Abstract
Analyses of the protein content and composition revealed dramatic changes in gene expression during in situ banana (Musa spp.) fruit formation/ripening. The total banana protein content rapidly increases during the first 60 to 70 d, but remains constant for the rest of fruit formation/ripening. During the phase of rapid protein accumulation, an inactive homolog of class III chitinases accounts for up to 40% (w/v) of the total protein. Concomitant with the arrest of net protein accumulation, the chitinase-related protein (CRP) progressively decreases and several novel proteins appear in the electropherograms. Hence, CRP behaves as a fruit-specific vegetative storage protein that accumulates during early fruit formation and serves as a source of amino acids for the synthesis of ripening-associated proteins. Analyses of individual proteins revealed that a thaumatin-like protein, a β-1,3-glucanase, a class I chitinase, and a mannose-binding lectin are the most abundant ripening-associated proteins. Because during the ripening of prematurely harvested bananas, similar changes take place as in the in situ ripening bananas, CRP present in immature fruits is a sufficient source of amino acids for a quasi-normal synthesis of ripening-associated proteins. However, it is evident that the conversion of CRP in ripening-associated proteins takes place at an accelerated rate, especially when climacteric ripening is induced by ethylene. The present report also includes a discussion of the accumulation of the major banana allergens and the identification of suitable promoters for the production of vaccines in transgenic bananas.
Fruit development and ripening are crucial physiological processes in the reproduction of flowering plants and are of great social and economical interest because fruits are often the edible part of crop plants. Therefore, intensive efforts have been undertaken for many decades to unravel the basic mechanisms of fruit formation and ripening and optimize the production, protection, quality, and postharvest treatment of various fruits. During the last decade, our general understanding of the molecular basis of the complex phenomena of fruit development and ripening rapidly has progressed (Brownleader et al., 1999; Bleecker and Kende, 2000; Giovannoni, 2001). However, because most research was focused on in-depth studies of the pre- and postharvest ripening process, relatively little attention has been given to the early phases of fruit formation and development. As a result, the molecular events taking place during the preharvest stage are still poorly documented. Numerous genes have been identified that are up- or down-regulated during ripening, but very little is known about differential gene expression in early developing fruits. This is well illustrated by our current insights in gene expression in pre- and postharvest bananas. Banana (Musa spp.) fruit ripening has been intensively studied because of the obvious interest in this globally important fruit as a worldwide dessert fruit and as a major carbohydrate staple crop in the tropics (Marriott, 1980). Apart from a study of the regulation of polyphenol oxidase in developing fruits (Gooding et al., 2001), most of these studies were done with detached bananas treated with ethylene to induce climacteric ripening. Two-dimensional SDS-PAGE of in vitro translation products and cDNA cloning of 25 different ripening-related mRNAs from the pulp led to the identification of up-regulated enzymes involved in ethylene biosynthesis, starch degradation, respiration, and cell wall degradation (e.g. pectate lyase and β-1, 3-glucanase; Medina-Suarez et al., 1997). At the same time, other proteins like starch synthetase, extensin, and a thaumatin-like protein (BanTLP) were down-regulated.
Differential screening of cDNA libraries representing banana pulp at different ripening stages also yielded a number of up-regulated (endochitinase, β-1, 3-glucanase, BanTLP, and methallothionin) as well as several down-regulated genes (starch synthase, class III chitinase, and jacalin-related lectin; Clendennen and May, 1997). Biochemical studies confirmed that several abundant pulp proteins like the BanTLP (Barre et al., 2000), the β-1,3-glucanase (Peumans et al., 2000a), and a class I chitinase that is considered the major banana allergen (Sanchez-Monge et al., 1999) are encoded by genes up-regulated during climacteric ripening. In addition, two banana pulp proteins were identified that are encoded by a down-regulated gene. One of these proteins, a Man-binding protein (lectin), is present in reasonable quantities in the pulp of ripe fruits (Peumans et al., 2000b), but is absent from immature fruits, which is difficult to reconcile with the presumed down-regulation of the corresponding gene during ripening. The second down-regulated protein is a class III chitinase that is absent from ripe fruits, but is very abundant in unripe bananas (Clendennen et al., 1998). It was postulated that this class III chitinase fulfills a storage role in the banana pulp. However, in the absence of any data on the developmental regulation during the preharvest stages of fruit development and the enzymatic activity, the presumed storage function of the putative class III chitinase remains to be demonstrated. Therefore, it is evident that the molecular and biochemical basis of the postharvest banana ripening cannot be fully understood without a minimal knowledge of differential gene expression in the preharvest stage of the fruit formation and development.
In this report, we describe the changes in the total protein composition and the concentration of five individual major proteins (i.e. a class III chitinase-related protein, a thaumatin-like protein, a β-1,3-glucanase, a class I chitinase, and a lectin) throughout the development and ripening of banana fruits. Experiments with fruits still attached to the plant revealed that young developing bananas accumulate large quantities of a catalytically inactive homolog of class III chitinases that is degraded during subsequent ripening. Similar changes take place in detached bananas but at an accelerated rate, especially when climacteric ripening is induced by ethephon. Our results not only demonstrate for the first time, to our knowledge, the occurrence of a transient vegetative storage protein (VSP) in fruits, but they also describe the accumulation of the major banana allergen and allow us to identify suitable promoters for the production of vaccines in transgenic bananas.
RESULTS
Changes in Protein Composition and Content of the Pulp and Peel during in Situ Development and Ripening
Though postharvest studies allowed for the identifying of genes that are up-regulated or down-regulated during fruit ripening, it is questionable whether and to what extent changes observed during climacteric ripening of detached bananas can be extrapolated to bananas attached on the plant. In addition, postharvest studies give no information about possible changes in gene expression during the preharvest developmental phases. To address these two important issues, changes in protein content and composition were followed throughout the development of banana fruit and ripening under normal physiological conditions (i.e. attached to the plant).
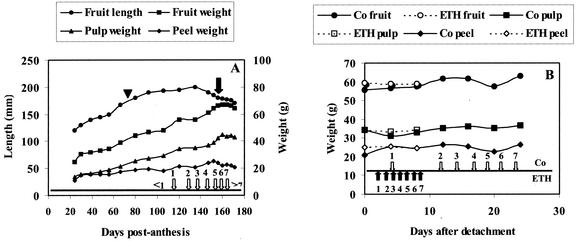
Under the conditions used in our experiment, the whole developmental cycle starting from flowering until complete maturity took approximately 170 d (Fig. 1A). To prevent spoilage of the ripening fruits on the plant (due to humid and warm conditions in the greenhouse), hands were cut from the bunch 156 d post-anthesis (DPA) and ripening was allowed to proceed under laboratory conditions. Samples were taken at regular intervals for determination of fruit size and weight, total protein content and composition, and quantification of some individual proteins. As shown in Figure 1A, fruit size and weight steadily increased for approximately 120 to 130 d. Afterward, the total fruit weight remained constant, whereas the length slightly decreased. Figure 1A also demonstrates that the contribution of the pulp and the peel to the total fruit weight changes during development and ripening. The slight decrease of the peel weight and concomitant increase of the pulp weight during the final 20 d is due to the softening of the peel, which results in a slightly altered distribution of the tissues when the peel is stripped from the pulp. To relate the developmental stage of the in situ ripening fruits to that of commercial bananas induced to ripen by ethylene, the time stages at which the different peel color indices (PCI: from 1 to 7) were reached are indicated in Figure 1A. It should be mentioned that the greenhouse-grown bananas are smaller than commercially grown bananas (which are approximately 20–25 cm in size and weigh approximately 150–200 g). However, the ratio between the weight of the pulp and the peel was similar (approximately 1.7 for fruits with PCI 4).
Figure 1.
A, Changes in size and weight during in situ development and ripening of banana fruits. Open arrows numbered 1 through 7 indicate at which stage the seven different PCI were reached. <1 means that the fruits did not yet reach the stage corresponding to PCI 1, whereas >7 refers to overripe bananas (peel color turning from brown to black). At 156 DPA (indicated by the black arrow), hands were cut and ripening continued under laboratory conditions. The time of harvest (70 DPA) of the immature fruits destined for the study of the ex planta ripening is indicated by a triangle. B, Changes in fruit, pulp, and peel weight of ex planta-ripening bananas in the absence (Co) and presence of ethephon (ETH). White and black arrows indicate when the seven different PCI were reached in control and ethephon-treated bananas, respectively.
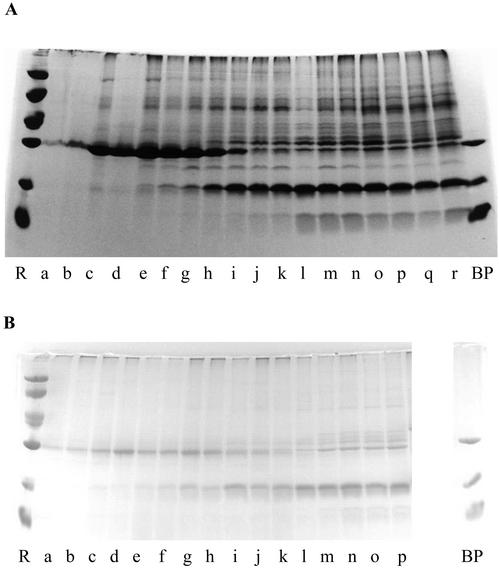
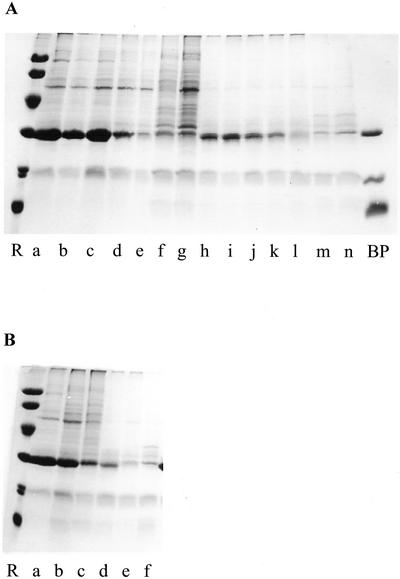
To trace major changes in the protein expression pattern as a function of fruit development, crude extracts were analyzed by SDS-PAGE and some of the most abundant polypeptides were quantified. As shown in Figure 2A, the protein pattern of the pulp drastically changed during fruit development. During the first phase, which lasts until approximately 50 DPA, the pulp accumulated large quantities of a single 30-kD protein. In the second phase spanning the period 50 to 120 DPA, the abundant 30-kD protein progressively decreased, and, at the same time, many novel polypeptides appeared. During the third phase, which begins when the fruits reach PCI 1, the SDS-PAGE pattern did not visibly change.
Figure 2.
SDS-PAGE of crude extracts from the pulp (A) and peel (B) from in situ-developing/ripening banana fruits. A mixture of BanGase (31 kD), BanTLP (20 kD), and BanLec (15 kD) was loaded in lane BP. Molecular mass reference proteins (lane R) were lysozyme (14 kD), soybean (Glycine max) trypsin inhibitor (20 kD), carbonic anhydrase (30 kD), ovalbumin (43 kD), bovine serum albumin (67 kD), and phosphorylase b (94 kD). A, Lanes a through r refer to samples of banana pulp taken 24, 30, 39, 48, 56, 66, 80, 92, 106, 120, 135, 147, 152, 156, 160, 164, 168, and 171 DPA, respectively. B, Lanes a through p show samples of banana peel taken after 24, 30, 39, 48, 56, 66, 80, 92, 106, 120, 135, 147, 152, 156, 160, and 164 DPA.
A similar change in the protein pattern was observed in the peel of developing bananas (Fig. 2B). Until 50 DPA, the peel accumulated relatively large quantities of a 30-kD protein. Between 50 and 135 DPA, the level of this 30-kD protein progressively decreased, whereas a 20-kD protein gradually accumulated. Unlike in the pulp, the protein pattern clearly changed in the peel during the final 40 d of the ripening process. After approximately 150 d, there was a rapid increase in the intensity of a 31-kD polypeptide and several other proteins in the Mr range between 30 and 40 kD. For the last two sampling times, no extracts could be made because the peel tissue was already decaying.
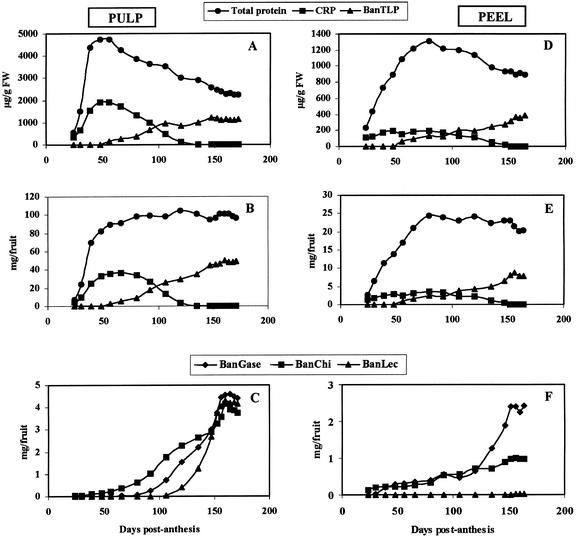
The results of the SDS-PAGE analysis clearly demonstrate dramatic changes in the protein composition of the pulp and peel of developing bananas, but do not provide detailed information about possible changes in total protein content of the respective tissues. Therefore, the total protein concentration of the extracts was determined and used to calculate the protein concentration (expressed as micrograms of protein per gram of tissue) and total protein content (expressed as milligrams of protein per fruit) of the pulp and peel tissue. As shown in Figure 3A and B, most of the protein in the pulp accumulates during early development of the fruit. No loss of protein occurs during the ripening process. The apparent decrease of the protein concentration after 60 d is the result of a “dilution” of the protein due to an increase of the pulp mass (by the accumulation of starch). Similar analyses indicated that the accumulation of protein in the peel lasts for about 80 d and that no net loss of protein occurs during the ripening period (Fig. 3, D and E).
Figure 3.
Changes in total protein, CRP, and BanTLP concentration (A and D), total protein, CRP, and BanTLP content (B and E), and BanGase, BanChi, and BanLec content (C and F) in the pulp (A–C) and peel (D–F) from in situ-developing/ripening banana fruits.
Identification of the Most Abundant Proteins in Pulp and Peel Tissue
To relate the obvious changes in gene expression in developing/ripening bananas to the appearance or disappearance of physiologically important proteins, the most prominent polypeptides present in the electropherograms of pulp and peel extracts were identified. The abundant 30-kD protein occurring in the pulp of unripe bananas was previously identified as a homolog of class III acidic chitinases (Clendennen et al., 1998). To check whether the 30-kD polypeptide (Fig. 2A, lanes a–h) corresponded to this chitinase homolog, the major protein in the pulp of young developing bananas (50 DPA) was purified to homogeneity and was characterized in some detail. N-terminal sequencing yielded an exact match (GRNSCIGVYWGQNTDEGSLADACATG) with the previously cloned class III chitinase homolog. Even upon prolonged incubation (up to 3 d), concentrated solutions of the protein (final concentration of 2 mg mL−1) failed to produce acid-soluble fragments from carboxymethyl-chitin-Remazol-Brilliant-Violet 5R, indicating that the chitinase homolog is devoid of chitinase activity. In accordance with this, the banana protein has to be considered a chitinase-related protein (CRP).
To trace the bands corresponding to the previously identified β-glucanase (BanGase), thaumatin-like protein (BanTLP), and Man-binding lectin (BanLec), a mixture of three purified proteins was run in a separate lane. As shown in Figure 2A, the predominant 20-kD polypeptide of ripe bananas comigrated with purified BanTLP, whereas two clearly visible bands of 31 and 15 kD exhibited the same mobility as the BanGase and BanLec, respectively. Sequencing of the respective polypeptides confirmed their identity (results not shown). The identity of the major 32-kD polypeptide was also checked by N-terminal sequencing, which confirmed that it corresponds to the 32-kD class I chitinase (BanChi) described by Sanchez-Monge et al. (1999).
N-terminal sequencing of the predominant 30-kD polypeptide in the peel of young developing fruits yielded a perfect match with CRP from pulp. Analysis of the purified protein revealed that it was devoid of chitinase activity. Extracts from the peel of ripe fruits also contain a predominant 20-kD polypeptide and a major 31-kD polypeptide comigrating with the BanTLP and BanGase, respectively. N-terminal sequencing confirmed the identity of both polypeptides (results not shown). Sequencing of the polypeptide band migrating just above the BanGase confirmed that it corresponds to the 32-kD BanChi.
Changes in the Level of Individual Proteins in the Pulp and Peel during in Situ Development and Ripening of Bananas
To quantify the obvious changes in the concentration of individual banana proteins during fruit formation and ripening the concentrations of CRP, BanTLP, BanChi, BanGase, and BanLec were determined for each developmental stage. Because CRP and BanTLP have no known biological activity, the quantification of these proteins was done by densitometric analysis of the gels shown in Figure 2, A and B. Changes in BanGase, BanChi, and BanLec were followed by determining the enzymatic/agglutinating activity and were quantified using solutions of known concentration of the respective purified proteins as standards. Based on these concentrations, the total content of the different proteins (expressed as milligrams of protein per fruit) was calculated. For the abundant CRP and BanTLP, changes in concentration and total content are given (to trace possible decreases in concentration due to a dilution of the protein by an increase of the pulp and peel mass). In case of the less abundant BanGase, BanChi, and BanLec, only changes in total content are shown.
The results of the quantification of the individual proteins shown in Figures 3 and 4 are based on an extensive analyses of single samples at each stage. This approach is adequate for the aims of the present work because preliminary experiments indicated that all fruits from a given hand yield a very similar protein pattern and exhibit virtually identical contents of all individual proteins studied here.
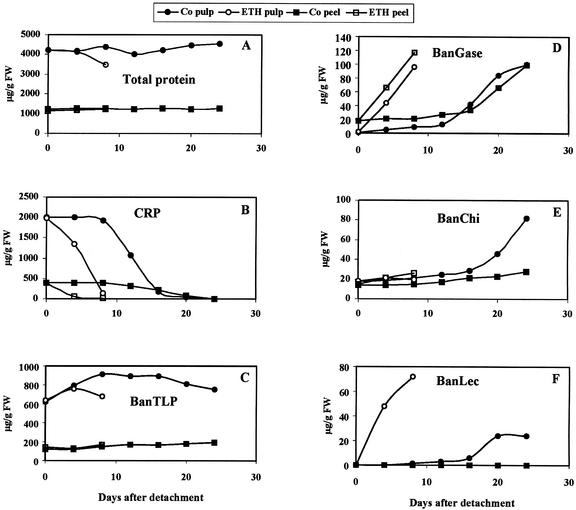
Figure 4.
Changes in total protein (A), CRP (B), BanTLP (C), BanGase (D), BanChi (E), and BanLec (F) concentration in the pulp and peel of ex planta-ripening bananas in the absence (Co) and presence of ethephon (ETH).
CRP accumulated in large quantities (up to 40% [w/v] of the total pulp protein) during the first 50 to 60 d of fruit development, but afterward, progressively decreased and eventually completely disappeared 120 DPA (Fig. 3, A and B). BanTLP, BanGase, BanChi, and BanLec exhibited similar patterns characterized by a progressive increase that comes to a halt approximately 155 DPA (corresponding to PCI 5; Fig. 3, B and C). During further ripening, the level of the proteins remained constant. Though the overall pattern is similar for BanTLP, BanGase, BanChi, and BanLec there are some important differences, especially with respect to the onset of their accumulation. BanGase, BanChi, and BanLec are far less abundant than BanTLP but they still account for approximately 4% (w/v) each of the total pulp protein of ripe bananas. Similar experiments with the peel of ripening bananas revealed that CRP accumulated in large quantities during early development (Fig. 3D). However, the accumulation phase lasted longer in the peel, and the relative abundance (15% [w/v] of the total protein) is considerably lower than in the pulp. At approximately 80 DPA, the concentration and content of CRP gradually decreased until the protein eventually completely disappeared 150 DPA. The accumulation of BanTLP in the peel started about the same time as in the pulp (50 DPA) and continued until 150 DPA (Fig. 3, D and E). BanChi was already present in the peel 24 DPA and slowly but steadily increased until 150 DPA (Fig. 3F). In a similar manner, BanGase became detectable 39 DPA and steadily increased until 120 DPA (Fig. 3F). After 120 DPA, the level of BanGase in the peel dramatically increased until 150 DPA. Though BanChi and BanGase are far less abundant than CRP and BanTLP, they still account for 4% and 10% (w/v), respectively, of the total peel protein. In contrast to the pulp, no lectin accumulation could be traced in the peel (Fig. 3F).
Changes in Protein Composition and Content of the Pulp and Peel during ex Planta Ripening of Detached Bananas
To check whether detached immature bananas also use CRP as a source of amino acids during artificial ripening, hands were cut from a bunch 70 DPA (coinciding with a maximal content of CRP in the pulp) and were allowed to ripe ex planta. Samples were taken at regular intervals for determination of fruit size and weight, total protein content and composition, and quantification of CRP, BanTLP, BanGase, BanChi, and BanLec in pulp and peel tissue. The ex planta ripening was not only followed under normal atmosphere, but also in the presence of ethephon to corroborate developmental changes induced or accelerated by ethylene.
Immature bananas harvested 70 DPA kept under a normal atmosphere at ambient temperature completed the whole ripening process (i.e. until PCI 7) in approximately 25 d. During this period fruit, pulp and peel weight remained virtually unchanged (Fig. 1B). The addition of ethephon accelerated the climacteric ripening to such an extent that PCI 7 was reached within 10 d after detachment of the hand from the plant.
The protein concentration in the pulp and peel remained fairly constant during the ex planta ripening (Fig. 4A). Because the weight of the fruits did not change, this constant protein concentration implies that the total protein content of the detached bananas also remains constant during the ripening process. In accordance with this, changes in the concentration of the individual proteins are directly proportional to changes in the total content of these proteins. Therefore, only changes in the concentration of the different proteins will be presented here.
Analysis of crude extracts by SDS-PAGE revealed that the protein composition of the pulp and the peel thoroughly changed during ex planta ripening. When ripening proceeded under a normal atmosphere, few changes occurred in the protein pattern of the pulp during the first 10 d (Fig. 5A). Thereafter, the abundant CRP rapidly decreased and eventually completely disappeared in less than a week. Concomitant with the decrease of the CRP content, the intensity of several polypeptide bands increased. A similar reasoning applies to the peel. Few changes could be observed in the electropherograms during the first 10 d of the ex planta ripening (Fig. 5A). Thereafter, the intensity of the CRP band rapidly decreased concomitant with an increase in the intensity of some other bands. Analysis of extracts from bananas ripening in the presence of ethephon revealed that CRP disappeared much faster in the pulp and the peel (Fig. 5B), indicating that ethylene accelerates the ripening-associated changes in the overall protein composition of the bananas.
Figure 5.
SDS-PAGE of crude extracts from pulp and peel of bananas ripening ex planta in the absence (A) and presence (B) of ethephon. A, Lanes a through g and h through n show crude extracts from pulp and peel of control bananas, respectively. Samples were taken 0, 4, 8, 12, 16, 20, and 24 d after detachment of the bananas from the tree. B, Lanes a through c and d through f show the protein pattern in pulp and peel of ethephon-treated bananas sampled 0, 4, and 8 d after detachment of the bananas. Lanes R and BP were as indicated in Figure 2.
Quantification of CRP, BanTLP, BanGase, BanChi, and BanLec allowed to follow the changes in the concentration of these proteins during ex planta ripening. As was observed in naturally ripening bananas, CRP eventually disappeared during the ripening of the detached bananas, whereas the concentration of BanTLP, BanGase, BanChi, and BanLec increased (Fig. 4). Ethephon had a drastic effect, resulting in an almost complete disappearance of CRP within 5 d. It should be noted that ethephon has a stronger effect on the decline of CRP in the peel than in the pulp. A comparison of the electropherograms shown in Figures 2 and 5 indicates that BanTLP is much less abundant in the pulp and peel of ex planta-ripened bananas than in the in situ-ripening fruits. Quantitative analyses revealed that the BanTLP concentration in the pulp increased during the first 10 d of the ex planta ripening (Fig. 4C). Thereafter, the concentration slowly decreased. This pattern strongly differs from that of the in situ-ripening bananas and explains why the final BanTLP concentration in the pulp of the ex planta-ripened bananas is considerably lower (750 versus 1,150 μg g−1 in the in situ-developing fruits). Ethephon reduced the accumulation period of BanTLP to 5 d and accelerated the subsequent decline of the protein. In the peel of the ex planta-ripening bananas, the concentration of BanTLP slowly but steadily increased until complete maturity (Fig. 4C). As a result, the BanTLP concentration in the peel reached the same level as that of the in situ-developing fruits. Ethephon caused no measurable effect on the changes in the BanTLP content of the peel.
At the time of the detachment of the immature bananas from the bunch, the concentration of BanGase in the pulp was still very low. After 10 d of the ex planta ripening, the concentration of the enzyme rapidly increased and eventually reached a level (approximately 100 μg g−1 fresh weight) comparable with that of the pulp of normally ripened bananas (Fig. 4D). The peel of the immature bananas already contained BanGase when the hands were cut from the bunch. During the ex planta ripening, the BanGase concentration increased (Fig. 4D) and eventually reached a level comparable with that of the in situ-developing fruits. Ethephon induced a very rapid increase of the BanGase concentration in the pulp and the peel.
BanChi was already present in the pulp and peel of the immature bananas. During ripening, the chitinase concentration in the pulp increased to roughly the same concentration (approximately 80 μg g−1 fresh weight; Fig. 4E) as that in the pulp of the in situ-ripening fruits. Ethephon did not accelerate the accumulation of BanChi. As a result, the final BanChi concentration in the pulp of the ethephon-treated bananas was approximately 4-fold lower than in the control fruits. In the peel, the BanChi concentration also increased, but the final concentration remained approximately 3-fold lower than in the peel of naturally ripened bananas.
The pulp and the peel of the immature bananas did not contain a detectable concentration of BanLec. Upon ex planta ripening, BanLec appears in the pulp (Fig. 4F), but the final concentration remained low as compared with the concentration in the pulp of the in situ-ripening fruits (24 versus 96 μg g−1). Ethephon not only accelerated the accumulation of BanLec but also enhanced the final lectin concentration 3-fold as compared with control fruits. No lectin could be detected in the peel of the ex planta-ripening fruits in the control or in the ethephon-treated bananas.
DISCUSSION
Young developing bananas accumulate large quantities of a catalytically inactive homolog of class III chitinases in pulp and peel tissue. Because this CRP has no enzymatic activity and completely disappears during fruit ripening, it can be considered a transient VSP. The results of the SDS-PAGE analysis and the quantification of the different proteins clearly indicate that the disappearance of CRP coincides with the onset of the synthesis of ripening-associated proteins. Taking into consideration that the total protein content of the pulp and peel does not increase once the maximal level of CRP is reached, the de novo synthesis of proteins after 80 DPA can in principle completely rely on the amino acids derived from the in situ degradation of CRP. Experiments with detached fruits confirmed that all ripening-associated proteins are normally synthesized without any import of nitrogen, which implies that the CRP present in the immature fruits is the major (or even sole) source of amino acids for the de novo protein synthesis in detached fruits. It is striking that apart from a reduced content of BanTLP and BanLec, the protein composition of the pulp and peel of detached fruits is similar to that of in situ-ripening fruits. This indicates that detachment of the fruits in se has no major effects on the developmental program that governs the ripening process. The only difference concerns the timing of the ripening process. Compared with in situ-developing fruits, detached bananas complete the (ex planta) ripening process in a much shorter time (24 versus 100 d). In other words the whole ripening process is compressed by a factor 4. Upon induction by ethephon, the ripening process is reduced to approximately 10 d corresponding to only one-tenth of the period required for the in situ ripening. A closer examination of the results indicates that ethephon not only strongly accelerates the ripening, but also affects the expression level of some proteins in detached fruits. For example, the BanLec concentration in the pulp increases upon ethephon treatment, whereas that of the BanChi decreases. Other proteins like BanTLP and BanGase are not (or very little) affected by ethephon.
Though the results presented here confirm part of the previously reported data on differential gene expression in ripening bananas, there are also some important discrepancies. First, our results leave no doubt that the peel also accumulates large quantities of CRP during early fruit formation. This finding contradicts the results of western-blot experiments showing that CRP is exclusively expressed in the pulp and is absent from the peel (Clendennen et al., 1998). Second, according to our results, BanLec accumulates in the pulp during the late stages of the ripening process in attached and detached fruits. This is difficult to reconcile with previously reported data based on northern-blot analysis showing that the banana lectin is down-regulated during ripening. The same holds true for the BanTLP, which, according to one report, is down-regulated (Clendennen et al., 1998). In this case, the discrepancy may be due to the fact that detached fruits accumulate less BanTLP than in situ-ripening fruits. However, the question remains as to why, in another report, BanTLP was considered an up-regulated protein in ethylene-treated bananas (Medina-Suarez et al., 1997).
The present study of changes in the protein composition of developing and ripening bananas also has a few important practical implications. First, it is shown that BanChi, which is the major banana allergen, is already present in the pulp and the peel of immature bananas. The concentration of the allergen strongly increases during normal but not during ethylene-induced ripening. Second, the quantification of the expression level of the most abundant proteins gives a fairly good idea about the strength of the different promoters under different ripening conditions. This information can be helpful in choosing suitable promoters for the production of vaccines in transgenic bananas. If the vaccines have to be produced in bananas that complete most of their ripening process in situ, the promoter of the BanTLP gene may be a suitable candidate. However, when the vaccines are produced in bananas that are detached and induced by ethylene, the BanTLP promoter may be far less effective than, for example, the promoter of the BanGase gene. Taking into consideration the fate of the CRP, the promoter of this gene (which must be very strong during the first phase of fruit development) is probably not a good candidate because a vaccine produced in the immature fruit may be degraded during ripening.
Based on its abundance, temporal regulation, and amino acid composition, it has been suggested that CRP is a storage protein homologous to class III acidic chitinases (Clendennen et al., 1998). Our results not only confirm the presumed storage role of CRP, but they also prove that the protein possesses no catalytic activity. This is important because the lack of enzymatic activity unambiguously demonstrates that CRP cannot play a role in plant defense but just acts as a transient VSP. The concept of CRP as a VSP is certainly not unique because many presumed storage proteins have been identified in various vegetative storage organs (Staswick, 1994; Van Damme et al., 2000). However, CRP is unique for three reasons. First, CRP is the first presumed VSP to be identified in fruits. Second, CRP is the first documented example of a developmentally regulated transient VSP. All other known VSPs are regulated by external stimuli. Third, CRP is the only example of a VSP that is degraded to provide the very same tissue with amino acids.
Some of the data reported here are also important in view of the widespread hypersensitivity to bananas (Blanco et al., 1999) that has been associated with the so-called “latex-fruit syndrome” and is merely caused by the presence in the pulp of panallergens belonging to the group of class I chitinases (Sanchez-Monge et al., 1999). These class I chitinases comprise an N-terminal hevein-like domain and accordingly are responsible for the allergen cross-reactivity observed between latex and banana (Mikkola et al., 1998). Similar proteins have also been identified as major allergens in avocado (Persea americana) and chestnut (Castanea sativa; Diaz-Perales et al., 1999). Besides class I chitinases, several other banana pulp proteins like BanGase (Breiteneder and Scheiner, 1998; Yagami, 1998) and BanTLP (Breiteneder and Ebner, 2000) are considered possible allergens. In addition, BanLec, which was shown to induce the production of IgG4 antibodies upon oral uptake (Koshte et al., 1992) is suspected to cause the development of allergic symptoms by susceptible individuals. According to the results shown in Figure 4, ethephon-treated ex planta-ripening bananas accumulate less BanChi and BanTLP, which are the most potent allergens of the pulp, than untreated fruits. Although ethylene has been reported to induce the accumulation of class I chitinases in the banana pulp (Sanchez-Monge et al., 2000), the final concentration of BanChi remains apparently much lower than in untreated fruits. Therefore, it can be concluded that ethylene treatment significantly reduces the content of the latex-fruit syndrome-associated allergen of ex planta-ripening bananas, but at the same time increases the content of the potentially allergenic protein BanLec.
MATERIALS AND METHODS
Plant Material
The banana variety Grande Naine (Musa sp.; AAA group, Cavendish subgroup; Daniells et al., 2001) was grown in the greenhouse of the Laboratory of Tropical Crop Improvement (Leuven, Belgium). Plants were grown in soil. From September to April the photoperiod was extended to 12 h by artificial light (14,000 Lux). The temperature was maintained at 28°C and 20°C during the light and dark period, respectively. The relative humidity ranged between 60% and 100%.
Sampling of in Situ Ripening Bananas
To follow changes in protein content and composition during the natural ripening process (i.e. attached to the plant), the banana fruits were allowed to develop until they reached PCI 4 (peel color more yellow than green). Samples were regularly taken starting from 24 DPA. To avoid heterogeneity due to differences in development, all samples were taken from a single hand (i.e. a pair of two adjacent rows of fruits). At each sampling time, individual fruits were cut from this particular hand. When the fruits reached PCI 4 (at 156 DPA), the remainder of the hand was cut from the bunch and was kept at room temperature for further ripening and sampling. Fruit size and weight was determined and the peel was separated from the pulp. The weight of the pulp and peel was determined and the material was frozen at −80°C until use.
Sampling during ex Planta Ripening of Prematurely Detached Bananas
To follow changes in protein content and composition during ex planta ripening of prematurely harvested bananas, hands were cut from a bunch 70 DPA and were kept at room temperature until the fruits reached full maturity (PCI 7). Samples were taken with regular intervals for 25 d and were processed as described above. The hands used for the control and ethephon treatment were cut from a single bunch (of a plant that was a clone of the plant used for the study of the in situ ripening).
Treatment of Prematurely Detached Bananas with Ethephon
Freshly cut hands were put in a transparent plastic bag (volume of approximately 5 L). Twenty microliters of a 0.1% (w/v) solution of ethephon in ethanol was spotted on a piece (5 × 5 cm) of filter paper (3MM; Whatman, Beverly, MA) and the bag was closed. At each sampling time, the bag was opened and a fresh solution (20 μL) of ethephon was added. Samples were taken and processed as described above.
Preparation of Extracts
Samples (1 g) of pulp and peel were extracted with mortar and pestle in 6 and 2 mL, respectively, of extraction medium. The homogenates were transferred to Eppendorf tubes and were centrifuged (12,000g for 10 min). Extracts used for SDS-PAGE and agglutination assays were prepared in 20 mm unbuffered 1,3-diaminopropane. Extracts destined for enzyme assays were prepared in 0.2 m NaCl.
SDS-PAGE of Crude Extracts from Pulp and Peel
Aliquots (100 μL) of the cleared extracts were mixed with 33.3 μL of 4-fold concentrated sample loading buffer (final concentration: 0.1 m Tris-HCl, pH 7.8, 4% [w/v] SDS, and 10% [w/v] glycerol containing 0.1 m β-mercaptoethanol) and were analyzed by SDS-PAGE using 12.5% to 25% (w/v) acrylamide gradient gels as described by Laemmli (1970).
For N-terminal amino acid sequencing, purified proteins were separated by SDS-PAGE and electroblotted on a polyvinylidene difluoride membrane. Polypeptides were excised from the blots and were sequenced on a protein sequencer interfaced with an online analyzer (models 477A and 120A, respectively; Applied Biosystems, Foster City, CA).
Enzyme and Hemagglutination Assays
The BanGase activity was assayed with an Azurine-cross-linked β-1,3-glucan substrate (AZCL-Pachyman tablets; Megazyme, Wicklow, Ireland). To 0.1 mL of extract, 0.9 mL of a suspension of AZCL-Pachyman (prepared by gently crushing a tablet in 25 mL of 0.1 m Na-acetate buffer, pH 5.0) was added in a small glass tube. The mixture was incubated at 37°C for 3 h and the reaction stopped by placing the tubes on ice. After settling of the Pachyman particles, aliquots of the supernatants were transferred to a microcuvette and the A590 was measured against the corresponding nonincubated control samples.
Chitinase activity was assayed using carboxymethyl-chitin-Remazol-Brilliant-Violet 5R as a substrate (Wirth and Wolf, 1990). Five microliters of extract was mixed with 695 μL 50 mm NaOAc buffer (pH 4.0) in an Eppendorf tube, and 100 μL of a solution of carboxymethyl-chitin-Remazol-Brilliant-Violet 5R (2 mg mL−1 in water) was added. After incubation for 1 h at 37°C, the reaction was stopped by the addition of 200 μL of a 4 n solution of HCl. The tubes were kept in ice for 20 min and were centrifuged (12,000g for 5 min). The supernatants were transferred to a microcuvette and the A520 was measured against the corresponding nonincubated control samples. Assays with a class III chitinase purified from leaves of Glechoma hederacea proved that the test is suitable for class III chitinases.
Because the banana lectin strongly reacts with endogenous polysaccharides (including starch; Goldstein et al., 2001; Mo et al., 2001), most of the lectin precipitates during centrifugation of the extracts unless extraction is done under nonbinding conditions (Peumans et al., 2000b). Therefore, the extracts used for the determination of the agglutination activity were prepared in 20 mm unbuffered 1,3-diaminopropane (pH of approximately 11). At this high pH, the lectin is reversibly inactivated and hence remains in solution. Upon lowering the pH, the lectin regains full activity.
Agglutination assays were carried out in 96-U-welled microtiter plates in a final volume of 50 μL containing 20 μL of a 2% (w/v) suspension of trypsin-treated rabbit erythrocytes (Peumans et al., 2000b), 20 μL of 1 m ammonium sulfate, and 10 μL of extract. Ammonium sulfate was added to lower the pH (to approximately 7.5) and to enhance the agglutination activity of the lectin. To determine the specific agglutination activity, the extracts were serially diluted with 2-fold increments in 20 mm unbuffered 1,3-diaminopropane. Agglutination was assessed visually after 1 h at room temperature. To estimate the lectin content, a parallel assay was included with known concentrations of pure lectin.
Purification of the Class III Chitinase Homolog from Unripe Bananas
Pulp (50 g) of freshly harvested bananas (50 DPA) was homogenized with mortar and pestle in 250 mL of 1% (w/v) ascorbic acid adjusted to pH 6 with NaOH, and the homogenate was centrifuged (8,000g for 10 min). Solid ammonium sulfate was added to the cleared extract to a final concentration of 1 m. After standing in the cold room (2°C) for 1 h, the extract was recentrifuged (8,000g for 10 min). The supernatant was taken off, filtered through paper (3MM; Whatman), and loaded onto a column (2.6 × 5 cm; approximately 25-mL bed volume) of phenyl-Sepharose equilibrated with 1 m ammonium sulfate. After loading, the column was washed with 1 m ammonium sulfate until the A280 fell below 0.01 and the proteins eluted with 20 mm Tris-HCl (pH 8.0). The peak fraction (8 mL) of this partially purified protein fraction was loaded onto a column (2.6 × 70 cm; approximately 350-mL bed volume) of Sephacryl 100 equilibrated with 20 mm Tris-HCl (pH 7.8) containing 0.2 m NaCl. The column was eluted with the same buffer and 4-mL fractions were collected. The bulk of the protein eluted in one major symmetrical peak with an apparent molecular mass of approximately 30 kD. SDS-PAGE showed that the protein present in the major peak yielded a single polypeptide band of 30 kD. To ensure complete purity, peak fractions from the gel filtration chromatography were dialyzed against 20 mm Tris-HCl (pH 8.7) and loaded onto a Mono-Q anion-exchange column (type HR 5/5) equilibrated with the same buffer for further purification using a fast-protein liquid chromatography system (Amersham Biosciences, Piscataway, NJ). After loading the proteins, the column was washed with 4 mL of Tris buffer and the proteins were eluted with a linear gradient (56 mL) of increasing NaCl concentration (from 0 to 0.5 m). Almost all of the protein eluted in a single peak at 0.1 m NaCl. SDS-PAGE confirmed that this peak contained a single polypeptide of about 30 kD. The top of the peak was collected, dialyzed against appropriate buffers, and used for further analysis. The same procedure was followed to purify the class III chitinase homolog from the peel of unripe bananas.
Footnotes
This work was supported in part by the Catholic University of Leuven (grant no. OT/98/17) and by the Fund for Scientific Research-Flanders (Belgium, grant no. G.0113.01). P.P. is a PostDoctoral Fellow of this fund.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006551.
LITERATURE CITED
- Barre A, Peumans WJ, Menu-Bouaouiche L, Van Damme EJM, May GD, Fernandez Herrera A, Van Leuven F, Rougé P. Purification and structural analysis of an abundant thaumatin-like protein from ripe banana fruit. Planta. 2000;211:791–799. doi: 10.1007/s004250000354. [DOI] [PubMed] [Google Scholar]
- Blanco C, Diaz-Perales A, Collada C, Sanchez-Monge R, Aragoncillo C, Castillo R, Ortega N, Alvarez M, Carrillo T, Salcedo G. Class I chitinases as potential panallergens involved in the latex-fruit syndrome. J Allergy Clin Immunol. 1999;103:507–513. doi: 10.1016/s0091-6749(99)70478-1. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106:27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Scheiner O. Molecular and immunological characteristics of latex allergens. Int Arch Allergy Immunol. 1998;116:83–92. doi: 10.1159/000023930. [DOI] [PubMed] [Google Scholar]
- Brownleader MD, Jackson P, Mobasheri A, Pantelides AT, Sumar S, Trevan M, Dey PM. Molecular aspects of cell wall modifications during fruit ripening. Crit Rev Food Sci Nutr. 1999;39:149–164. doi: 10.1080/10408399908500494. [DOI] [PubMed] [Google Scholar]
- Clendennen SK, Lopez-Gomez R, Gomez-Lim M, Arntzen CJ, May GD. The abundant 31-kilodalton banana pulp protein is homologous to class-III acidic chitinases. Phytochemistry. 1998;47:613–619. doi: 10.1016/s0031-9422(97)00616-x. [DOI] [PubMed] [Google Scholar]
- Clendennen SK, May GD. Differential gene expression in ripening banana fruit. Plant Physiol. 1997;115:463–469. doi: 10.1104/pp.115.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniells J, Jenny C, Karamura D, Tompeke K. Musalogue: a catalogue of Musa germplasm. Diversity in the genus Musa. Montpellier, France: INIBAP; 2001. http://www.inibap.org/publications/musalogue_eng.htm [Google Scholar]
- Diaz-Perales A, Collada C, Blanco C, Sanchez-Monge R, Carrillo T, Aragoncillo C, Salcedo G. Cross-reactions in the latex-fruit syndrome: a relevant role of chitinases but not of complex asparagine-linked glycans. J Allergy Clin Immunol. 1999;104:681–687. doi: 10.1016/s0091-6749(99)70342-8. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Goldstein IJ, Winter HC, Mo H, Misaki A, Van Damme EJM, Peumans WJ. Carbohydrate binding properties of banana (Musa acuminata) lectin: binding of laminaribiose oligosaccharides and β-glucans containing β-1,6-glucosyl end groups. Eur J Biochem. 2001;268:2616–2619. doi: 10.1046/j.1432-1327.2001.02149.x. [DOI] [PubMed] [Google Scholar]
- Gooding PS, Bird C, Robinson SP. Molecular cloning and characterization of banana fruit polyphenol oxidase. Planta. 2001;213:748–757. doi: 10.1007/s004250100553. [DOI] [PubMed] [Google Scholar]
- Koshte VL, Aalbers M, Calkhoven PG, Aalberse RC. The potent IgG4-inducing antigen in banana is a mannose-binding lectin, BanLec-I. Int Arch Allergy Immunol. 1992;97:17–24. doi: 10.1159/000236090. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marriott J. Bananas: physiology and biochemistry of storage and ripening for optimum quality. Crit Rev Nutr Food Sci. 1980;13:41–88. doi: 10.1080/10408398009527284. [DOI] [PubMed] [Google Scholar]
- Medina-Suarez R, Manning K, Fletcher J, Aked J, Bird CR, Seymour GB. Gene expression in the pulp of ripening bananas: two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis of in vitro translation products and cDNA cloning of 25 different ripening-related mRNAs. Plant Physiol. 1997;115:453–461. doi: 10.1104/pp.115.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola JH, Alenius H, Kalkkinen N, Turjanmaa K, Palosuo T, Reunala T. Hevein-like protein domains as a possible cause for allergen cross-reactivity between latex and banana. J Allergy Clin Immunol. 1998;102:1005–1012. doi: 10.1016/s0091-6749(98)70339-2. [DOI] [PubMed] [Google Scholar]
- Mo H, Winter HC, Van Damme EJM, Peumans WJ, Misaki A, Goldstein IJ. Carbohydrate binding properties of banana (Musa acuminata) lectin: novel recognition of internal 1, 3-linked glucosyl residues. Eur J Biochem. 2001;268:2609–2615. doi: 10.1046/j.1432-1327.2001.02148.x. [DOI] [PubMed] [Google Scholar]
- Peumans WJ, Barre A, Derycke V, Rougé P, Zhang W, May GD, Delcour JA, Van Leuven F, Van Damme EJM. Purification, characterization and structural analysis of an abundant β-1,3-glucanase from banana fruit. Eur J Biochem. 2000a;267:1188–1195. doi: 10.1046/j.1432-1327.2000.01117.x. [DOI] [PubMed] [Google Scholar]
- Peumans WJ, Zhang W, Barre A, Houles-Astoul C, Balint-Kurti P, Rovira P, Rougé P, May GD, Van Leuven F, Truffa-Bachi P et al. Fruit-specific lectins from banana and plantain. Planta. 2000b;211:546–554. doi: 10.1007/s004250000307. [DOI] [PubMed] [Google Scholar]
- Sanchez-Monge R, Blanco C, Diaz-Perales A, Collada C, Carrillo T, Aragoncillo C, Salcedo G. Isolation and characterization of major banana allergens: identification as fruit class I chitinases. Clin Exp Allergy. 1999;29:673–680. doi: 10.1046/j.1365-2222.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Monge R, Blanco C, Perales AD, Collada C, Carrillo T, Aragoncillo C, Salcedo G. Class I chitinases, the panallergens responsible for the latex-fruit syndrome, are induced by ethylene treatment and inactivated by heating. J Allergy Clin Immunol. 2000;106:190–195. doi: 10.1067/mai.2000.107599. [DOI] [PubMed] [Google Scholar]
- Staswick PE. Storage proteins of vegetative plant tissues. Annu Rev Plant Physiol Mol Biol. 1994;45:303–322. [Google Scholar]
- Van Damme EJM, Hao Q, Barre A, Rougé P, Van Leuven F, Peumans WJ. Major protein of resting rhizomes of Calystegia sepium (hedge bindweed) closely resembles plant RNases but has no enzymatic activity. Plant Physiol. 2000;122:433–445. doi: 10.1104/pp.122.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth SJ, Wolf GA. Dye-labeled substrates for the assay and detection of chitinase and lysozyme activity. J Microbiol Methods. 1990;12:197–205. [Google Scholar]
- Yagami T. Plant defense-related proteins as latex allergens. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 1998;116:4662. [article in Japanese] [PubMed] [Google Scholar]