsmBI restriction site (underlined) was present in each primer. The 3N-3:sCAX1 mutant (ANV to PSI change) was produced using the primers 5′-GAA TTC CGT CTC AAC GGA AAC CCA AGC ATA ACC GCG AAA GGC-3′ (forward) and 5′-GAA TTC CGT CTC TCC GTT CTC GGC GAT TGC TGC CCA TGG CTC-3′ (reverse) and the 3N-1:sCAX1 mutant (His-30 to Leu change) was produced using the primers 5′-GAA TTC CGT CTC GAG CTG CGA CTT GGG AGA ACA GCA CAC AAC-3′ (forward) and 5′-GAA TTC CGT CTC CAG CTC CCT GCT CGA GCC TTT CGC GGT CAC-3′ (reverse). The VN-4:sCAX1 mutant (the addition of SITA after Pro-16) was produced using the primers: forward, 5′-GAA TTC CGT CTC AAC CCG AGC ATA ACA GCA AAG GTG TTA ACA AGG GAA ATG-3′; and reverse, 5′-GAA TTC CGT CTC CGG GTT TCC GTT CTC CAG-3′. The VN-2:sCAX1 mutant (MRH to LRL change) was produced using the primers: forward, 5′-GAA TTC CGT CTC CGG TCG CAC TGC GCA CAG CAT GTC TTC-3′; and reverse, 5′-GAA TTC CGT CTC CGA CCC AGT CTT AGT TCC CTT GTC AAC ACC TTC GG-3′. The VN-3:sCAX1 mutant (VLT to GSS change) was produced using the primers: forward, 5′-GAA TTC CGT CTC CAG GGA AAT GAG ACA TGG TCG CAC TG-3′; and reverse, 5′-GAA TTC CGT CTC CCC CTG CTG CTT CCC TTG GGG TTT CCG TTC TCC AG-3′. The CAX1-L30H mutant was produced using the primers: forward, 5′-GAA TTC CGT CTC CTA AGA CAC GGC CGA ACC GCT CAC AAC ATG TCT TCT TC-3′; and reverse, 5′-GAA TTC CGT CTC TCT TAG TTC TCT GCT CGA-3′. All constructs were completely sequenced before they were subcloned into the yeast expression vector piHGpd.
Yeast Growth and Transformation
The yeast strain K667 (cnb1::LEU2 pmc1::TRP1 vcx1Δ; Cunningham and Fink, 1996) was transformed and grown as described previously (Pittman and Hirschi, 2001; Shigaki et al., 2001).
Protein Isolation and Western Analysis of Epitope-Tagged CAX Constructs
Total protein was isolated from yeast expressing c-myc-tagged CAX constructs using the glass bead method (Ausubel et al., 1998). Protein concentration was determined by protein assay (Bio-Rad, Hercules, CA). Protein samples were separated by SDS-PAGE on a 12% (w/v) precast gel (Bio-Rad) and transferred to polyvinylidene fluoride membrane (Pall Gelman, Ann Arbor, MI). The blots were blocked in 5% (w/v) nonfat dried milk in phosphate-buffered saline with Tween (PBS-T; 10 mm NaH2PO4/NaOH [pH 7.2] and 150 mm NaCl with 0.1% [v/v] Tween 20) for 1 h, and then reacted with a 1:1,000 (v/v) dilution of anti-c-myc monoclonal primary antibody (Berkely Antibody Co., Richmond, CA) in PBS-T for 1 h at room temperature. The blots were washed in PBS-T before incubating for 1 h in PBS-T containing a 1:10,000 (v/v) dilution of horseradish peroxidase-coupled anti-mouse secondary antibody (Amersham, Little Chalfont, UK). The blots were then washed in PBS-T. ECL Plus reagents (Amersham) were used to develop the blots, which were then exposed to Hyperfilm photographic film (Amersham).
Preparation of Endomembrane Vesicles and Ca2+ Transport Assay
Yeast vacuolar-enriched membrane vesicles were prepared as previously described (Pittman and Hirschi, 2001). Measurements of time-dependent 45Ca2+/H+ transport into endomembrane vesicles were performed as previously described (Pittman and Hirschi, 2001; Shigaki et al., 2001). Synthetic peptides were used from a previous study (Pittman et al., 2002). Ca2+ transport was measured in the presence of the synthetic peptides as previously described (Pittman et al., 2002).
Distribution of Materials
All novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material.
ACKNOWLEDGMENTS
We are grateful to Masayoshi Maeshima (Nagoya University, Japan) for the VCAX1 cDNA in pBluescript. We also thank Bonnie Bartel (Rice University, Houston) for the pT7-5Xmyc plasmid. We thank Ning-Hui Cheng (Baylor College of Medicine, Houston, TX) for critical reading of the manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture-Agricultural Research Service (Cooperative Agreement No. 58–6250–6001) and by the National Institutes of Health (grant nos. CHRC 5 P30 and 1R01 GM57427).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008193.
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates/Wiley Interscience; 1998. [Google Scholar]
- Blackford S, Rea PA, Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990;265:9617–9620. [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ. Kinetics of Ca2+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris L. Plant Physiol. 1986;80:727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N-H, Pittman JK, Shigaki T, Hirschi KD. Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol. 2002;128:1245–1254. doi: 10.1104/pp.010857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A, Penniston JT. Autoinhibitory domains of various Ca2+ transporters cross-react. J Biol Chem. 1993;268:17120–17125. [PubMed] [Google Scholar]
- Harper JF. Dissecting calcium oscillators in plant cells. Trends Plant Sci. 2001;6:395–397. doi: 10.1016/s1360-1385(01)02023-4. [DOI] [PubMed] [Google Scholar]
- Hirschi K. Vacuolar H+/Ca2+ transport: Who's directing the traffic? Trends Plant Sci. 2001;6:100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000;124:125–133. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000;122:157–168. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- Li L, Guerini D, Carafoli E. Calcineurin controls the transcription of Na+/Ca2+ exchanger isoforms in developing cerebellar neurons. J Biol Chem. 2000;275:20903–20910. doi: 10.1074/jbc.M000995200. [DOI] [PubMed] [Google Scholar]
- Li Z, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD. Identification of a peptide inhibitor of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1991;266:1014–1020. [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D et al. Phylogenetic relationships within cation-transporter families of Arabidopsis thaliana. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA. 1999;96:1409–1414. doi: 10.1073/pnas.96.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD. Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter. Identification of an N-terminal autoinhibitory domain. Plant Physiol. 2001;127:1020–1029. [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Cheng N-H, Hirschi KD. Mechanism of N-terminal autoinhibition in the Arabidopsis Ca2+/H+ antiporter CAX1. J Biol Chem. 2002;277:26452–26459. doi: 10.1074/jbc.M202563200. [DOI] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Ca2+/H+ antiport system driven by the proton electrochemical gradient of a tonoplast H+-ATPase from oat roots. Plant Physiol. 1985;79:1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T, Cheng N-H, Pittman JK, Hirschi K. Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J Biol Chem. 2001;276:43152–43159. doi: 10.1074/jbc.M106637200. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi K. Characterization of CAX-like genes in plants: implications for functional diversity. Gene. 2000;257:291–298. doi: 10.1016/s0378-1119(00)00390-5. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD. Use of class IIS restriction enzymes for site directed mutagenesis: variations on Phoenix mutagenesis. Anal Biochem. 2001;298:118–120. doi: 10.1006/abio.2001.5341. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Sreevidya C, Hirschi K. Analysis of the Ca2+ domain in the Arabidopsis H+/Ca2+ antiporters CAX1 and CAX3. Plant Mol Biol. 2002;50:475–483. doi: 10.1023/a:1019880006606. [DOI] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Maeshima M. Quantification of Ca2+/H+ antiporter VCAX1p in vacuolar membranes and its absence in roots of mung bean. Plant Cell Physiol. 2000;41:1067–1071. doi: 10.1093/pcp/pcd023. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Nakanishi Y, Tanaka Y, Maeshima M. Properties and molecular cloning of a Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur J Biochem. 1999;262:417–425. doi: 10.1046/j.1432-1327.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M. Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem. 2000;267:3090–3098. doi: 10.1046/j.1432-1033.2000.01343.x. [DOI] [PubMed] [Google Scholar]